Application of Pluronics for Enhancing Aqueous Solubility of Lipophilic Microtubule Destabilizing Compounds on the Sea Urchin Embryo Model
Abstract
:1. Introduction
2. Results and Discussion
2.1. Solubilization of Chalcones III and IV Using Pluronics P85, P123, and F127
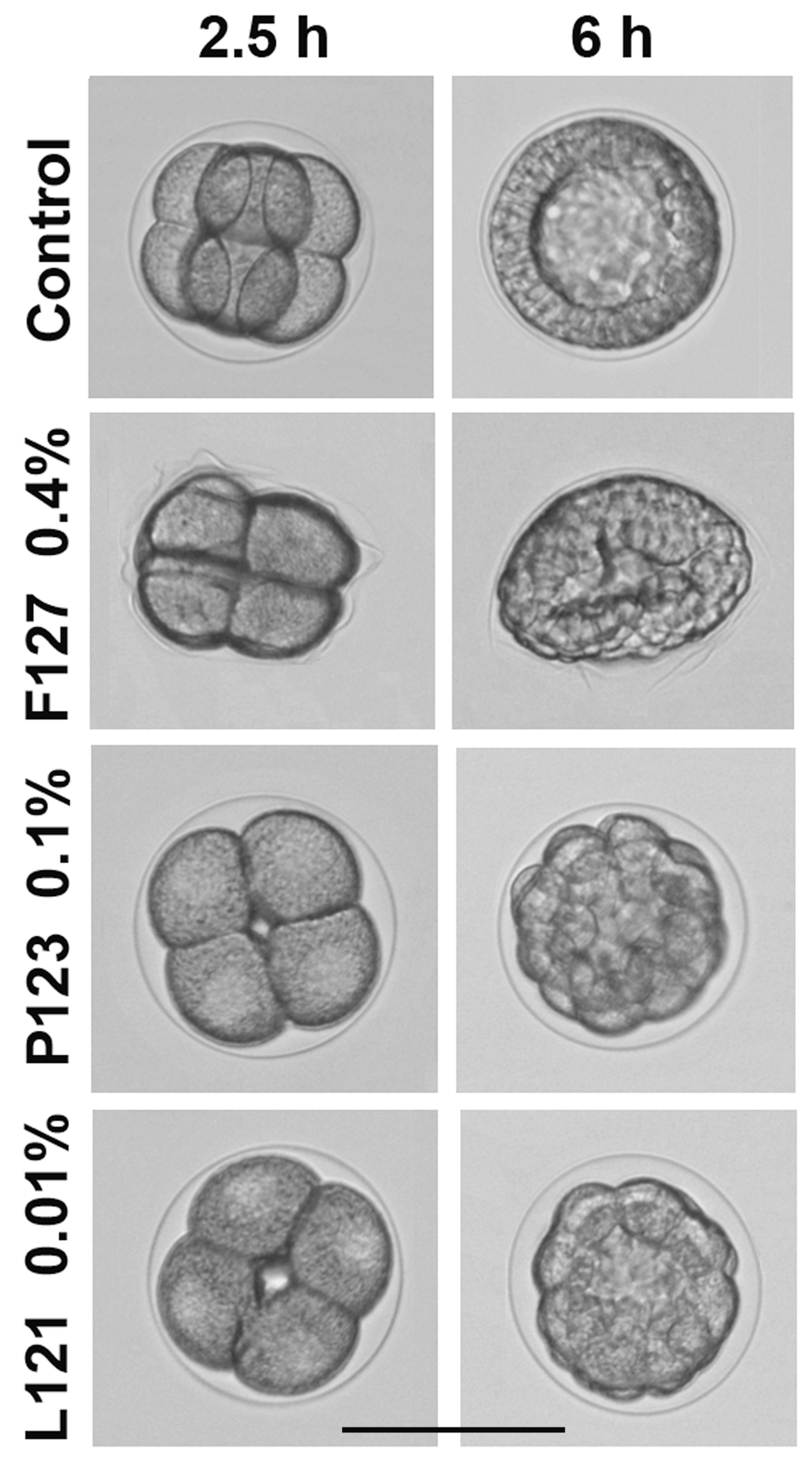
2.2. Study of Pluronic Toxicity on the Sea Urchin Embryo Model
2.3. Evaluation of Pluronic F127 and Pluronic P123 CMC in Seawater at Ambient Temperatures

2.4. Application of Pluronics to Enhance the Solubility of Lipophilic Tubulin-Targeting Antimitotics in the Sea Urchin Embryo Assay
3. Materials and Methods
3.1. Materials
3.2. Evaluation of Aqueous Solubility of Lipophilic Microtubule Destabilizing Compounds
3.3. CMC Determination Procedure
3.4. Phenotypic Sea Urchin Embryo Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMC | critical micelle concentration |
| DPH | 1,6-diphenyl-1,3,5-hexatriene |
| FSW | filtered seawater |
| HLB | hydrophilic-lipophilic balance |
| MDR | multidrug resistant |
| MEC | minimal effective concentration |
| MLC | minimal lethal concentration |
| NOEC | no observed effect concentration |
| PEO | polyethylene oxide |
| PPO | polypropylene oxide |
References
- Popa-Burke, I.G.; Issakova, O.; Arroway, J.D.; Bernasconi, P.; Chen, M.; Coudurier, L.; Galasinski, S.; Jadhav, A.P.; Janzen, W.P.; Lagasca, D.; et al. Streamlined system for purifying and quantifying a diverse library of compounds and the effect of compound concentration measurements on the accurate interpretation of biological assay results. Anal. Chem. 2004, 76, 7278–7287. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C. Molecular Drug Properties. Measurement and Prediction; Mannhold, R., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 257–282. [Google Scholar]
- Semenova, M.N.; Demchuk, D.V.; Tsyganov, D.V.; Chernysheva, N.B.; Samet, A.V.; Silyanova, E.A.; Kislyi, V.P.; Maksimenko, A.S.; Varakutin, A.E.; Konyushkin, L.D.; et al. Sea urchin embryo model as a reliable in vivo phenotypic screen to characterize selective antimitotic molecules. Comparative evaluation of combretapyrazoles, -isoxazoles, -1,2,3-triazoles, and -pyrroles as tubulin-binding agents. ACS Comb. Sci. 2018, 20, 700–721. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.; Lee, S.; Li, S.; Venne, A.; Alakhov, V.; Kabanov, A. Fundamental relationships between the composition of pluronic block copolymers and their hypersensitization effect in MDR cancer cells. Pharm. Res. 1999, 16, 1373–1379. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Li, S.; Alakhov, V.Y.; Miller, D.W.; Kabanov, A.V. Optimal structure requirements for pluronic block copolymers in modifying P-glycoprotein drug efflux transporter activity in bovine brain microvessel endothelial cells. J. Pharmacol. Exp. Ther. 2003, 304, 845–854. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kabanov, A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 2008, 130, 98–106. [Google Scholar] [CrossRef]
- Apte, S. Selecting surfactants for the maximum inhibition of the activity of the multidrug resistance efflux pump transporter, P-glycoprotein: Conceptual development. J. Excip. Food Chem. 2010, 1, 51–59. Available online: https://ojs.abo.fi/ojs/index.php/jefc/article/view/43 (accessed on 5 June 2022).
- Russo, E.; Villa, C. Poloxamer hydrogels for biomedical applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Singh-Joy, S.D.; McLain, V.C. Safety assessment of poloxamers 101, 105, 108, 122, 123, 124, 181, 182, 183, 184, 185, 188, 212, 215, 217, 231, 234, 235, 237, 238, 282, 284, 288, 331, 333, 334, 335, 338, 401, 402, 403, and 407, poloxamer 105 benzoate, and poloxamer 182 dibenzoate as used in cosmetics. Int. J. Toxicol. 2008, 27 (Suppl. S2), 93–128. [Google Scholar] [CrossRef] [PubMed]
- FDA. Inactive Ingredient Search for Approved Drug Products. Available online: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm (accessed on 18 February 2022).
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Jarak, I.; Varela, C.L.; Tavares da Silva, E.; Roleira, F.F.M.; Veiga, F.; Figueiras, A. Pluronic-based nanovehicles: Recent advances in anticancer therapeutic applications. Eur. J. Med. Chem. 2020, 206, 112526. [Google Scholar] [CrossRef]
- Kozlov, M.Y.; Melik-Nubarov, N.S.; Batrakova, E.V.; Kabanov, A.V. Relationship between Pluronic block copolymer structure, critical micellization concentration and partitioning coefficients of low molecular mass solutes. Macromolecules 2000, 33, 3305–3313. [Google Scholar] [CrossRef]
- Guo, X.; Rong, Z.; Ying, X. Calculation of hydrophile–lipophile balance for polyethoxylated surfactants by group contribution method. J. Colloid Interf. Sci. 2006, 298, 441–450. [Google Scholar] [CrossRef]
- Ash, M.; Ash, I. Handbook of Industrial Surfactants; Routledge: New York, NY, USA, 2019. [Google Scholar]
- Alexandridis, P.; Hatton, T.A. Poly(ethylene oxide) poly(propylene oxide) poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloid Surface A 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Su, Y.-L.; Liu, H.-Z. Temperature-dependent solubilization of PEO-PPO-PEO block copolymers and their application for extraction trace organics from aqueous solutions. Korean J. Chem. Eng. 2003, 20, 343–346. [Google Scholar] [CrossRef]
- Desai, P.R.; Jain, N.J.; Sharma, R.K.; Bahadur, P. Effect of additives on the micellization of PEO/PPO/PEO block copolymer F127 in aqueous solution. Colloid Surface A 2001, 178, 57–69. [Google Scholar] [CrossRef]
- Su, Y.-L.; Wei, X.-F.; Liu, H.-Z. Effect of sodium chloride on association behavior of poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) block copolymer in aqueous solutions. J. Colloid Interf. Sci. 2003, 264, 526–531. [Google Scholar] [CrossRef]
- Chiappetta, D.A.; Sosnik, A. Poly(ethylene oxide)–poly(propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs. Eur. J. Pharm. Biopharm. 2007, 66, 303–317. [Google Scholar] [CrossRef]
- Raval, A.; Pillai, S.A.; Bahadur, A.; Bahadur, P. Systematic characterization of Pluronic® micelles and their application for solubilization and in vitro release of some hydrophobic anticancer drugs. J. Mol. Liq. 2017, 230, 473–481. [Google Scholar] [CrossRef]
- Paterson, I.F.; Chowdhry, B.Z.; Leharne, S.A. Investigations of naphthalene solubilization in aqueous solutions of ethylene oxide-b-propylene oxide-b-ethylene oxide copolymers. Langmuir 1999, 15, 6187–6194. [Google Scholar] [CrossRef]
- Budkina, O.A.; Demina, T.V.; Dorodnykh, T.Y.; Melik-Nubarov, N.S.; Grozdova, I.D. Cytotoxicity of nonionic amphiphilic copolymers. Polym. Sci. Ser. A 2012, 54, 707–717. [Google Scholar] [CrossRef]
- Grozdova, I.D.; Melik-Nubarov, N.S. Concentration control of chemosensitizing, cell protectiveness, and cytotoxic properties of Pluronics. ACS Appl. Polym. Mater. 2022, 4, 8764–8773. [Google Scholar] [CrossRef]
- Wanka, G.; Hoffmann, H.; Ulbricht, W. Phase diagrams and aggregation behavior of poly(oxyethylene)-poly(oxypropylene)-poly(oxyethylene) triblock copolymers in aqueous solutions. Macromolecules 1994, 27, 4145–4159. [Google Scholar] [CrossRef]
- Exner, A.A.; Krupka, T.M.; Scherrer, K.; Teets, J.M. Enhancement of carboplatin toxicity by Pluronic block copolymers. J. Control. Release 2005, 106, 188–197. [Google Scholar] [CrossRef]
- Khattak, S.F.; Bhatia, S.R.; Roberts, S.C. Pluronic F127 as a cell encapsulation material: Utilization of membrane-stabilizing agents. Tissue Eng. 2005, 11, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.; Braunbeck, T. Bioavailability of microplastic-bound pollutants in vitro: The role of adsorbate lipophilicity and surfactants. Comp. Biochem. Phys. C 2019, 221, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Hering, I.; Eilebrecht, E.; Parnham, M.J.; Günday-Türeli, N.; Türeli, A.E.; Weiler, M.; Schäfers, C.; Fenske, M.; Wacker, M.G. Evaluation of potential environmental toxicity of polymeric nanomaterials and surfactants. Environ. Toxicol. Phar. 2020, 76, 103353. [Google Scholar] [CrossRef]
- Kier, L.D.; Wagner, L.M.; Wilson, T.V.; Li, A.P.; Short, R.D.; Kennedy, G.L. Cytotoxicity of Ethylene Oxide/Propylene Oxide Copolymers in Cultured Mammalian Cells. Drug Chem. Toxicol. 1995, 18, 29–41. [Google Scholar] [CrossRef]
- Redhead, M.; Mantovani, G.; Nawaz, S.; Carbone, P.; Gorecki, D.C.; Alexander, C.; Bosquillon, C. Relationship between the affinity of PEO-PPO-PEO block copolymers for biological membranes and their cellular effects. Pharm. Res. 2012, 29, 1908–1918. [Google Scholar] [CrossRef]
- Arranja, A.; Denkova, A.G.; Morawska, K.; Waton, G.; van Vlierberghe, S.; Dubruel, P.; Schosseler, F.; Mendes, E. Interactions of Pluronic nanocarriers with 2D and 3D cell cultures: Effects of PEO block length and aggregation state. J. Control. Release 2016, 224, 126–135. [Google Scholar] [CrossRef]
- DRUGBANK Online. Albendazole. Available online: https://go.drugbank.com/drugs/DB00518 (accessed on 12 June 2022).
- Semenov, V.V.; Semenova, M.N. Polyalkoxyflavonoids as inhibitors of cell division. Russ. Chem. Rev. 2015, 84, 134–158. [Google Scholar] [CrossRef]
- Ducki, S.; Rennison, D.; Woo, M.; Kendall, A.; Chabert, J.F.; McGown, A.T.; Lawrence, N.J. Combretastatin-like chalcones as inhibitors of microtubule polymerization. Part 1: Synthesis and biological evaluation of antivascular activity. Bioorg. Med. Chem. 2009, 17, 7698–7710. [Google Scholar] [CrossRef] [PubMed]
- Newby, G.E.; Hamley, I.W.; King, S.M.; Martin, C.M.; Terrill, N.J. Structure, rheology and shear alignment of Pluronic block copolymer mixtures. J. Colloid Interf. Sci. 2009, 329, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ruthstein, S.; Potapov, A.; Raitsimring, A.M.; Goldfarb, D. Double electron electron resonance as a method for characterization of micelles. J. Phys. Chem. B 2005, 109, 22843–22851. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, K.; Brown, W. Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous solution. The influence of relative block size. Macromolecules 1993, 26, 4128–4135. [Google Scholar] [CrossRef]
- Gu, Z.; Alexandridis, P. Osmotic stress measurements of iIntermolecular forces in ordered assemblies formed by solvated block copolymers. Macromolecules 2004, 37, 912–924. [Google Scholar] [CrossRef]
- Le-Deygen, I.M.; Musatova, O.E.; Orlov, V.N.; Melik-Nubarov, N.S.; Grozdova, I.D. Poly(ethylene glycol) interacts with hyaluronan in aqueous media. Biomacromolecules 2021, 22, 681–689. [Google Scholar] [CrossRef]
- Larabell, C.; Chandler, D.E. Fertilization-induced changes in the vitelline envelope of echinoderm and amphibian eggs: Self-assembly of an extracellular matrix. J. Electron Micr. Tech. 1991, 17, 294–318. [Google Scholar] [CrossRef]
- Bryskhe, K.; Schillén, K.; Löfroth, J.-E.; Olsson, U. Lipid–block copolymer immiscibility. Phys. Chem. Chem. Phys. 2001, 3, 1303–1309. [Google Scholar] [CrossRef]
- Yang, Y.-W.; Wu, C.-A.; Morrow, W.J.W. Cell death induced by vaccine adjuvants containing surfactants. Vaccine 2004, 22, 1524–1536. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Release 2002, 82, 189–212. [Google Scholar] [CrossRef]
- Pillai, K.; Akhter, J.; Morris, D.L. Super aqueous solubility of albendazole in β-cyclodextrin for parenteral application in cancer therapy. J. Cancer 2017, 8, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Ezhov, A.A.; Ferberg, A.S.; Krylov, S.S.; Semenova, M.N.; Semenov, V.V.; Kudryashova, E.V. Polymeric micelles formulation of combretastatin derivatives with enhanced solubility, cytostatic activity and selectivity against cancer cells. Pharmaceutics 2023, 15, 1613. [Google Scholar] [CrossRef] [PubMed]
- Hyder, F.; Petroff, O.A.; Mattson, R.H.; Rothman, D.L. Localized 1H NMR measurements of 2-pyrrolidinone in human brain in vivo. Magn. Reson. Med. 1999, 41, 889–896. [Google Scholar] [CrossRef]
- Jain, P.; Yalkowsky, S.H. Solubilization of poorly soluble compounds using 2-pyrrolidone. Int. J. Pharmaceut. 2007, 342, 1–5. [Google Scholar] [CrossRef] [PubMed]
- BASF Pharma. Available online: https://pharma.basf.com/products/kollisolv-pyr (accessed on 27 April 2022).
- The European Agency for the Evaluation of Medicinal Products, Committee for Veterinary Medicinal Products. 2-Pyrrolidone. Summary Report. 1998. Available online: https://www.ema.europa.eu/documents/mrl-report/2-pyrrolidone-summary-report-committee-veterinary-medicinal-products_en.pdf (accessed on 27 April 2022).
- Oren, Y.S.; Biesheuvel, P.M. Theory of ion and water transport in reverse-osmosis membranes. Phys. Rev. Appl. 2018, 9, 024034. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; London, E. Fluorimetric determination of critical micelle concentration avoiding interference from detergent charge. Anal. Biochem. 1984, 139, 408–412. [Google Scholar] [CrossRef]



 | |||||
| Pluronic | Poloxamer | MW | Average m b | Average n c | HLB d |
| Pluronics studied in this work | |||||
| P85 | P235 | 4600 | 39.7 | 52.3 | 16 |
| L121 | P401 | 4400 | 68.3 | 10 | 1 |
| P123 | P403 | 5750 | 69.4 | 39.2 | 8 |
| F127 | P407 | 12600 | 65.2 | 200.4 | 22 |
| Pluronics from the literature cited in this work | |||||
| L31 | P101 | 1100 | 17 | 2.5 | 5 |
| F38 | P108 | 4700 | 17.1 | 84.3 | 31 |
| L61 | P181 | 2000 | 31 | 4.5 | 3 |
| L64 | P184 | 2900 | 30 | 26.4 | 15 |
| F68 | P188 | 8400 | 28.9 | 152.7 | 29 |
| F77 | P217 | 6600 | 34.1 | 104.9 | 25 |
| F87 | P237 | 7700 | 39.8 | 122.5 | 24 |
| P94 | P284 | 4600 | 47 | 42 | 14 |
| P103 | P333 | 4950 | 59.7 | 33.8 | 9 |
| P105 | P335 | 6500 | 56 | 73.9 | 15 |
| Pluronic | Mean Temperature, °C | NOEC | MEC | MLC | CMC, μM b | ||||
|---|---|---|---|---|---|---|---|---|---|
| % w/v | μM | % w/v | μM | % w/v | μM | % w/v | μM | ||
| F127 | 23 | 0.05 | 39.7 | 0.1; 0.4 c | 79; 316 c | 5 | 3970 | 0.023 | 18.7 ± 3 |
| P123 | 24 | 0.005 | 8.6 | 0.01–0.02 | 17.25–34.5 | 0.1 | 172.5 | 0.0017 | 3.0 ± 0.5 |
| 23 | 0.005 | 8.6 | 0.01–0.02 | 17.25–34.5 | 0.05 | 86 | 0.0017 | 3.0 ± 0.5 | |
| 21 | 0.00125 | 2.15 | 0.0025–0.005 | 4.3–8.6 | 0.01–0.02 | 17.25–34.5 | 0.0025 | 4.3 ± 1 | |
| 16 | 0.00125 | 2.15 | 0.0025 | 4.3 | 0.005 | 8.6 | 0.023 | 40 ± 10 | |
| L121 d | 23 | 0.000625 | 1.42 | 0.00125 | 2.84 | 0.01 | 22.7 | <0.0004 | <1 |
| Pluronic | CMC/Seawater | |||||||
|---|---|---|---|---|---|---|---|---|
| 25 °C | 18 °C | 13 °C | 10 °C | |||||
| % w/v | μM | % w/v | μM | % w/v | μM | % w/v | μM | |
| F127 | 0.0139 ± 0.001 | 11 ± 1.3 | 0.095 ± 0.01 | 75 ± 10 | 1.3 ± 0.1 | 1025 ± 120 | 1.90 ± 0.17 | 1520 ± 130 |
| P123 | 0.0011 ± 0.0001 | 1.9 ± 0.2 | 0.018 ± 0.002 | 31.1 ± 3 | 0.063 ± 0.1 | 108.9 ± 12 | 0.273 ± 0.03 | 412 ± 50 |
| Cmpd | Solvent a | Cosolvent | MEC, μM | |
|---|---|---|---|---|
| Cleavage Alteration | Cleavage Arrest | |||
| 1 | DMSO | FSW | >0.01 | >0.01 |
| 96% EtOH | 0.002 | 0.005 | ||
| 5% F127 | 0.002 | 0.005 | ||
| 1.25% P123 | 0.002 | 0.005 | ||
| 2-Pyrrolidone | FSW | 0.005 | >0.01 | |
| 96% EtOH | 0.002 | 0.005 | ||
| 5% F127 | 0.002 | 0.005 | ||
| 2 | DMSO | FSW | 0.005 | 0.01 |
| 96% EtOH | 0.001 | 0.005 | ||
| 5% F127 | 0.001 | 0.005 | ||
| 1.25% P123 | 0.001 | 0.005 | ||
| 2-Pyrrolidone | FSW | 0.01 | >0.01 | |
| 96% EtOH | 0.001 | 0.005 | ||
| 5% F127 | 0.001 | 0.005 | ||
| 3 | DMSO | FSW | 0.02 | 0.2 |
| 96% EtOH | 0.01 | 0.05 | ||
| 5% F127 | 0.01 | 0.05 | ||
| 1.25% P123 | 0.01 | 0.05 | ||
| 4 | DMSO | FSW b | >0.2 | Not available |
| 96% EtOH | 0.1 | 1 | ||
| 5% F127 | 0.1 | 1 | ||
| 1.25% P123 | 0.1 | 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenova, M.N.; Melik-Nubarov, N.S.; Semenov, V.V. Application of Pluronics for Enhancing Aqueous Solubility of Lipophilic Microtubule Destabilizing Compounds on the Sea Urchin Embryo Model. Int. J. Mol. Sci. 2023, 24, 14695. https://doi.org/10.3390/ijms241914695
Semenova MN, Melik-Nubarov NS, Semenov VV. Application of Pluronics for Enhancing Aqueous Solubility of Lipophilic Microtubule Destabilizing Compounds on the Sea Urchin Embryo Model. International Journal of Molecular Sciences. 2023; 24(19):14695. https://doi.org/10.3390/ijms241914695
Chicago/Turabian StyleSemenova, Marina N., Nikolay S. Melik-Nubarov, and Victor V. Semenov. 2023. "Application of Pluronics for Enhancing Aqueous Solubility of Lipophilic Microtubule Destabilizing Compounds on the Sea Urchin Embryo Model" International Journal of Molecular Sciences 24, no. 19: 14695. https://doi.org/10.3390/ijms241914695
APA StyleSemenova, M. N., Melik-Nubarov, N. S., & Semenov, V. V. (2023). Application of Pluronics for Enhancing Aqueous Solubility of Lipophilic Microtubule Destabilizing Compounds on the Sea Urchin Embryo Model. International Journal of Molecular Sciences, 24(19), 14695. https://doi.org/10.3390/ijms241914695







