Anhydrous Alum Inhibits α-MSH-Induced Melanogenesis by Down-Regulating MITF via Dual Modulation of CREB and ERK
Abstract
:1. Introduction
2. Results
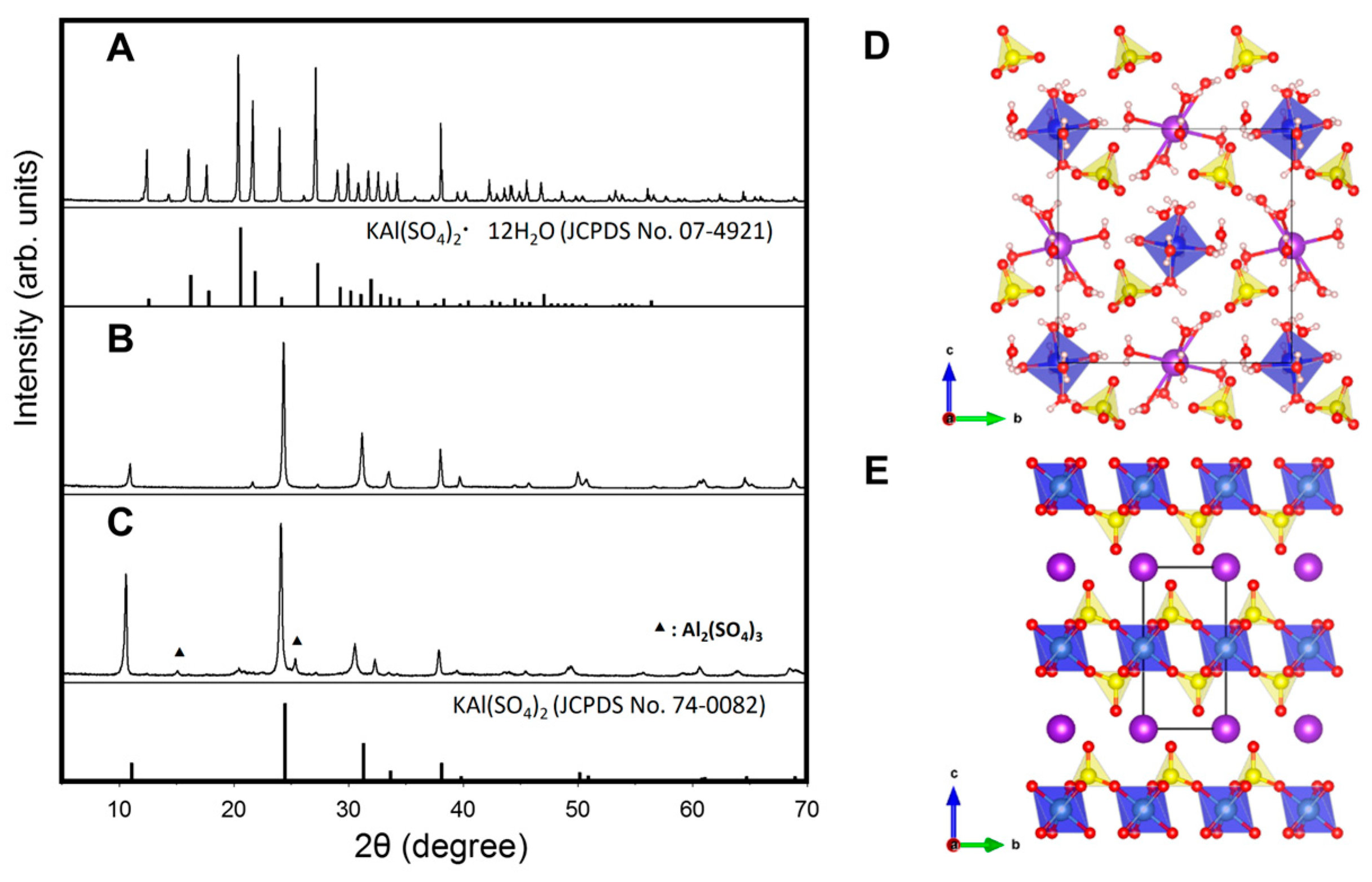
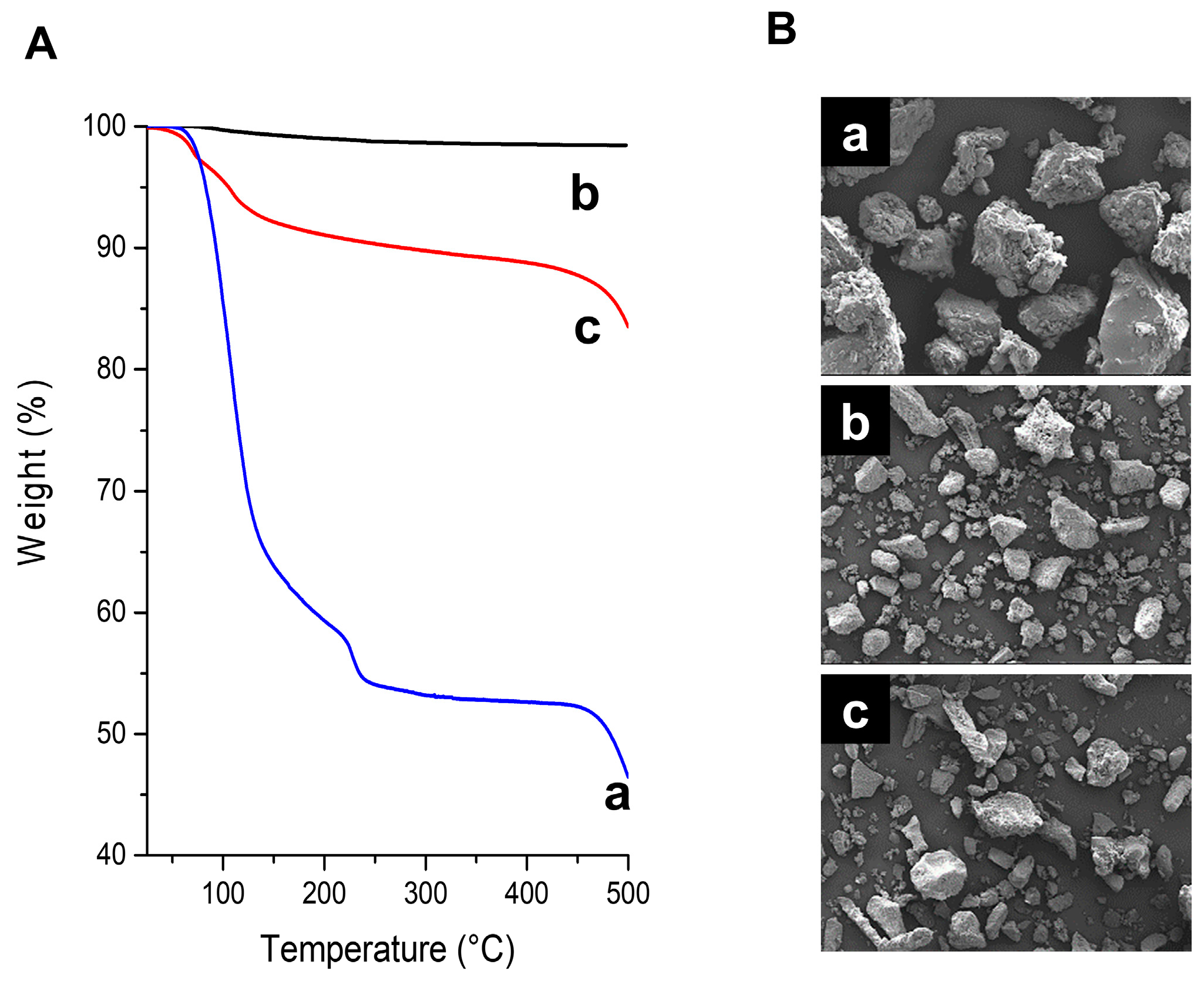
2.1. Structural Characterization of Pure Anhydrous Alum
2.2. Pure Anhydrous Alum Inhibited Melanogenesis with Little Cytotoxicity
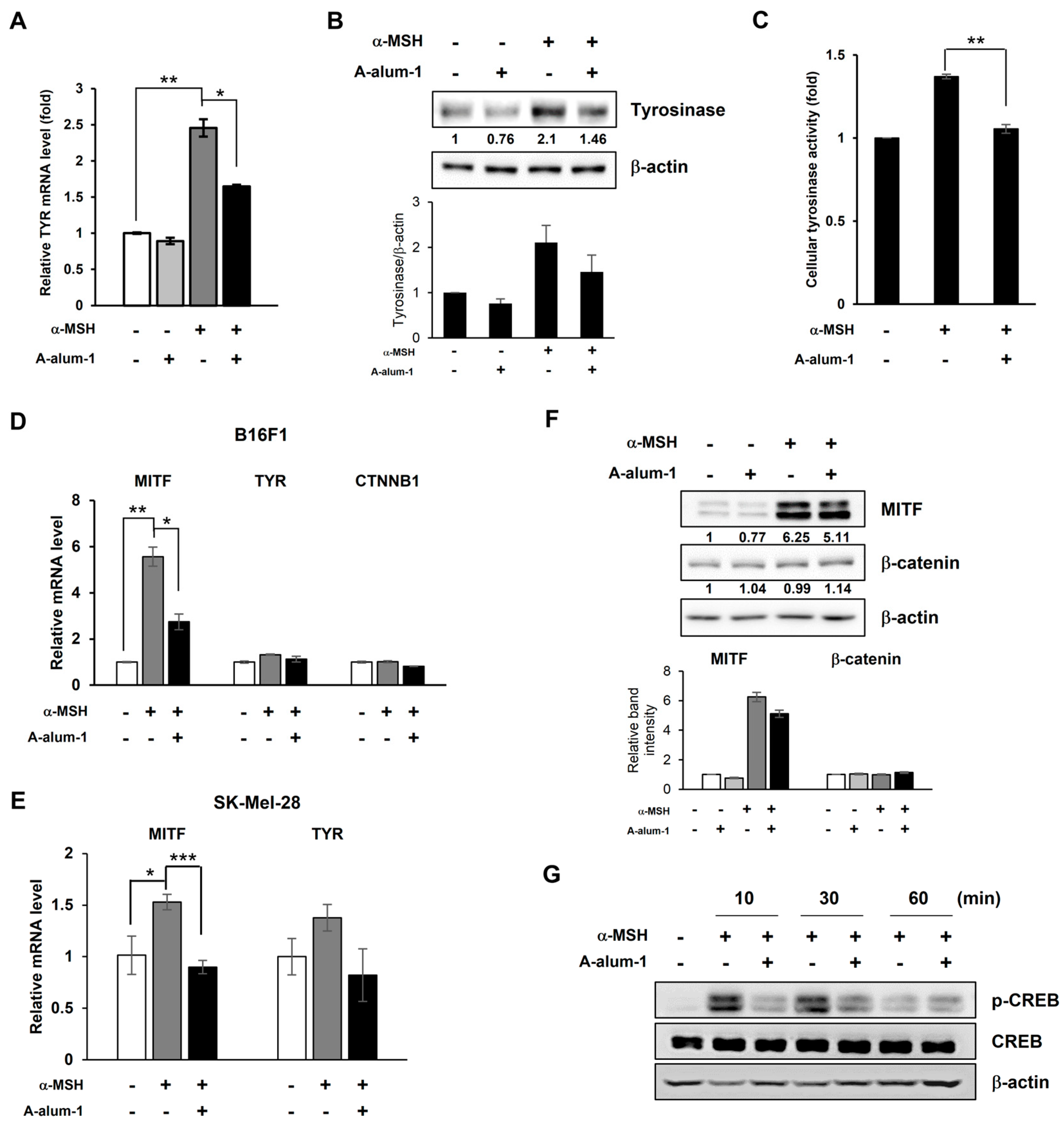
2.3. A-Alum-1 Suppresses Tyrosinase Gene Expression by Reducing MITF Expression in α-MSH-Induced B16F1 Cells
2.4. A-Alum-1 Decreases α-MSH-Induced MITF Expression via Inhibiting CREB Phosphorylation in B16F1 Cells
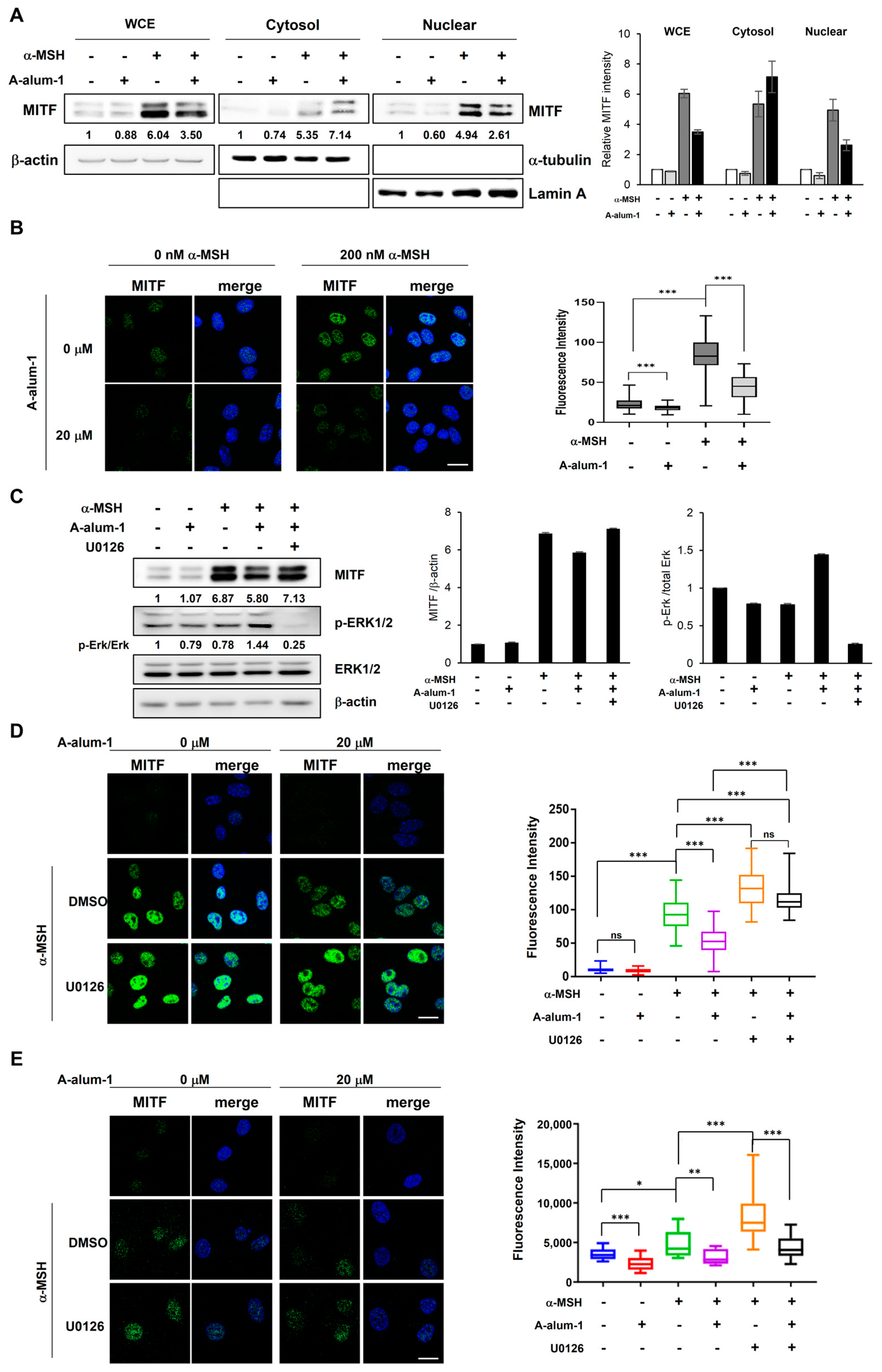
2.5. A-Alum-1 Inhibits Nuclear Localization of MITF in α-MSH-Induced Melanoma Cells
2.6. A-Alum-1 Inhibits the Nuclear Localization of MITF through Activation of Erk1/2 Signal Pathway in α-MSH-Induced Melanoma Cells
3. Discussion
4. Materials and Methods
4.1. Preparation and Characterization of Anhydrous Alum
4.2. Cells and Reagents
4.3. Cell Viability Assay
4.4. Melanin Content and Tyrosinase Activity Assays
4.5. RNA Isolation and Quantitative Reverse Transcription PCR (qRT-PCR)
4.6. Western Blot Analysis
4.7. Nuclear and Cytoplasm Subcellular Fractionation
4.8. Immunofluorescence
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Aramwit, P.; Luplertlop, N.; Kanjanapruthipong, T.; Ampawong, S. Effect of urea-extracted sericin on melanogenesis: Potential applications in post-inflammatory hyperpigmentation. Biol. Res. 2018, 51, 54. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Thng, S.T.G.; Verma, N.K.; Gautam, H.K. Melanogenesis inhibitors. Acta Derm. Venereol. 2018, 98, 924–931. [Google Scholar] [CrossRef]
- Lee, A.Y. Skin Pigmentation Abnormalities and Their Possible Relationship with Skin Aging. Int. J. Mol. Sci. 2021, 22, 3727. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Recent development of signaling pathways inhibitors of melanogenesis. Cell. Signal 2017, 40, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef] [PubMed]
- Jimbow, K.; Obata, H.; Pathak, M.A.; Fitzpatrick, T.B. Mechanism of depigmentation by hydroquinone. J. Investig. Dermatol. 1974, 62, 436–449. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef]
- Xu, K.X.; Xue, M.G.; Li, Z.; Ye, B.C.; Zhang, B. Recent Progress on Feasible Strategies for Arbutin Production. Front. Bioeng. Biotechnol. 2022, 10, 914280. [Google Scholar] [CrossRef]
- Jang, E.J.; Shin, Y.; Park, H.J.; Kim, D.; Jung, C.; Hong, J.-Y.; Kim, S.; Lee, S.K. Anti-melanogenic activity of phytosphingosine via the modulation of the microphthalmia-associated transcription factor signaling pathway. J. Dermatol. Sci. 2017, 87, 19–28. [Google Scholar] [CrossRef]
- del Marmol, V.; Beermann, F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef]
- Qu, Y.; Zhan, Q.; Du, S.; Ding, Y.; Fang, B.; Du, W.; Wu, Q.; Yu, H.; Li, L.; Huang, W. Catalysis-based specific detection and inhibition of tyrosinase and their application. J. Pharm. Anal. 2020, 10, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Orlow, S.J.; Zhou, B.K.; Chakraborty, A.K.; Drucker, M.; Pifko-Hirst, S.; Pawelek, J.M. High-molecular-weight forms of tyrosinase and the tyrosinase-related proteins: Evidence for a melanogenic complex. J. Investig. Dermatol. 1994, 103, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-S. Natural Melanogenesis Inhibitors Acting Through the Down-Regulation of Tyrosinase Activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Kadekaro, A.L.; Kavanagh, R.; Kanto, H.; Terzieva, S.; Hauser, J.; Kobayashi, N.; Schwemberger, S.; Cornelius, J.; Babcock, G.; Shertzer, H.G.; et al. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005, 65, 4292–4299. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Slominski, R.M.; Raman, C.; Chen, J.Y.; Athar, M.; Elmets, C. Neuroendocrine signaling in the skin with a special focus on the epidermal neuropeptides. Am. J. Physiol. Cell Physiol. 2022, 323, C1757–C1776. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef]
- Lee, S.-H. Attenuation of melanogenesis by Nymphaea nouchali (Burm. f) flower extract through the regulation of cAMP/CREB/MAPKs/MITF and proteasomal degradation of tyrosinase. Sci. Rep. 2018, 8, 13928. [Google Scholar] [CrossRef]
- Yun, C.-Y.; You, S.-T.; Kim, J.-H.; Chung, J.H.; Han, S.-B.; Shin, E.-Y.; Kim, E.-G. p21-activated kinase 4 critically regulates melanogenesis via activation of the CREB/MITF and β-catenin/MITF pathways. J. Investig. Dermatol. 2015, 135, 1385–1394. [Google Scholar] [CrossRef]
- Cho, M.; Ryu, M.; Jeong, Y.; Chung, Y.H.; Kim, D.E.; Cho, H.S.; Kang, S.; Han, J.S.; Chang, M.Y.; Lee, C.K.; et al. Cardamonin suppresses melanogenesis by inhibition of Wnt/beta-catenin signaling. Biochem. Biophys. Res. Commun. 2009, 390, 500–505. [Google Scholar] [CrossRef]
- Shiino, M.; Watanabe, Y.; Umezawa, K. Synthesis and tyrosinase inhibitory activity of novel N-hydroxybenzyl-N-nitrosohydroxylamines. Bioorg. Chem. 2003, 31, 129–135. [Google Scholar] [CrossRef]
- Brtko, J. Biological functions of kojic acid and its derivatives in medicine, cosmetics, and food industry: Insights into health aspects. Arch. Pharm. 2022, 355, e2200215. [Google Scholar] [CrossRef] [PubMed]
- Hasssan, M.; Shahzadi, S.; Kloczkowski, A. Tyrosinase Inhibitors Naturally Present in Plants and Synthetic Modifications of These Natural Products as Anti-Melanogenic Agents: A Review. Molecules 2023, 28, 378. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Kinst-Hori, I.; Kubo, Y.; Yamagiwa, Y.; Kamikawa, T.; Haraguchi, H. Molecular design of antibrowning agents. J. Agric. Food Chem. 2000, 48, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, Z.R.; Chen, P.; Yang, L.; Deng, W.R.; Wang, Y.Q.; Li, H.Y. Effect of the tyrosinase inhibitor (S)-N-trans-feruloyloctopamine from garlic skin on tyrosinase gene expression and melanine accumulation in melanoma cells. Bioorg. Med. Chem. Lett. 2015, 25, 1476–1478. [Google Scholar] [CrossRef]
- Parvez, S.; Kang, M.; Chung, H.S.; Cho, C.; Hong, M.C.; Shin, M.K.; Bae, H. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. 2006, 20, 921–934. [Google Scholar] [CrossRef]
- Luo, L.; Jiang, L.; Geng, C.; Cao, J.; Zhong, L. Hydroquinone-induced genotoxicity and oxidative DNA damage in HepG2 cells. Chem. Biol. Interact. 2008, 173, 1–8. [Google Scholar] [CrossRef]
- Enguita, F.J.; Leitão, A.L. Hydroquinone: Environmental pollution, toxicity, and microbial answers. BioMed Res. Int. 2013, 2013, 542168. [Google Scholar] [CrossRef]
- Chae, J.K.; Subedi, L.; Jeong, M.; Park, Y.U.; Kim, C.Y.; Kim, H.; Kim, S.Y. Gomisin N inhibits melanogenesis through regulating the PI3K/Akt and MAPK/ERK signaling pathways in melanocytes. Int. J. Mol. Sci. 2017, 18, 471. [Google Scholar] [CrossRef]
- Nyburg, S.C.; Steed, J.W.; Aleksovska, S.; Petrusevski, V. Structure of the alums. I. On the sulfate group disorder in the α-alums. Acta Crystallogr. B Struct. Sci. Crist. Eng. Mater. 2000, 56, 204–209. [Google Scholar] [CrossRef]
- West, D.; Huang, Q.; Zandbergen, H.; McQueen, T.; Cava, R. Structural disorder, octahedral coordination and two-dimensional ferromagnetism in anhydrous alums. J. Solid State Chem. 2008, 181, 2768–2775. [Google Scholar] [CrossRef]
- Kishimura, H.; Matsumoto, H. Structural changes on hydrous and anhydrous potash alum caused by mechanical milling. Part. Sci. Technol. 2018, 37, 820–826. [Google Scholar] [CrossRef]
- Souza, R.; Navarro, R.; Grillo, A.V.; Brocchi, E. Potassium alum thermal decomposition study under non-reductive and reductive conditions. J. Mater. Res. Technol. 2019, 8, 745–751. [Google Scholar] [CrossRef]
- Seo, H.-S. An Experimental Study of the Anti-oxidant and the Anti-inflammatory Effects of Alum and Burnt Alum. J. Pharmacopunct. 2012, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Ahn, S.S.; Kim, J.B.; Lim, Y.; Lee, Y.H.; Shin, S.Y. Downregulation of α-Melanocyte-Stimulating Hormone-Induced Activation of the Pax3-MITF-Tyrosinase Axis by Sorghum Ethanolic Extract in B16F10 Melanoma Cells. Int. J. Mol. Sci. 2018, 19, 1640. [Google Scholar] [CrossRef]
- Shibahara, S.; Takeda, K.; Yasumoto, K.; Udono, T.; Watanabe, K.; Saito, H.; Takahashi, K. Microphthalmia-associated transcription factor (MITF): Multiplicity in structure, function, and regulation. J. Investig. Dermatol. Symp. Proc. 2001, 6, 99–104. [Google Scholar] [CrossRef]
- Ngeow, K.C.; Friedrichsen, H.J.; Li, L.; Zeng, Z.; Andrews, S.; Volpon, L.; Brunsdon, H.; Berridge, G.; Picaud, S.; Fischer, R.; et al. BRAF/MAPK and GSK3 signaling converges to control MITF nuclear export. Proc. Natl. Acad. Sci. USA 2018, 115, E8668–E8677. [Google Scholar] [CrossRef]
- Lim, J.W.; Ha, J.H.; Jeong, Y.J.; Park, S.N. Anti-melanogenesis effect of dehydroglyasperin C through the downregulation of MITF via the reduction of intracellular cAMP and acceleration of ERK activation in B16F1 melanoma cells. Pharmacol. Rep. 2018, 70, 930–935. [Google Scholar] [CrossRef]
- Yang, Y.M.; Son, Y.O.; Lee, S.A.; Jeon, Y.M.; Lee, J.C. Quercetin inhibits α-MSH-stimulated melanogenesis in B16F10 melanoma cells. Phytother. Res. 2011, 25, 1166–1173. [Google Scholar] [CrossRef]
- Saha, B.; Singh, S.K.; Sarkar, C.; Bera, R.; Ratha, J.; Tobin, D.J.; Bhadra, R. Activation of the Mitf promoter by lipid-stimulated activation of p38-stress signalling to CREB. Pigment. Cell Res. 2006, 19, 595–605. [Google Scholar] [CrossRef]
- O’brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Ko, S.C.; Lee, S.H. Protocatechuic Aldehyde Inhibits α-MSH-Induced Melanogenesis in B16F10 Melanoma Cells via PKA/CREB-Associated MITF Downregulation. Int. J. Mol. Sci. 2021, 22, 3861. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kang, B.; Kim, J.C.; Park, T.J.; Kang, H.Y. Senescent fibroblast-derived GDF15 induces skin pigmentation. J. Investig. Dermatol. 2020, 140, 2478–2486. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
In, K.-R.; Kang, M.A.; Kim, S.D.; Shin, J.; Kang, S.U.; Park, T.J.; Kim, S.-J.; Lee, J.-S. Anhydrous Alum Inhibits α-MSH-Induced Melanogenesis by Down-Regulating MITF via Dual Modulation of CREB and ERK. Int. J. Mol. Sci. 2023, 24, 14662. https://doi.org/10.3390/ijms241914662
In K-R, Kang MA, Kim SD, Shin J, Kang SU, Park TJ, Kim S-J, Lee J-S. Anhydrous Alum Inhibits α-MSH-Induced Melanogenesis by Down-Regulating MITF via Dual Modulation of CREB and ERK. International Journal of Molecular Sciences. 2023; 24(19):14662. https://doi.org/10.3390/ijms241914662
Chicago/Turabian StyleIn, Kyu-Ree, Mi Ae Kang, Su Dong Kim, Jinho Shin, Sung Un Kang, Tae Jun Park, Seung-Joo Kim, and Jong-Soo Lee. 2023. "Anhydrous Alum Inhibits α-MSH-Induced Melanogenesis by Down-Regulating MITF via Dual Modulation of CREB and ERK" International Journal of Molecular Sciences 24, no. 19: 14662. https://doi.org/10.3390/ijms241914662
APA StyleIn, K.-R., Kang, M. A., Kim, S. D., Shin, J., Kang, S. U., Park, T. J., Kim, S.-J., & Lee, J.-S. (2023). Anhydrous Alum Inhibits α-MSH-Induced Melanogenesis by Down-Regulating MITF via Dual Modulation of CREB and ERK. International Journal of Molecular Sciences, 24(19), 14662. https://doi.org/10.3390/ijms241914662






