Exposing Salmonella Senftenberg and Escherichia coli Strains Isolated from Poultry Farms to Formaldehyde and Lingonberry Extract at Low Concentrations
Abstract
:1. Introduction
2. Results
2.1. Assessment of Biofilm Formation by Salmonella Senftenberg and Escherichia coli Strains
2.2. Evaluation of the Effectiveness of Formaldehyde in the Eradication of Biofilms Formed by Salmonella Senftenberg and Escherichia coli Strains
2.3. The Influence of Formaldehyde on the Survival of S. Senftenberg and E. coli Strains in Biofilms
- survival 0.0–5.0%—total inhibition
- survival 5.1–25.0%—slight inhibition
- survival 25.1–75.0%—moderate inhibition
- survival 75.1–95.0%—strong inhibition
- survival 95.1–100%—no inhibition
2.4. Antibiofilm Properties of V. vitis-idaea Extract in Biofilms Formed by S. Senftenberg and E. coli
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Chemical and Plant Material
4.3. Preparation of V. vitis-idaea Extract
4.4. Biofilm Formation
4.5. Biofilm-Oriented Aseptic Test (BOAT)
4.6. Quantitative Measurements of the Number of Colony-Forming Units in BOAT Assay
4.7. Antibiofilm Properties of Lingonberry (V. vitis-idaea) Extract
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and Contamination Routes of Microbial Pathogens to Fresh Produce during Field Cultivation: A Review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; García, P. Bacteriophages as Weapons against Bacterial Biofilms in the Food Industry. Front Microbiol. 2016, 7, 825. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, e06971. [Google Scholar] [CrossRef]
- Rosińska, M.; Czarkowski, M.P.; Sadkowska-Todys, M. Infectious Diseases in Poland in 2020. Przegl. Epidemiol. 2022, 76, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Ehuwa, O.; Jaiswal, A.K.; Jaiswal, S. Salmonella, Food Safety and Food Handling Practices. Foods 2021, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Srednik, M.E.; Morningstar-Shaw, B.R.; Hicks, J.A.; Mackie, T.A.; Schlater, L.K. Antimicrobial Resistance and Genomic Characterization of Salmonella enterica Serovar Senftenberg Isolates in Production Animals from the United States. Front Microbiol. 2022, 13, 979790. [Google Scholar] [CrossRef] [PubMed]
- Kathayat, D.; Lokesh, D.; Ranjit, S.; Rajashekara, G. Avian Pathogenic Escherichia coli (APEC): An Overview of Virulence and Pathogenesis Factors, Zoonotic Potential, and Control Strategies. Pathogens 2021, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Jechalke, S.; Schierstaedt, J.; Becker, M.; Flemer, B.; Grosch, R.; Smalla, K.; Schikora, A. Salmonella Establishment in Agricultural Soil and Colonization of Crop Plants Depend on Soil Type and Plant Species. Front Microbiol. 2019, 10, 967. [Google Scholar] [CrossRef]
- Szejniuk, B.; Wasilewski, P.; Kubisz, L.; Szrajda, P.; Wronski, G. Elimination of Salmonella Senftenberg W775 bacteria in the cultivation of selected agricultural plants. Acta Sci. Pol. 2007, 6, 73–81. (In Polish) [Google Scholar]
- Chen, S.; Feng, Z.; Sun, H.; Zhang, R.; Qin, T.; Peng, D. Biofilm-Formation-Related Genes CsgD and BcsA Promote the Vertical Transmission of Salmonella Enteritidis in Chicken. Front. Vet. Sci. 2021, 7, 625049. [Google Scholar] [CrossRef]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef]
- Course, C.E.; Boerlin, P.; Slavic, D.; Vaillancourt, J.-P.; Guerin, M.T. Factors Associated with Salmonella enterica and Escherichia coli during Downtime in Commercial Broiler Chicken Barns in Ontario. Poult. Sci. 2021, 100, 101065. [Google Scholar] [CrossRef]
- Cadena, M.; Kelman, T.; Marco, M.L.; Pitesky, M. Understanding Antimicrobial Resistance (AMR) Profiles of Salmonella Biofilm and Planktonic Bacteria Challenged with Disinfectants Commonly Used During Poultry Processing. Foods 2019, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Kim, Y.-M. Antibiotics Application Strategies to Control Biofilm Formation in Pathogenic Bacteria. Curr. Pharm. Biotechnol. 2020, 21, 270–286. [Google Scholar] [CrossRef]
- Książczyk, M.; Krzyżewska, E.; Futoma-Kołoch, B.; Bugla-Płoskońska, G. Disinfectants—Bacterial Cells Interactions in the View of Hygiene and Public Health. Adv. Hyg. Exp. Med. 2015, 69, 1042–1055. [Google Scholar]
- Mammo, F.K.; Amoah, I.D.; Gani, K.M.; Pillay, L.; Ratha, S.K.; Bux, F.; Kumari, S. Microplastics in the Environment: Interactions with Microbes and Chemical Contaminants. Sci. Total Environ. 2020, 743, 140518. [Google Scholar] [CrossRef] [PubMed]
- Tyski, S.; Bocian, E.; Laudy, A.E. Application of Normative Documents for Determination of Biocidal Activity of Disinfectants and Antiseptics Dedicated to the Medical Area: A Narrative Review. J. Hosp. Infect. 2022, 125, 75–91. [Google Scholar] [CrossRef]
- Tian, L.; Luo, Y.; Wen, T.; Yang, W.; Zhao, Y.; Huang, P.; He, H.; Wu, J.; Li, Z.; Pan, C. A Quadruple Protection Procedure for Resuming Pig Production in Small-Scale ASFV-Positive Farms in China. Curr. Res. Microb. Sci. 2021, 2, 100014. [Google Scholar] [CrossRef]
- Dzieciołowski, T.; Boqvist, S.; Rydén, J.; Hansson, I. Cleaning and Disinfection of Transport Crates for Poultry—Comparison of Four Treatments at Slaughter Plant. Poult. Sci. 2022, 101, 101521. [Google Scholar] [CrossRef]
- Document 32020R1763; Commission Implementing Regulation (EU) 2020/1763 of 25 November 2020 Approving Formaldehyde as an Existing Active Substance for Use in Biocidal Products of Product-Types 2 and 3 (Text with EEA Relevance). Official Journal of the European Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R1763 (accessed on 3 September 2023).
- Cioch, M.; Satora, P.; Skotniczny, M.; Semik-Szczurak, D.; Tarko, T. Characterisation of Antimicrobial Properties of Extracts of Selected Medicinal Plants. Pol. J. Microbiol. 2017, 66, 463–472. [Google Scholar] [CrossRef]
- Wojnicz, D.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kicia, M.; Tichaczek-Goska, D. Medicinal Plants Extracts Affect Virulence Factors Expression and Biofilm Formation by the Uropathogenic Escherichia coli. Urol. Res. 2012, 40, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Bujor, O.-C.; Ginies, C.; Popa, V.I.; Dufour, C. Phenolic Compounds and Antioxidant Activity of Lingonberry (Vaccinium vitis-idaea L.) Leaf, Stem and Fruit at Different Harvest Periods. Food Chem. 2018, 252, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Kryvtsova, M.V.; Salamon, I.; Koscova, J.; Spivak, M.Y. Antibiofilm Forming, Antimicrobial Activity and Some Biochemical Properties of Vaccinium vitis idaea Leaf and Berry Extracts on Staphylococcus aureus. Biosyst. Divers. 2020, 28, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Shamilov, A.A.; Bubenchikova, V.N.; Chernikov, M.V.; Pozdnyakov, D.I.; Garsiya, E.R. Vaccinium vitis-idaea L.: Chemical Contents, Pharmacological Activities. Pharm. Sci. 2020, 26, 344–362. [Google Scholar] [CrossRef]
- Tsemenko, K.V. Antibacterial activity of phytosubstants from Vaccinium vitis-idaea leaves. Ann. Mechnikov Inst. 2018, 3, 23–26. [Google Scholar]
- Ștefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. Chemical Composition and Biological Activities of the Nord-West Romanian Wild Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.) Leaves. Antioxidants 2020, 9, 495. [Google Scholar] [CrossRef]
- Feliciano, R.P.; Meudt, J.J.; Shanmuganayagam, D.; Krueger, C.G.; Reed, J.D. Ratio of “A-Type” to “B-Type” Proanthocyanidin Interflavan Bonds Affects Extra-Intestinal Pathogenic Escherichia coli Invasion of Gut Epithelial Cells. J. Agric. Food Chem. 2014, 62, 3919–3925. [Google Scholar] [CrossRef]
- Pärnänen, P.; Lähteenmäki, H.; Tervahartiala, T.; Räisänen, I.T.; Sorsa, T. Lingonberries-General and Oral Effects on the Microbiome and Inflammation. Nutrients 2021, 13, 3738. [Google Scholar] [CrossRef]
- Kowalska, K. Lingonberry (Vaccinium vitis-idaea L.) Fruit as a Source of Bioactive Compounds with Health-Promoting Effects—A Review. Int. J. Mol. Sci. 2021, 22, 5126. [Google Scholar] [CrossRef]
- Dróżdż, P.; Šėžienė, V.; Pyrzynska, K. Phytochemical Properties and Antioxidant Activities of Extracts from Wild Blueberries and Lingonberries. Plant Foods Hum. Nutr. 2017, 72, 360–364. [Google Scholar] [CrossRef]
- Dittoe, D.K.; Ricke, S.C.; Kiess, A.S. Organic Acids and Potential for Modifying the Avian Gastrointestinal Tract and Reducing Pathogens and Disease. Front. Vet. Sci. 2018, 5, 216. [Google Scholar] [CrossRef] [PubMed]
- Ricke, S.C.; Richardson, K.; Dittoe, D.K. Formaldehydes in Feed and Their Potential Interaction with the Poultry Gastrointestinal Tract Microbial Community—A Review. Front. Vet. Sci. 2019, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, R.A.; Huss, A.R.; Aldrich, G.C.; Stark, C.R.; Jones, C.K. Evaluating Chemical Mitigation of Salmonella Typhimurium ATCC 14028 in Animal Feed Ingredients. J. Food Prot. 2016, 79, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Wales, A.D.; Allen, V.M.; Davies, R.H. Chemical Treatment of Animal Feed and Water for the Control of Salmonella. Foodborne Pathog. Dis. 2010, 7, 3–15. [Google Scholar] [CrossRef]
- Gosling, R.J.; Mawhinney, I.; Vaughan, K.; Davies, R.H.; Smith, R.P. Efficacy of Disinfectants and Detergents Intended for a Pig Farm Environment Where Salmonella Is Present. Vet. Microbiol. 2017, 204, 46–53. [Google Scholar] [CrossRef]
- Carey, D.E.; McNamara, P.J. The Impact of Triclosan on the Spread of Antibiotic Resistance in the Environment. Front. Microbiol. 2015, 5, 780. [Google Scholar] [CrossRef]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance Toward Chlorhexidine in Oral Bacteria—Is There Cause for Concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef]
- Rozman, U.; Pušnik, M.; Kmetec, S.; Duh, D.; Šostar Turk, S. Reduced Susceptibility and Increased Resistance of Bacteria against Disinfectants: A Systematic Review. Microorganisms 2021, 9, 2550. [Google Scholar] [CrossRef]
- Wand, M.E.; Bock, L.J.; Bonney, L.C.; Sutton, J.M. Mechanisms of Increased Resistance to Chlorhexidine and Cross-Resistance to Colistin Following Exposure of Klebsiella pneumoniae Clinical Isolates to Chlorhexidine. Antimicrob. Agents Chemother. 2017, 61, e01162-16. [Google Scholar] [CrossRef]
- Futoma-Kołoch, B.; Dudek, B.; Kapczyńska, K.; Krzyżewska, E.; Wańczyk, M.; Korzekwa, K.; Rybka, J.; Klausa, E.; Bugla-Płoskońska, G. Relationship of Triamine-Biocide Tolerance of Salmonella enterica Serovar Senftenberg to Antimicrobial Susceptibility, Serum Resistance and Outer Membrane Proteins. Int. J. Mol. Sci. 2017, 18, 1459. [Google Scholar] [CrossRef]
- Davin-Regli, A.; Pagès, J.M. Cross-Resistance between Biocides and Antimicrobials: An Emerging Question. Rev. Sci. Tech. 2012, 31, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Baucheron, S.; Mouline, C.; Praud, K.; Chaslus-Dancla, E.; Cloeckaert, A. TolC but not AcrB is essential for multidrug-resistant Salmonella enterica serotype Typhimurium colonization of chicks. J. Antimicrob. Chemother. 2005, 55, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Karatzas, K.A.G.; Randall, L.P.; Webber, M.; Piddock, L.J.V.; Humphrey, T.J.; Woodward, M.J.; Coldham, N.G. Phenotypic and Proteomic Characterization of Multiply Antibiotic-Resistant Variants of Salmonella enterica Serovar Typhimurium Selected Following Exposure to Disinfectants. Appl. Environ. Microbiol. 2008, 74, 1508–1516. [Google Scholar] [CrossRef]
- Oosterik, L.H.; Peeters, L.; Mutuku, I.; Goddeeris, B.M.; Butaye, P. Susceptibility of Avian Pathogenic Escherichia Coli from Laying Hens in Belgium to Antibiotics and Disinfectants and Integron Prevalence. Avian Dis. 2014, 58, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Romeu, M.J.; Rodrigues, D.; Azeredo, J. Effect of Sub-Lethal Chemical Disinfection on the Biofilm Forming Ability, Resistance to Antibiotics and Expression of Virulence Genes of Salmonella Enteritidis Biofilm-Surviving Cells. Biofouling 2020, 36, 101–112. [Google Scholar] [CrossRef]
- Chen, N.H.; Djoko, K.Y.; Veyrier, F.J.; McEwan, A.G. Formaldehyde Stress Responses in Bacterial Pathogens. Front. Microbiol. 2016, 7, 257. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef]
- Kauffmann, A.C.; Castro, V.S. Phenolic Compounds in Bacterial Inactivation: A Perspective from Brazil. Antibiotics 2023, 12, 645. [Google Scholar] [CrossRef]
- Dasiman, R.; Md Nor, N.; Eshak, Z.; Mohd Mutalip, S.S.; Suwandi, N.R.; Bidin, H. A Review of Procyanidin: Updates on Current Bioactivities and Potential Health Benefits. Biointerface Res. Appl. Chem. 2021, 12, 5918–5940. [Google Scholar] [CrossRef]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm Activity of Plant Polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef]
- Lahiri, D.; Dash, S.; Dutta, R.; Nag, M. Elucidating the Effect of Anti-Biofilm Activity of Bioactive Compounds Extracted from Plants. J. Biosci. 2019, 44, 52. [Google Scholar] [CrossRef]
- Laslo, É.; Kobolkuti, Z.A. Total Phenol Content and Antimicrobial Activity of Lingonberry (Vaccinium vitis-idaea L.) from Several Areas in the Eastern Carpathians. Not. Sci. Biol. 2017, 9, 77–83. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Nohynek, L.; Hartmann-Schmidlin, S.; Kahkonen, M.; Heinonen, M.; Maatta-Riihinen, K.; Oksman-Caldentey, K.-M. Berry Phenolics Selectively Inhibit the Growth of Intestinal Pathogens. J. Appl. Microbiol. 2005, 98, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Vernigorova, M.N.; Buzuk, G.N. Chromatodensitometric Study of the Common Lingonberry Leaves (Vaccinium vitis-idaea L.). Vestn. Vitebsk. State Med. Univ. 2019, 18, 107–113. [Google Scholar] [CrossRef]
- Fontaine, B.M.; Nelson, K.; Lyles, J.T.; Jariwala, P.B.; García-Rodriguez, J.M.; Quave, C.L.; Weinert, E.E. Identification of Ellagic Acid Rhamnoside as a Bioactive Component of a Complex Botanical Extract with Anti-Biofilm Activity. Front. Microbiol. 2017, 8, 496. [Google Scholar] [CrossRef]
- Mokhtar, R.A.; Rahim, R.H.; Amin, Z. Extraction methods for Escherichia coli antibacterial assay. Borneo Int. J. Biotechnol. (BIJB) 2022, 2, 95–107. [Google Scholar] [CrossRef]
- Sarowska, J.; Olszak, T.; Jama-Kmiecik, A.; Frej-Mądrzak, M.; Futoma-Kołoch, B.; Gaweł, A.; Drulis-Kawa, Z.; Choroszy-Król, I. Comparative Characteristics and Pathogenic Potential of Escherichia coli Isolates Originating from Poultry Farms, Retail Meat, and Human Urinary Tract Infection. Life 2022, 12, 845. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Initiation of Biofilm Formation in Pseudomonas fluorescens WCS365 Proceeds via Multiple, Convergent Signalling Pathways: A Genetic Analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef]
- Lamas, A.; Fernandez-No, I.C.; Miranda, J.M.; Vázquez, B.; Cepeda, A.; Franco, C.M. Biofilm Formation and Morphotypes of Salmonella enterica Subsp. arizonae Differs from Those of Other Salmonella enterica Subspecies in Isolates from Poultry Houses. J. Food Prot. 2016, 79, 1127–1134. [Google Scholar] [CrossRef]
- Junka, A.; Bartoszewicz, M.; Smutnicka, D.; Secewicz, A.; Szymczyk, P. Efficacy of Antiseptics Containing Povidone-Iodine, Octenidine Dihydrochloride and Ethacridine Lactate against Biofilm Formed by Pseudomonas aeruginosa and Staphylococcus aureus Measured with the Novel Biofilm-Oriented Antiseptics Test. Int. Wound J. 2014, 11, 730–734. [Google Scholar] [CrossRef]
- Zalewska, M.; Błażejewska, A.; Czapko, A.; Popowska, M. Antibiotics and Antibiotic Resistance Genes in Animal Manure—Consequences of Its Application in Agriculture. Front. Microbiol. 2021, 12, 610656. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, G.; Li, S.; Zhou, L.; Yang, H. Dual-Species Biofilms Formed by Escherichia coli and Salmonella Enhance Chlorine Tolerance. Appl. Environ. Microbiol. 2022, 88, e0148222. [Google Scholar] [CrossRef] [PubMed]
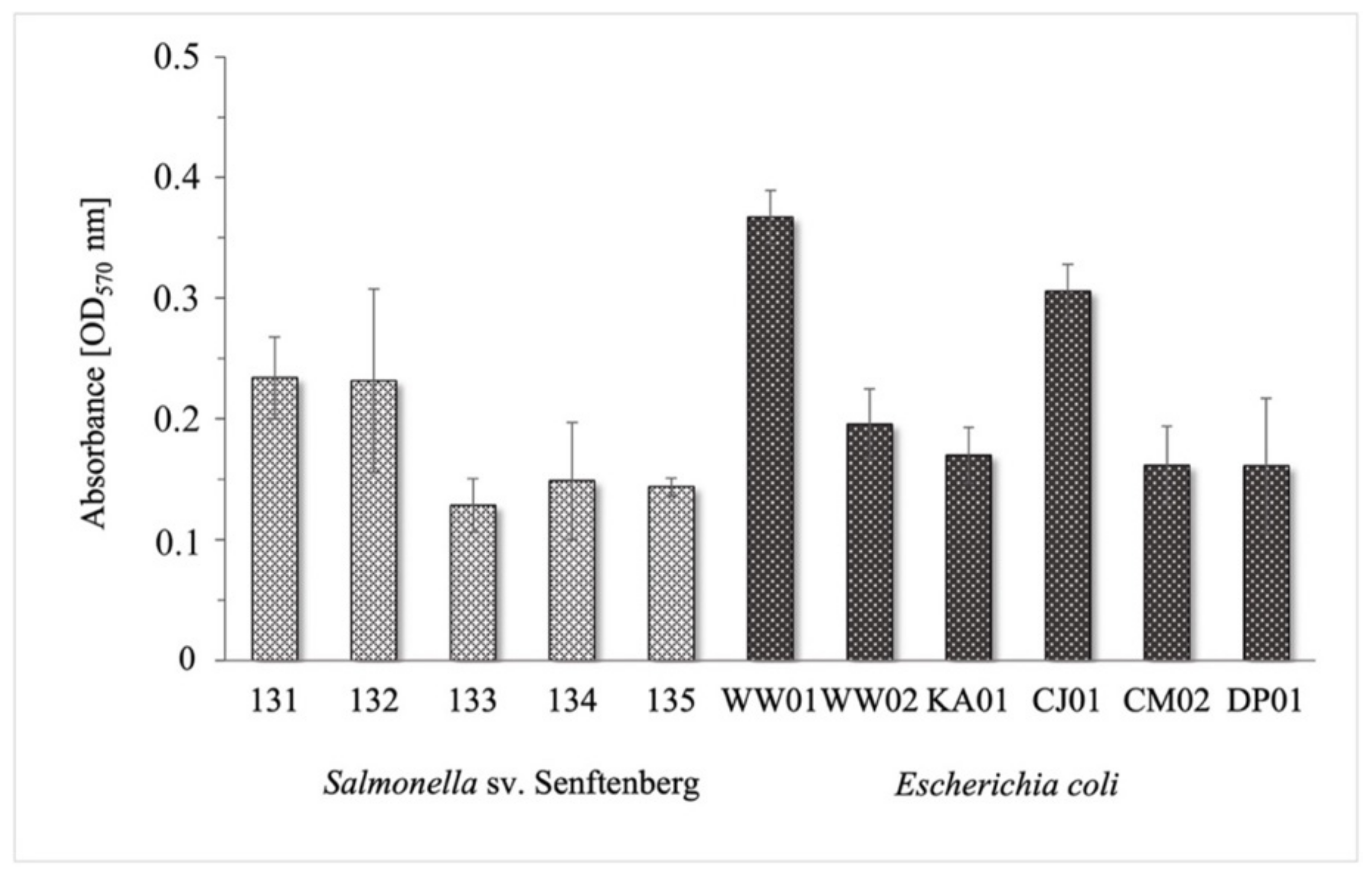
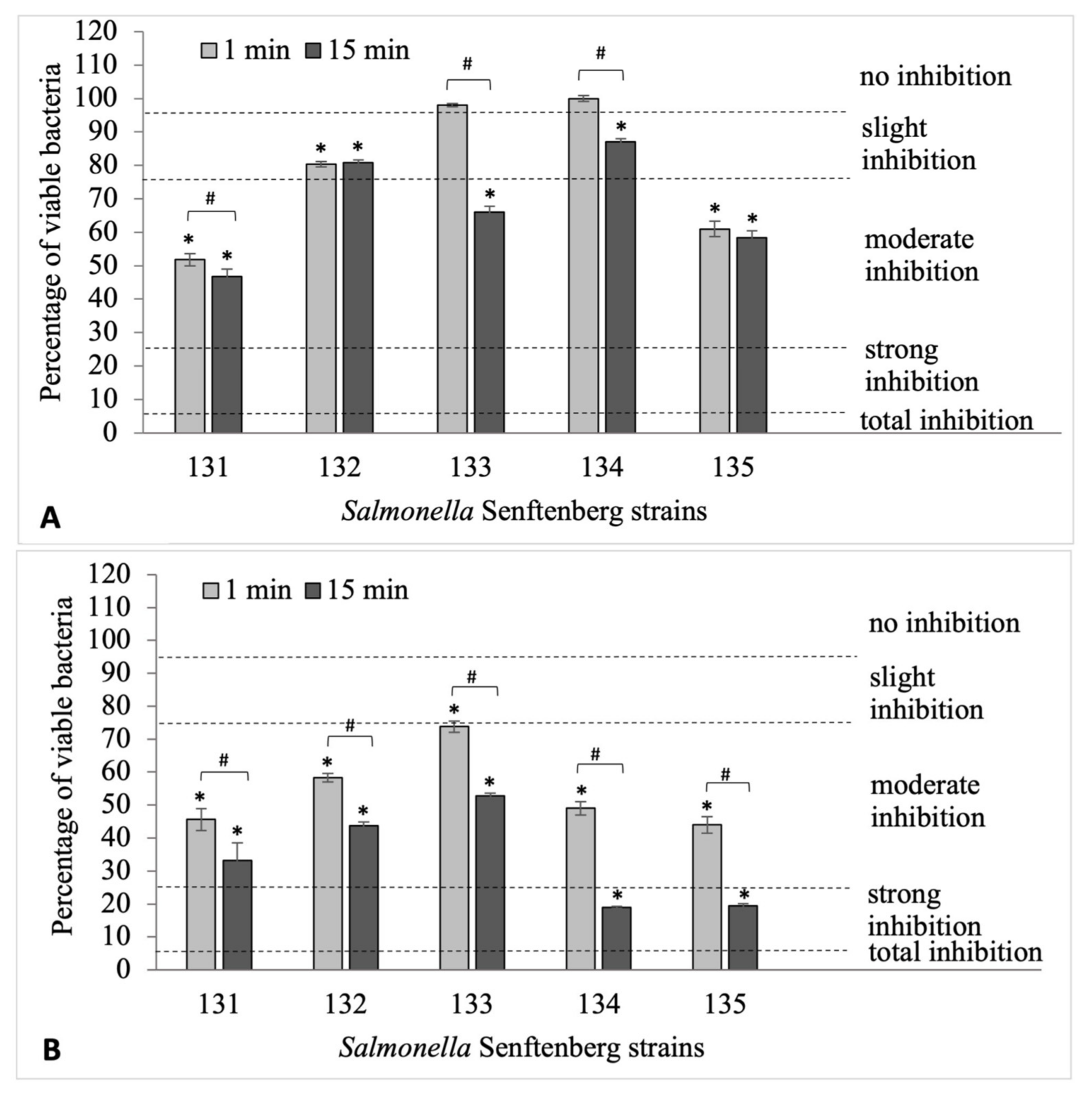
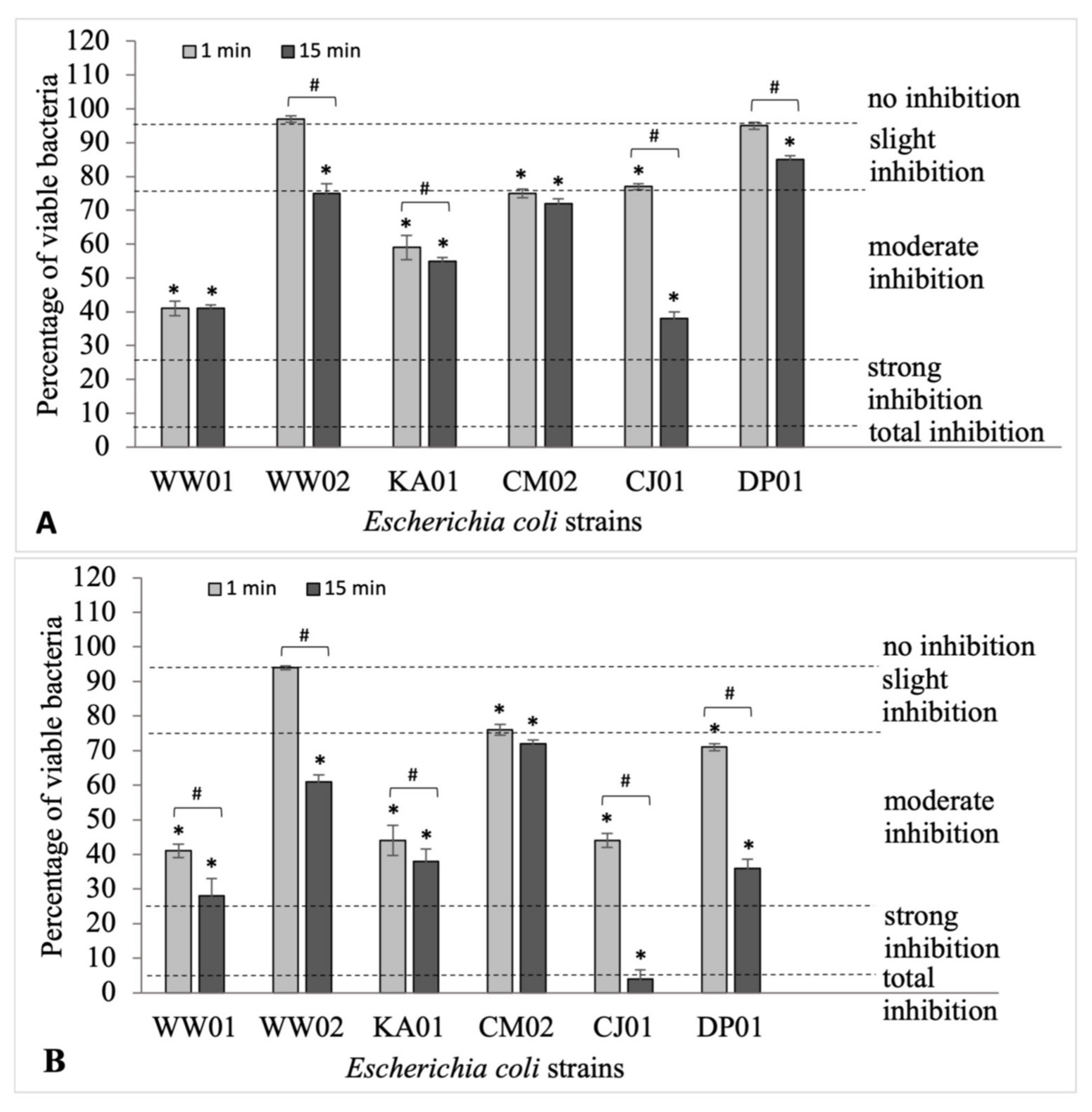
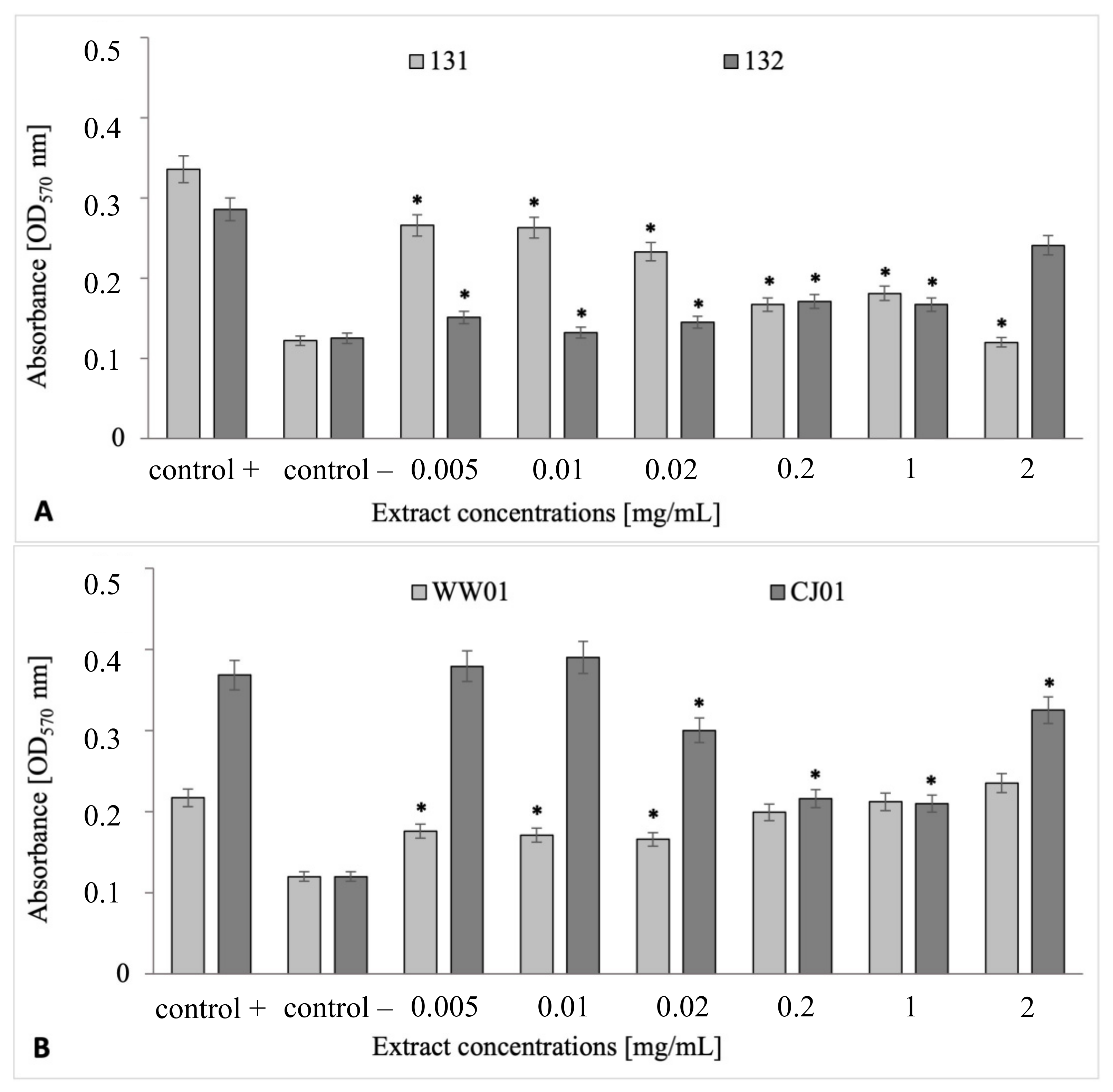
| Strain No. | 131 | 132 | 133 | 134 | 135 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Incubation Time (Min) | ||||||||||
| Formaldehyde concentration (%) | 1 | 15 | 1 | 15 | 1 | 15 | 1 | 15 | 1 | 15 |
| 0.0 (positive control) | + | + | + | + | + | + | + | + | + | + |
| 0.02 | + | + | + | + | + | + | + | + | + | + |
| 0.2 | + | + | + | + | + | + | + | + | + | + |
| 2.0 | − | − | − | − | − | − | − | − | − | − |
| Strain No. | WW01 | WW02 | KA01 | CM02 | CJ01 | DP01 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incubation Time (Min) | ||||||||||||
| Formaldehyde concentration (%) | 1 | 15 | 1 | 15 | 1 | 15 | 1 | 15 | 1 | 15 | 1 | 15 |
| 0.0 (positive control) | + | + | + | + | + | + | + | + | + | + | + | + |
| 0.02 | + | + | + | + | + | + | + | + | + | + | + | + |
| 0.2 | + | + | + | + | + | + | + | + | + | + | + | + |
| 2.0 | + | − | − | − | − | − | − | − | + | − | − | − |
| Species | Subspecies | Serovar | Internal Collection No. | Antibiotic Resistance [41] |
|---|---|---|---|---|
| Salmonella enterica | enterica | Senftenberg | 131 | not found |
| enterica | Senftenberg | 132 | not found | |
| enterica | Senftenberg | 133 | not found | |
| enterica | Senftenberg | 134 | not found | |
| enterica | Senftenberg | 135 | not found |
| E. coli Strain | Phylogenetic Group | Antibiotic Resistance [58] |
|---|---|---|
| KAO1 | A | not found |
| CJ01 | B1 | not found |
| DP01 | C | not found |
| CM02 | D | AMP/TET/SXT/PRL |
| WW01 | B1 | AMP |
| WW02 | A | not found |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choroszy-Król, I.; Futoma-Kołoch, B.; Kuźnik, K.; Wojnicz, D.; Tichaczek-Goska, D.; Frej-Mądrzak, M.; Jama-Kmiecik, A.; Sarowska, J. Exposing Salmonella Senftenberg and Escherichia coli Strains Isolated from Poultry Farms to Formaldehyde and Lingonberry Extract at Low Concentrations. Int. J. Mol. Sci. 2023, 24, 14579. https://doi.org/10.3390/ijms241914579
Choroszy-Król I, Futoma-Kołoch B, Kuźnik K, Wojnicz D, Tichaczek-Goska D, Frej-Mądrzak M, Jama-Kmiecik A, Sarowska J. Exposing Salmonella Senftenberg and Escherichia coli Strains Isolated from Poultry Farms to Formaldehyde and Lingonberry Extract at Low Concentrations. International Journal of Molecular Sciences. 2023; 24(19):14579. https://doi.org/10.3390/ijms241914579
Chicago/Turabian StyleChoroszy-Król, Irena, Bożena Futoma-Kołoch, Klaudia Kuźnik, Dorota Wojnicz, Dorota Tichaczek-Goska, Magdalena Frej-Mądrzak, Agnieszka Jama-Kmiecik, and Jolanta Sarowska. 2023. "Exposing Salmonella Senftenberg and Escherichia coli Strains Isolated from Poultry Farms to Formaldehyde and Lingonberry Extract at Low Concentrations" International Journal of Molecular Sciences 24, no. 19: 14579. https://doi.org/10.3390/ijms241914579
APA StyleChoroszy-Król, I., Futoma-Kołoch, B., Kuźnik, K., Wojnicz, D., Tichaczek-Goska, D., Frej-Mądrzak, M., Jama-Kmiecik, A., & Sarowska, J. (2023). Exposing Salmonella Senftenberg and Escherichia coli Strains Isolated from Poultry Farms to Formaldehyde and Lingonberry Extract at Low Concentrations. International Journal of Molecular Sciences, 24(19), 14579. https://doi.org/10.3390/ijms241914579







