The Effect of Hole Geometry on the Nonlinear Nanomechanics of γ-Graphyne Structures: A Finite Element Analysis
Abstract
:1. Introduction
2. Computational Model
2.1. Geometric Characteristics
2.2. Force Field Description
2.3. Finite Element Model
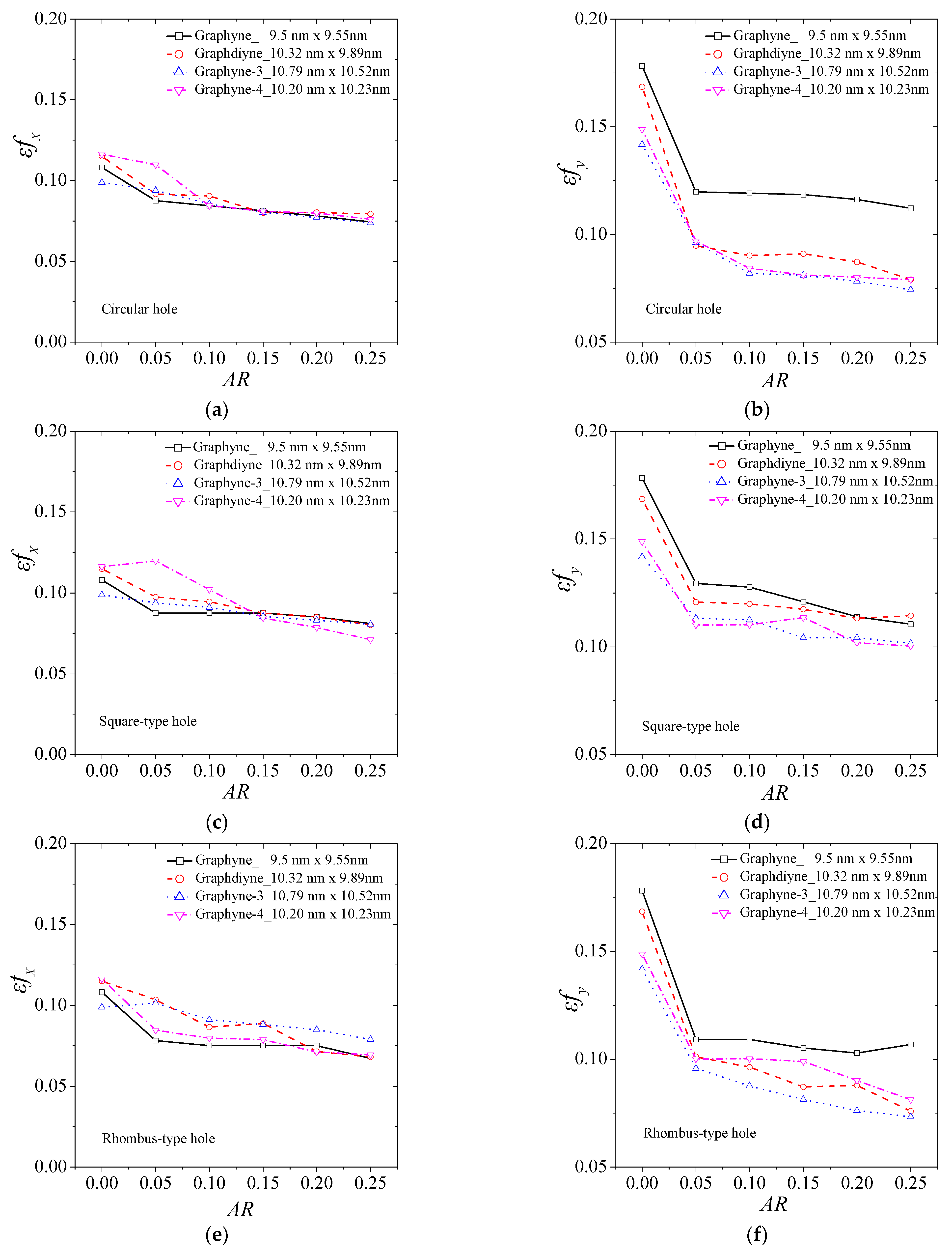
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

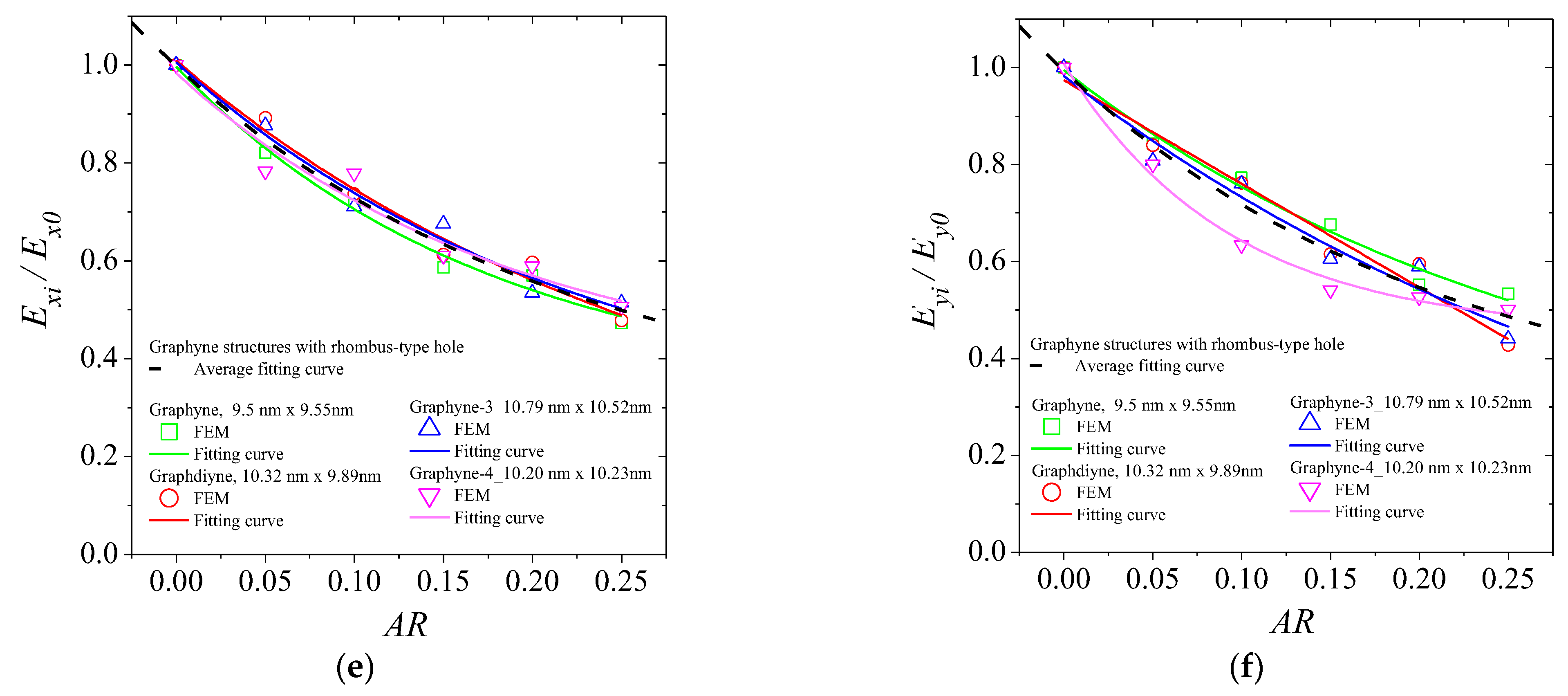

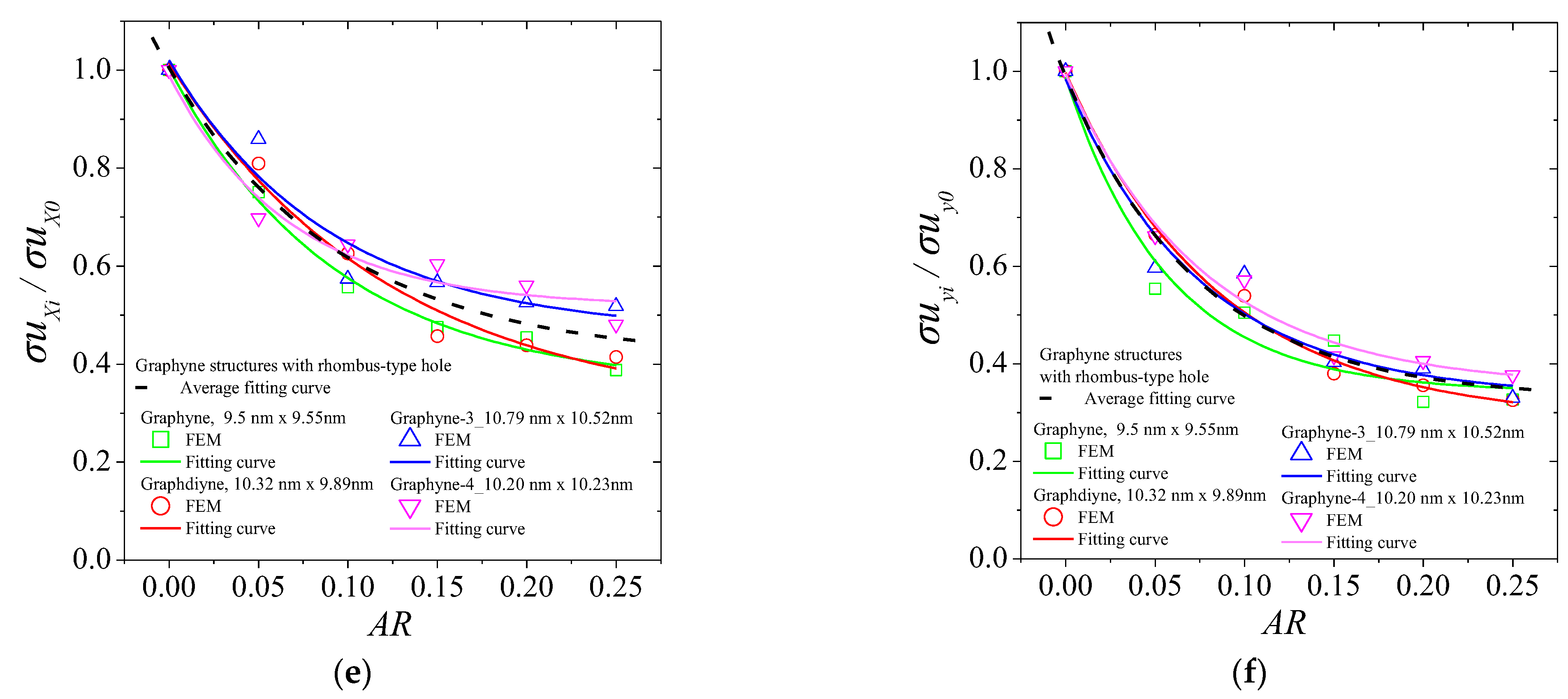
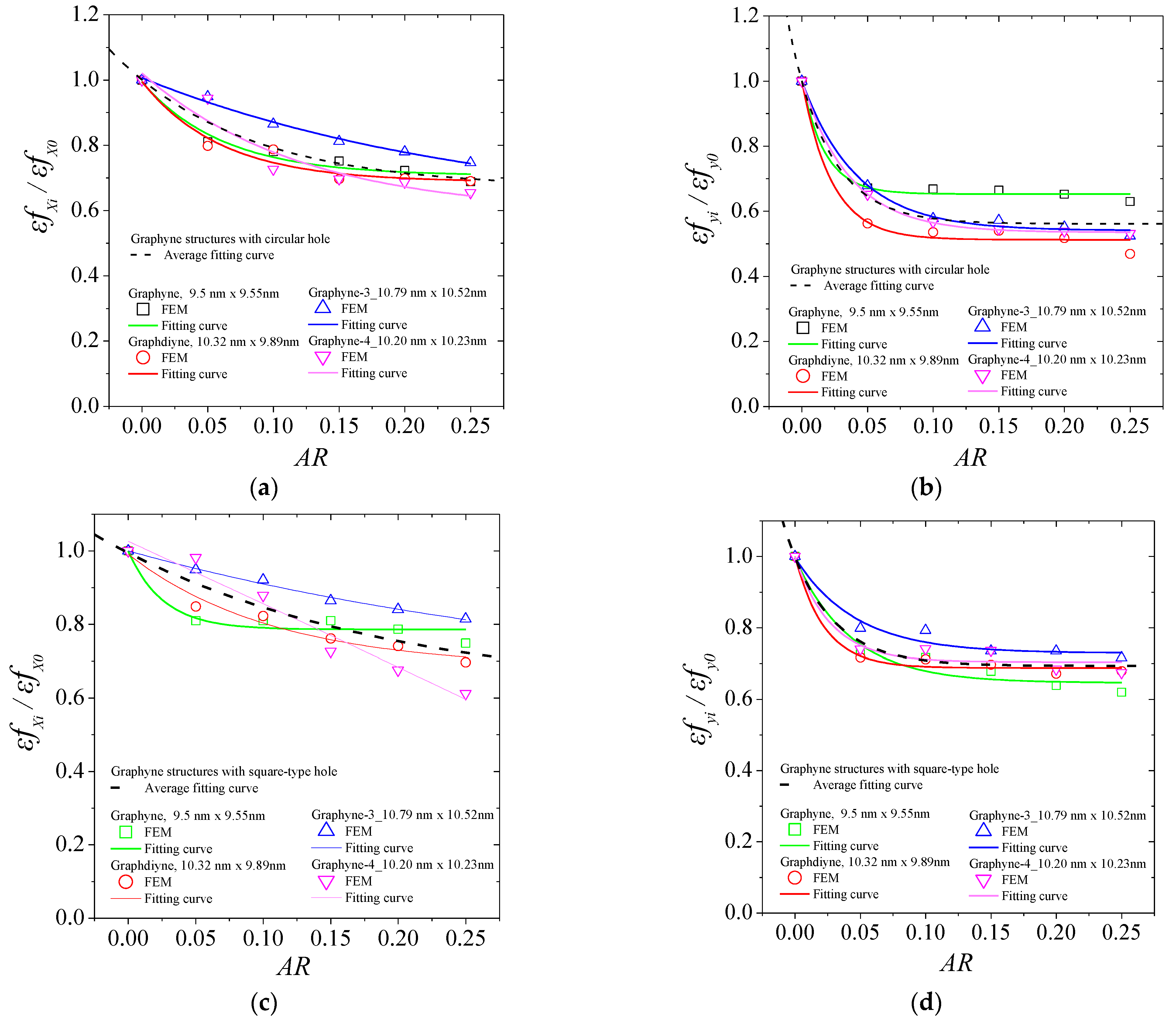

| Ex | Ey | σux | σuy | εfx | εfy | ||
|---|---|---|---|---|---|---|---|
| Circular hole | a | −0.01328 | −0.02175 | 0.31215 | 0.39201 | 0.70679 | 0.65288 |
| b | 1.01861 | 1.02460 | 0.68999 | 0.59942 | 0.28739 | 0.34707 | |
| c | 0.39087 | 0.42385 | 0.15879 | 0.06569 | 0.06158 | 0.01771 | |
| R2 | 0.99000 | 0.99000 | 0.99000 | 0.98000 | 0.95000 | 0.98000 | |
| Square hole | a | 0.19684 | 0.15620 | 0.47900 | 0.49592 | 0.78609 | 0.64616 |
| b | 0.80854 | 0.83635 | 0.52249 | 0.49939 | 0.21336 | 0.34956 | |
| c | 0.27754 | 0.34094 | 0.10267 | 0.04676 | 0.02598 | 0.04227 | |
| R2 | 0.98 | 0.99 | 0.99 | 0.97 | 0.90 | 0.94 | |
| Rhombic hole | a | 0.32312 | 0.18802 | 0.35359 | 0.34209 | 0.67028 | 0.59384 |
| b | 0.6737 | 0.80686 | 0.65038 | 0.64546 | 0.32904 | 0.40612 | |
| c | 0.17649 | 0.28148 | 0.09313 | 0.05726 | 0.02908 | 0.01662 | |
| R2 | 0.98 | 0.97 | 0.99 | 0.94 | 0.93 | 0.99 |
| Ex | Ey | σux | σuy | εfx | εfy | ||
|---|---|---|---|---|---|---|---|
| Circular hole | a | 0.52877 | 0.56265 | 0.53169 | 0.41842 | 0.68781 | 0.51250 |
| b | 0.44434 | 0.43016 | 0.45601 | 0.57879 | 0.30756 | 0.48722 | |
| c | 0.11159 | 0.08445 | 0.05557 | 0.03396 | 0.06053 | 0.02279 | |
| R2 | 0.82000 | 0.98000 | 0.87000 | 0.96000 | 0.94000 | 0.97000 | |
| Square hole | a | −1.22782 | 0.30336 | 0.34185 | 0.48296 | 0.68108 | 0.68787 |
| b | 2.21081 | 0.68163 | 0.64310 | 0.50637 | 0.31021 | 0.31189 | |
| c | 1.08266 | 0.22266 | 0.21818 | 0.05221 | 0.10768 | 0.02238 | |
| R2 | 0.96 | 0.96 | 0.94 | 0.89 | 0.95 | 0.98 | |
| Rhombic hole | a | 0.11455 | 61,266 | 0.29567 | 0.28294 | −96,147 | 0.50061 |
| b | 0.89455 | −61,265 | 0.7189 | 0.71424 | 96,148 | 0.49665 | |
| c | 0.28715 | −28,643 | 0.12369 | 0.08568 | 58,937 | 0.03542 | |
| R2 | 0.97 | 0.95 | 0.97 | 0.99 | 0.9 | 0.96 |
| Ex | Ey | σux | σuy | εfx | εfy | ||
|---|---|---|---|---|---|---|---|
| Circular hole | a | 0.47251 | 0.49490 | 0.39114 | 0.42952 | 0.58107 | 0.54080 |
| b | 0.51969 | 0.49912 | 0.60804 | 0.55934 | 0.42564 | 0.45870 | |
| c | 0.16870 | 0.11669 | 0.16182 | 0.05451 | 0.26025 | 0.04193 | |
| R2 | 0.91 | 0.98 | 0.90 | 0.93 | 0.98 | 0.99 | |
| Square hole | a | 0.01813 | 0.18307 | −0.03830 | 0.36855 | 0.61464 | 0.72907 |
| b | 0.96220 | 0.81386 | 1.01187 | 0.62103 | 0.38556 | 0.26762 | |
| c | 0.42336 | 0.25694 | 0.47241 | 0.11561 | 0.37605 | 0.04605 | |
| R2 | 0.91 | 0.99 | 0.91 | 0.97 | 0.98 | 0.95 | |
| Rhombic hole | a | 0.24141 | −0.0613 | 0.46377 | 0.33207 | 1.38875 | 0.53561 |
| b | 0.76417 | 1.04379 | 0.5563 | 0.65178 | −0.39214 | 0.46029 | |
| c | 0.23271 | 0.36545 | 0.09018 | 0.07463 | −0.62357 | 0.04771 | |
| R2 | 0.97 | 0.96 | 0.9 | 0.93 | 0.98 | 0.98 |
| Ex | Ey | σux | σuy | εfx | εfy | ||
|---|---|---|---|---|---|---|---|
| Circular hole | a | −2.31049 | 0.20093 | 0.32807 | 0.39183 | 0.58986 | 0.53511 |
| b | 3.30293 | 0.79905 | 0.65845 | 0.59124 | 0.43111 | 0.46471 | |
| c | 1.57087 | 0.25039 | 0.19239 | 0.08284 | 0.12130 | 0.03638 | |
| R2 | 0.99000 | 0.93000 | 0.96000 | 0.94000 | 0.87000 | 0.99000 | |
| Square hole | a | 2.34985 | 0.30658 | 0.43378 | 0.45300 | 14.08941 | 0.70433 |
| b | −1.36430 | 0.68101 | 0.56028 | 0.53464 | −13.06307 | 0.29473 | |
| c | −0.82667 | 0.17919 | 0.17312 | 0.07094 | −7.71899 | 0.02722 | |
| R2 | 0.82 | 0.91 | 0.97 | 0.93 | 0.94 | 0.93 | |
| Rhombic hole | a | 0.34482 | 0.45448 | 0.51764 | 0.35273 | 0.62012 | 0.61046 |
| b | 0.63847 | 0.55286 | 0.47121 | 0.64212 | 0.37527 | 0.38635 | |
| c | 0.19142 | 0.09276 | 0.06646 | 0.07679 | 0.04731 | 0.03378 | |
| R2 | 0.92 | 0.99 | 0.93 | 0.98 | 0.94 | 0.88 |
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.; Jiang, D.; Zhang, Y.; Dubonos, S.; Grigorieva, I.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Balaban, A.T.; Rentia, C.C.; Ciupitu, E. Chemical graphs. 6. Estimation of relative stability of several planar and tridimensional lattices for elementary carbon. Rev. Roum. Chim. 1968, 13, 231–247. [Google Scholar]
- Narita, N.; Nagai, S.; Suzuki, S.; Nakao, K. Optimized geometries and electronic structures of graphyne and its family. Phys. 1998, 58, 11009–11014. [Google Scholar] [CrossRef]
- Gong, Y.; Shen, L.; Kang, Z.; Liu, K.; Du, Q.; Ye, D.; Zhao, H.; Sun, X.A.; Zhang, J. Progress in energy-related graphyne-based materials: Advanced synthesis, functional mechanisms and applications. J. Mater. Chem. A Mater. 2020, 8, 21408–21433. [Google Scholar] [CrossRef]
- Peng, Q.; Crean, J.; Han, L.; Liu, S.; Wen, X.; De, S.; Dearden, A. New materials graphyne, graphdiyne, graphone, and graphane: Review of properties, synthesis, and application in nanotechnology. Nanotechnol. Sci. Appl. 2014, 7, 1–29. [Google Scholar] [CrossRef]
- Srinivasu, K.; Ghosh, S.K. Graphyne and Graphdiyne: Promising Materials for Nanoelectronics and Energy Storage Applications. J. Phys. Chem. C 2012, 116, 5951–5956. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Liu, H.; Guo, Y.; Li, Y.; Zhu, D. Architecture of graphdiyne nanoscale films. Chem. Commun. 2010, 46, 3256. [Google Scholar] [CrossRef]
- Ivanovskii, A.L. Graphynes and graphdyines. Progress. Solid. State Chem. 2013, 41, 1–19. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Li, Z.; Zhao, R.; Tong, L.; Liu, Z.; Zhang, J.; Liu, Z. Raman Spectra and Corresponding Strain Effects in Graphyne and Graphdiyne. J. Phys. Chem. C 2016, 120, 10605–10613. [Google Scholar] [CrossRef]
- Xiao, K.; Li, J.; Wu, X.; Liu, H.; Huang, C.; Li, Y. Nanoindentation of Thin Graphdiyne Films: Experiments and Molecular Dynamics Simulation. Carbon 2019, 144, 72–80. [Google Scholar] [CrossRef]
- Ghorbani, K.; Rajabpour, A.; Ghadiri, M. Determination of carbon nanotubes size-dependent parameters: Molecular dynamics simulation and nonlocal strain gradient continuum shell model. Mech. Based Des. Struct. Mach. 2021, 49, 103–120. [Google Scholar] [CrossRef]
- Rafiee, R.; Eskandariyun, A. Estimating Young’s modulus of graphene/polymer composites using stochastic multi-scale modeling. Compos. B Eng. 2019, 173, 106842. [Google Scholar] [CrossRef]
- Ansari, R.; Ajori, S.; Malakpour, S. Characterization of Elastic Properties of Porous Graphene Using an Ab Initio Study. J. Ultrafine Grained Nanostruct. Mater. 2016, 49, 97–102. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, X.-L.; Wang, C.-X.; Wei, N.; Rabczuk, T. Thermal conductivity of graphene nanoribbons under shear deformation: A molecular dynamics simulation. Sci. Rep. 2017, 7, 41398. [Google Scholar] [CrossRef]
- Volgin, I.; Larin, S.; Abad, E.; Lyulin, S. Molecular Dynamics Simulations of Fullerene Diffusion in Polymer Melts. Macromolecules 2017, 50, 2207–2218. [Google Scholar] [CrossRef]
- Cruz-Silva, R.; Araki, T.; Hayashi, T.; Terrones, H.; Terrones, M.; Endo, M. Fullerene and nanotube growth: New insights using first principles and molecular dynamics. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150327. [Google Scholar] [CrossRef]
- Hsu, J.-C.; Chang, R.-P.; Chang, W.-J. Resonance frequency of chiral single-walled carbon nanotubes using Timoshenko beam theory. Phys. Lett. A 2008, 372, 2757–2759. [Google Scholar] [CrossRef]
- Li, C.; Chou, T.-W. A structural mechanics approach for the analysis of carbon nanotubes. Int. J. Solids Struct. 2003, 40, 2487–2499. [Google Scholar] [CrossRef]
- Ansari, R.; Rouhi, S. Atomistic finite element model for axial buckling of single-walled carbon nanotubes. Phys. E Low. Dimens. Syst. Nanostruct. 2010, 43, 58–69. [Google Scholar] [CrossRef]
- Georgantzinos, S.K. A New Finite Element for an Efficient Mechanical Analysis of Graphene Structures Using Computer Aided Design/Computer Aided Engineering Techniques. J. Comput. Theor. Nanosci. 2017, 14, 5347–5354. [Google Scholar] [CrossRef]
- Georgantzinos, S.K.; Giannopoulos, G.I.; Anifantis, N.K. Coupled thermomechanical behavior of graphene using the spring-based finite element approach. J. Appl. Phys. 2016, 120, 014305. [Google Scholar] [CrossRef]
- Georgantzinos, S.K.; Giannopoulos, G.I.; Anifantis, N.K. On the coupling of axial and shear deformations of single-walled carbon nanotubes and graphene: A numerical study. Proc. Inst. Mech. Eng. Part N J. Nanoeng. Nanosyst. 2010, 224, 163–172. [Google Scholar] [CrossRef]
- Theodosiou, T.C.; Saravanos, D.A. Numerical investigation of mechanisms affecting the piezoresistive properties of CNT-doped polymers using multi-scale models. Compos. Technol. 2010, 70, 1312–1320. [Google Scholar] [CrossRef]
- Ru, C.Q. Elastic buckling of single-walled carbon nanotube ropes under high pressure. Phys. Rev. B 2000, 62, 10405–10408. [Google Scholar] [CrossRef]
- Giannopoulos, G.I.; Tsiros, A.P.; Georgantzinos, S.K. Prediction of Elastic Mechanical Behavior and Stability of Single-Walled Carbon Nanotubes Using Bar Elements. Mech. Adv. Mater. Struct. 2013, 20, 730–741. [Google Scholar] [CrossRef]
- Georgantzinos, S.K.; Giannopoulos, G.I. Thermomechanical buckling of single walled carbon nanotubes by a structural mechanics method. Diam. Relat. Mater. 2017, 80, 27–37. [Google Scholar] [CrossRef]
- Couto, R.; Silvestre, N. Finite Element Modelling and Mechanical Characterization of Graphyne. J. Nanomater. 2016, 2016, 7487049. [Google Scholar] [CrossRef]
- Lee, H.L.; Yang, Y.C.; Chang, W.J. Atomic-scale finite element method for analyzing the sensitivity of graphyne-based resonators. J. Nanomater. 2018, 2018, 2580171. [Google Scholar] [CrossRef]
- Georgantzinos, S.K.; Siampanis, S.G. Size-dependent elastic mechanical properties of γ-graphyne structures: A comprehensive finite element investigation. Mater. Des. 2021, 202, 109524. [Google Scholar] [CrossRef]
- Georgantzinos, S.K.; Siampanis, S.G.; Baldoukas, A.; Giannopoulos, G.I. Designing the nonlinear mechanical response of graphyne structures: A finite element structural mechanics approach. Mater. Today Commun. 2022, 31, 103386. [Google Scholar] [CrossRef]
- Galhofo, D.; Silvestre, N. Computational simulation of γ-graphynes under monotonic and hysteretic loading. Mech. Adv. Mater. Struct. 2021, 28, 495–505. [Google Scholar] [CrossRef]
- Siampanis, S.G.; Giannopoulos, G.I.; Lagaros, N.D.; Hatziefremidis, A.; Georgantzinos, S.K. Nonlinear Finite Element Analysis of γ-Graphyne Structures under Shearing. Molecules 2022, 27, 1729. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Cooper, V.R.; Dai, S. Porous Graphene as the Ultimate Membrane for Gas Separation. Nano Lett. 2009, 9, 4019–4024. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Tanugi, D.; Grossman, J.C. Water Desalination across Nanoporous Graphene. Nano Lett. 2012, 12, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, H.-F.; Huang, J.-Q.; Qian, W.; Wei, F.; Qiao, S.-Z.; Zhang, Q. 3D Hierarchical Porous Graphene-Based Energy Materials: Synthesis, Functionalization, and Application in Energy Storage and Conversion. Electrochem. Energy Rev. 2019, 2, 332–371. [Google Scholar] [CrossRef]
- Tao, Y.; Sui, Z.-Y.; Han, B.-H. Advanced porous graphene materials: From in-plane pore generation to energy storage applications. J. Mater. Chem. A Mater. 2020, 8, 6125–6143. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A.; Demin, V.A.; Bellucci, S. Bilayered graphene/h-BN with folded holes as new nanoelectronic materials: Modeling of structures and electronic properties. Sci. Rep. 2016, 6, 38029. [Google Scholar] [CrossRef]
- Genoese, A.; Genoese, A.; Salerno, G. In-plane and out-of-plane tensile behaviour of single-layer graphene sheets: A new interatomic potential. Acta Mech. 2020, 231, 2915–2930. [Google Scholar] [CrossRef]
- Muraru, S.; Burns, J.S.; Ionita, M. GOPY: A tool for building 2D graphene-based computational models. SoftwareX 2020, 12, 100586. [Google Scholar] [CrossRef]
- Yoon, J.; Kwon, M.H.; Shin, D.H.; Lee, S.W. Mechanical resonance properties of porous graphene membrane; simulation study and proof of concept experiment. Curr. Appl. Phys. 2021, 23, 30–35. [Google Scholar] [CrossRef]
- Lee, H.L.; Wang, Y.M.; Yang, Y.C.; Chang, W.J. Young’s modulus of nanoporous γ-graphyne membrane using an atomistic finite element model. Solid. State Commun. 2018, 280, 1–5. [Google Scholar] [CrossRef]
- Rodrigues, F.C.; Silvestre, N.; Deus, A.M. Nonlinear mechanical behaviour of γ-graphyne through an atomistic finite element model. Comput. Mater. Sci. 2015, 134, 171–183. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, X. Mechanical properties of graphyne and its family—A molecular dynamics investigation. Comput. Mater. Sci. 2012, 61, 83–88. [Google Scholar] [CrossRef]
- Yue, Q.; Chang, S.; Kang, J.; Qin, S.; Li, J. Mechanical and electronic properties of graphyne and its family under elastic strain: Theoretical predictions. J. Phys. Chem. C 2013, 117, 14804–14811. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Y.; Liu, J. A Domain-Decomposition Generalized Finite Difference Method for Stress Analysis in Three-Dimensional Composite Materials. Appl. Math. Lett. 2020, 104, 106226. [Google Scholar] [CrossRef]
- Kabir, H.; Aghdam, M.M. A Robust Bézier Based Solution for Nonlinear Vibration and Post-Buckling of Random Checkerboard Graphene Nano-Platelets Reinforced Composite Beams. Compos. Struct. 2019, 212, 184–198. [Google Scholar] [CrossRef]
- Bert, C.W.; Malik, M. Differential Quadrature: A Powerful New Technique for Analysis of Composite Structures. Compos. Struct. 1997, 39, 179–189. [Google Scholar] [CrossRef]
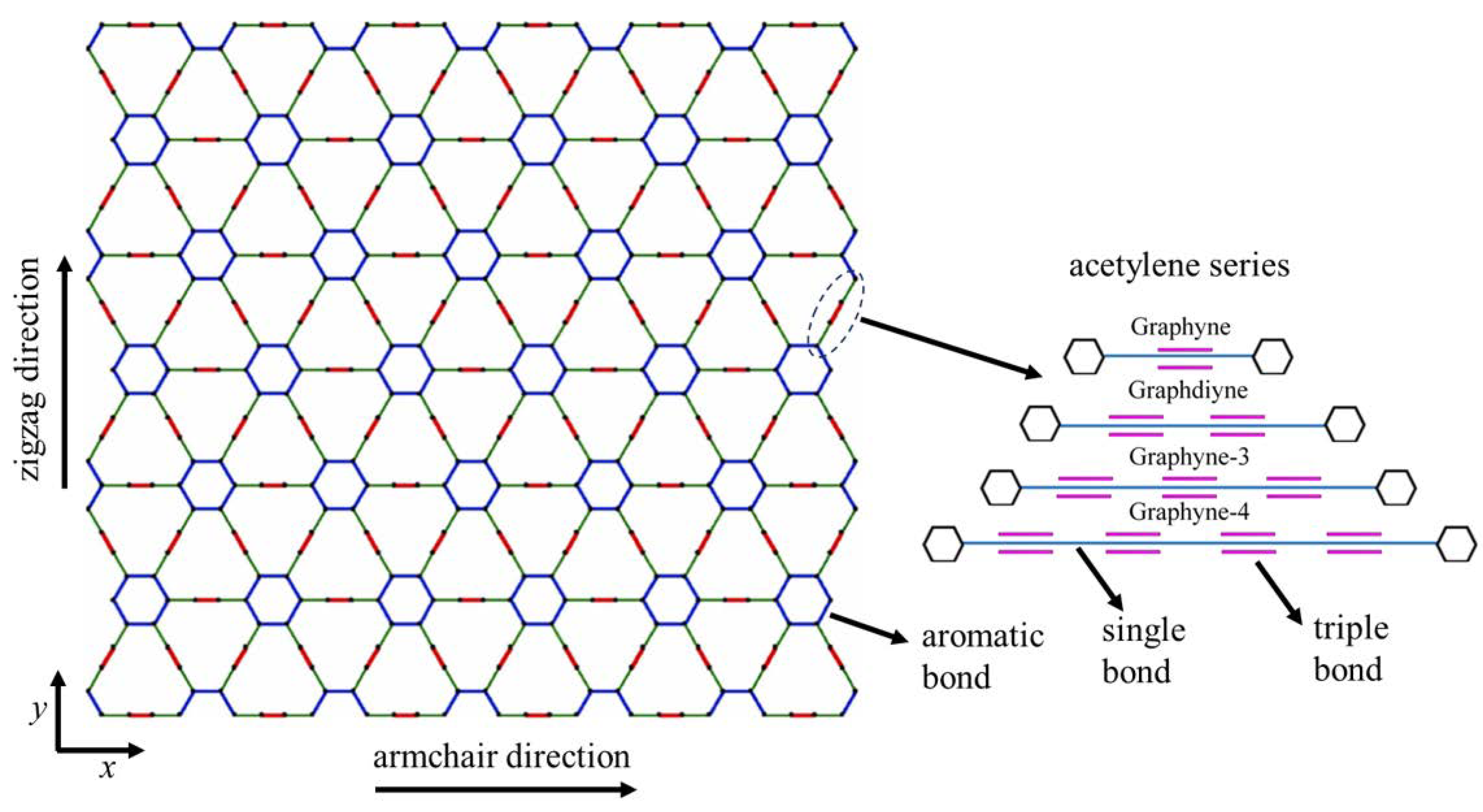
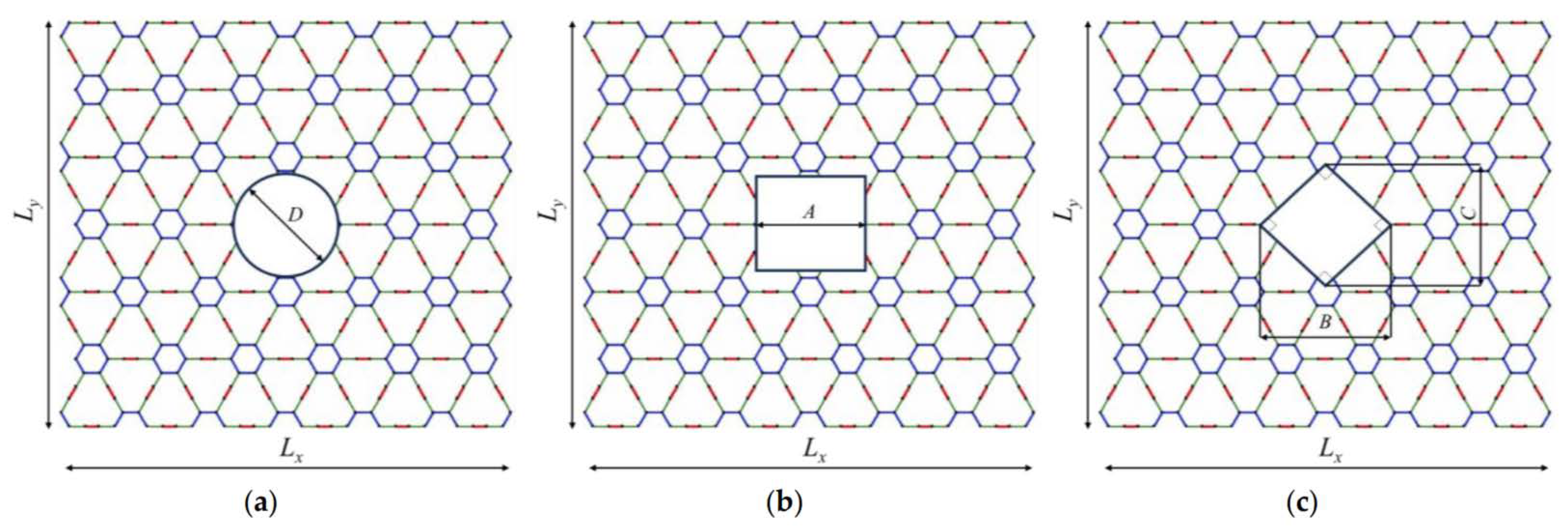

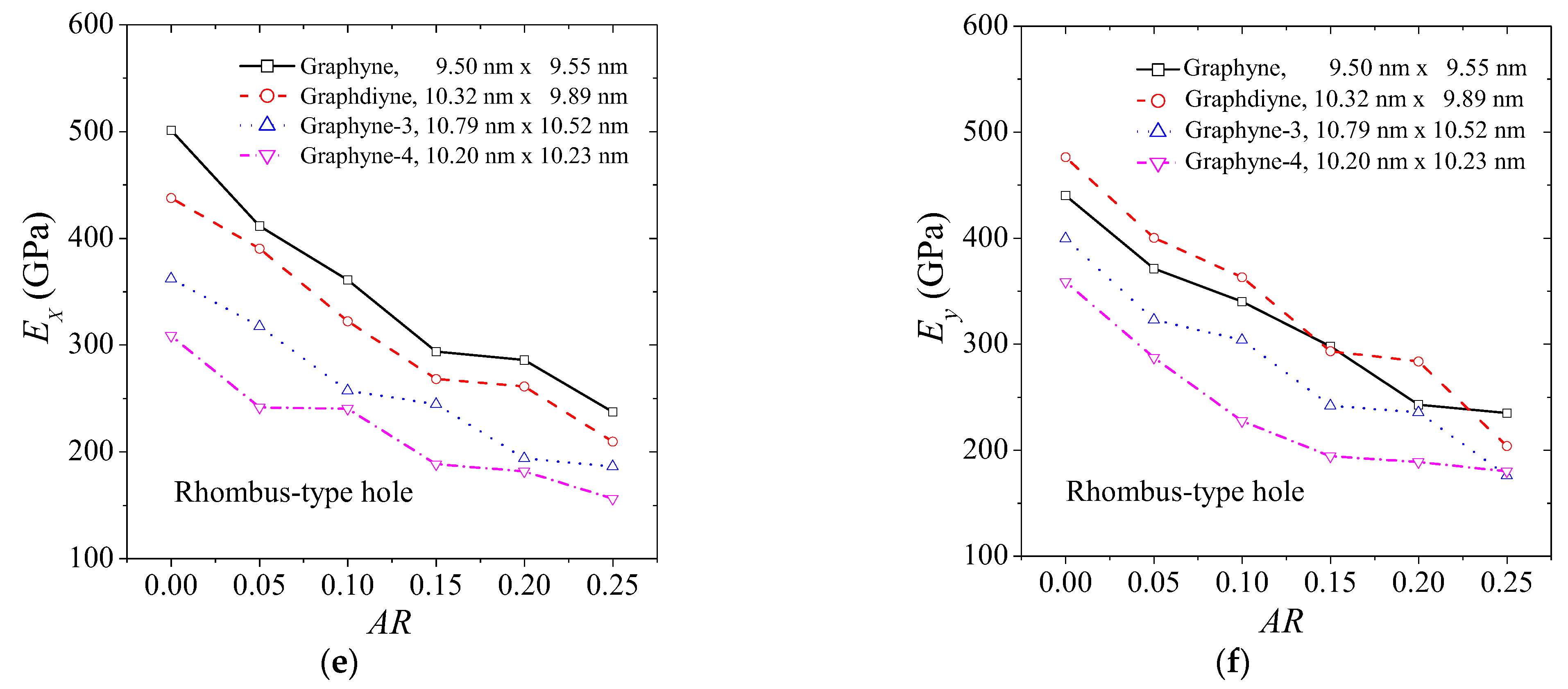
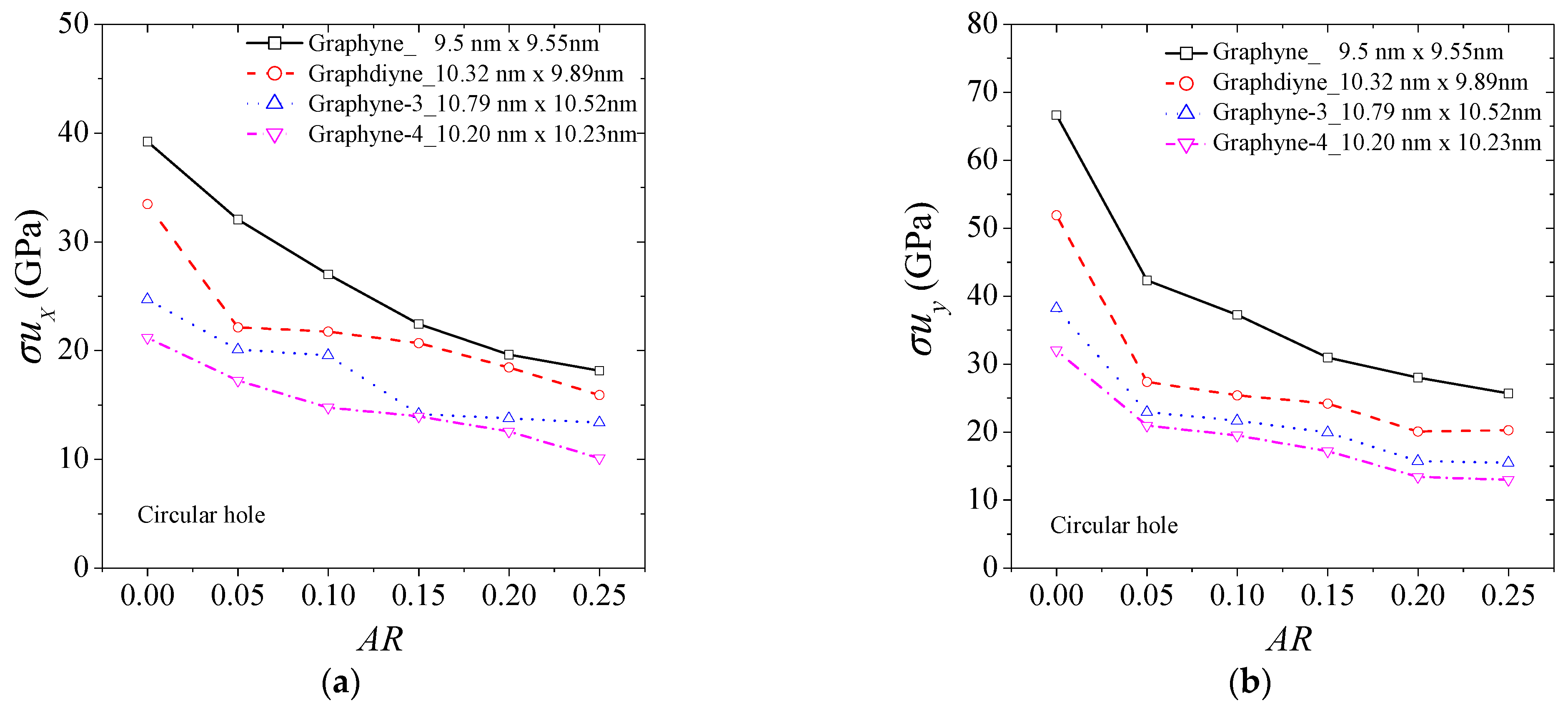
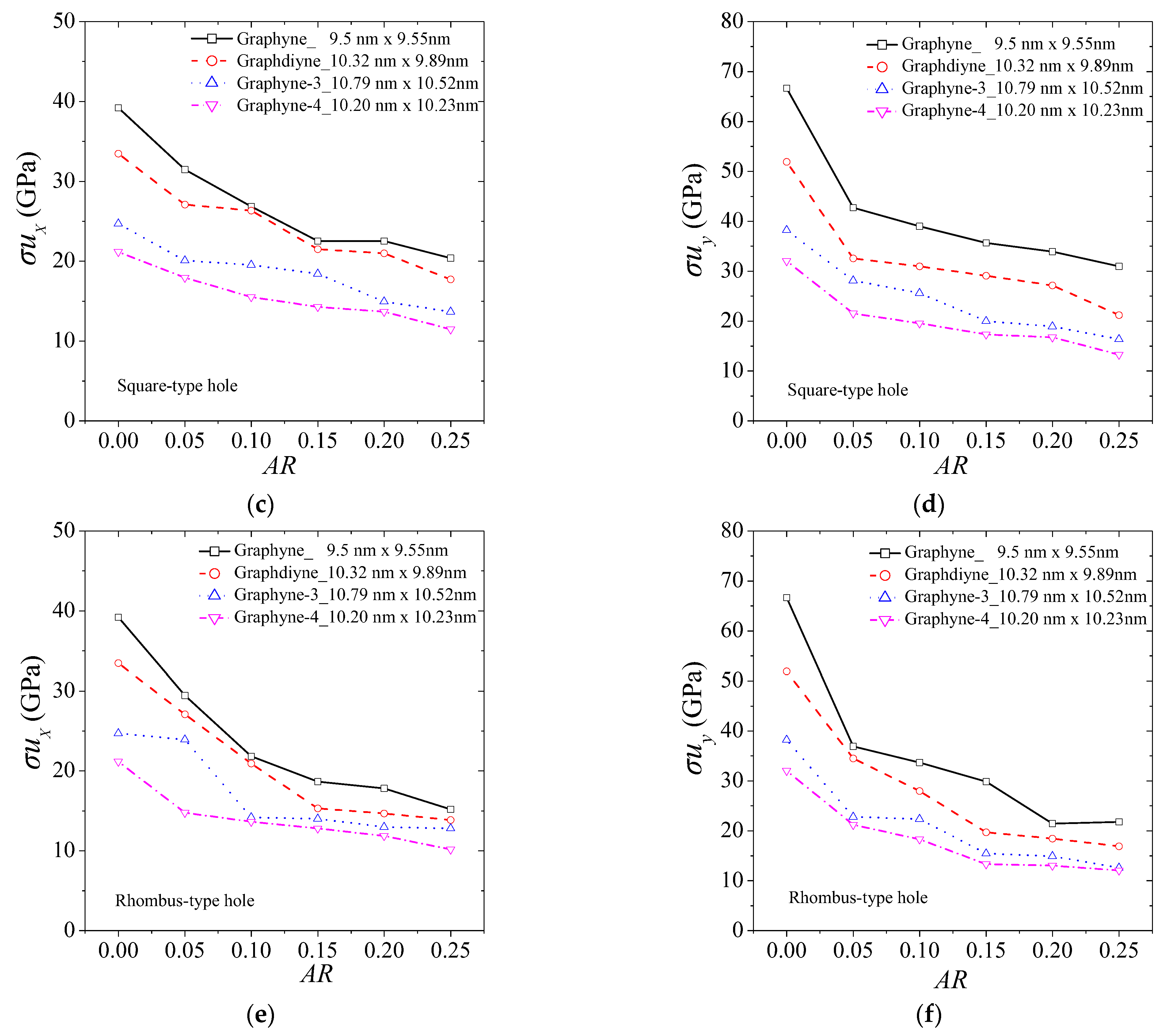
| Method | Lx × Ly (nm × nm) | Ex (GPa) | Ey (GPa) | σux (GPa) | σuy (GPa) | εfx | εfy | |
|---|---|---|---|---|---|---|---|---|
| Graphyne | FEM (present) | 9.5 × 9.55 | 501.2 | 440.1 | 39.2 | 66.6 | 0.108 | 0.178 |
| FEM [31] | 10.9 × 9.8 | 512.6 | 507.8 | 48.6 | 72 | 0.13 | 0.17 | |
| FEM [43] | 9.5 × 9.55 | 481.6 | 419.0 | - | - | - | - | |
| MD [5] | 10 × 10 | - | - | 45 | 63.96 | 0.11 | 0.177 | |
| Graph- diyne | FEM (present) | 10.32 × 9.89 | 439.2 | 476.4 | 33.4 | 51.8 | 0.115 | 0.168 |
| FEM [3] | 10.7 × 10.1 | 360.6 | 383.6 | - | - | - | - | |
| MD [44] | 10 × 10 | 312.5 | 270.3 | 29.8 | 65.1 | 0.109 | 0.208 | |
| DFT [45] | 7 × 7 | - | 384.8 | - | - | - | - | |
| Graphyne-3 | FEM (present) | 10.8 × 10.5 | 364.9 | 399.5 | 24.8 | 38.2 | 0.096 | 0.141 |
| FEM [43] | 11.2 × 10.7 | 288.9 | 307.8 | - | - | - | - | |
| MD [5] | 10 × 10 | 243.1 | 212.3 | 22.8 | 65.3 | 0.109 | 0.223 | |
| Graphene-4 | FEM (present) | 10.2 × 10.23 | 310.2 | 345.8 | 21.15 | 32.5 | 0.116 | 0.166 |
| FEM [31] | 10.8 × 10.5 | 364.9 | 399.5 | 24.8 | 38.2 | 0.096 | 0.141 | |
| FEM [43] | 10.6 × 10.4 | 239.7 | 257.4 | - | - | - | - | |
| MD [44] | 10 × 10 | 199.5 | 168.3 | 18.4 | 65.3 | 0.108 | 0.224 |
| Ex | Ey | σux | σuy | εfx | εfy | ||
|---|---|---|---|---|---|---|---|
| Circular hole | a | 0.2655 | 0.41855 | 0.41263 | 0.41566 | 0.66951 | 0.56134 |
| b | 0.72223 | 0.57663 | 0.57642 | 0.57436 | 0.33157 | 0.4381 | |
| c | 0.26581 | 0.16479 | 0.13383 | 0.05611 | 0.10066 | 0.03045 | |
| R2 | 0.94 | 0.95 | 0.93 | 0.95 | 0.84 | 0.91 | |
| Square hole | a | −0.40512 | 0.25461 | 0.41804 | 0.45973 | 0.60684 | 0.69359 |
| b | 1.39502 | 0.73541 | 0.57141 | 0.52862 | 0.38811 | 0.30464 | |
| c | 0.62309 | 0.23804 | 0.17159 | 0.06837 | 0.20698 | 0.03318 | |
| R2 | 0.94 | 0.95 | 0.94 | 0.95 | 0.77 | 0.91 | |
| Rhombic hole | a | 0.26796 | 0.26272 | 0.40943 | 0.32775 | 0.64213 | 0.56321 |
| b | 0.73041 | 0.73113 | 0.59529 | 0.66189 | 0.34733 | 0.43487 | |
| c | 0.21701 | 0.21103 | 0.09505 | 0.07381 | 0.10297 | 0.0326 | |
| R2 | 0.97 | 0.95 | 0.94 | 0.97 | 0.78 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgantzinos, S.K.; Siampanis, S.G.; Rogkas, N.; Spitas, V. The Effect of Hole Geometry on the Nonlinear Nanomechanics of γ-Graphyne Structures: A Finite Element Analysis. Int. J. Mol. Sci. 2023, 24, 14524. https://doi.org/10.3390/ijms241914524
Georgantzinos SK, Siampanis SG, Rogkas N, Spitas V. The Effect of Hole Geometry on the Nonlinear Nanomechanics of γ-Graphyne Structures: A Finite Element Analysis. International Journal of Molecular Sciences. 2023; 24(19):14524. https://doi.org/10.3390/ijms241914524
Chicago/Turabian StyleGeorgantzinos, Stelios K., Sotirios G. Siampanis, Nikolaos Rogkas, and Vasilios Spitas. 2023. "The Effect of Hole Geometry on the Nonlinear Nanomechanics of γ-Graphyne Structures: A Finite Element Analysis" International Journal of Molecular Sciences 24, no. 19: 14524. https://doi.org/10.3390/ijms241914524
APA StyleGeorgantzinos, S. K., Siampanis, S. G., Rogkas, N., & Spitas, V. (2023). The Effect of Hole Geometry on the Nonlinear Nanomechanics of γ-Graphyne Structures: A Finite Element Analysis. International Journal of Molecular Sciences, 24(19), 14524. https://doi.org/10.3390/ijms241914524







