Atopic Dermatitis: Molecular Alterations between Lesional and Non-Lesional Skin Determined Noninvasively by In Vivo Confocal Raman Microspectroscopy
Abstract
1. Introduction
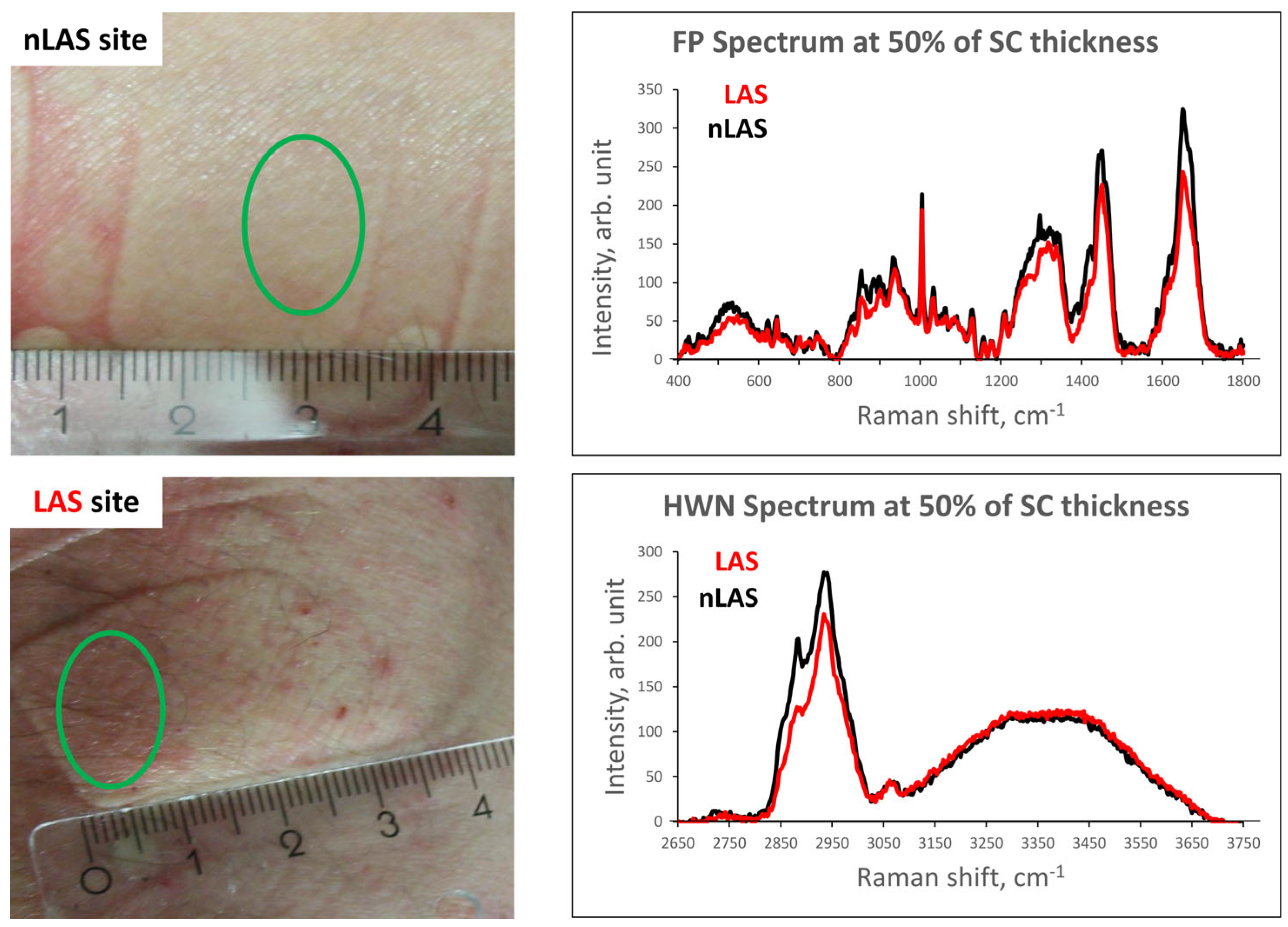
2. Results
2.1. SC Thickness
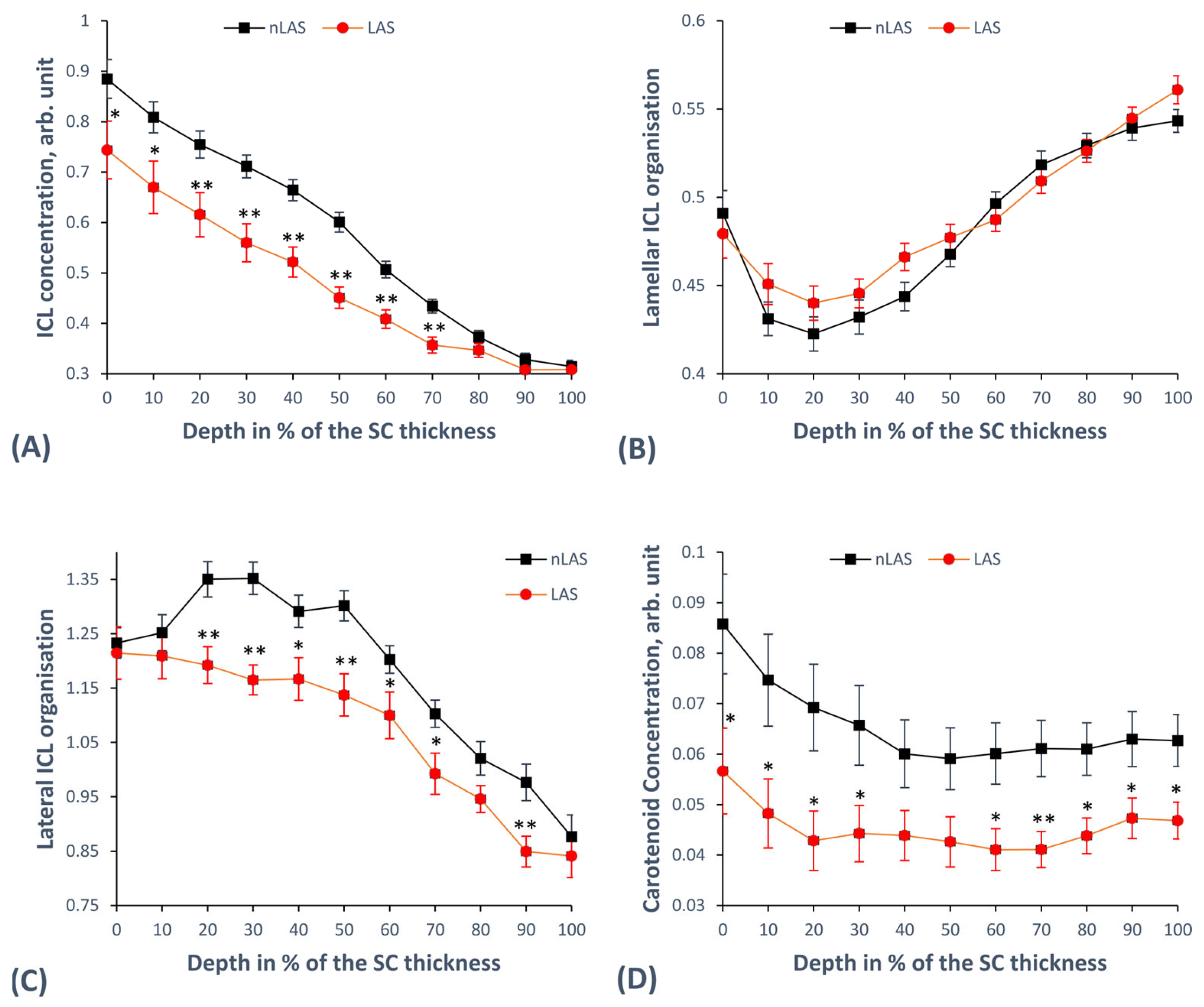
2.2. Concentration and Organisation of Intercellular Lipids
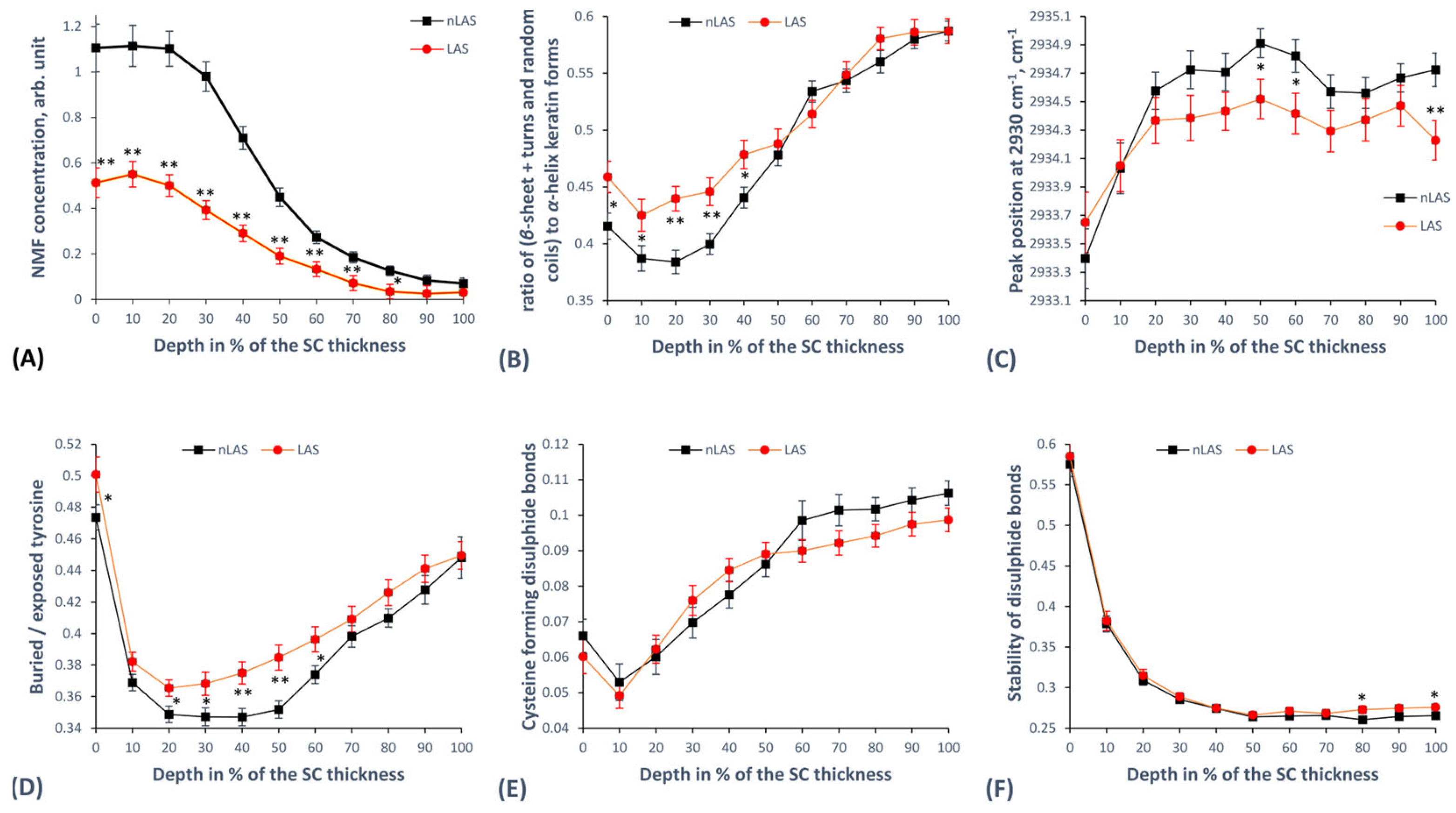
2.3. Concentration of Natural Moisturising Factor (NMF) Molecules
2.4. Keratin Structure
2.4.1. Secondary Keratin Structure
2.4.2. Tertiary Keratin Structure
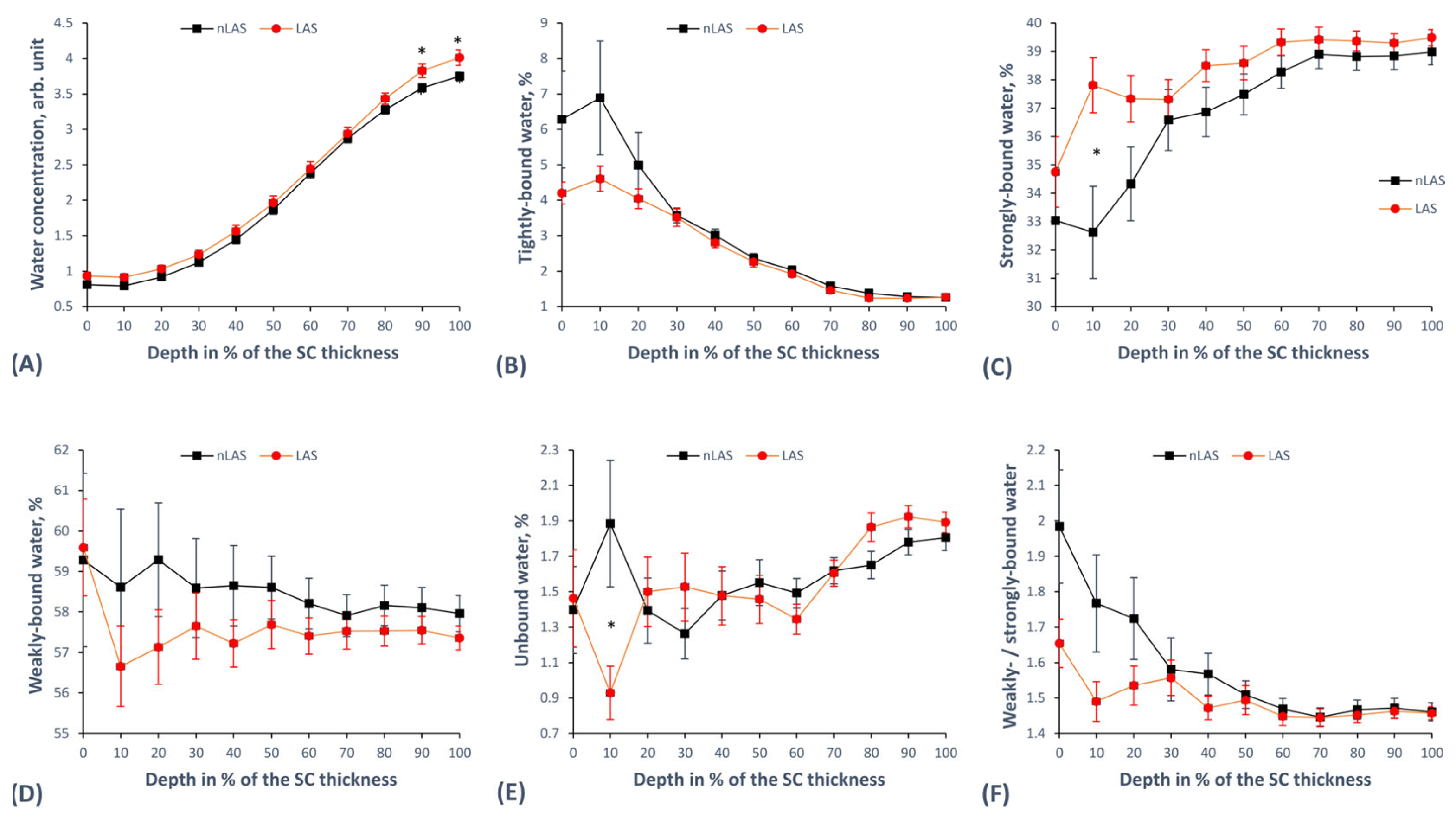
2.5. Concentration and Mobility States of Water
3. Discussion
4. Materials and Methods
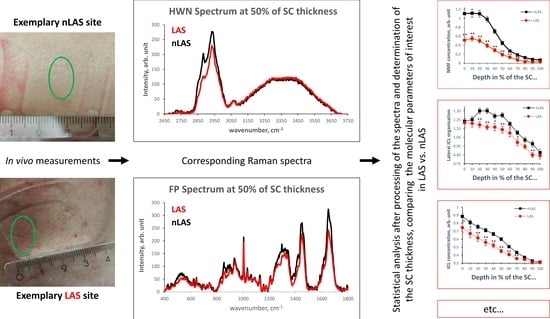
4.1. Confocal Raman Microspectroscopy (CRM)
4.2. Subjects and Measurements
4.3. Data Processing and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | atopic dermatitis |
| CRM | confocal Raman microspectroscopy |
| DA | single donor—single acceptor |
| DAA | single donor—double acceptor |
| DDA | double donor—single acceptor |
| DDAA | double donor—double acceptor |
| EASI | eczema area and severity index |
| FFA(s) | free fatty acids |
| FP | fingerprint region |
| HWN | high wavenumber region |
| HS | healthy skin, i.e., skin of healthy subjects |
| Ix | intensity at Raman peak position “x” (in cm−1) |
| IL | interleukin |
| ICL(s) | intercellular lipid(s) |
| Kx | keratin type “x” |
| LAS | lesional atopic skin |
| nLAS | non-lesional atopic skin |
| NMF(s) | natural moisturizing factor(s) |
| SC | stratum corneum |
| SBF | skin barrier function |
| SG | stratum granulosum |
| SP | serine protease(s) |
| TEWL | transepidermal water loss |
| TH2 | type 2 T-helper cells |
References
- Nettis, E.; Ortoncelli, M.; Pellacani, G.; Foti, C.; Di Leo, E.; Patruno, C.; Rongioletti, F.; Argenziano, G.; Ferrucci, S.M.; Macchia, L.; et al. A Multicenter Study on the Prevalence of Clinical Patterns and Clinical Phenotypes in Adult Atopic Dermatitis. J. Investig. Allergol. Clin. Immunol. 2020, 30, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Silvestre Salvador, J.; Romero-Pérez, D.; Encabo-Durán, B. Atopic Dermatitis in Adults: A Diagnostic Challenge. J. Investig. Allergol. Clin. Immunol. 2017, 27, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Heratizadeh, A.; Haufe, E.; Stölzl, D.; Abraham, S.; Heinrich, L.; Kleinheinz, A.; Wollenberg, A.; Weisshaar, E.; Augustin, M.; Wiemers, F.; et al. Baseline Characteristics, Disease Severity and Treatment History of Patients with Atopic Dermatitis Included in the German AD Registry TREATgermany. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef]
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular Mechanisms of Atopic Dermatitis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef]
- Cork, M.J.; Danby, S.G.; Vasilopoulos, Y.; Hadgraft, J.; Lane, M.E.; Moustafa, M.; Guy, R.H.; MacGowan, A.L.; Tazi-Ahnini, R.; Ward, S.J. Epidermal Barrier Dysfunction in Atopic Dermatitis. J. Investig. Dermatol. 2009, 129, 1892–1908. [Google Scholar] [CrossRef]
- Bin, L.; Leung, D.Y.M. Genetic and Epigenetic Studies of Atopic Dermatitis. Allergy Asthma Clin. Immunol. 2016, 12, 52. [Google Scholar] [CrossRef]
- Levin, J.; Friedlander, S.F.; Del Rosso, J.Q. Atopic Dermatitis and the Stratum Corneum: Part 1: The Role of Filaggrin in the Stratum Corneum Barrier and Atopic Skin. J. Clin. Aesthet. Dermatol. 2013, 6, 16–22. [Google Scholar]
- Levin, J.; Friedlander, S.F.; Del Rosso, J.Q. Atopic Dermatitis and the Stratum Corneum: Part 2: Other Structural and Functional Characteristics of the Stratum Corneum Barrier in Atopic Skin. J. Clin. Aesthet. Dermatol. 2013, 6, 49–54. [Google Scholar]
- Sabat, R.; Wolk, K.; Loyal, L.; Döcke, W.-D.; Ghoreschi, K. T Cell Pathology in Skin Inflammation. Semin. Immunopathol. 2019, 41, 359–377. [Google Scholar] [CrossRef]
- Worm, M.; Glatzel, V.; Baumgart, S.; Mei, H.E.; Sörensen, T.; Grützkau, A.; Heine, G. Immune Cell Profiling Reveals Natural Killer and T Cell Subpopulations to Be Associated with Atopic Dermatitis Severity. Clin. Exp. Allergy 2023, 53, 105–108. [Google Scholar] [CrossRef]
- Callard, R.E.; Harper, J.I. The Skin Barrier, Atopic Dermatitis and Allergy: A Role for Langerhans Cells? Trends Immunol. 2007, 28, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, M.; Iordanidou, M.; Vasileiou, E.; Trypsianis, G.; Chatzimichael, A.; Paraskakis, E. A Short Period of Breastfeeding in Infancy, Excessive House Cleaning, Absence of Older Sibling, and Passive Smoking Are Related to More Severe Atopic Dermatitis in Children. Eur. J. Dermatol. 2018, 28, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Schmuth, M.; Blunder, S.; Dubrac, S.; Gruber, R.; Moosbrugger-Martinz, V. Epidermal Barrier in Hereditary Ichthyoses, Atopic Dermatitis, and Psoriasis. JDDG J. Der Dtsch. Dermatol. Ges. 2015, 13, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.; Fallon Friedlander, S.; Del Rosso, J.Q. Atopic Dermatitis and the Stratum Corneum: Part 3: The Immune System in Atopic Dermatitis. J. Clin. Aesthet. Dermatol. 2013, 6, 37–44. [Google Scholar]
- Elias, P.M.; Schmuth, M. Abnormal Skin Barrier in the Etiopathogenesis of Atopic Dermatitis. Curr. Allergy Asthma Rep. 2009, 9, 265–272. [Google Scholar] [CrossRef]
- Elias, P.M.; Hatano, Y.; Williams, M.L. Basis for the Barrier Abnormality in Atopic Dermatitis: Outside-inside-Outside Pathogenic Mechanisms. J. Allergy Clin. Immunol. 2008, 121, 1337–1343. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The Skin: An Indispensable Barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Matsui, T.; Amagai, M. Dissecting the Formation, Structure and Barrier Function of the Stratum Corneum. Int. Immunol. 2015, 27, 269–280. [Google Scholar] [CrossRef]
- Pouillot, A.; Dayan, N.; Polla, A.S.; Polla, L.L.; Polla, B.S. The Stratum Corneum: A Double Paradox. J. Cosmet. Dermatol. 2008, 7, 143–148. [Google Scholar] [CrossRef]
- O’Regan, G.M.; Irvine, A.D. The Role of Filaggrin in the Atopic Diathesis. Clin. Exp. Allergy 2010, 40, 965–972. [Google Scholar] [CrossRef]
- Nemes, Z.; Steinert, P.M. Bricks and Mortar of the Epidermal Barrier. Exp. Mol. Med. 1999, 31, 5–19. [Google Scholar] [CrossRef]
- Weerheim, A.; Ponec, M. Determination of Stratum Corneum Lipid Profile by Tape Stripping in Combination with High-Performance Thin-Layer Chromatography. Arch. Dermatol. Res. 2001, 293, 191–199. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Ponec, M. The Skin Barrier in Healthy and Diseased State. Bba-Biomembr. 2006, 1758, 2080–2095. [Google Scholar] [CrossRef] [PubMed]
- Damien, F.; Boncheva, M. The Extent of Orthorhombic Lipid Phases in the Stratum Corneum Determines the Barrier Efficiency of Human Skin in Vivo. J. Investig. Dermatol. 2010, 130, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Choe, C.; Lademann, J.; Darvin, M.E. A Depth-Dependent Profile of the Lipid Conformation and Lateral Packing Order of the Stratum Corneum in Vivo Measured Using Raman Microscopy. Analyst 2016, 141, 1981–1987. [Google Scholar] [CrossRef]
- Ri, J.-S.; Choe, C.-S.; Choe, S.-H.; Jong, K.-H.; Hong, S.-N.; Schleusener, J.; Lademann, J.; Darvin, M.E. Lycopene, but Not Zeaxanthin, Serves as a Skeleton for the Formation of an Orthorhombic Organization of Intercellular Lipids within the Lamellae in the Stratum Corneum: Molecular Dynamics Simulations of the Hydrated Ceramide NS Bilayer Model. Biochim. Biophys. Acta (BBA)—Biomembr. 2023, 1865, 184081. [Google Scholar] [CrossRef]
- Darvin, M.E.; Lademann, J.; von Hagen, J.; Lohan, S.B.; Kolmar, H.; Meinke, M.C.; Jung, S. Carotenoids in Human Skin In Vivo: Antioxidant and Photo-Protectant Role against External and Internal Stressors. Antioxidants 2022, 11, 1451. [Google Scholar] [CrossRef]
- Miajlovic, H.; Fallon, P.G.; Irvine, A.D.; Foster, T.J. Effect of Filaggrin Breakdown Products on Growth of and Protein Expression by Staphylococcus Aureus. J. Allergy Clin. Immunol. 2010, 126, 1184–1190.e3. [Google Scholar] [CrossRef]
- Hata, M.; Tokura, Y.; Takigawa, M.; Sato, M.; Shioya, Y.; Fujikura, Y.; Imokawa, G. Assessment of Epidermal Barrier Function by Photoacoustic Spectrometry in Relation to Its Importance in the Pathogenesis of Atopic Dermatitis. Lab. Investig. 2002, 82, 1451–1461. [Google Scholar] [CrossRef]
- Voegeli, R.; Rawlings, A.V.; Breternitz, M.; Doppler, S.; Schreier, T.; Fluhr, J.W. Increased Stratum Corneum Serine Protease Activity in Acute Eczematous Atopic Skin. Br. J. Dermatol. 2009, 161, 70–77. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Segura-Fernández-Nogueras, M.-V.; Pérez-Rodríguez, I.; Soler-Gongora, M.; Martinez-Lopez, A.; Fernández-González, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin Barrier Function in Psoriasis and Atopic Dermatitis: Transepidermal Water Loss and Temperature as Useful Tools to Assess Disease Severity. J. Clin. Med. 2021, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- van Smeden, J.; Janssens, M.; Kaye, E.C.J.; Caspers, P.J.; Lavrijsen, A.P.; Vreeken, R.J.; Bouwstra, J.A. The Importance of Free Fatty Acid Chain Length for the Skin Barrier Function in Atopic Eczema Patients. Exp. Dermatol. 2014, 23, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Knor, T.; Meholjić-Fetahović, A.; Mehmedagić, A. Stratum Corneum Hydration and Skin Surface PH in Patients with Atopic Dermatitis. Acta Dermatovenerol. Croat. 2011, 19, 242–247. [Google Scholar] [PubMed]
- Matsuki, H.; Kiyokane, K.; Matsuki, T.; Sato, S.; Imokawa, G. Recharacterization of the Nonlesional Dry Skin in Atopic Dermatitis through Disrupted Barrier Function. Exog. Dermatol. 2004, 3, 282–292. [Google Scholar] [CrossRef]
- Eberlein-König, B.; Schäfer, T.; Huss-Marp, J.; Darsow, U.; Möhrenschlager, M.; Herbert, O.; Abeck, D.; Krämer, U.; Behrendt, H.; Ring, J. Skin Surface PH, Stratum Corneum Hydration, Trans-Epidermal Water Loss and Skin Roughness Related to Atopic Eczema and Skin Dryness in a Population of Primary School Children. Acta Derm. Venereol. 2000, 80, 188–191. [Google Scholar] [CrossRef]
- Kezic, S.; Kemperman, P.M.J.H.; Koster, E.S.; de Jongh, C.M.; Thio, H.B.; Campbell, L.E.; Irvine, A.D.; McLean, W.H.I.; Puppels, G.J.; Caspers, P.J. Loss-of-Function Mutations in the Filaggrin Gene Lead to Reduced Level of Natural Moisturizing Factor in the Stratum Corneum. J. Investig. Dermatol. 2008, 128, 2117–2119. [Google Scholar] [CrossRef]
- Palmer, C.N.A.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.D.; et al. Common Loss-of-Function Variants of the Epidermal Barrier Protein Filaggrin Are a Major Predisposing Factor for Atopic Dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef]
- Irvine, A.D. Fleshing Out Filaggrin Phenotypes. J. Investig. Dermatol. 2007, 127, 504–507. [Google Scholar] [CrossRef]
- van den Bogaard, E.H.; Elias, P.M.; Goleva, E.; Berdyshev, E.; Smits, J.P.H.; Danby, S.G.; Cork, M.J.; Leung, D.Y.M. Targeting Skin Barrier Function in Atopic Dermatitis. J. Allergy Clin. Immunol. Pract. 2023, 11, 1335–1346. [Google Scholar] [CrossRef]
- De Benedetto, A.; Rafaels, N.M.; McGirt, L.Y.; Ivanov, A.I.; Georas, S.N.; Cheadle, C.; Berger, A.E.; Zhang, K.; Vidyasagar, S.; Yoshida, T.; et al. Tight Junction Defects in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2011, 127, 773–786.e7. [Google Scholar] [CrossRef] [PubMed]
- Darlenski, R.; Kozyrskyj, A.L.; Fluhr, J.W.; Caraballo, L. Association between Barrier Impairment and Skin Microbiota in Atopic Dermatitis from a Global Perspective: Unmet Needs and Open Questions. J. Allergy Clin. Immunol. 2021, 148, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Francuzik, W.; Franke, K.; Schumann, R.R.; Heine, G.; Worm, M. Propionibacterium Acnes Abundance Correlates Inversely with Staphylococcus Aureus: Data from Atopic Dermatitis Skin Microbiome. Acta Derm.-Venereol. 2018, 98, 490–495. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef]
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The Microbiome in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2019, 143, 26–35. [Google Scholar] [CrossRef]
- Simpson, E.L.; Villarreal, M.; Jepson, B.; Rafaels, N.; David, G.; Hanifin, J.; Taylor, P.; Boguniewicz, M.; Yoshida, T.; De Benedetto, A.; et al. Patients with Atopic Dermatitis Colonized with Staphylococcus Aureus Have a Distinct Phenotype and Endotype. J. Investig. Dermatol. 2018, 138, 2224–2233. [Google Scholar] [CrossRef]
- Choi, M.J.; Maibach, H.I. Role of Ceramides in Barrier Function of Healthy and Diseased Skin. Am. J. Clin. Dermatol. 2005, 6, 215–223. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Zuberbier, T.; Darlenski, R. Noninvasive Measures in Atopic Dermatitis. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 417. [Google Scholar] [CrossRef] [PubMed]
- Caspers, P.J.; Lucassen, G.W.; Carter, E.A.; Bruining, H.A.; Puppels, G.J. In Vivo Confocal Raman Microspectroscopy of the Skin: Noninvasive Determination of Molecular Concentration Profiles. J. Investig. Dermatol. 2001, 116, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, B.P.; Venets, A.V.; Schleusener, J.; Fadeev, V.V.; Lademann, J.; Shirshin, E.A.; Darvin, M.E. Blind Source Separation of Molecular Components of the Human Skin in Vivo: Non-Negative Matrix Factorization of Raman Microspectroscopy Data. Analyst 2021, 146, 3185–3196. [Google Scholar] [CrossRef]
- Darvin, M.E.; Schleusener, J.; Lademann, J.; Choe, C.-S. Current Views on Noninvasive in Vivo Determination of Physiological Parameters of the Stratum Corneum Using Confocal Raman Microspectroscopy. Ski. Pharmacol. Physiol. 2022, 35, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Vyumvuhore, R.; Tfayli, A.; Duplan, H.; Delalleau, A.; Manfait, M.; Baillet-Guffroy, A. Raman Spectroscopy: A Tool for Biomechanical Characterization of Stratum Corneum. J. Raman Spectrosc. 2013, 44, 1077–1083. [Google Scholar] [CrossRef]
- Choe, C.; Schleusener, J.; Lademann, J.; Darvin, M.E. Human Skin in Vivo Has a Higher Skin Barrier Function than Porcine Skin Ex Vivo-Comprehensive Raman Microscopic Study of the Stratum Corneum. J. Biophotonics 2018, 11, e201700355. [Google Scholar] [CrossRef] [PubMed]
- Rigal, A.; Michael-Jubeli, R.; Nkengne, A.; Baillet-Guffroy, A.; Bigouret, A.; Tfayli, A. Raman Confocal Microscopy and Biophysics Multiparametric Characterization of the Skin Barrier Evolution with Age. J. Biophotonics 2021, 14, e202100107. [Google Scholar] [CrossRef]
- Choe, C.; Schleusener, J.; Lademann, J.; Darvin, M.E. Age Related Depth Profiles of Human Stratum Corneum Barrier-Related Molecular Parameters by Confocal Raman Microscopy in Vivo. Mech. Ageing Dev. 2018, 172, 6–12. [Google Scholar] [CrossRef]
- Wohlrab, J.; Vollmann, A.; Wartewig, S.; Marsch, W.C.; Neubert, R. Noninvasive Characterization of Human Stratum Corneum of Undiseased Skin of Patients with Atopic Dermatitis and Psoriasis as Studied by Fourier Transform Raman Spectroscopy. Biopolymers 2001, 62, 141–146. [Google Scholar] [CrossRef]
- Egawa, M.; Kunizawa, N.; Hirao, T.; Yamamoto, T.; Sakamoto, K.; Terui, T.; Tagami, H. In Vivo Characterization of the Structure and Components of Lesional Psoriatic Skin from the Observation with Raman Spectroscopy and Optical Coherence Tomography: A Pilot Study. J. Dermatol. Sci. 2010, 57, 66–69. [Google Scholar] [CrossRef]
- Zolotas, M.; Schleusener, J.; Lademann, J.; Meinke, M.C.; Kokolakis, G.; Darvin, M.E. Altered Structure Indicating Reduced Barrier Function of Lesional Compared to Non-Lesional Psoriatic Skin—A Non-Invasive in Vivo Study of the Human Stratum Corneum with Confocal Raman Micro-Spectroscopy. Exp. Dermatol. 2023. [Google Scholar] [CrossRef]
- O’Regan, G.M.; Kemperman, P.M.; Sandilands, A.; Chen, H.; Campbell, L.E.; Kroboth, K.; Watson, R.; Rowland, M.; Puppels, G.J.; McLean, W.H.; et al. Raman Profiles of the Stratum Corneum Define 3 Filaggrin Genotype-Determined Atopic Dermatitis Endophenotypes. J. Allergy Clin. Immunol. 2010, 126, 574–580.e1. [Google Scholar] [CrossRef]
- González, F.J.; Alda, J.; Moreno-Cruz, B.; Martínez-Escanamé, M.; Ramírez-Elías, M.G.; Torres-Álvarez, B.; Moncada, B. Use of Raman Spectroscopy for the Early Detection of Filaggrin-Related Atopic Dermatitis. Ski. Res. Technol. 2011, 17, 45–50. [Google Scholar] [CrossRef]
- Mlitz, V.; Latreille, J.; Gardinier, S.; Jdid, R.; Drouault, Y.; Hufnagl, P.; Eckhart, L.; Guinot, C.; Tschachler, E. Impact of Filaggrin Mutations on Raman Spectra and Biophysical Properties of the Stratum Corneum in Mild to Moderate Atopic Dermatitis. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Richters, R.J.; Falcone, D.; Uzunbajakava, N.E.; Varghese, B.; Caspers, P.J.; Puppels, G.J.; van Erp, P.E.; van de Kerkhof, P.C. Sensitive Skin: Assessment of the Skin Barrier Using Confocal Raman Microspectroscopy. Ski. Pharmacol. Physiol. 2017, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dinish, U.S.; Yik Weng, Y.; Ram, K.V.; Bi, R.; Attia, A.B.E.; Valerie, T.X.; Rajarahm, P.; Tien Guan Thng, S.; Olivo, M. Non-Invasive Biochemical Analysis and Comparison of Atopic Dermatitis and Psoriasis Skin Using Handheld Confocal Raman Spectroscopy. J. Biophotonics 2023, e202300191. [Google Scholar] [CrossRef] [PubMed]
- Dev, K.; Ho, C.J.H.; Bi, R.; Yew, Y.W.; Dinish, U.S.; Attia, A.B.E.; Moothanchery, M.; Guan, S.T.T.; Olivo, M. Machine Learning Assisted Handheld Confocal Raman Micro-Spectroscopy for Identification of Clinically Relevant Atopic Eczema Biomarkers. Sensors 2022, 22, 4674. [Google Scholar] [CrossRef] [PubMed]
- Janssens, M.; van Smeden, J.; Puppels, G.J.; Lavrijsen, A.P.; Caspers, P.J.; Bouwstra, J.A. Lipid to Protein Ratio Plays an Important Role in the Skin Barrier Function in Patients with Atopic Eczema. Br. J. Dermatol. 2014, 170, 1248–1255. [Google Scholar] [CrossRef]
- Verzeaux, L.; Vyumvuhore, R.; Boudier, D.; Le Guillou, M.; Bordes, S.; Essendoubi, M.; Manfait, M.; Closs, B. Atopic Skin: In Vivo Raman Identification of Global Molecular Signature, a Comparative Study with Healthy Skin. Exp. Dermatol. 2018, 27, 403–408. [Google Scholar] [CrossRef]
- Choe, C.; Lademann, J.; Darvin, M.E. Depth Profiles of Hydrogen Bound Water Molecule Types and Their Relation to Lipid and Protein Interaction in the Human Stratum Corneum in Vivo. Analyst 2016, 141, 6329–6337. [Google Scholar] [CrossRef]
- Choe, C.; Schleusener, J.; Lademann, J.; Darvin, M.E. Keratin-Water-NMF Interaction as a Three Layer Model in the Human Stratum Corneum Using in Vivo Confocal Raman Microscopy. Sci. Rep. 2017, 7, 15900. [Google Scholar] [CrossRef]
- Choe, C.; Ri, J.; Schleusener, J.; Lademann, J.; Darvin, M.E. The Non-Homogenous Distribution and Aggregation of Carotenoids in the Stratum Corneum Correlates with the Organization of Intercellular Lipids in Vivo. Exp. Dermatol. 2019, 28, 1237–1243. [Google Scholar] [CrossRef]
- Paquin, R.; Colomban, P. Nanomechanics of Single Keratin Fibres: A Raman Study of the Alpha-Helix ->beta-Sheet Transition and the Effect of Water. J. Raman Spectrosc. 2007, 38, 504–514. [Google Scholar] [CrossRef]
- Vyumvuhore, R.; Tfayli, A.; Duplan, H.; Delalleau, A.; Manfait, M.; Baillet-Guffroy, A. Effects of Atmospheric Relative Humidity on Stratum Corneum Structure at the Molecular Level: Ex Vivo Raman Spectroscopy Analysis. Analyst 2013, 138, 4103–4111. [Google Scholar] [CrossRef] [PubMed]
- Bragulla, H.H.; Homberger, D.G. Structure and Functions of Keratin Proteins in Simple, Stratified, Keratinized and Cornified Epithelia. J. Anat. 2009, 214, 516–559. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, W.; Edwards, H.G. Fourier-Transform Raman Spectroscopy of Mammalian and Avian Keratotic Biopolymers. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1997, 53A, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q. Local Statistical Interpretation for Water Structure. Chem. Phys. Lett. 2013, 568, 90–94. [Google Scholar] [CrossRef]
- Schleusener, J.; Salazar, A.; von Hagen, J.; Lademann, J.; Darvin, M.E. Retaining Skin Barrier Function Properties of the Stratum Corneum with Components of the Natural Moisturizing Factor-A Randomized, Placebo-Controlled Double-Blind In Vivo Study. Molecules 2021, 26, 1649. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Fölster-Holst, R.; Jensen, J.-M. Skin Barrier Function, Epidermal Proliferation and Differentiation in Eczema. J. Dermatol. Sci. 2006, 43, 159–169. [Google Scholar] [CrossRef]
- Zhang, L.; Cambron, T.; Niu, Y.; Xu, Z.; Su, N.; Zheng, H.; Wei, K.; Ray, P. MCR Approach Revealing Protein, Water, and Lipid Depth Profile in Atopic Dermatitis Patients’ Stratum Corneum via in Vivo Confocal Raman Spectroscopy. Anal. Chem. 2019, 91, 2784–2790. [Google Scholar] [CrossRef]
- Imokawa, G. Cutting Edge of the Pathogenesis of Atopic Dermatitis: Sphingomyelin Deacylase, the Enzyme Involved in Its Ceramide Deficiency, Plays a Pivotal Role. Int. J. Mol. Sci. 2021, 22, 1613. [Google Scholar] [CrossRef]
- Clausen, M.-L.; Kezic, S.; Olesen, C.M.; Agner, T. Cytokine Concentration across the Stratum Corneum in Atopic Dermatitis and Healthy Controls. Sci. Rep. 2020, 10, 21895. [Google Scholar] [CrossRef]
- Danso, M.; Boiten, W.; van Drongelen, V.; Gmelig Meijling, K.; Gooris, G.; El Ghalbzouri, A.; Absalah, S.; Vreeken, R.; Kezic, S.; van Smeden, J.; et al. Altered Expression of Epidermal Lipid Bio-Synthesis Enzymes in Atopic Dermatitis Skin Is Accompanied by Changes in Stratum Corneum Lipid Composition. J. Dermatol. Sci. 2017, 88, 57–66. [Google Scholar] [CrossRef]
- Sho, Y.; Sakai, T.; Sato, T.; Sonezaki, M.; Taima, H.; Taguchi, H.; Kaizu, K.; Nishizaka, T.; Takagi, Y.; Hatano, Y. Stratum Corneum Ceramide Profiles Provide Reliable Indicators of Remission and Potential Flares in Atopic Dermatitis. J. Investig. Dermatol. 2022, 142, 3184–3191.e7. [Google Scholar] [CrossRef] [PubMed]
- Ho, Q.W.C.; Zheng, X.; Ali, Y. Ceramide Acyl Chain Length and Its Relevance to Intracellular Lipid Regulation. Int. J. Mol. Sci. 2022, 23, 9697. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fang, H.; Dang, E.; Wang, G. The Role of Ceramides in Skin Homeostasis and Inflammatory Skin Diseases. J. Dermatol. Sci. 2020, 97, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Pullmannová, P.; Pavlíková, L.; Kováčik, A.; Sochorová, M.; Školová, B.; Slepička, P.; Maixner, J.; Zbytovská, J.; Vávrová, K. Permeability and Microstructure of Model Stratum Corneum Lipid Membranes Containing Ceramides with Long (C16) and Very Long (C24) Acyl Chains. Biophys. Chem. 2017, 224, 20–31. [Google Scholar] [CrossRef]
- Mojumdar, E.H.; Helder, R.W.J.; Gooris, G.S.; Bouwstra, J.A. Monounsaturated Fatty Acids Reduce the Barrier of Stratum Corneum Lipid Membranes by Enhancing the Formation of a Hexagonal Lateral Packing. Langmuir 2014, 30, 6534–6543. [Google Scholar] [CrossRef]
- Kake, T.; Imai, M.; Takahashi, N. Effects of β-Carotene on Oxazolone-Induced Atopic Dermatitis in Hairless Mice. Exp. Dermatol. 2019, 28, 1044–1050. [Google Scholar] [CrossRef]
- Zhang, Y.; Heinemann, N.; Rademacher, F.; Darvin, M.E.; Raab, C.; Keck, C.M.; Vollert, H.; Fluhr, J.W.; Gläser, R.; Harder, J.; et al. Skin Care Product Rich in Antioxidants and Anti-Inflammatory Natural Compounds Reduces Itching and Inflammation in the Skin of Atopic Dermatitis Patients. Antioxidants 2022, 11, 1071. [Google Scholar] [CrossRef]
- Nouwen, A.E.M.; Karadavut, D.; Pasmans, S.G.M.A.; Elbert, N.J.; Bos, L.D.N.; Nijsten, T.E.C.; Arends, N.J.T.; Pijnenburg, M.W.H.; Koljenović, S.; Puppels, G.J.; et al. Natural Moisturizing Factor as a Clinical Marker in Atopic Dermatitis. Allergy 2020, 75, 188–190. [Google Scholar] [CrossRef]
- Ekanayake-Mudiyanselage, S.; Jensen, J.-M.; Proksch, E.; Aschauer, H.; Schmook, F.P.; Meingassner, J.G. Expression of Epidermal Keratins and the Cornified Envelope Protein Involucrin Is Influenced by Permeability Barrier Disruption. J. Investig. Dermatol. 1998, 111, 517–523. [Google Scholar] [CrossRef]
- Totsuka, A.; Omori-Miyake, M.; Kawashima, M.; Yagi, J.; Tsunemi, Y. Expression of Keratin 1, Keratin 10, Desmoglein 1 and Desmocollin 1 in the Epidermis: Possible Downregulation by Interleukin-4 and Interleukin-13 in Atopic Dermatitis. Eur. J. Dermatol. 2017, 27, 247–253. [Google Scholar] [CrossRef]
- Altrichter, S.; Kriehuber, E.; Moser, J.; Valenta, R.; Kopp, T.; Stingl, G. Serum IgE Autoantibodies Target Keratinocytes in Patients with Atopic Dermatitis. J. Investig. Dermatol. 2008, 128, 2232–2239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choe, C.; Choe, S.; Schleusener, J.; Lademann, J.; Darvin, M.E. Modified Normalization Method in in Vivo Stratum Corneum Analysis Using Confocal Raman Microscopy to Compensate Nonhomogeneous Distribution of Keratin. J. Raman Spectrosc. 2019, 50, 945–957. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B. The Validity and Practicality of Sun-Reactive Skin Types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Hanifin, J.M.; Thurston, M.; Omoto, M.; Cherill, R.; Tofte, S.J.; Graeber, M.; Evaluator Group, T.E. The Eczema Area and Severity Index (EASI): Assessment of Reliability in Atopic Dermatitis. Exp. Dermatol. 2001, 10, 11–18. [Google Scholar] [CrossRef]
- Crowther, J.M.; Sieg, A.; Blenkiron, P.; Marcott, C.; Matts, P.J.; Kaczvinsky, J.R.; Rawlings, A.V. Measuring the Effects of Topical Moisturizers on Changes in Stratum Corneum Thickness, Water Gradients and Hydration in Vivo. Br. J. Dermatol. 2008, 159, 567–577. [Google Scholar] [CrossRef]
| Local EASI | Erythema | Oedema/Papulation | Excoriation | Lichenification |
|---|---|---|---|---|
| median | 2 | 1 | 0.5 | 0 |
| maximum | 3 | 1 | 3 | 1 |
| minimum | 1 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zolotas, M.; Schleusener, J.; Lademann, J.; Meinke, M.C.; Kokolakis, G.; Darvin, M.E. Atopic Dermatitis: Molecular Alterations between Lesional and Non-Lesional Skin Determined Noninvasively by In Vivo Confocal Raman Microspectroscopy. Int. J. Mol. Sci. 2023, 24, 14636. https://doi.org/10.3390/ijms241914636
Zolotas M, Schleusener J, Lademann J, Meinke MC, Kokolakis G, Darvin ME. Atopic Dermatitis: Molecular Alterations between Lesional and Non-Lesional Skin Determined Noninvasively by In Vivo Confocal Raman Microspectroscopy. International Journal of Molecular Sciences. 2023; 24(19):14636. https://doi.org/10.3390/ijms241914636
Chicago/Turabian StyleZolotas, Michael, Johannes Schleusener, Jürgen Lademann, Martina C. Meinke, Georgios Kokolakis, and Maxim E. Darvin. 2023. "Atopic Dermatitis: Molecular Alterations between Lesional and Non-Lesional Skin Determined Noninvasively by In Vivo Confocal Raman Microspectroscopy" International Journal of Molecular Sciences 24, no. 19: 14636. https://doi.org/10.3390/ijms241914636
APA StyleZolotas, M., Schleusener, J., Lademann, J., Meinke, M. C., Kokolakis, G., & Darvin, M. E. (2023). Atopic Dermatitis: Molecular Alterations between Lesional and Non-Lesional Skin Determined Noninvasively by In Vivo Confocal Raman Microspectroscopy. International Journal of Molecular Sciences, 24(19), 14636. https://doi.org/10.3390/ijms241914636