Gene Expression Profiling Reveals Potential Players of Sex Determination and Asymmetrical Development in Chicken Embryo Gonads
Abstract
1. Introduction
2. Results
2.1. Sex-Biased Transcript Expression Profile of Chicken Gonads at E4.5 Revealed via PacBio Sequencing
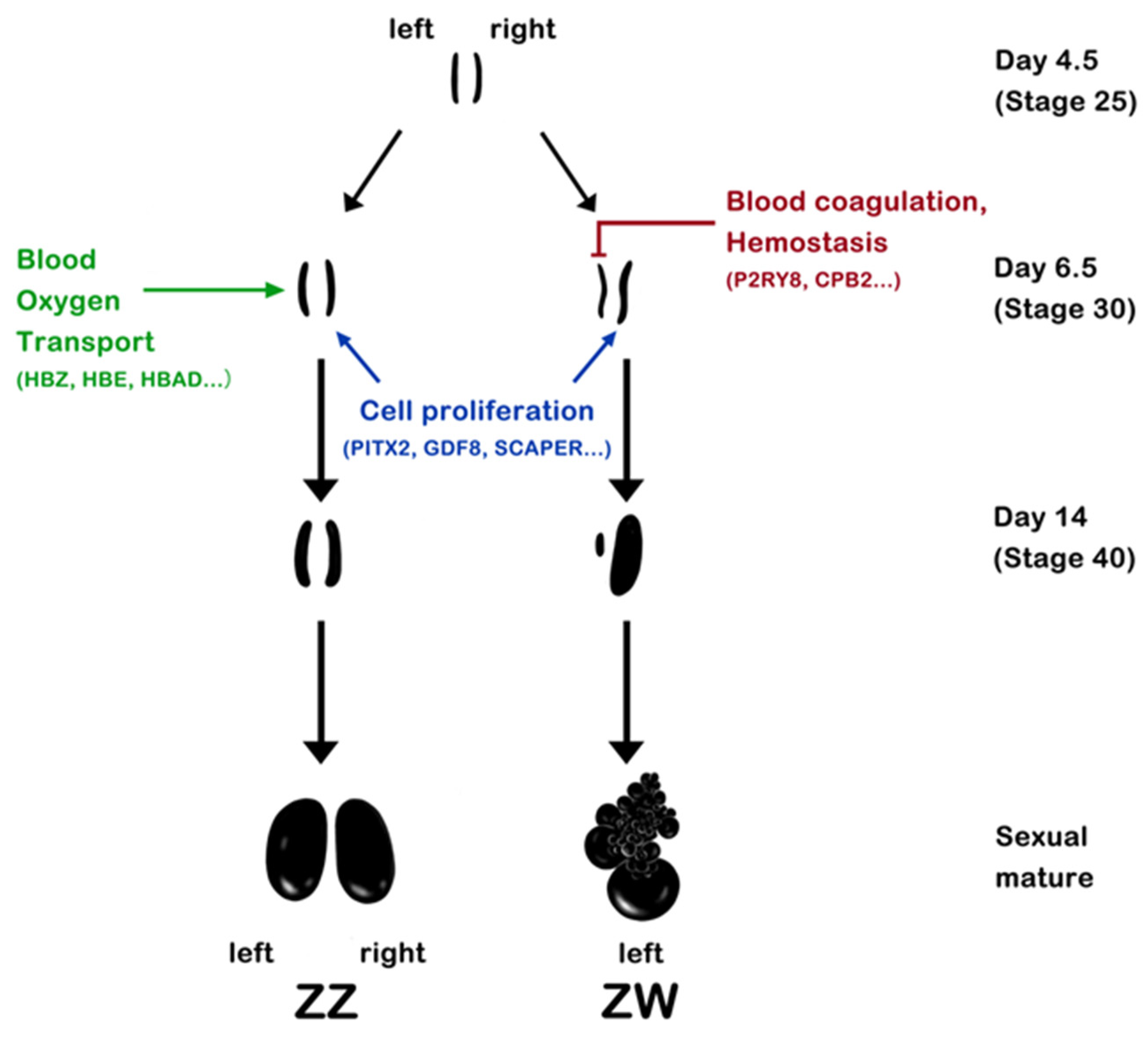
2.2. Dynamic Changes in Gene Expression in Key Stages of Chicken Sex Differentiation
2.3. Left–Right Asymmetric Gene Expression in Embryonic Chicken Gonads
2.4. Validation of Differentially Expressed Genes by qPCR
3. Discussions
3.1. Sex Determination and Gonadal Differentiation-Related Genes in Chicken Embryos
3.2. Left–Right Asymmetric Development of Chicken Gonads
4. Materials and Methods
4.1. Chicken Egg Incubation and Gonad Sampling
4.2. Sexing and RNA Extraction
4.3. Library Construction and Transcriptome Sequencing
4.4. Data Filtering and Alignment
4.5. Differentially Expressed Gene Analysis
4.6. Gene Ontology (GO) and Pathway Analysis
4.7. Quantitative Real-Time PCR (qRT-PCR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fridolfsson, A.K.; Cheng, H.; Copeland, N.G.; Jenkins, N.A.; Liu, H.C.; Raudsepp, T.; Woodage, T.; Chowdhary, B.; Halverson, J.; Ellegren, H. Evolution of the avian sex chromosomes from an ancestral pair of autosomes. Proc. Natl. Acad. Sci. USA 1998, 95, 8147–8152. [Google Scholar] [CrossRef]
- Hirst, C.E.; Major, A.T.; Smith, C.A. Sex determination and gonadal sex differentiation in the chicken model. Int. J. Dev. Biol. 2018, 62, 153–166. [Google Scholar] [CrossRef]
- Smith, C.A.; Sinclair, A.H. Sex determination: Insights from the chicken. BioEssays 2004, 26, 120–132. [Google Scholar] [CrossRef]
- McQueen, H.A.; Clinton, M. Avian sex chromosomes: Dosage compensation matters. Chromosom. Res. 2009, 17, 687–697. [Google Scholar] [CrossRef]
- Nanda, I.; Schlegelmilch, K.; Haaf, T.; Schartl, M.; Schmid, M. Synteny conservation of the Z chromosome in 14 avian species (11 families) supports a role for Z dosage in avian sex determination. Cytogenet. Genome Res. 2008, 122, 150–156. [Google Scholar] [CrossRef]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef]
- Li, B.C.; Chen, G.H.; Xiao, X.J.; Qin, J.; Wu, S.X.; Xie, K.Z.; Olojide, O. Relationship between PGCs Settle and Gonad Development in the Early Chicken Embryo. Asian-Australas. J. Anim. Sci. 2004, 17, 453–459. [Google Scholar] [CrossRef]
- Nagai, H.; Sezaki, M.; Bertocchini, F.; Fukuda, K.; Sheng, G. HINTW, a W-chromosome HINT gene in chick, is expressed ubiquitously and is a robust female cell marker applicable in intraspecific chimera studies. Genesis 2014, 52, 424–430. [Google Scholar] [CrossRef]
- Major, A.T.; Ayers, K.L.; Chue, J.; Roeszler, K.N.; Smith, C.A. FOXL2 antagonises the male developmental pathway in embryonic chicken gonads. J. Endocrinol. 2019, 243, 211–228. [Google Scholar] [CrossRef]
- Uhlenhaut, N.H.; Treier, M. Foxl2 function in ovarian development. Mol. Genet. Metab. 2006, 88, 225–234. [Google Scholar] [CrossRef]
- Pannetier, M.; Fabre, S.; Batista, F.; Kocer, A.; Renault, L.; Jolivet, G.; Mandon-Pépin, B.; Cotinot, C.; Veitia, R.; Pailhoux, E. FOXL2 activates P450 aromatase gene transcription: Towards a better characterization of the early steps of mammalian ovarian development. J. Mol. Endocrinol. 2006, 36, 399–413. [Google Scholar] [CrossRef]
- Ayers, K.; Sinclair, A.; Smith, C. The Molecular Genetics of Ovarian Differentiation in the Avian Model. Sex. Dev. 2013, 7, 80–94. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Q.; Liu, Z.; Guo, X.; Du, Y.; Yuan, Z.; Guo, M.; Kang, L.; Sun, Y.; Jiang, Y. Transcriptome Analysis on Single Small Yellow Follicles Reveals That Wnt4 Is Involved in Chicken Follicle Selection. Front. Endocrinol. 2017, 8, 317. [Google Scholar] [CrossRef]
- Lambeth, L.S.; Raymond, C.S.; Roeszler, K.N.; Kuroiwa, A.; Nakata, T.; Zarkower, D.; Smith, C.A. Over-expression of DMRT1 induces the male pathway in embryonic chicken gonads. Dev. Biol. 2014, 389, 160–172. [Google Scholar] [CrossRef]
- Da Silva, S.M.; Hacker, A.; Harley, V.; Goodfellow, P.; Swain, A.; Lovell-Badge, R. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat. Genet. 1996, 14, 62–68. [Google Scholar] [CrossRef]
- Takada, S.; Mano, H.; Koopman, P. Regulation of Amh during sex determination in chickens: Sox gene expression in male and female gonads. Cell. Mol. Life Sci. 2005, 62, 2140–2146. [Google Scholar] [CrossRef]
- Smith, C.A.; Katz, M.; Sinclair, A.H. DMRT1 is upregulated in the gonads during female-to-male sex reversal in ZW chicken embryos. Biol. Reprod. 2003, 68, 560–570. [Google Scholar] [CrossRef]
- Nakata, T.; Ishiguro, M.; Aduma, N.; Izumi, H.; Kuroiwa, A. Chicken hemogen homolog is involved in the chicken-specific sex-determining mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 3417–3422. [Google Scholar] [CrossRef]
- Ioannidis, J.; Taylor, G.; Zhao, D.; Liu, L.; Idoko-Akoh, A.; Gong, D.; Lovell-Badge, R.; Guioli, S.; McGrew, M.J.; Clinton, M. Primary sex determination in birds depends on DMRT1 dosage, but gonadal sex does not determine adult secondary sex characteristics. Proc. Natl. Acad. Sci. USA 2021, 118, e2020909118. [Google Scholar] [CrossRef]
- Ayers, K.L.; Lambeth, L.S.; Davidson, N.M.; Sinclair, A.H.; Oshlack, A.; Smith, C.A. Identification of candidate gonadal sex differentiation genes in the chicken embryo using RNA-seq. BMC Genom. 2015, 16, 704. [Google Scholar] [CrossRef]
- Ukeshima, A.; Fujimoto, T. A fine morphological study of germ cells in asymmetrically developing right and left ovaries of the chick. Anat. Rec. 1991, 230, 378–386. [Google Scholar] [CrossRef]
- Wan, Z.; Lu, Y.; Rui, L.; Yu, X.; Yang, F.; Tu, C.; Li, Z. Gene Expression Profiling Reveals Potential Players of Left-Right Asymmetry in Female Chicken Gonads. Int. J. Mol. Sci. 2017, 18, 1299. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Komatsu, T.; Kasahara, M.; Katoh-Fukui, Y.; Ogawa, H.; Toyama, Y.; Maekawa, M.; Toshimori, K.; Chandraratna, R.A.S.; Morohashi, K.-I.; et al. Mechanism of asymmetric ovarian development in chick embryos. Development 2008, 135, 677–685. [Google Scholar] [CrossRef]
- Intarapat, S.; Stern, C.D. Sexually Dimorphic and Sex-Independent Left-Right Asymmetries in Chicken Embryonic Gonads. PLoS ONE 2013, 8, e69893. [Google Scholar] [CrossRef]
- Przyklenk, M.; Georgieva, V.S.; Metzen, F.; Mostert, S.; Kobbe, B.; Callewaert, B.; Sengle, G.; Brachvogel, B.; Mecham, R.P.; Paulsson, M.; et al. LTBP1 promotes fibrillin incorporation into the extracellular matrix. Matrix Biol. 2022, 110, 60–75. [Google Scholar] [CrossRef]
- Hasegawa, H.; Noguchi, J.; Yamashita, M.; Okada, R.; Sugimoto, R.; Furuya, M.; Unoki, T.; Funakoshi, Y.; Baba, T.; Kanaho, Y. Phosphatidylinositol 4-Phosphate 5-Kinase Is Indispensable for Mouse Spermatogenesis1. Biol. Reprod. 2012, 86, 136. [Google Scholar] [CrossRef]
- Estermann, M.A.; Williams, S.; Hirst, C.E.; Roly, Z.Y.; Serralbo, O.; Adhikari, D.; Powell, D.; Major, A.T.; Smith, C.A. Insights into Gonadal Sex Differentiation Provided by Single-Cell Transcriptomics in the Chicken Embryo. Cell Rep. 2020, 31, 107491. [Google Scholar] [CrossRef]
- Nemeth, M.J.; Curtis, D.J.; Kirby, M.R.; Garrett-Beal, L.J.; Seidel, N.E.; Cline, A.P.; Bodine, D.M. Hmgb3: An HMG-box family member expressed in primitive hematopoietic cells that inhibits myeloid and B-cell differentiation. Blood 2003, 102, 1298–1306. [Google Scholar] [CrossRef]
- Gu, J.; Xu, T.; Huang, Q.-H.; Zhang, C.-M.; Chen, H.-Y. HMGB3 silence inhibits breast cancer cell proliferation and tumor growth by interacting with hypoxia-inducible factor 1α. Cancer Manag. Res. 2019, 11, 5075–5089. [Google Scholar] [CrossRef]
- Escalier, D.; Bai, X.-Y.; Silvius, D.; Xu, P.-X.; Xu, X. Spermatid nuclear and sperm periaxonemal anomalies in the mouseUbe2b null mutant. Mol. Reprod. Dev. 2003, 65, 298–308. [Google Scholar] [CrossRef]
- Bellott, D.W.; Skaletsky, H.; Cho, T.-J.; Brown, L.; Locke, D.; Chen, N.; Galkina, S.; Pyntikova, T.; Koutseva, N.; Graves, T.; et al. Avian W and mammalian Y chromosomes convergently retained dosage-sensitive regulators. Nat. Genet. 2017, 49, 387–394. [Google Scholar] [CrossRef]
- Nanda, I.; Zend-Ajusch, E.; Shan, Z.; Grützner, F.; Schartl, M.; Burt, D.; Koehler, M.; Fowler, V.; Goodwin, G.; Schneider, W.; et al. Conserved synteny between the chicken Z sex chromosome and human chromosome 9 includes the male regulatory gene DMRT1: A comparative (re)view on avian sex determination. Cytogenet. Genome Res. 2000, 89, 67–78. [Google Scholar] [CrossRef]
- Ayers, K.L.; Davidson, N.M.; Demiyah, D.; Roeszler, K.N.; Grützner, F.; Sinclair, A.H.; Oshlack, A.; Smith, C.A. RNA sequencing reveals sexually dimorphic gene expression before gonadal differentiation in chicken and allows comprehensive annotation of the W-chromosome. Genome Biol. 2013, 14, R26. [Google Scholar] [CrossRef]
- Raymond, C.S.; Kettlewell, J.R.; Hirsch, B.; Bardwell, V.J.; Zarkower, D. Expression of Dmrt1 in the Genital Ridge of Mouse and Chicken Embryos Suggests a Role in Vertebrate Sexual Development. Dev. Biol. 1999, 215, 208–220. [Google Scholar] [CrossRef]
- Bole-Feysot, C.; Goffin, V.; Edery, M.; Binart, N.; Kelly, P.A. Prolactin (PRL) and its receptor: Actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 1998, 19, 225–268. [Google Scholar] [CrossRef]
- Zhang, L.; Li, D.; Liu, Y.; Wang, Y.; Zhao, X.; Zhu, Q. Genetic effect of the prolactin receptor gene on egg production traits in chickens. Genet. Mol. Res. 2012, 11, 4307–4315. [Google Scholar] [CrossRef]
- Smith, C.A.; Roeszler, K.N.; Sinclair, A.H. Genetic evidence against a role for W-linked histidine triad nucleotide binding protein (HINTW) in avian sex determination. Int. J. Dev. Biol. 2009, 53, 59–67. [Google Scholar] [CrossRef]
- Bäckström, N.; Ceplitis, H.; Berlin, S.; Ellegren, H. Gene Conversion Drives the Evolution of HINTW, an Ampliconic Gene on the Female-Specific Avian W Chromosome. Mol. Biol. Evol. 2005, 22, 1992–1999. [Google Scholar] [CrossRef]
- Witschi, E. Origin of asymmetry in the reproductive system of birds. Am. J. Anat. 1935, 56, 119–141. [Google Scholar] [CrossRef]
- Lin, C.R.; Kioussi, C.; O’Connell, S.; Briata, P.; Szeto, D.; Liu, F.; Izpisúa-Belmonte, J.C.; Rosenfeld, M.G. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature 1999, 401, 279–282. [Google Scholar] [CrossRef]
- Guioli, S.; Lovell-Badge, R. PITX2 controls asymmetric gonadal development in both sexes of the chick and can rescue the degeneration of the right ovary. Development 2007, 134, 4199–4208. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Koide, M.; Ono, T.; Yasugi, S. Sex-specific and left-right asymmetric expression pattern of Bmp7 in the gonad of normal and sex-reversed chicken embryos. Dev. Growth Differ. 2005, 47, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Carré-Eusèbe, D.; Coudouel, N.; Magre, S. OVEX1, a novel chicken endogenous retrovirus with sex-specific and left-right asymmetrical expression in gonads. Retrovirology 2009, 6, 59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patricia, H.O.A.; Palma-Irizarry, M.; Carlos, S.H.J.; Carlos, L.G. Amplification of the CHD1 Gene for Molecular Sexing of Birds using Touchdown-PCR. Arch. Biochem. Mol. Biol. 2020, 1, 17–26. [Google Scholar]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Tardaguila, M.; De La Fuente, L.; Marti, C.; Pereira, C.; Pardo-Palacios, F.J.; Del Risco, H.; Ferrell, M.; Mellado, M.; Macchietto, M. SQANTI: Extensive characterization of long-read transcript sequences for quality control in full-length transcriptome identification and quantification. Genome Res. 2018, 3, 396–411. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Pheatmap. Available online: https://cran.r-project.org/web/packages/pheatmap/index.html (accessed on 4 January 2019).
- Huerta-Cepas, J.; Forslund, K.; Coelho, L.P.; Szklarczyk, D.; Jensen, L.J.; Von Mering, C.; Bork, P. Fast Genome-Wide Functional Annotation through Orthology Assignment by eggNOG-Mapper. Mol. Biol. Evol. 2017, 34, 2115–2122. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]




| Gene Name | Transcript ID | F:fpkm | M:fpkm | FC: M/F | p-Value | Gene Location |
|---|---|---|---|---|---|---|
| CETN3 | ENSGALT00000023609 | 0 | 60.7 | Inf | 0.00 | Z(+)59940636-59957144 |
| POLR1D | novel | 0 | 84.83 | Inf | 0.00 | 4(−)11170879-11177016 |
| RPL34 | ENSGALT00000085406 | 0 | 146.21 | Inf | 0.00 | 4(−)37708386-37711694 |
| NDUFV2 | ENSGALT00000071937 | 0.02 | 94.36 | Inf | 0.00 | 2(−)98648739-98662576 |
| SEC62 | ENSGALT00000015276 | 0.04 | 20.32 | 621.52 | 0.00 | 9(−)20031613-20047157 |
| EMC2 | ENSGALT00000101212 | 0.17 | 69.67 | 379.42 | 0.00 | 2(+)132291685-132326563 |
| ISCA1 | ENSGALT00000020582 | 0.18 | 29.01 | 158.32 | 0.00 | Z(−)40832095-40838966 |
| HMGB3 | ENSGALT00000014763 | 0.64 | 94.11 | 149.77 | 0.00 | 4(−)17645770-17651412 |
| IDH3A | ENSGALT00000087067 | 2.05 | 51.19 | 24.97 | 0.00 | 10(−)4228399-4240203 |
| EIF4E | ENSGALT00000051025 | 4.24 | 67.17 | 15.78 | 0.00 | 4(−)59890563-59909041 |
| REEP5 | ENSGALT00000089219 | 6.55 | 60.7 | 9.28 | 0.00 | Z(+)46073109-46090480 |
| HMGN4 | ENSGALT00000056521 | 16.03 | 70.96 | 4.39 | 0.00 | 23(−)269675-272833 |
| PSMB7 | ENSGALT00000001657 | 139.01 | 68.59 | 0.49 | 0.00 | 17(−)9673940-9693735 |
| SNRPB2 | ENSGALT00000044185 | 140.25 | 51.2 | 0.36 | 0.00 | 3(+)5652610-5658848 |
| HMGB3 | ENSGALT00000014763 | 136.13 | 44.6 | 0.33 | 0.00 | 4(−)17645769-17651424 |
| ENO1 | ENSGALT00000089190 | 575.01 | 178.4 | 0.31 | 0.00 | 21(−)3220610-3233013 |
| POLR1D | Novel | 113.88 | 18.75 | 0.16 | 0.00 | 4(−)11170880-11177013 |
| - | Novel | 180.1 | 9.41 | 0.05 | 0.00 | W(+)2134880-2136766 |
| ATP5F1AW | ENSGALT00000088356 | 215.12 | 9.38 | 0.04 | 0.00 | W(+)1391550-1415936 |
| HINTW | ENSGALT00000051715 | 2508.64 | 105.82 | 0.04 | 0.00 | W(+)1840711-1843135 |
| HINTW | novel | 158.03 | 5.86 | 0.04 | 0.00 | W(+)1840727-1843634 |
| HINTW | novel | 178.42 | 5.35 | 0.03 | 0.00 | W(+)1840716-1843899 |
| LOC107049046 | ENSGALT00000058877 | 736.13 | 24.84 | 0.03 | 0.00 | W(+)2403711-2407146 |
| RPS25 | ENSGALT00000012476 | 176.43 | 0 | 0 | 0.00 | 24(−)5664049-5665730 |
| Gene Name | Gene ID | Chr | E4.5 Gonads | E5.5 Gonads | E6.5 Gonads | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F:fpkm | M:fpkm | FC: M/F | p-Value | F:fpkm | M:fpkm | FC: M/F | p-Value | F:fpkm | M:fpkm | FC: M/F | p-Value | |||
| HINTW | ENSGALG00000035998 | W | 590.99 | 24.23 | 0.04 | 0.00 | 445.06 | 7.12 | 0.02 | 0.00 | 338.51 | 1.26 | 0.00 | 0.00 |
| LOC107049046 | ENSGALG00000040263 | W | 185.19 | 5.84 | 0.03 | 0.00 | 171.05 | 1.95 | 0.01 | 0.00 | 159.39 | 0.24 | 0.00 | 0.00 |
| HNRNPKL | ENSGALG00000040086 | W | 142.17 | 3.67 | 0.03 | 0.00 | 177.48 | 1.01 | 0.01 | 0.00 | 179.81 | 0.08 | 0.00 | 0.00 |
| ATP5F1AW | ENSGALG00000043758 | W | 99.09 | 2.87 | 0.03 | 0.00 | 131.88 | 0.61 | 0.00 | 0.00 | 154.00 | 0.10 | 0.00 | 0.00 |
| UBE2R2L | ENSGALG00000048542 | W | 28.17 | 0.65 | 0.02 | 0.00 | 38.85 | 0.17 | 0.00 | 0.00 | 42.17 | 0.02 | 0.00 | 0.00 |
| SPIN1W | ENSGALG00000040704 | W | 16.67 | 0.46 | 0.03 | 0.00 | 27.25 | 0.09 | 0.00 | 0.00 | 33.04 | 0.04 | 0.00 | 0.00 |
| UBAP2 | ENSGALG00000040780 | W | 9.62 | 0.23 | 0.02 | 0.00 | 11.05 | 0.07 | 0.01 | 0.00 | 17.55 | 0.00 | 0.00 | 0.00 |
| SMAD7B | ENSGALG00000029545 | W | 4.56 | 0.10 | 0.02 | 0.00 | 5.90 | 0.06 | 0.01 | 0.00 | 8.82 | 0.00 | 0.00 | 0.00 |
| CYP19A1 | ENSGALG00000013294 | 10 | 0.02 | 0.03 | 1.70 | 0.95 | 0.13 | 0 | 0 | 0.29 | 229.02 | 0.03 | 0.00 | 0.00 |
| FOXL2 | ENSGALG00000029282 | 9 | 0.34 | 0.61 | 1.79 | 0.48 | 3.46 | 0.33 | 0.09 | 0.00 | 17.16 | 0.45 | 0.03 | 0.00 |
| FSHR | ENSGALG00000009100 | 3 | 0.74 | 0.77 | 1.04 | 0.90 | 1.89 | 1.23 | 0.64 | 0.12 | 30.72 | 2.17 | 0.07 | 0.00 |
| ATPIF1 | ENSGALG00000038672 | 23 | 742.38 | 643.27 | 0.86 | 0.46 | 328.95 | 140.52 | 0.42 | 0.00 | 140.52 | 179.86 | 1.19 | 0.48 |
| TDH | ENSGALG00000016651 | 3 | 52.71 | 55.59 | 1.05 | 0.60 | 62.04 | 53.17 | 0.84 | 0.35 | 139.97 | 30.50 | 0.23 | 0.00 |
| PISD | ENSGALG00000006872 | 15 | 22.71 | 23.11 | 1.02 | 0.86 | 32.68 | 36.80 | 1.11 | 0.47 | 114.20 | 36.59 | 0.33 | 0.00 |
| GXYLT2 | ENSGALG00000007804 | 12 | 34.73 | 35.23 | 1.02 | 0.95 | 44.38 | 44.12 | 0.98 | 0.84 | 113.08 | 42.62 | 0.39 | 0.00 |
| SOX9 | ENSGALG00000004386 | 18 | 2.52 | 3.24 | 1.27 | 0.42 | 1.38 | 2.02 | 1.44 | 0.59 | 1.51 | 4.58 | 3.15 | 0.00 |
| AMH | ENSGALG00000036346 | 28 | 0.54 | 0.99 | 1.86 | 0.14 | 8.18 | 50.98 | 6.14 | 0.00 | 131.06 | 945.91 | 7.55 | 0.00 |
| DMRT1 | ENSGALG00000010160 | Z | 27.89 | 45.88 | 1.66 | 0.00 | 65.15 | 122.66 | 1.85 | 0.00 | 65.63 | 167.66 | 2.66 | 0.00 |
| ZFAND5 | ENSGALG00000015144 | Z | 59.77 | 119.55 | 2.01 | 0.00 | 52.50 | 82.33 | 1.54 | 0.19 | 53.93 | 58.84 | 1.13 | 0.49 |
| SMAD2Z | ENSGALG00000036001 | Z | 12.96 | 26.98 | 2.08 | 0.00 | 17.61 | 39.04 | 2.18 | 0.00 | 25.20 | 37.35 | 1.53 | 0.01 |
| PRLR | ENSGALG00000003446 | Z | 0.15 | 0.71 | 4.84 | 0.00 | 1.47 | 5.10 | 3.42 | 0.00 | 8.74 | 22.65 | 2.67 | 0.00 |
| SMC2 | ENSGALG00000015691 | Z | 87.04 | 163.27 | 1.88 | 0.00 | 108.77 | 224.85 | 2.03 | 0.00 | 123.64 | 205.75 | 1.72 | 0.00 |
| HSDL2 | ENSGALG00000015663 | Z | 108.36 | 197.35 | 1.82 | 0.00 | 160.49 | 283.46 | 1.74 | 0.00 | 175.57 | 339.56 | 2.01 | 0.00 |
| CLTA | ENSGALG00000015326 | Z | 152.30 | 268.87 | 1.76 | 0.00 | 147.40 | 250.55 | 1.67 | 0.00 | 165.76 | 393.64 | 2.47 | 0.00 |
| TAX1BP3 | ENSGALG00000004618 | 19 | 132.53 | 135.62 | 1.02 | 0.79 | 139.17 | 183.24 | 1.30 | 0.03 | 143.78 | 340.41 | 2.46 | 0.00 |
| HSPA5 | ENSGALG00000001000 | 17 | 126.60 | 137.33 | 1.09 | 0.38 | 147.36 | 203.10 | 1.36 | 0.01 | 179.84 | 348.97 | 2.02 | 0.00 |
| RPL17 | ENSGALG00000036774 | Z | 865.21 | 1518.39 | 1.75 | 0.00 | 667.40 | 1056.16 | 1.56 | 0.00 | 646.65 | 1315.70 | 2.11 | 0.00 |
| RPS6 | ENSGALG00000015082 | Z | 2416.42 | 4163.01 | 1.72 | 0.00 | 2147.00 | 3052.78 | 1.40 | 0.00 | 1866.34 | 3788.96 | 2.11 | 0.01 |
| RPL22L1 | ENSGALG00000009312 | 9 | 726.94 | 712.95 | 0.98 | 0.82 | 562.73 | 463.01 | 0.81 | 0.08 | 469.58 | 1041.19 | 2.31 | 0.05 |
| RPS23 | ENSGALG00000015617 | Z | 1715.83 | 2843.80 | 1.65 | 0.00 | 1093.88 | 1395.79 | 1.26 | 0.05 | 783.93 | 1605.17 | 2.13 | 0.05 |
| Sequence Technology | Comparison Content | Incubation Day | Gonad Sample |
|---|---|---|---|
| Illumina | DEG of male and female gonads | E4.5 | Female vs. Male |
| E5.5 | Female vs. Male | ||
| E6.5 | Female vs. Male | ||
| DEG of left and right gonads | E4.5 | Female left vs. Female right | |
| Male left vs. Male right | |||
| E5.5 | Female left vs. Female right | ||
| Male left vs. Male right | |||
| E6.5 | Female left vs. Female right | ||
| Male left vs. Male right | |||
| PacBio | DEG of male and female gonads | E4.5 | Female vs. Male |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.; Zhou, H.; Jiang, S.; He, C.; Xu, K.; Ding, J.; Liu, J.; Qin, C.; Chen, K.; Zhou, W.; et al. Gene Expression Profiling Reveals Potential Players of Sex Determination and Asymmetrical Development in Chicken Embryo Gonads. Int. J. Mol. Sci. 2023, 24, 14597. https://doi.org/10.3390/ijms241914597
Luo H, Zhou H, Jiang S, He C, Xu K, Ding J, Liu J, Qin C, Chen K, Zhou W, et al. Gene Expression Profiling Reveals Potential Players of Sex Determination and Asymmetrical Development in Chicken Embryo Gonads. International Journal of Molecular Sciences. 2023; 24(19):14597. https://doi.org/10.3390/ijms241914597
Chicago/Turabian StyleLuo, Huaixi, Hao Zhou, Shengyao Jiang, Chuan He, Ke Xu, Jinmei Ding, Jiajia Liu, Chao Qin, Kangchun Chen, Wenchuan Zhou, and et al. 2023. "Gene Expression Profiling Reveals Potential Players of Sex Determination and Asymmetrical Development in Chicken Embryo Gonads" International Journal of Molecular Sciences 24, no. 19: 14597. https://doi.org/10.3390/ijms241914597
APA StyleLuo, H., Zhou, H., Jiang, S., He, C., Xu, K., Ding, J., Liu, J., Qin, C., Chen, K., Zhou, W., Wang, L., Yang, W., Zhu, W., & Meng, H. (2023). Gene Expression Profiling Reveals Potential Players of Sex Determination and Asymmetrical Development in Chicken Embryo Gonads. International Journal of Molecular Sciences, 24(19), 14597. https://doi.org/10.3390/ijms241914597





