The Function and Regulation Mechanism of Non-Coding RNAs in Muscle Development
Abstract
:1. Introduction
2. Overview of Skeletal Muscle Growth and Development in Animals
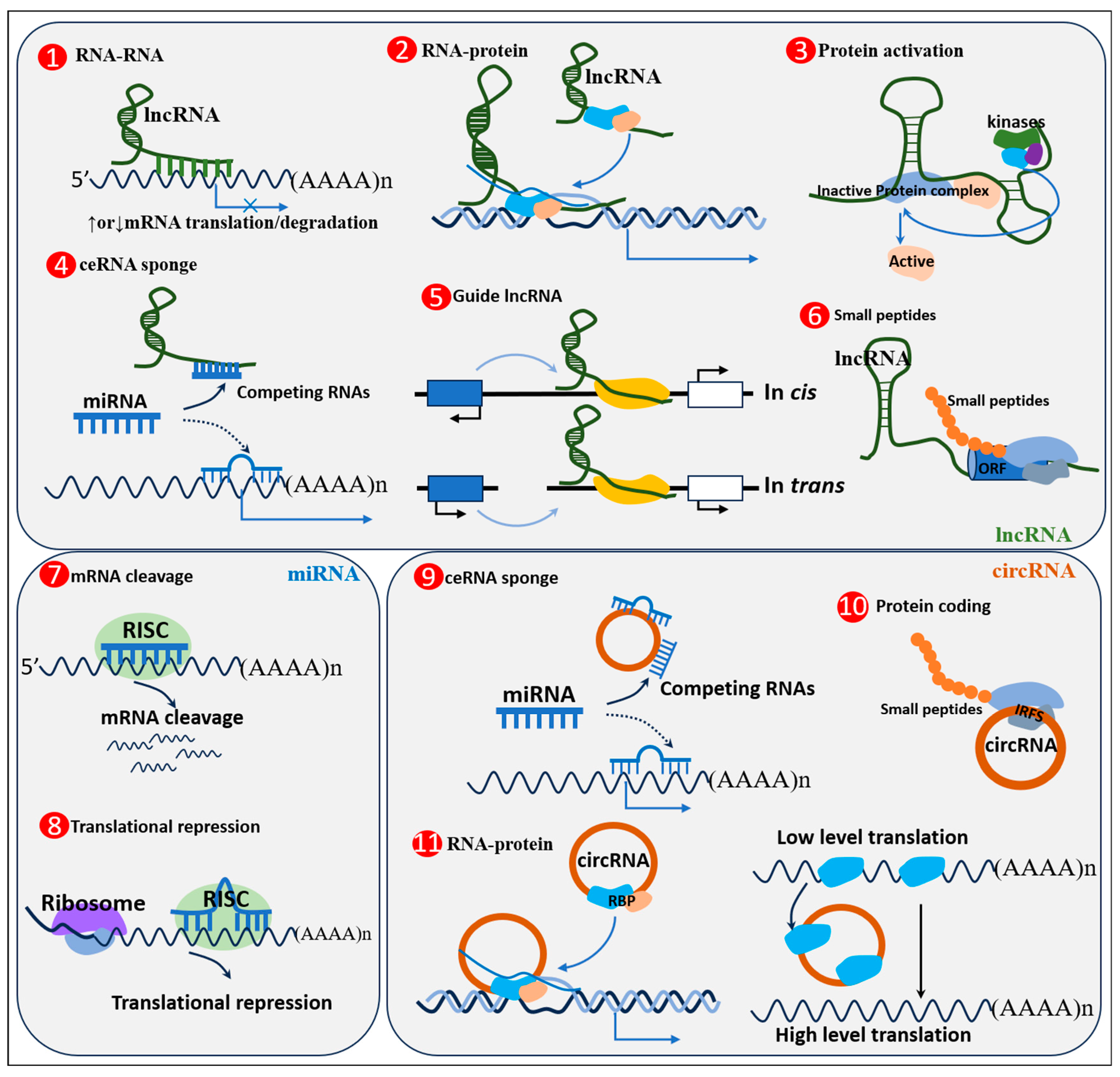
3. ncRNAs and Their Mechanisms of Action
4. Regulation of Skeletal Muscle Growth and Development by ncRNAs
4.1. Regulation of Skeletal Muscle Growth and Development by miRNAs
4.1.1. miRNAs Are Involved in Skeletal Muscle Development in Satellite Cells
4.1.2. MiRNA Modulates Skeletal Muscle through Regulating Myoblasts
4.1.3. Specification and Maintenance of Fiber Type-Associated miRNAs
4.2. Regulation of Skeletal Muscle Growth and Development by lncRNA
4.2.1. lncRNAs Regulate Skeletal Muscle through Sponge miRNAs
4.2.2. LncRNAs Regulate Skeletal Muscle through Cis or Trans Gene Expression
4.3. Regulation of Skeletal Muscle Growth and Development by circRNA
4.3.1. CircRNA Regulates Skeletal Muscle Growth through Sponge miRNAs
4.3.2. CircRNA Is Involved in Different Growth and Developmental Processes in Skeletal Muscle
4.3.3. CircRNAs Are Directly Converted into Proteins Involved in Skeletal Muscle Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ge, F.; Ma, X.; Dai, R.; Dingkao, R.; Zhaxi, Z.; Burenchao, G.; Bao, P.; Wu, X.; Guo, X.; et al. Comprehensive Analysis of mRNA, lncRNA, circRNA, and miRNA Expression Profiles and Their ceRNA Networks in the Longissimus Dorsi Muscle of Cattle-Yak and Yak. Front. Genet. 2021, 12, 772557. [Google Scholar] [CrossRef]
- Tüncel, Ö.; Kara, M.; Yaylak, B.; Erdoğan, İ.; Akgül, B. Noncoding RNAs in apoptosis: Identification and function. Turk. J. Biol. 2022, 46, 1–40. [Google Scholar] [PubMed]
- Wohlwend, M.; Laurila, P.-P.; Williams, K.; Romani, M.; Lima, T.; Pattawaran, P.; Benegiamo, G.; Salonen, M.; Schneider, B.L.; Lahti, J.; et al. The exercise-induced long noncoding RNA CYTOR promotes fast-twitch myogenesis in aging. Sci. Transl. Med. 2021, 13, eabc7367. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, M.; Kong, L.; Cao, M.; Zhang, M.; Wang, Y.; Song, C.; Fang, X.; Chen, H.; Zhang, C. CircARID1A regulates mouse skeletal muscle regeneration by functioning as a sponge of miR-6368. FASEB J. 2021, 35, e21324. [Google Scholar] [CrossRef]
- Du, M.; Zhu, M. Fetal programming of skeletal muscle development. Appl. Musc. Biol. Meat Sci. 2009, 81–96. [Google Scholar]
- Buckingham, M.; Bajard, L.; Chang, T.; Daubas, P.; Hadchouel, J.; Meilhac, S.; Montarras, D.; Rocancourt, D.; Relaix, F. The formation of skeletal muscle: From somite to limb. J. Anat. 2003, 202, 59–68. [Google Scholar] [CrossRef]
- Mohammadabadi, M.; Bordbar, F.; Jensen, J.; Du, M.; Guo, W. Key genes regulating skeletal muscle development and growth in farm animals. Animals 2021, 11, 835. [Google Scholar] [CrossRef]
- Braun, T.; Gautel, M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell Biol. 2011, 12, 349–361. [Google Scholar] [CrossRef]
- Ran, J.; Li, J.; Yin, L.; Zhang, D.; Yu, C.; Du, H.; Jiang, X.; Yang, C.; Liu, Y. Comparative Analysis of Skeletal Muscle DNA Methylation and Transcriptome of the Chicken Embryo at Different Developmental Stages. Front. Physiol. 2021, 12, 697121. [Google Scholar] [CrossRef] [PubMed]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.-L.; Ye, J.-L.; Yang, J.; Gao, C.-Q.; Yan, H.-C.; Li, H.-C.; Wang, X.-Q. mTORC1 mediates lysine-induced satellite cell activation to promote skeletal muscle growth. Cells 2019, 8, 1549. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.J.; Pannérec, A.; Cadot, B.; Parlakian, A.; Besson, V.; Gomes, E.R.; Marazzi, G.; Sassoon, D.A. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat. Cell Biol. 2010, 12, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Du, Y.; Trakooljul, N.; Brand, B.; Muráni, E.; Krischek, C.; Wicke, M.; Schwerin, M.; Wimmers, K.; Ponsuksili, S. Muscle Transcriptional Profile Based on Muscle Fiber, Mitochondrial Respiratory Activity, and Metabolic Enzymes. Int. J. Biol. Sci. 2015, 11, 1348–1362. [Google Scholar] [CrossRef] [PubMed]
- Blaauw, B.; Schiaffino, S.; Reggiani, C. Mechanisms Modulating Skeletal Muscle Phenotype. Compr. Physiol. 2013, 3, 1645–1687. [Google Scholar] [CrossRef]
- Khor, S.C.; Razak, A.M.; Wan Ngah, W.Z.; Mohd Yusof, Y.A.; Abdul Karim, N.; Makpol, S. The Tocotrienol-Rich Fraction Is Superior to Tocopherol in Promoting Myogenic Differentiation in the Prevention of Replicative Senescence of Myoblasts. PLoS ONE 2016, 11, e0149265. [Google Scholar] [CrossRef]
- Chargé, S.B.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef]
- Dhawan, J.; Rando, T.A. Stem cells in postnatal myogenesis: Molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005, 15, 666–673. [Google Scholar] [CrossRef]
- Hernández-Hernández, J.M.; García-González, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef]
- Taylor, M.V.; Hughes, S.M. Mef2 and the skeletal muscle differentiation program. Semin. Cell Dev. Biol. 2017, 72, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kanisicak, O.; Mendez, J.J.; Yamamoto, S.; Yamamoto, M.; Goldhamer, D.J. Progenitors of skeletal muscle satellite cells express the muscle determination gene, MyoD. Dev. Biol. 2009, 332, 131–141. [Google Scholar] [CrossRef]
- Adhikari, A.; Kim, W.; Davie, J. Myogenin is required for assembly of the transcription machinery on muscle genes during skeletal muscle differentiation. PLoS ONE 2021, 16, e0245618. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.-K.; Bang, J.S.; Lee, J.E.; Tran, H.-D.; Park, G.; Lee, D.R.; Jo, J. Generation of Skeletal Muscle Organoids from Human Pluripotent Stem Cells to Model Myogenesis and Muscle Regeneration. Int. J. Mol. Sci. 2022, 23, 5108. [Google Scholar] [CrossRef] [PubMed]
- Lagha, M.; Sato, T.; Bajard, L.; Daubas, P.; Esner, M.; Montarras, D.; Relaix, F.; Buckingham, M. Regulation of Skeletal Muscle Stem Cell Behaviorby Pax3 and Pax7. Cold Harb. Symp. Quant. Biol. 2008, 73, 307–315. [Google Scholar] [CrossRef]
- Xia, Y.; Zhong, X.; Zhang, X.; Zhang, X.; Yuan, J.; Liu, C.; Sha, Z.; Li, F. Gene Structure, Expression and Function Analysis of MEF2 in the Pacific White Shrimp Litopenaeus vannamei. Int. J. Mol. Sci. 2023, 24, 5832. [Google Scholar] [CrossRef]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Li, F.; Yang, C.; Xie, Y.; Gao, X.; Zhang, Y.; Ning, H.; Liu, G.; Chen, Z.; Shan, A. Maternal nutrition altered embryonic MYOD1, MYF5, and MYF6 gene expression in genetically fat and lean lines of chickens. Anim. Biosci. 2022, 35, 1223–1234. [Google Scholar] [CrossRef]
- Mattick, J.S.; Makunin, I.V. Non-coding RNA. Hum. Mol. Genet. 2006, 15 (Suppl. 1), R17–R29. [Google Scholar] [CrossRef]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: lncRNA localization and function. J. Cell Biol. 2021, 220, e202009045. [Google Scholar] [CrossRef]
- Herman, A.B.; Tsitsipatis, D.; Gorospe, M. Integrated lncRNA function upon genomic and epigenomic regulation. Mol. Cell 2022, 82, 2252–2266. [Google Scholar] [CrossRef] [PubMed]
- Asl, E.R.; Amini, M.; Najafi, S.; Mansoori, B.; Mokhtarzadeh, A.; Mohammadi, A.; Lotfinejad, P.; Bagheri, M.; Shirjang, S.; Lotfi, Z.; et al. Interplay between MAPK/ERK signaling pathway and MicroRNAs: A crucial mechanism regulating cancer cell metabolism and tumor progression. Life Sci. 2021, 278, 119499. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Lee, K.-P.; Kwon, K.-S.; Suh, Y. MicroRNAs in Skeletal Muscle Aging: Current Issues and Perspectives. J. Gerontol. Ser. A 2018, 74, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Rahman, F.; Akand, S.K.; Faiza, M.; Tabrez, S.; Rub, A. miRNA Target Prediction: Overview and Applications. In Integrated Omics Approaches to Infectious Diseases; Springer: Berlin/Heidelberg, Germany, 2021; pp. 241–253. [Google Scholar]
- Bukhari, S.I.A.; Truesdell, S.S.; Lee, S.; Kollu, S.; Classon, A.; Boukhali, M.; Jain, E.; Mortensen, R.D.; Yanagiya, A.; Sadreyev, R.I.; et al. A Specialized Mechanism of Translation Mediated by FXR1a-Associated MicroRNP in Cellular Quiescence. Mol. Cell 2016, 61, 760–773. [Google Scholar] [CrossRef]
- De Freitas, J.H.; Bragato, J.P.; Rebech, G.T.; Costa, S.F.; Dos Santos, M.O.; Soares, M.F.; Eugênio, F.R.; Dos Santos, P.S.P.; De Lima, V.M.F. MicroRNA-21 and microRNA-148a affects PTEN, NO and ROS in canine leishmaniasis. Front. Genet. 2023, 14, 1106496. [Google Scholar] [CrossRef]
- Hangauer, M.J.; Vaughn, I.W.; McManus, M.T. Pervasive transcription of the human genome produces thousands of previously unidentified long intergenic noncoding RNAs. PLoS Genet. 2013, 9, e1003569. [Google Scholar] [CrossRef]
- Zhao, B.; Li, J.; Liu, M.; Hu, S.; Yang, N.; Liang, S.; Zhang, X.; Dai, Y.; Bao, Z.; Chen, Y.; et al. lncRNA2919 Suppresses Rabbit Dermal Papilla Cell Proliferation via trans-Regulatory Actions. Cells 2022, 11, 2443. [Google Scholar] [CrossRef]
- Xia, L.; Li, P.; Bi, W.; Yang, R.; Zhang, Y. LncRNA HAGLR promotes the proliferation, migration, and neurotrophic factor production of Schwann cells via miR-204/CDK5R1 after sciatic nerve injury. J. Neuropathol. Exp. Neurol. 2023, 82, 324–332. [Google Scholar] [CrossRef]
- Tran, K.; Brown, E.; Desouza, T.; Jespersen, N.; Nandrup-Bus, C.; Yang, Q.; Yang, Z.; Desai, A.; Min, S.; Rojas-Rodriguez, R. Human thermogenic adipocyte regulation by the long noncoding RNA LINC00473. Nat. Metab. 2020, 2, 397–412. [Google Scholar] [CrossRef]
- Fu, Y.; Li, B.; Huang, R.; Ji, X.; Bai, W.-K. Long noncoding RNA DLEU2 promotes growth and invasion of hepatocellular carcinoma by regulating miR-30a-5p/PTP4A1 axis. Pathol. Res. Pract. 2022, 238, 154078. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.J.; Chang, H.Y. Long noncoding RNAs: Cellular address codes in development and disease. Cell 2013, 152, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Chang, H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xia, L.Q.; Lu, W.W.; Zhang, J.; Zhu, J.S. LncRNAs and cancer. Oncol. Lett. 2016, 12, 1233–1239. [Google Scholar] [CrossRef]
- Yang, L.; Lin, C.; Liu, W.; Zhang, J.; Ohgi, K.A.; Grinstein, J.D.; Dorrestein, P.C.; Rosenfeld, M.G. ncRNA- and Pc2 methylation-dependent gene relocation between nuclear structures mediates gene activation programs. Cell 2011, 147, 773–788. [Google Scholar] [CrossRef]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef]
- Meng, X.; Li, X.; Zhang, P.; Wang, J.; Zhou, Y.; Chen, M. Circular RNA: An emerging key player in RNA world. Brief. Bioinform. 2017, 18, 547–557. [Google Scholar] [CrossRef]
- Guarnerio, J.; Bezzi, M.; Jeong, J.C.; Paffenholz, S.V.; Berry, K.; Naldini, M.M.; Lo-Coco, F.; Tay, Y.; Beck, A.H.; Pandolfi, P.P. Oncogenic role of fusion-circRNAs derived from cancer-associated chromosomal translocations. Cell 2016, 165, 289–302. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Li, J.; Hu, S.; Deng, Y.; Yin, H.; Bao, X.; Zhang, Q.C.; Wang, G.; Wang, B. Identification of mecciRNAs and their roles in the mitochondrial entry of proteins. Sci. China Life Sci. 2020, 63, 1429–1449. [Google Scholar] [CrossRef]
- Das, A.; Das, A.; Das, D.; Abdelmohsen, K.; Panda, A.C. Circular RNAs in myogenesis. Biochim. Et Biophys. Acta Gene Regul. Mech. 2020, 1863, 194372. [Google Scholar] [CrossRef]
- Zheng, T.; Gan, M.L.; Shen, L.Y.; Niu, L.L.; Guo, Z.Y.; Wang, J.Y.; Zhang, S.H.; Zhu, L. circRNA on animal skeletal muscle development regulation. Yi Chuan Hered. 2020, 42, 1178–1191. [Google Scholar] [CrossRef]
- Wei, H.U.; Meng, X.; Tiancheng, L.U.; Lei, W.U.; Ting, L.I. MicroRNA1 inhibits the proliferation of Chinese sika deerderived cartilage cells by binding to the 3′-untranslated region of IGF1. Mol. Med. Rep. 2013, 8, 523–538. [Google Scholar]
- Shen, X.; Tang, J.; Huang, Y.; Lan, X.; Lei, C.; Chen, H. CircRNF111 Contributes to Adipocyte Differentiation by Elevating PPARγ Expression via miR-27a-3p. Epigenetics 2023, 18, 2145058. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hussain, T.; Yue, R.; Liao, Y.; Li, Q.; Yao, J.; Song, Y.; Sun, X.; Wang, N.; Xu, L.; et al. MicroRNA-199a Inhibits Cellular Autophagy and Downregulates IFN-β Expression by Targeting TBK1 in Mycobacterium bovis Infected Cells. Front. Cell. Infect. Microbiol. 2018, 8, 238. [Google Scholar] [CrossRef]
- Krek, A.; Grün, D.; Poy, M.N.; Wolf, R.; Rosenberg, L.; Epstein, E.J.; MacMenamin, P.; da Piedade, I.; Gunsalus, K.C.; Stoffel, M.; et al. Combinatorial microRNA target predictions. Nat. Genet. 2005, 37, 495–500. [Google Scholar] [CrossRef]
- Cui, M.; Yao, X.; Lin, Y.; Zhang, D.; Cui, R.; Zhang, X. Interactive functions of microRNAs in the miR-23a-27a-24-2 cluster and the potential for targeted therapy in cancer. J. Cell. Physiol. 2020, 235, 6–16. [Google Scholar] [CrossRef]
- Cao, X.; Tang, S.; Du, F.; Li, H.; Shen, X.; Li, D.; Wang, Y.; Zhang, Z.; Xia, L.; Zhu, Q.; et al. miR-99a-5p Regulates the Proliferation and Differentiation of Skeletal Muscle Satellite Cells by Targeting MTMR3 in Chicken. Genes 2020, 11, 369. [Google Scholar] [CrossRef]
- Zhang, D.; Ran, J.; Li, J.; Yu, C.; Cui, Z.; Amevor, F.K.; Wang, Y.; Jiang, X.; Qiu, M.; Du, H.; et al. miR-21-5p Regulates the Proliferation and Differentiation of Skeletal Muscle Satellite Cells by Targeting KLF3 in Chicken. Genes 2021, 12, 814. [Google Scholar] [CrossRef]
- Yin, H.; He, H.; Cao, X.; Shen, X.; Han, S.; Cui, C.; Zhao, J.; Wei, Y.; Chen, Y.; Xia, L.; et al. MiR-148a-3p Regulates Skeletal Muscle Satellite Cell Differentiation and Apoptosis via the PI3K/AKT Signaling Pathway by Targeting Meox2. Front. Genet. 2020, 11, 512. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, F.; Wu, P.; Li, T.; He, M.; Yin, X.; Shi, H.; Duan, Y.; Zhang, T.; Wang, J.; et al. MicroRNA-7 Targets the KLF4 Gene to Regulate the Proliferation and Differentiation of Chicken Primary Myoblasts. Front. Genet. 2020, 11, 842. [Google Scholar] [CrossRef]
- Dong, X.; Cheng, Y.; Qiao, L.; Wang, X.; Zeng, C.; Feng, Y. Male-Biased gga-miR-2954 Regulates Myoblast Proliferation and Differentiation of Chicken Embryos by Targeting YY1. Genes 2021, 12, 1325. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Wu, Y.; Yin, X.; Li, T.; Chen, F.; Wu, P.; Zhang, S.; Wang, J.; Zhang, G. MicroRNA-214 Inhibits Chicken Myoblasts Proliferation, Promotes Their Differentiation, and Targets the TRMT61A Gene. Genes 2020, 11, 1400. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhai, B.; Yuan, P.; Fan, S.; Jin, W.; Li, W.; Sun, G.; Tian, Y.; Liu, X.; Kang, X.; et al. MiR-29b-1-5p regulates the proliferation and differentiation of chicken primary myoblasts and analysis of its effective targets. Poult. Sci. 2022, 101, 101557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, P.; Dan, X.; Kang, X.; Ma, Y.; Shi, Y. miR-377 Inhibits Proliferation and Differentiation of Bovine Skeletal Muscle Satellite Cells by Targeting FHL2. Genes 2022, 13, 947. [Google Scholar] [CrossRef]
- Hu, X.; Xing, Y.; Ren, L.; Wang, Y.; Li, Q.; Yang, Q.; Du, M.; Xu, L.; Willems, L.; Li, J.; et al. bta-miR-23a Regulates the Myogenic Differentiation of Fetal Bovine Skeletal Muscle-Derived Progenitor Cells by Targeting MDFIC Gene. Genes 2020, 11, 1232. [Google Scholar] [CrossRef]
- Tong, H.L.; Jiang, R.Y.; Zhang, W.W.; Yan, Y.Q. MiR-2425-5p targets RAD9A and MYOG to regulate the proliferation and differentiation of bovine skeletal muscle-derived satellite cells. Sci. Rep. 2017, 7, 418. [Google Scholar] [CrossRef]
- Elsaeid Elnour, I.; Dong, D.; Wang, X.; Zhansaya, T.; Khan, R.; Jian, W.; Jie, C.; Chen, H. Bta-miR-885 promotes proliferation and inhibits differentiation of myoblasts by targeting MyoD1. J. Cell. Physiol. 2020, 235, 6625–6636. [Google Scholar] [CrossRef]
- He, M.; Zhang, W.; Wang, S.; Ge, L.; Cao, X.; Wang, S.; Yuan, Z.; Lv, X.; Getachew, T.; Mwacharo, J.M.; et al. MicroRNA-181a Regulates the Proliferation and Differentiation of Hu Sheep Skeletal Muscle Satellite Cells and Targets the YAP1 Gene. Genes 2022, 13, 520. [Google Scholar] [CrossRef]
- Greene, M.A.; Powell, R.R.; Bruce, T.; Bridges, W.C.; Duckett, S.K. miRNA transcriptome and myofiber characteristics of lamb skeletal muscle during hypertrophic growth. Front. Genet. 2022, 13, 988756. [Google Scholar] [CrossRef]
- Liao, R.; Lv, Y.; Dai, J.; Zhang, D.; Zhu, L.; Lin, Y. chi-miR-99b-3p Regulates the Proliferation of Goat Skeletal Muscle Satellite Cells In Vitro by Targeting Caspase-3 and NCOR1. Animals 2022, 12, 2368. [Google Scholar] [CrossRef]
- Kyei, B.; Odame, E.; Li, L.; Yang, L.; Zhan, S.; Li, J.; Chen, Y.; Dai, D.; Cao, J.; Guo, J.; et al. Knockdown of CDR1as Decreases Differentiation of Goat Skeletal Muscle Satellite Cells via Upregulating miR-27a-3p to Inhibit ANGPT1. Genes 2022, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Deng, K.; Kang, Z.; Wang, F.; Fan, Y. MicroRNA profiling reveals miR-145-5p inhibits goat myoblast differentiation by targeting the coding domain sequence of USP13. FASEB J. 2022, 36, e22370. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cao, X.; Ge, L.; Gu, Y.; Lv, X.; Getachew, T.; Mwacharo, J.M.; Haile, A.; Sun, W. MiR-22-3p Inhibits Proliferation and Promotes Differentiation of Skeletal Muscle Cells by Targeting IGFBP3 in Hu Sheep. Animals 2022, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, Y.; Song, C.; Chen, Y.; Wang, Y.; Lai, M.; Zhang, C.; Fang, X. MiR-424-5p targets HSP90AA1 to facilitate proliferation and restrain differentiation in skeletal muscle development. Anim. Biotechnol. 2022, 33, 1–13. [Google Scholar] [CrossRef]
- Yang, L.; Qi, Q.; Wang, J.; Song, C.; Wang, Y.; Chen, X.; Chen, H.; Zhang, C.; Hu, L.; Fang, X. MiR-452 Regulates C2C12 Myoblast Proliferation and Differentiation via Targeting ANGPT1. Front. Genet. 2021, 12, 640807. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Feng, X.; Jia, Y.; Luo, S.; Jiang, M.; Luo, J.; Hua, Y.; Zhang, J.; Lin, Y.; Li, J.; et al. miR-424(322)-5p targets Ezh1 to inhibit the proliferation and differentiation of myoblasts. Acta Biochim. Et Biophys. Sin. 2023, 55, 472–483. [Google Scholar] [CrossRef]
- Song, C.; Wang, Q.; Qi, Q.; Chen, X.; Wang, Y.; Zhang, C.; Fang, X. MiR-495-3p regulates myoblasts proliferation and differentiation through targeting cadherin 2. Anim. Biotechnol. 2022, 33, 1–9. [Google Scholar] [CrossRef]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Chen, H.; Luo, Y.; He, J.; Zheng, P.; Yu, J.; Yu, B. Resveratrol regulates muscle fiber type conversion via miR-22-3p and AMPK/SIRT1/PGC-1α pathway. J. Nutr. Biochem. 2020, 77, 108297. [Google Scholar] [CrossRef]
- Shi, Y.; Mao, X.; Cai, M.; Hu, S.; Lai, X.; Chen, S.; Jia, X.; Wang, J.; Lai, S. miR-194-5p negatively regulates the proliferation and differentiation of rabbit skeletal muscle satellite cells. Mol. Cell. Biochem. 2021, 476, 425–433. [Google Scholar] [CrossRef]
- Dang, H.Q.; Xu, G.-L.; Hou, L.-J.; Xu, J.; Hong, G.-L.; Hu, C.; Wang, C. MicroRNA-22 inhibits proliferation and promotes differentiation of satellite cells in porcine skeletal muscle. J. Integr. Agric. 2020, 19, 225–233. [Google Scholar] [CrossRef]
- Wu, N.; Gu, T.; Lu, L.; Cao, Z.; Song, Q.; Wang, Z.; Zhang, Y.; Chang, G.; Xu, Q.; Chen, G. Roles of miRNA-1 and miRNA-133 in the proliferation and differentiation of myoblasts in duck skeletal muscle. J. Cell. Physiol. 2019, 234, 3490–3499. [Google Scholar] [CrossRef] [PubMed]
- Callis, T.E.; Deng, Z.; Chen, J.F.; Wang, D.Z. Muscling through the microRNA world. Exp. Biol. Med. 2008, 233, 131–138. [Google Scholar] [CrossRef]
- Oikawa, S.; Akimoto, T. Functional Analysis of MicroRNAs in Skeletal Muscle. Methods Mol. Biol. 2023, 2640, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Scharner, J.; Zammit, P.S. The muscle satellite cell at 50: The formative years. Skelet. Muscle 2011, 1, 28. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Sabourin, L.A.; Girgis-Gabardo, A.; Mansouri, A.; Gruss, P.; Rudnicki, M.A. Pax7 is required for the specification of myogenic satellite cells. Cell 2000, 102, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Millay, D.P. Regulation of the myoblast fusion reaction for muscle development, regeneration, and adaptations. Exp. Cell Res. 2022, 415, 113134. [Google Scholar] [CrossRef]
- Xie, S.-J.; Li, J.-H.; Chen, H.-F.; Tan, Y.-Y.; Liu, S.-R.; Zhang, Y.; Xu, H.; Yang, J.-H.; Liu, S.; Zheng, L.-L. Inhibition of the JNK/MAPK signaling pathway by myogenesis-associated miRNAs is required for skeletal muscle development. Cell Death Differ. 2018, 25, 1581–1597. [Google Scholar] [CrossRef]
- Rao, P.K.; Kumar, R.M.; Farkhondeh, M.; Baskerville, S.; Lodish, H.F. Myogenic factors that regulate expression of muscle-specific microRNAs. Proc. Natl. Acad. Sci. USA 2006, 103, 8721–8726. [Google Scholar] [CrossRef]
- Nakasa, T.; Ishikawa, M.; Shi, M.; Shibuya, H.; Adachi, N.; Ochi, M. Acceleration of muscle regeneration by local injection of muscle-specific microRNAs in rat skeletal muscle injury model. J. Cell. Mol. Med. 2010, 14, 2495–2505. [Google Scholar] [CrossRef]
- Luo, W.; Li, E.; Nie, Q.; Zhang, X. Myomaker, Regulated by MYOD, MYOG and miR-140-3p, Promotes Chicken Myoblast Fusion. Int. J. Mol. Sci. 2015, 16, 26186–26201. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Min, K.-H.; Lee, W. MiR-96-5p Induced by Palmitic Acid Suppresses the Myogenic Differentiation of C2C12 Myoblasts by Targeting FHL1. Int. J. Mol. Sci. 2020, 21, 9445. [Google Scholar] [CrossRef]
- Huang, W.; Guo, L.; Zhao, M.; Zhang, D.; Xu, H.; Nie, Q. The Inhibition on MDFIC and PI3K/AKT Pathway Caused by miR-146b-3p Triggers Suppression of Myoblast Proliferation and Differentiation and Promotion of Apoptosis. Cells 2019, 8, 656. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-L.; Xian, G.; Liang, C.-N.; Min, C.; WU, X.-Y.; Ping, Y. MicroRNA transcriptome of skeletal muscle during yak development reveals miR-652 regulates myoblasts differentiation and survival by targeting ISL1. J. Integr. Agric. 2022, 22, 1502–1513. [Google Scholar] [CrossRef]
- Ismail, I.; Joo, S.T. Poultry Meat Quality in Relation to Muscle Growth and Muscle Fiber Characteristics. Korean J. Food Sci. Anim. Resour. 2017, 37, 873–883. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C.; Murgia, M. Fiber type diversity in skeletal muscle explored by mass spectrometry-based single fiber proteomics. Histol. Histopathol. 2020, 35, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, M.; Shan, Y.; Ji, G.; Ju, X.; Tu, Y.; Sheng, Z.; Xie, J.; Zou, J.; Shu, J. miRNA-mRNA network regulation in the skeletal muscle fiber phenotype of chickens revealed by integrated analysis of miRNAome and transcriptome. Sci. Rep. 2020, 10, 10619. [Google Scholar] [CrossRef]
- van Rooij, E.; Quiat, D.; Johnson, B.A.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Kelm, R.J., Jr.; Olson, E.N. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev. Cell 2009, 17, 662–673. [Google Scholar] [CrossRef]
- Liu, M.; Li, B.; Peng, W.; Ma, Y.; Huang, Y.; Lan, X.; Lei, C.; Qi, X.; Liu, G.E.; Chen, H. LncRNA-MEG3 promotes bovine myoblast differentiation by sponging miR-135. J. Cell. Physiol. 2019, 234, 18361–18370. [Google Scholar] [CrossRef]
- Przanowska, R.K.; Weidmann, C.A.; Saha, S.; Cichewicz, M.A.; Jensen, K.N.; Przanowski, P.; Irving, P.S.; Janes, K.A.; Guertin, M.J.; Weeks, K.M.; et al. Distinct MUNC lncRNA structural domains regulate transcription of different promyogenic factors. Cell Rep. 2022, 38, 110361. [Google Scholar] [CrossRef]
- Chang, M.W.; Yang, J.H.; Tsitsipatis, D.; Yang, X.; Martindale, J.L.; Munk, R.; Pandey, P.R.; Banskota, N.; Romero, B.; Batish, M.; et al. Enhanced myogenesis through lncFAM-mediated recruitment of HNRNPL to the MYBPC2 promoter. Nucleic Acids Res. 2022, 50, 13026–13044. [Google Scholar] [CrossRef]
- Wang, D.; Pu, Y.; Li, Y.; Pan, D.; Wang, S.; Tian, W.; Ma, Y.; Jiang, L. Comprehensive analysis of lncRNAs involved in skeletal muscle development in ZBED6-knockout Bama pigs. BMC Genom. 2021, 22, 593. [Google Scholar] [CrossRef] [PubMed]
- Nolte, W.; Weikard, R.; Brunner, R.M.; Albrecht, E.; Hammon, H.M.; Reverter, A.; Kühn, C. Biological Network Approach for the Identification of Regulatory Long Non-Coding RNAs Associated with Metabolic Efficiency in Cattle. Front. Genet. 2019, 10, 1130. [Google Scholar] [CrossRef] [PubMed]
- Puttabyatappa, M.; Saadat, N.; Elangovan, V.R.; Dou, J.; Bakulski, K.; Padmanabhan, V. Developmental programming: Impact of prenatal bisphenol-A exposure on liver and muscle transcriptome of female sheep. Toxicol. Appl. Pharmacol. 2022, 451, 116161. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Zheng, Q.; Sui, M.; Zhu, L.; Xu, L.; Zhang, Y.; Liu, Y.; Fang, F.; Chu, M.; Ma, Y.; et al. Comprehensive Analysis of LncRNA Reveals the Temporal-Specific Module of Goat Skeletal Muscle Development. Int. J. Mol. Sci. 2019, 20, 3950. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, S.; Wang, L.; Zhang, W.; Chen, W.; Lv, X.; Li, Y.; Hussain, Z.; Sun, W. Long Noncoding RNA (lncRNA) CTTN-IT1 Elevates Skeletal Muscle Satellite Cell Proliferation and Differentiation by Acting as ceRNA for YAP1 through Absorbing miR-29a in Hu Sheep. Front. Genet 2020, 11, 843. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, Z.; Chen, Y.; Liu, L.; Wang, L.; Yi, G.; Yang, Y.; Wang, D.; Li, K.; Tang, Z. Single-cell analysis reveals the lncRNA-MEG3/miRNA-133a-3p/PRRT2 axis regulates skeletal muscle regeneration and myogenesis. Genes Dis. 2022, 10, 359–362. [Google Scholar] [CrossRef]
- Li, J.; Su, T.; Zou, C.; Luo, W.; Shi, G.; Chen, L.; Fang, C.; Li, C. Long non-coding RNA H19 regulates porcine satellite cell differentiation through miR-140-5p/SOX4 and DBN1. Front. Cell Dev. Biol. 2020, 8, 518724. [Google Scholar] [CrossRef]
- Lin, J.; Luo, Z.; Liu, S.; Chen, Q.; Liu, S.; Chen, J. Long non-coding RNA H19 promotes myoblast fibrogenesis via regulating the miR-20a-5p-Tgfbr2 axis. Clin. Exp. Pharmacol. Physiol. 2021, 48, 921–931. [Google Scholar] [CrossRef]
- Lin, J.; Yang, X.; Liu, S.; Luo, Z.; Chen, Q.; Sun, Y.; Ding, Z.; Chen, J. Long non-coding RNA MFAT1 promotes skeletal muscle fibrosis by modulating the miR-135a-5p-Tgfbr2/Smad4 axis as a ceRNA. J. Cell. Mol. Med. 2021, 25, 4420–4433. [Google Scholar] [CrossRef]
- Zhang, J.; Sheng, H.; Zhang, L.; Li, X.; Guo, Y.; Wang, Y.; Guo, H.; Ding, X. Bta-miR-206 and a Novel lncRNA-lncA2B1 Promote Myogenesis of Skeletal Muscle Satellite Cells via Common Binding Protein HNRNPA2B1. Cells 2023, 12, 1028. [Google Scholar] [CrossRef]
- Zhan, S.; Zhang, Y.; Yang, C.; Li, D.; Zhong, T.; Wang, L.; Li, L.; Zhang, H. LncR-133a Suppresses Myoblast Differentiation by Sponging miR-133a-3p to Activate the FGFR1/ERK1/2 Signaling Pathway in Goats. Genes 2022, 13, 818. [Google Scholar] [CrossRef] [PubMed]
- Militello, G.; Hosen, M.R.; Ponomareva, Y.; Gellert, P.; Weirick, T.; John, D.; Hindi, S.M.; Mamchaoui, K.; Mouly, V.; Döring, C.; et al. A novel long non-coding RNA Myolinc regulates myogenesis through TDP-43 and Filip1. J. Mol. Cell Biol. 2018, 10, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Li, Z.; Ma, M.; Wang, Z.; Han, P.; Abdalla, B.A.; Nie, Q.; Zhang, X. LncRNA-Six1 Encodes a Micropeptide to Activate Six1 in Cis and Is Involved in Cell Proliferation and Muscle Growth. Front. Physiol. 2017, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, J.; Jiang, R.; Wei, X.; Song, C.; Huang, Y.; Lan, X.; Lei, C.; Ma, Y.; Hu, L.; et al. Long Non-coding RNA Profiling Reveals an Abundant MDNCR that Promotes Differentiation of Myoblasts by Sponging miR-133a. Mol. Ther. Nucleic Acids 2018, 12, 610–625. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, M.; Liu, X.; Zhang, L.; Ding, X.; Guo, Y.; Li, X.; Guo, H. A novel lncRNA, lnc403, involved in bovine skeletal muscle myogenesis by mediating KRAS/Myf6. Gene 2020, 751, 144706. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Elnour, I.E.; Wang, X.; Zhansaya, T.; Akhatayeva, Z.; Khan, R.; Cheng, J.; Hung, Y.; Lan, X.; Lei, C.; Chen, H. Circular RNA circMYL1 Inhibit Proliferation and Promote Differentiation of Myoblasts by Sponging miR-2400. Cells 2021, 10, 176. [Google Scholar] [CrossRef]
- Yang, Z.; Song, C.; Jiang, R.; Huang, Y.; Lan, X.; Lei, C.; Qi, X.; Zhang, C.; Huang, B.; Chen, H. CircNDST1 Regulates Bovine Myoblasts Proliferation and Differentiation via the miR-411a/Smad4 Axis. J. Agric. Food Chem. 2022, 70, 10044–10057. [Google Scholar] [CrossRef]
- Shen, X.; Wei, Y.; Liu, W.; You, G.; Tang, S.; Su, Z.; Du, M.; He, J.; Zhao, J.; Tian, Y. A novel circular RNA circITSN2 targets the miR-218-5p/LMO7 axis to promote chicken embryonic myoblast proliferation and differentiation. Front. Cell Dev. Biol. 2021, 9, 748844. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, X.; Shen, X.; Zhang, Y.; Zhang, Y.; Ye, L.; Li, D.; Zhu, Q.; Yin, H. CircCCDC91 regulates chicken skeletal muscle development by sponging miR-15 family via activating IGF1-PI3K/AKT signaling pathway. Poult. Sci. 2022, 101, 101803. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, Y.; Deng, K.; Liang, Y.; Zhang, G.; Gao, X.; El-Samahy, M.; Zhang, Y.; Deng, M.; Wang, F. Circular RNA circUSP13 sponges miR-29c to promote differentiation and inhibit apoptosis of goat myoblasts by targeting IGF1. FASEB J. 2022, 36, e22097. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, Z.; Deng, K.; Kang, Z.; Guo, J.; Zhang, G.; Zhang, Y.; Wang, F. CircUBE3A promotes myoblasts proliferation and differentiation by sponging miR-28-5p to enhance expression. Int. J. Biol. Macromol. 2023, 226, 730–745. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, N.; Li, J.; Ji, M.; Zhao, T.; An, J.; Cai, C.; Yang, Y.; Gao, P.; Cao, G.; et al. CircRNA Profiling of Skeletal Muscle in Two Pig Breeds Reveals CircIGF1R Regulates Myoblast Differentiation via miR-16. Int. J. Mol. Sci. 2023, 24, 3779. [Google Scholar] [CrossRef]
- Das, A.; Shyamal, S.; Sinha, T.; Mishra, S.S.; Panda, A.C. Identification of Potential circRNA-microRNA-mRNA Regulatory Network in Skeletal Muscle. Front. Mol. Biosci. 2021, 8, 762185. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhu, C.; Jing, J.; Ling, Y.; Qin, S.; Wang, J.; Zha, L.; Liu, Y.; Fang, F. Morphological changes and functional circRNAs screening of rabbit skeletal muscle development. BMC Genom. 2021, 22, 469. [Google Scholar] [CrossRef]
- Wei, Y.-H.; Zhao, X.-Y.; Shen, X.-X.; Ye, L.; Zhang, Y.; Wang, Y.; Li, D.-Y.; Zhu, Q.; Yin, H.-D. The expression, function, and coding potential of circular RNA circEDC3 in chicken skeletal muscle development. J. Integr. Agric. 2022, 21, 1444–1456. [Google Scholar] [CrossRef]
- Hong, L.; Gu, T.; He, Y.; Zhou, C.; Hu, Q.; Wang, X.; Zheng, E.; Huang, S.; Xu, Z.; Yang, J.; et al. Genome-Wide Analysis of Circular RNAs Mediated ceRNA Regulation in Porcine Embryonic Muscle Development. Front. Cell Dev. Biol. 2019, 7, 289. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, T.; Lei, S.; She, Y.; Shi, H.; Zhou, S.; Ou, J.; Liu, Y. Expression of circular RNAs during C2C12 myoblast differentiation and prediction of coding potential based on the number of open reading frames and N6-methyladenosine motifs. Cell Cycle 2018, 17, 1832–1845. [Google Scholar] [CrossRef]
- Yin, H.; Shen, X.; Zhao, J.; Cao, X.; He, H.; Han, S.; Chen, Y.; Cui, C.; Wei, Y.; Wang, Y.; et al. Circular RNA CircFAM188B Encodes a Protein That Regulates Proliferation and Differentiation of Chicken Skeletal Muscle Satellite Cells. Front. Cell Dev. Biol. 2020, 8, 522588. [Google Scholar] [CrossRef]
- Wong, C.H.; Lou, U.K.; Li, Y.; Chan, S.L.; Tong, J.H.; To, K.-F.; Chen, Y. CircFOXK2 Promotes Growth and Metastasis of Pancreatic Ductal Adenocarcinoma by Complexing with RNA-Binding Proteins and Sponging MiR-942. Cancer Res. 2020, 80, 2138–2149. [Google Scholar] [CrossRef]
- Pandey, P.R.; Yang, J.-H.; Tsitsipatis, D.; Panda, A.C.; Noh, J.H.; Kim, K.M.; Munk, R.; Nicholson, T.; Hanniford, D.; Argibay, D.; et al. circSamd4 represses myogenic transcriptional activity of PUR proteins. Nucleic Acids Res. 2020, 48, 3789–3805. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wei, X.; Song, M.; Jiang, R.; Li, H. Circular RNA circMYBPC1 promotes skeletal muscle differentiation by targeting MyHC. Mol. Ther. Nucleic Acids 2021, 24, 352–368. [Google Scholar] [CrossRef] [PubMed]
- Yue, B.; Yang, H.; Wu, J.; Wang, J.; Ru, W.; Cheng, J.; Huang, Y.; Lan, X.; Lei, C.; Chen, H. circSVIL regulates bovine myoblast development by inhibiting STAT1 phosphorylation. Sci. China Life Sci. 2022, 65, 376–386. [Google Scholar] [CrossRef] [PubMed]

| Species | miRNA | Proliferation | Differentiation | Target | Source |
|---|---|---|---|---|---|
| Chicken | miR-99a-5p | Promote | Inhibition | MTMR3 | [58] |
| Chicken | miR-21-5p | Promote | Promote | KLF3 | [59] |
| Chicken | miR-148a-3p | No influence | Promote | Meox2 | [60] |
| Chicken | miR-7 | Inhibition | Inhibition | KLF4 | [61] |
| Chicken | miR-2954 | Promote | Inhibition | YY1 | [62] |
| Chicken | miR-214 | Inhibition | Promote | TRMT61A | [63] |
| Chicken | miR-29b-1-5p | Inhibition | Promote | ANKRD9 | [64] |
| Bovine | miR-377 | Inhibition | Inhibition | FHL2 | [65] |
| Bovine | miR-23a | Unknown | Promote | MDFIC | [66] |
| Bovine | miR-2425-5p | Promote | Inhibition | RAD9A and MYOG | [67] |
| Bovine | miR-885 | Promote | Inhibition | MyoD1 | [68] |
| Sheep | miR-181a | Inhibition | Promote | YAP1 | [69] |
| Sheep | miR-29a | Inhibition | Inhibition | Unknown | [70] |
| Goat | miR-99b-3p | Promote | Promote | Caspase-3 and NCOR1 | [71] |
| Goat | miR-27a-3p | Unknown | Inhibition | ANGPT1 | [72] |
| Goat | miR-145-5p | Unknown | Inhibition | USP13 | [73] |
| Sheep | miR-22-3p | Inhibition | Promote | IGFBP3 | [74] |
| Mouse | miR-424-5p | Promote | Inhibition | HSP90AA1 | [75] |
| Mouse | miR-452 | Promote | Inhibition | ANGPT1 | [76] |
| Mouse | miR-424(322)-5p | Inhibition | Inhibition | Ezh1 | [77] |
| Mouse | miR-495-3p | Inhibition | Promote | CDH2 | [78] |
| Mouse | miR-22-3p | Inhibition | Promote | Unknown | [79] |
| Rabbit | miR-194-5p | Inhibition | Inhibition | Mef2c | [80] |
| Pig | miR-22 | Inhibition | Promote | Unknown | [81] |
| Duck | miR-1 miR-133 | No influence Promote | Promote No influence | HDAC4 SRF, TGFBR1 | [82] |
| Regulating Method | lncRNA | Ce RNA | Target Genes | Functions | Species | Source |
|---|---|---|---|---|---|---|
| Sponges miRNAs | lncRNA-MEG3 | miR-133 a-3 p | PRRT 2 | Regulation of the skeletal muscle regeneration | Mouse | [107] |
| LncRNA H19 | miR-140-5p | Drebrin 1 | Inhibition of skeletal muscle satellite cell differentiation | Pig | [108] | |
| LncRNA H19 | miR-20a-5p | TGFBR2 | Promotes skeletal muscle fibrosis | Mouse | [109] | |
| lncMFAT1 | miR-135a-5p | TGFBR135/SMAD5 | Promotes skeletal muscle fibrosis | Mouse | [110] | |
| lncA2B1 | miR-206 | HNRNPA2B1 | Promotes myogenic cell differentiation and myogenesis | Bovine | [111] | |
| lncR-133a | miR-133a-3p | FGFR1/ERK1/2 | Inhibition of myogenic cell differentiation | Goat | [112] | |
| Regulation of Gene expression in cis or trans | MUNC lncRNA | Myod1 | Promotes myogenic cell differentiation | Mouse | [100] | |
| LncMyolinc | Filip1 | Inhibits myoblast differentiation into myotubes | Mouse | [113] | ||
| LncRNA-Six1 | Six1 | Promotes myoblast proliferation | Chicken | [114] |
| circRNA | Regulation Method | Function | Species | Source |
|---|---|---|---|---|
| circ-FoxO3 | miR-2400 sponge | Inhibition of differentiation of myogenic cells | Mouse | [118] |
| circNfix | miR-204-5p sponge | Promotes myoblast differentiation | Mouse | [125] |
| circNDST1 | miR-411a sponge | Promotes proliferation and inhibits cell differentiation of bovine myogenic cells | Bovine | [119] |
| circITSN2 | miR-218-5p sponge | Promotion of skeletal muscle development in chicken embryos | Chicken | [120] |
| CircCCDC91 | binding to the miR-15 family | Promotes myogenic proliferation and differentiation, and relieves skeletal muscle atrophy | Chicken | [121] |
| circUSP13 | miR-29c sponge | Promotes differentiation and inhibit apoptosis of goat myogenic cells | Goat | [122] |
| CircUBE3A | miR-28-5p sponge | Promotes myogenic cell proliferation and differentiation | Goat | [123] |
| circIGF1R | miR-16 sponge | Promotes myogenic cell differentiation | Pig | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wu, J.; Liu, W.; Zhao, Y.; Chen, H. The Function and Regulation Mechanism of Non-Coding RNAs in Muscle Development. Int. J. Mol. Sci. 2023, 24, 14534. https://doi.org/10.3390/ijms241914534
Yang Y, Wu J, Liu W, Zhao Y, Chen H. The Function and Regulation Mechanism of Non-Coding RNAs in Muscle Development. International Journal of Molecular Sciences. 2023; 24(19):14534. https://doi.org/10.3390/ijms241914534
Chicago/Turabian StyleYang, Yaling, Jian Wu, Wujun Liu, Yumin Zhao, and Hong Chen. 2023. "The Function and Regulation Mechanism of Non-Coding RNAs in Muscle Development" International Journal of Molecular Sciences 24, no. 19: 14534. https://doi.org/10.3390/ijms241914534




