The Pulmonary Endothelial Glycocalyx Modifications in Glypican 1 Knockout Mice Do Not Affect Lung Endothelial Function in Physiological Conditions
Abstract
:1. Introduction
2. Results
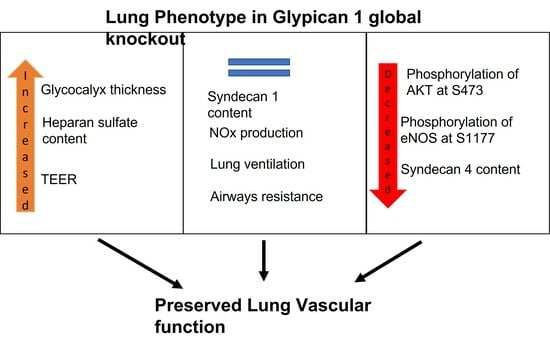
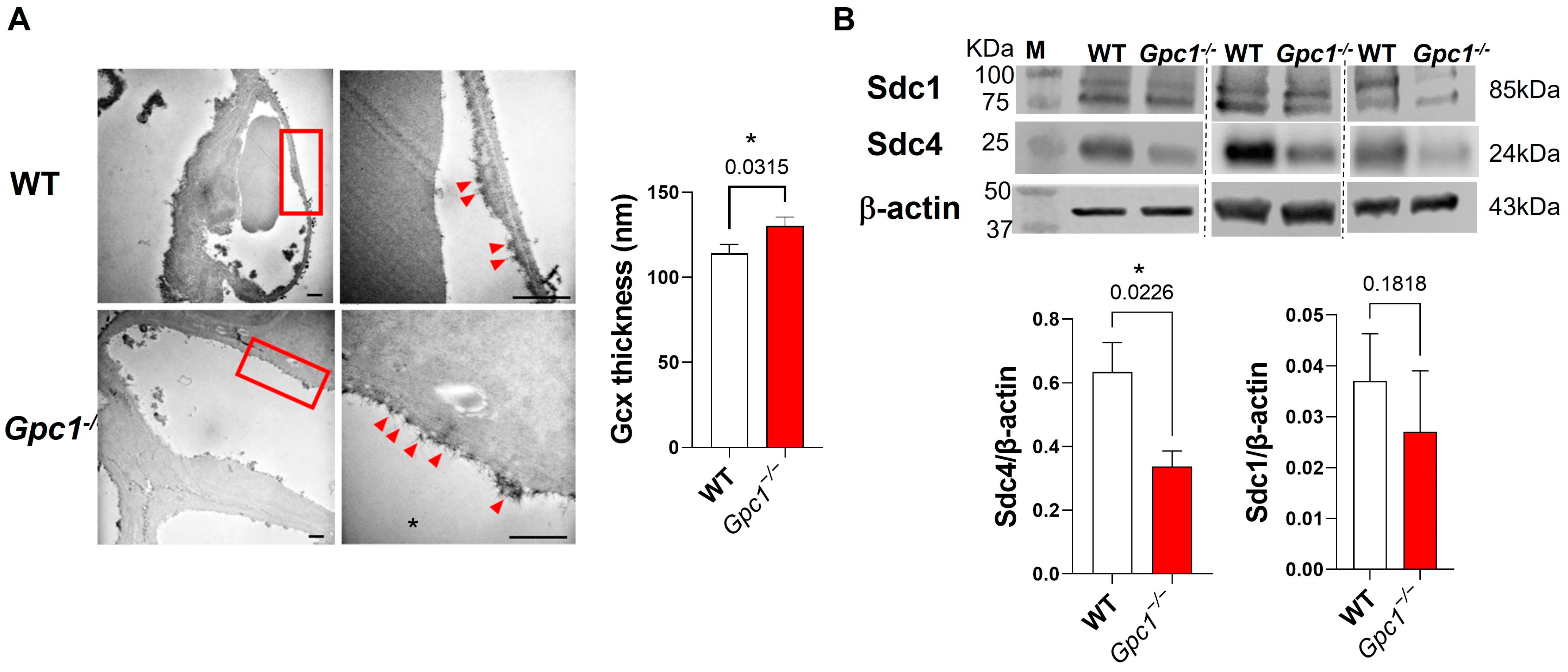
2.1. Global Deletion of Gpc1 Leads to Increased Glycocalyx Thickness in the Lung Vasculature
2.2. Global Deletion of Gpc1 Modified the Chemical Composition of the Glycocalyx
2.3. Glypican 1 Knockout Mice Show Decreased eNOS Activity with Preserved Nitric Oxide Production
2.4. Decreased eNOS Activity Does Not Affect Endothelial Barrier Formation In Vitro
2.5. Pulmonary Ventilation and Mechanics in Glypican 1 Knockout Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Whole-Body Plethysmography
4.4. Pulmonary Mechanics
4.5. Mean Linear Intercept
4.6. Transmission Electron Microscopy
4.7. Western Blotting
4.8. Colorimetric Griess Reaction
4.9. Myeloperoxidase Activity
4.10. LPS-Induced Lung Edema
4.11. Primary Isolation of Mouse Lung Endothelial Cells
4.12. Transendothelial Electrical Resistance
4.13. Immunofluorescence
4.13.1. Lung Tissue
4.13.2. Mouse Lung Endothelial Cells
4.14. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almahayni, K.; Mockl, L. Setting the stage for universal pharmacological targeting of the glycocalyx. Curr. Top. Membr. 2023, 91, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Luft, J.H. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed. Proc. 1966, 25, 1773–1783. [Google Scholar] [PubMed]
- Curry, F.E.; Michel, C.C. A fiber matrix model of capillary permeability. Microvasc. Res. 1980, 20, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo, L.F.; Lomakina, E.B.; Kuebel, J.; Waugh, R.E. Changes in endothelial glycocalyx layer protective ability after inflammatory stimulus. Am. J. Physiol. Cell. Physiol. 2021, 320, C216–C224. [Google Scholar] [CrossRef] [PubMed]
- Levick, J.R.; Michel, C.C. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 2010, 87, 198–210. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Z.; Ji, Z.; Wu, Y.; He, Y.; Liu, K.; Chang, Y.; Peng, Y.; Lin, Z.; Wang, S.; et al. Glycocalyx is critical for blood-brain barrier integrity by suppressing caveolin1-dependent endothelial transcytosis following ischemic stroke. Brain Pathol. 2022, 32, e13006. [Google Scholar] [CrossRef]
- Dull, R.O.; Chignalia, A.Z. The Glycocalyx and Pressure-Dependent Transcellular Albumin Transport. Cardiovasc. Eng. Technol. 2020, 11, 655–662. [Google Scholar] [CrossRef]
- Chappell, D.; Brettner, F.; Doerfler, N.; Jacob, M.; Rehm, M.; Bruegger, D.; Conzen, P.; Jacob, B.; Becker, B.F. Protection of glycocalyx decreases platelet adhesion after ischaemia/reperfusion: An animal study. Eur. J. Anaesthesiol. 2014, 31, 474–481. [Google Scholar] [CrossRef]
- Britten, M.W.; Lumers, L.; Tominaga, K.; Peters, J.; Dirkmann, D. Glycocalyx components affect platelet function, whole blood coagulation, and fibrinolysis: An in vitro study suggesting a link to trauma-induced coagulopathy. BMC Anesthesiol. 2021, 21, 83. [Google Scholar] [CrossRef]
- Mulivor, A.W.; Lipowsky, H.H. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1282–H1291. [Google Scholar] [CrossRef]
- Chappell, D.; Dorfler, N.; Jacob, M.; Rehm, M.; Welsch, U.; Conzen, P.; Becker, B.F. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock 2010, 34, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Ding, C.; Huang, J.; Chen, Y.; Chen, Y. Pulmonary Vascular Endothelial Glycocalyx Degradation Contributes to Acute Lung Injury in Experiencing Heatstroke. Shock 2023, 59, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.P.; Yang, Y.; Janssen, W.J.; Gandjeva, A.; Perez, M.J.; Barthel, L.; Zemans, R.L.; Bowman, J.C.; Koyanagi, D.E.; Yunt, Z.X.; et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 2012, 18, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Machin, D.R.; Phuong, T.T.; Donato, A.J. The role of the endothelial glycocalyx in advanced age and cardiovascular disease. Curr. Opin. Pharmacol. 2019, 45, 66–71. [Google Scholar] [CrossRef]
- Askari, H.; Sadeghinejad, M.; Fancher, I.S. Mechanotransduction and the endothelial glycocalyx: Interactions with membrane and cytoskeletal proteins to transduce force. Curr. Top. Membr. 2023, 91, 43–60. [Google Scholar] [CrossRef]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.; oude Egbrink, M.G. The endothelial glycocalyx: Composition, functions, and visualization. Pflugers Arch. 2007, 454, 345–359. [Google Scholar] [CrossRef]
- Lord, M.S.; Chuang, C.Y.; Melrose, J.; Davies, M.J.; Iozzo, R.V.; Whitelock, J.M. The role of vascular-derived perlecan in modulating cell adhesion, proliferation and growth factor signaling. Matrix Biol. 2014, 35, 112–122. [Google Scholar] [CrossRef]
- Potje, S.R.; Isbatan, A.; Tostes, R.C.; Bendhack, L.M.; Dull, R.O.; Carvalho-de-Souza, J.L.; Chignalia, A.Z. Glypican 1 and syndecan 1 differently regulate noradrenergic hypertension development: Focus on IP3R and calcium. Pharmacol. Res. 2021, 172, 105813. [Google Scholar] [CrossRef]
- Parnigoni, A.; Viola, M.; Karousou, E.; Rovera, S.; Giaroni, C.; Passi, A.; Vigetti, D. Hyaluronan in pathophysiology of vascular diseases: Specific roles in smooth muscle cells, endothelial cells, and macrophages. Am. J. Physiol. Cell Physiol. 2022, 323, C505–C519. [Google Scholar] [CrossRef]
- Borland, S.J.; Morris, T.G.; Borland, S.C.; Morgan, M.R.; Francis, S.E.; Merry, C.L.R.; Canfield, A.E. Regulation of vascular smooth muscle cell calcification by syndecan-4/FGF-2/PKCalpha signalling and cross-talk with TGFbeta. Cardiovasc. Res. 2017, 113, 1639–1652. [Google Scholar] [CrossRef]
- Chaterji, S.; Lam, C.H.; Ho, D.S.; Proske, D.C.; Baker, A.B. Syndecan-1 regulates vascular smooth muscle cell phenotype. PLoS ONE 2014, 9, e89824. [Google Scholar] [CrossRef] [PubMed]
- Voyvodic, P.L.; Min, D.; Liu, R.; Williams, E.; Chitalia, V.; Dunn, A.K.; Baker, A.B. Loss of syndecan-1 induces a pro-inflammatory phenotype in endothelial cells with a dysregulated response to atheroprotective flow. J. Biol. Chem. 2014, 289, 9547–9559. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.S.; Chignalia, A.Z.; Carvalho-de-Souza, J.L. Modulation of cardiac voltage-activated K(+) currents by glypican 1 heparan sulfate proteoglycan. Life Sci. 2022, 308, 120916. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, A.M.W.; Mathews, R.; Mahmoud, M.M.; Cancel, L.M.; Haq, Z.S.; Tarbell, J.M. Heparan sulfate proteoglycan glypican-1 and PECAM-1 cooperate in shear-induced endothelial nitric oxide production. Sci. Rep. 2021, 11, 11386. [Google Scholar] [CrossRef]
- Mahmoud, M.; Mayer, M.; Cancel, L.M.; Bartosch, A.M.; Mathews, R.; Tarbell, J.M. The glycocalyx core protein Glypican 1 protects vessel wall endothelial cells from stiffness-mediated dysfunction and disease. Cardiovasc. Res. 2021, 117, 1592–1605. [Google Scholar] [CrossRef]
- Ebong, E.E.; Lopez-Quintero, S.V.; Rizzo, V.; Spray, D.C.; Tarbell, J.M. Shear-induced endothelial NOS activation and remodeling via heparan sulfate, glypican-1, and syndecan-1. Integr. Biol. 2014, 6, 338–347. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, J. Role of glypican-1 in endothelial NOS activation under various steady shear stress magnitudes. Exp. Cell Res. 2016, 348, 184–189. [Google Scholar] [CrossRef]
- Bartosch, A.M.W.; Mathews, R.; Tarbell, J.M. Endothelial Glycocalyx-Mediated Nitric Oxide Production in Response to Selective AFM Pulling. Biophys. J. 2017, 113, 101–108. [Google Scholar] [CrossRef]
- Gosens, R.; Gross, N. The mode of action of anticholinergics in asthma. Eur. Respir. J. 2018, 52, 1701247. [Google Scholar] [CrossRef]
- Dull, R.O.; Mecham, I.; McJames, S. Heparan sulfates mediate pressure-induced increase in lung endothelial hydraulic conductivity via nitric oxide/reactive oxygen species. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L1452–L1458. [Google Scholar] [CrossRef]
- Dull, R.O.; Cluff, M.; Kingston, J.; Hill, D.; Chen, H.; Hoehne, S.; Malleske, D.T.; Kaur, R. Lung heparan sulfates modulate K(fc) during increased vascular pressure: Evidence for glycocalyx-mediated mechanotransduction. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L816–L828. [Google Scholar] [CrossRef] [PubMed]
- Thota, L.N.R.; Chignalia, A.Z. The Role of the Glypican and Syndecan Families of Heparan Sulfate Proteoglycans in Cardiovascular Function and Disease. Am. J. Physiol. Cell Physiol. 2022, 323, C1052–C1060. [Google Scholar] [CrossRef]
- van den Berg, B.M.; Spaan, J.A.; Rolf, T.M.; Vink, H. Atherogenic region and diet diminish glycocalyx dimension and increase intima-to-media ratios at murine carotid artery bifurcation. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H915–H920. [Google Scholar] [CrossRef] [PubMed]
- Florian, J.A.; Kosky, J.R.; Ainslie, K.; Pang, Z.; Dull, R.O.; Tarbell, J.M. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ. Res. 2003, 93, e136–e142. [Google Scholar] [CrossRef]
- Mochizuki, S.; Vink, H.; Hiramatsu, O.; Kajita, T.; Shigeto, F.; Spaan, J.A.; Kajiya, F. Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H722–H726. [Google Scholar] [CrossRef] [PubMed]
- Vink, H.; Constantinescu, A.A.; Spaan, J.A. Oxidized lipoproteins degrade the endothelial surface layer: Implications for platelet-endothelial cell adhesion. Circulation 2000, 101, 1500–1502. [Google Scholar] [CrossRef]
- Constantinescu, A.A.; Vink, H.; Spaan, J.A. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Adamson, R.H. Permeability of frog mesenteric capillaries after partial pronase digestion of the endothelial glycocalyx. J. Physiol. 1990, 428, 1–13. [Google Scholar] [CrossRef]
- Huxley, V.H.; Williams, D.A. Role of a glycocalyx on coronary arteriole permeability to proteins: Evidence from enzyme treatments. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H1177–H1185. [Google Scholar] [CrossRef]
- Chignalia, A.Z.; Isbatan, A.; Patel, M.; Ripper, R.; Sharlin, J.; Shosfy, J.; Borlaug, B.A.; Dull, R.O. Pressure-dependent NOS activation contributes to endothelial hyperpermeability in a model of acute heart failure. Biosci. Rep. 2018, 38, BSR20181239. [Google Scholar] [CrossRef]
- Pratap, A.; Li, A.; Westbrook, L.; Gergen, A.K.; Mitra, S.; Chauhan, A.; Cheng, L.; Weyant, M.J.; McCarter, M.; Wani, S.; et al. Glypican 1 promotes proliferation and migration in esophagogastric adenocarcinoma via activating AKT/GSK/beta-catenin pathway. J. Gastrointest. Oncol. 2022, 13, 2082–2104. [Google Scholar] [CrossRef]
- Pratap, A.; Qualman, A.; Garrett, H.; Westbrook, L.; The, E.; Mitra, S.; Cordero, M.; Monge, K.M.; Idrovo, J.P.; Chauhan, A.; et al. Silencing Glypican-1 enhances the antitumor effects of Pictilisib via downregulating PI3K/Akt/ERK signaling in chemo-resistant esophageal adenocarcinoma. Mol. Cell. Oncol. 2023, 10, 2238873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, J.T.; Pan, Y.; Liu, X.F.; Xu, J.W.; Cui, W.J.; Qiao, X.R.; Dong, L. Syndecan-1 Shedding by Matrix Metalloproteinase-9 Signaling Regulates Alveolar Epithelial Tight Junction in Lipopolysaccharide-Induced Early Acute Lung Injury. J. Inflamm. Res. 2021, 14, 5801–5816. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wu, D.; Gao, F.; Yan, J.; Liang, F.; Hui, J. Evaluation of extravascular lung water in patients with acute respiratory distress syndrome by syndecan-1 combined with lung ultrasonography. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2021, 33, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Quindry, J.C.; Ballmann, C.G.; Epstein, E.E.; Selsby, J.T. Plethysmography measurements of respiratory function in conscious unrestrained mice. J. Physiol. Sci. 2016, 66, 157–164. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Oliveira, M.A.; Tsujita, M.; Nunes, F.P.B.; Casagrande, F.B.; Gomes, E.; Russo, M.; Tavares de Lima, W.; Martins, J.O. Insulin Modulates the Immune Cell Phenotype in Pulmonary Allergic Inflammation and Increases Pulmonary Resistance in Diabetic Mice. Front. Immunol. 2020, 11, 84. [Google Scholar] [CrossRef]
- Chignalia, A.Z.; Vogel, S.M.; Reynolds, A.B.; Mehta, D.; Dull, R.O.; Minshall, R.D.; Malik, A.B.; Liu, Y. p120-catenin expressed in alveolar type II cells is essential for the regulation of lung innate immune response. Am. J. Pathol. 2015, 185, 1251–1263. [Google Scholar] [CrossRef]
- Inagawa, R.; Okada, H.; Takemura, G.; Suzuki, K.; Takada, C.; Yano, H.; Ando, Y.; Usui, T.; Hotta, Y.; Miyazaki, N.; et al. Ultrastructural Alteration of Pulmonary Capillary Endothelial Glycocalyx During Endotoxemia. Chest 2018, 154, 317–325. [Google Scholar] [CrossRef]
- Wang, J.; Niu, N.; Xu, S.; Jin, Z.G. A simple protocol for isolating mouse lung endothelial cells. Sci. Rep. 2019, 9, 1458. [Google Scholar] [CrossRef]
- Lu, Q.; Zemskov, E.A.; Sun, X.; Wang, H.; Yegambaram, M.; Wu, X.; Garcia-Flores, A.; Song, S.; Tang, H.; Kangath, A.; et al. Activation of the mechanosensitive Ca(2+) channel TRPV4 induces endothelial barrier permeability via the disruption of mitochondrial bioenergetics. Redox Biol. 2021, 38, 101785. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thota, L.N.R.; Lopez Rosales, J.E.; Placencia, I.; Zemskov, E.A.; Tonino, P.; Michael, A.N.; Black, S.M.; Chignalia, A.Z. The Pulmonary Endothelial Glycocalyx Modifications in Glypican 1 Knockout Mice Do Not Affect Lung Endothelial Function in Physiological Conditions. Int. J. Mol. Sci. 2023, 24, 14568. https://doi.org/10.3390/ijms241914568
Thota LNR, Lopez Rosales JE, Placencia I, Zemskov EA, Tonino P, Michael AN, Black SM, Chignalia AZ. The Pulmonary Endothelial Glycocalyx Modifications in Glypican 1 Knockout Mice Do Not Affect Lung Endothelial Function in Physiological Conditions. International Journal of Molecular Sciences. 2023; 24(19):14568. https://doi.org/10.3390/ijms241914568
Chicago/Turabian StyleThota, Lakshmi N. R., Joaquin E. Lopez Rosales, Ivan Placencia, Evgeny A. Zemskov, Paola Tonino, Ashley N. Michael, Stephen M. Black, and Andreia Z. Chignalia. 2023. "The Pulmonary Endothelial Glycocalyx Modifications in Glypican 1 Knockout Mice Do Not Affect Lung Endothelial Function in Physiological Conditions" International Journal of Molecular Sciences 24, no. 19: 14568. https://doi.org/10.3390/ijms241914568
APA StyleThota, L. N. R., Lopez Rosales, J. E., Placencia, I., Zemskov, E. A., Tonino, P., Michael, A. N., Black, S. M., & Chignalia, A. Z. (2023). The Pulmonary Endothelial Glycocalyx Modifications in Glypican 1 Knockout Mice Do Not Affect Lung Endothelial Function in Physiological Conditions. International Journal of Molecular Sciences, 24(19), 14568. https://doi.org/10.3390/ijms241914568