Time-Resolved Fluorescence Spectroscopy of Blood, Plasma and Albumin as a Potential Diagnostic Tool for Acute Inflammation in COVID-19 Pneumonia Patients
Abstract
:1. Introduction
2. Results
2.1. Time-Resolved Fluorescence Spectroscopy Measurements
2.2. Clinical and Correlation Study
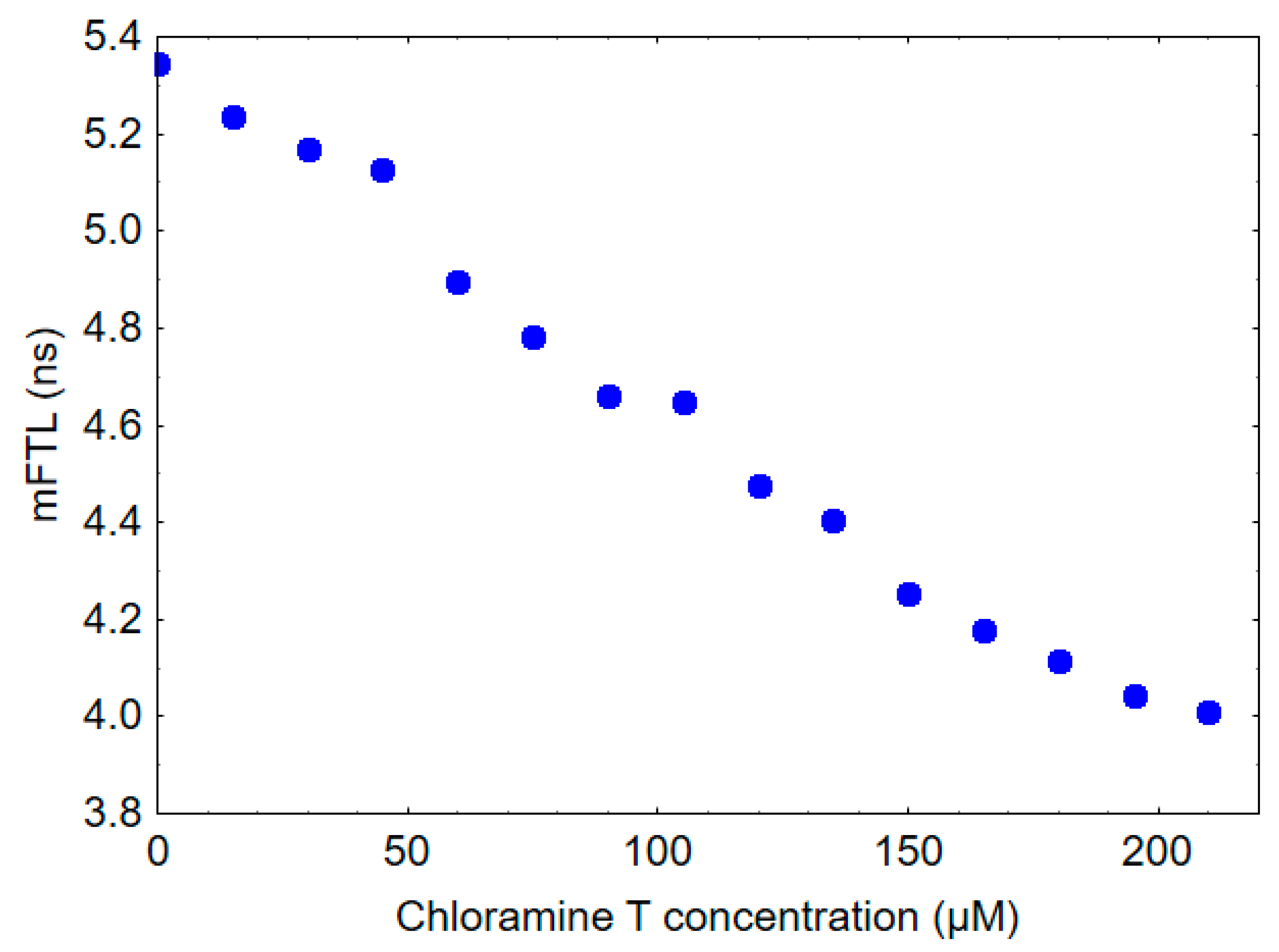
2.3. In Vitro Study
3. Discussion
4. Materials and Methods
4.1. General Characteristics of the Study Patients
4.2. Sample Preparation
4.3. AOPPs Measurements
4.4. In Vitro Study
4.5. Time-Resolved Fluorescence Spectroscopy Measurements
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masilamani, V.; Al-Zhrani, K.; Al-Salhi, M.; Al-Diab, A.; Al-Ageily, M. Cancer Diagnosis by Autofluorescence of Blood Components. J. Lumin. 2004, 109, 143–154. [Google Scholar] [CrossRef]
- Sikora, J.; Cyrankiewicz, M.; Wybranowski, T.; Ziomkowska, B.; Kasprzak, M.; Krintus, M.; Odrowąż-Sypniewska, G.; Augustyńska, B.; Kruszewski, S.; Kubica, J. Fluorescence Lifetime of Collagen Degradation Products in Plasma of Patients with Left Ventricular Remodeling. Med. Res. J. 2014, 3, 20–25. [Google Scholar]
- Devanesan, S.; AlShebly, M.; Kalaivani, R.; Sivaji, K.; Farhat, K.; AlSalhi, M.S.; AlAtawi, M.; Rabah, D.; Masilamani, V. Fluorescence Spectral Features of Blood Components of Pregnant Women. Curr. Sci. 2017, 113, 457–461. [Google Scholar] [CrossRef]
- Shrirao, A.B.; Schloss, R.S.; Fritz, Z.; Shrirao, M.V.; Rosen, R.; Yarmush, M.L. Autofluorescence of Blood and Its Application in Biomedical and Clinical Research. Biotechnol. Bioeng. 2021, 118, 4550–4576. [Google Scholar] [CrossRef]
- Wybranowski, T.; Pyskir, J.; Bosek, M.; Napiórkowska, M.; Cyrankiewicz, M.; Ziomkowska, B.; Pilaczyńska-Cemel, M.; Pyskir, M.; Rogańska, M.; Kruszewski, S.; et al. The Mortality Risk and Pulmonary Fibrosis Investigated by Time-Resolved Fluorescence Spectroscopy from Plasma in COVID-19 Patients. J. Clin. Med. 2022, 11, 5081. [Google Scholar] [CrossRef]
- O’Connor, D.V.; Phillips, D. Time-Correlated Single Photon Counting; Academic Press: London, UK, 1984. [Google Scholar]
- Millar, D.P. Time-Resolved Fluorescence Spectroscopy. Curr. Opin. Struct. Biol. 1996, 6, 637–642. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006; ISBN 9780387463124. [Google Scholar]
- Li, B.-H.; Zhang, Z.-X.; Xie, S.-S.; Chen, R. Fluorescence Spectral Characteristics of Human Blood and Its Endogenous Fluorophores. Guang Pu Xue Yu Guang Pu Fen Xi 2006, 26, 1310–1313. [Google Scholar]
- Li, C.; Pastila, R.K.; Pitsillides, C.; Runnels, J.M.; Puoris’haag, M.; Côté, D.; Lin, C.P. Imaging Leukocyte Trafficking in Vivo with Two-Photon-Excited Endogenous Tryptophan Fluorescence. Opt. Express 2010, 18, 988–999. [Google Scholar] [CrossRef]
- Zhdanova, N.G.; Shirshin, E.A.; Maksimov, E.G.; Panchishin, I.M.; Saletsky, A.M.; Fadeev, V.V. Tyrosine Fluorescence Probing of the Surfactant-Induced Conformational Changes of Albumin. Photochem. Photobiol. Sci. 2015, 14, 897–908. [Google Scholar] [CrossRef]
- Gayer, A.V.; Yakimov, B.P.; Sluchanko, N.N.; Shirshin, E.A. Multifarious Analytical Capabilities of the UV/Vis Protein Fluorescence in Blood Plasma. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 286, 122028. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Achilefu, S. Fluorescence Lifetime Measurements and Biological Imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [PubMed]
- Belinskaia, D.A.; Voronina, P.A.; Popova, P.I.; Voitenko, N.G.; Shmurak, V.I.; Vovk, M.A.; Baranova, T.I.; Batalova, A.A.; Korf, E.A.; Avdonin, P.V.; et al. Albumin Is a Component of the Esterase Status of Human Blood Plasma. Int. J. Mol. Sci. 2023, 24, 10383. [Google Scholar] [CrossRef] [PubMed]
- Temple, A.; Yen, T.Y.; Gronert, S. Identification of Specific Protein Carbonylation Sites in Model Oxidations of Human Serum Albumin. J. Am. Soc. Mass. Spectrom. 2006, 17, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Descamps-Latscha, B.; Witko-Sarsat, V. Importance of Oxidatively Modified Proteins in Chronic Renal Failure. Kidney Int. Suppl. 2001, 78, S108–S113. [Google Scholar] [CrossRef]
- Ulfig, A.; Leichert, L.I. The Effects of Neutrophil-Generated Hypochlorous Acid and Other Hypohalous Acids on Host and Pathogens. Cell. Mol. Life Sci. 2020, 78, 385–414. [Google Scholar] [CrossRef]
- Ulfig, A.; Bader, V.; Varatnitskaya, M.; Lupilov, N.; Winklhofer, K.F.; Leichert, L.I. Hypochlorous Acid-Modified Human Serum Albumin Suppresses MHC Class II—Dependent Antigen Presentation in pro-Inflammatory Macrophages. Redox Biol. 2021, 43, 101981. [Google Scholar] [CrossRef]
- Salavej, P.; Spalteholz, H.; Arnhold, J. Modification of amino acid residues in human serum albumin by myeloperoxidase. Free Radic. Biol. Med. 2006, 40, 516–525. [Google Scholar] [CrossRef]
- Gorudko, I.V.; Grigorieva, D.V.; Shamova, E.V.; Kostevich, V.A.; Sokolov, A.V.; Mikhalchik, E.V.; Cherenkevich, S.N.; Arnhold, J.; Panasenko, O.M. Hypohalous acid-modified human serum albumin induces neutrophil NADPH oxidase activation, degranulation, and shape change. Free Radic. Biol. Med. 2014, 68, 326–334. [Google Scholar] [CrossRef]
- Laforge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.J.; Becker, C. Tissue Damage from Neutrophil-Induced Oxidative Stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Yin, Y.; Zhang, Y.; Cao, Y.; Lin, X.; Huang, L.; Hoffmann, D.; Lu, M.; Qiu, Y. Excessive Neutrophils and Neutrophil Extracellular Traps in COVID-19. Front. Immunol. 2020, 11, 2063. [Google Scholar] [CrossRef]
- Benfield, T.; Bodilsen, J.; Brieghel, C.; Harboe, Z.B.; Helleberg, M.; Holm, C.; Israelsen, S.B.; Jensen, J.; Jensen, T.Ø.; Johansen, I.S.; et al. Correction to: Increased Peripheral Blood Neutrophil Activation Phenotypes and Neutrophil Extracellular Trap Formation in Critically Ill Coronavirus Disease 2019 Patients: A Case Series and Review of the Literature. Clin. Infect. Dis. 2022, 74, 1889–1890. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, R.; Cecchini, A.L. SARS-CoV-2 Infection Pathogenesis Is Related to Oxidative Stress as a Response to Aggression. Med. Hypotheses 2020, 143, 110102. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, B.V.; Popova, E.N.; Prikhodko, A.S.; Grebenchikov, O.A.; Zinovkina, L.A.; Zinovkin, R.A. COVID-19 and Oxidative Stress. Biochemistry 2020, 85, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, H.; Alp, H.H.; Ekin, S.; Arisoy, A.; Gunbatar, H.; Asker, S.; Cilingir, B.M.; Sunnetcioglu, A.; Celikel, M.; Esen, N.; et al. Analysis of Endogenous Oxidative Damage Markers and Association with Pulmonary Involvement Severity in Patients with SARS-CoV-2 Pneumonia. Infect. Dis. Now. 2021, 51, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Norouzi, P.; Aazami, H.; Moosavi-Movahedi, A.A. Review on Oxidative Stress Relation on COVID-19: Biomolecular and Bioanalytical Approach. Int. J. Biol. Macromol. 2021, 189, 802–818. [Google Scholar] [CrossRef]
- Wieczfinska, J.; Kleniewska, P.; Pawliczak, R. Oxidative Stress-Related Mechanisms in SARS-CoV-2 Infections. Oxid. Med. Cell. Longev. 2022, 2022, 5589089. [Google Scholar] [CrossRef]
- Wybranowski, T.; Napiórkowska, M.; Bosek, M.; Pyskir, J.; Ziomkowska, B.; Cyrankiewicz, M.; Pyskir, M.; Pilaczyńska-Cemel, M.; Rogańska, M.; Kruszewski, S.; et al. Study of Albumin Oxidation in COVID-19 Pneumonia Patients: Possible Mechanisms and Consequences. Int. J. Mol. Sci. 2022, 23, 10103. [Google Scholar] [CrossRef]
- Vollbracht, C.; Kraft, K. Oxidative Stress and Hyper-Inflammation as Major Drivers of Severe COVID-19 and Long COVID: Implications for the Benefit of High-Dose Intravenous Vitamin C. Front. Pharmacol. 2022, 13, 899198. [Google Scholar] [CrossRef]
- McGarry, T.; Biniecka, M.; Veale, D.J.; Fearon, U. Hypoxia, Oxidative Stress and Inflammation. Free Radic. Biol. Med. 2018, 125, 15–24. [Google Scholar] [CrossRef]
- Luo, Z.; Tian, M.; Yang, G.; Tan, Q.; Chen, Y.; Li, G.; Zhang, Q.; Li, Y.; Wan, P.; Wu, J. Hypoxia Signaling in Human Health and Diseases: Implications and Prospects for Therapeutics. Signal Transduct. Target Ther. 2022, 7, 218. [Google Scholar] [CrossRef]
- Wybranowski, T.; Ziomkowska, B.; Cyrankiewicz, M.; Bosek, M.; Pyskir, J.; Napiórkowska, M.; Kruszewski, S. A Study of the Oxidative Processes in Human Plasma by Time-Resolved Fluorescence Spectroscopy. Sci. Rep. 2022, 12, 9012. [Google Scholar] [CrossRef] [PubMed]
- Gallo Marin, B.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.J.; Chiang, S.S.; Salazar-Mather, T.P.; Dumenco, L.; Savaria, M.C.; Aung, S.N.; et al. Predictors of COVID-19 Severity: A Literature Review. Rev. Med. Virol. 2021, 31, 1–10. [Google Scholar] [CrossRef]
- René Albani, J. Fluorescence Lifetimes of Tryptophan: Structural Origin and Relation with So --> 1Lb and So --> 1La Transitions. J. Fluoresc. 2009, 19, 1061–1071. [Google Scholar] [CrossRef]
- Amiri, M.; Jankeje, K.; Albani, J.R. Characterization of Human Serum Albumin Forms with PH. Fluorescence Lifetime Studies. J. Pharm. Biomed. Anal. 2010, 51, 1097–1102. [Google Scholar] [CrossRef]
- Amiri, M.; Jankeje, K.; Albani, J.R. Origin of Fluorescence Lifetimes in Human Serum Albumin. Studies on Native and Denatured Protein. J. Fluoresc. 2010, 20, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Albani, J.R. Origin of Tryptophan Fluorescence Lifetimes. Part 2: Fluorescence Lifetimes Origin of Tryptophan in Proteins. J. Fluoresc. 2014, 24, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wool, G.D.; Miller, J.L. The Impact of COVID-19 Disease on Platelets and Coagulation. Pathobiology 2021, 88, 15–27. [Google Scholar] [CrossRef]
- Colombo, G.; Clerici, M.; Altomare, A.; Rusconi, F.; Giustarini, D.; Portinaro, N.; Garavaglia, M.L.; Rossi, R.; Dalle-Donne, I.; Milzani, A. Thiol Oxidation and Di-Tyrosine Formation in Human Plasma Proteins Induced by Inflammatory Concentrations of Hypochlorous Acid. J. Proteom. 2017, 152, 22–32. [Google Scholar] [CrossRef]
- Pattison, D.I.; Hawkins, C.L.; Davies, M.J. Hypochlorous Acid-Mediated Protein Oxidation: How Important Are Chloramine Transfer Reactions and Protein Tertiary Structure? Biochemistry 2007, 46, 9853–9864. [Google Scholar] [CrossRef]
- Žarković, N.; Orehovec, B.; Milković, L.; Baršić, B.; Tatzber, F.; Wonisch, W.; Tarle, M.; Kmet, M.; Mataić, A.; Jakovčević, A.; et al. Preliminary Findings on the Association of the Lipid Peroxidation Product 4-Hydroxynonenal with the Lethal Outcome of Aggressive COVID-19. Antioxidants 2021, 10, 1341. [Google Scholar] [CrossRef] [PubMed]
- Martín-Fernández, M.; Aller, R.; Heredia-Rodríguez, M.; Gómez-Sánchez, E.; Martínez-Paz, P.; Gonzalo-Benito, H.; Sánchez-de Prada, L.; Gorgojo, Ó.; Carnicero-Frutos, I.; Tamayo, E.; et al. Lipid Peroxidation as a Hallmark of Severity in COVID-19 Patients. Redox Biol. 2021, 48, 102181. [Google Scholar] [CrossRef] [PubMed]
- Monici, M.; Pratesi, R.; Bernabei, P.A.; Caporale, R.; Ferrini, P.R.; Croce, A.C.; Balzarini, P.; Bottiroli, G. Natural Fluorescence of White Blood Cells: Spectroscopic and Imaging Study. J. Photochem. Photobiol. B. 1995, 30, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Cheng, A.; Yuan, X.; Zhong, X.; Wang, H.; Zhou, W.; Xu, X.; Li, Y. Characteristics of Peripheral White Blood Cells in COVID-19 Patients Revealed by a Retrospective Cohort Study. BMC Infect. Dis. 2021, 21, 1236. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Feng, X.; Jiang, C.; Mi, S.; Yang, L.; Zhao, Z.; Zhang, Y.; Zhang, L. Correlation between White Blood Cell Count at Admission and Mortality in COVID-19 Patients: A Retrospective Study. BMC Infect. Dis. 2021, 21, 574. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red Blood Cell Oxidative Stress Impairs Oxygen Delivery and Induces Red Blood Cell Aging. Front. Physiol. 2014, 28, 84. [Google Scholar] [CrossRef]
- Thomas, T.; Stefanoni, D.; Dzieciatkowska, M.; Issaian, A.; Nemkov, T.; Hill, R.C.; Francis, R.O.; Hudson, K.E.; Buehler, P.W.; Zimring, J.C.; et al. Evidence for Structural Protein Damage and Membrane Lipid Remodeling in Red Blood Cells from COVID-19 Patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Russo, A.; Tellone, E.; Barreca, D.; Ficarra, S.; Laganà, G. Implication of COVID-19 on Erythrocytes Functionality: Red Blood Cell Biochemical Implications and Morpho-Functional Aspects. Int. J. Mol. Sci. 2022, 23, 2171. [Google Scholar] [CrossRef]
- Zavodnik, I.B.; Lapshina, E.A.; Zavodnik, L.B.; Soszyński, M.; Bartosz, G.; Bryszewska, M. Hypochlorous acid-induced oxidative damage of human red blood cells: Effects of tert-butyl hydroperoxide and nitrite on the HOCl reaction with erythrocytes. Bioelectrochemistry 2002, 58, 127–135. [Google Scholar] [CrossRef]
- Zavodnik, L.B.; Zavodnik, I.B.; Lapshyna, E.A.; Buko, V.U.; Bryszewska, M.J. Hypochlorous acid-induced membrane pore formation in red blood cells. Bioelectrochemistry 2002, 58, 157–161. [Google Scholar] [CrossRef]
- Vissers, M.C.; Stern, A.; Kuypers, F.; van den Berg, J.; Winterbourn, C.C. Membrane changes associated with lysis of red blood cells by hypochlorous acid. Free Radic. Biol. Med. 1994, 16, 703–712. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease Is Suspected: Interim Guidance. 2020. Available online: https://apps.who.int/iris/handle/10665/331446 (accessed on 14 November 2022).
- Witko-Sarsat, V.; Friedlander, M.; Capeillere-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced Oxidation Protein Products as a Novel Marker of Oxidative Stress in Uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Hanasand, M.; Omdal, R.; Norheim, K.B.; Gøransson, L.G.; Brede, C.; Jonsson, G. Improved Detection of Advanced Oxidation Protein Products in Plasma. Clin. Chim. Acta 2012, 413, 901–906. [Google Scholar] [CrossRef] [PubMed]
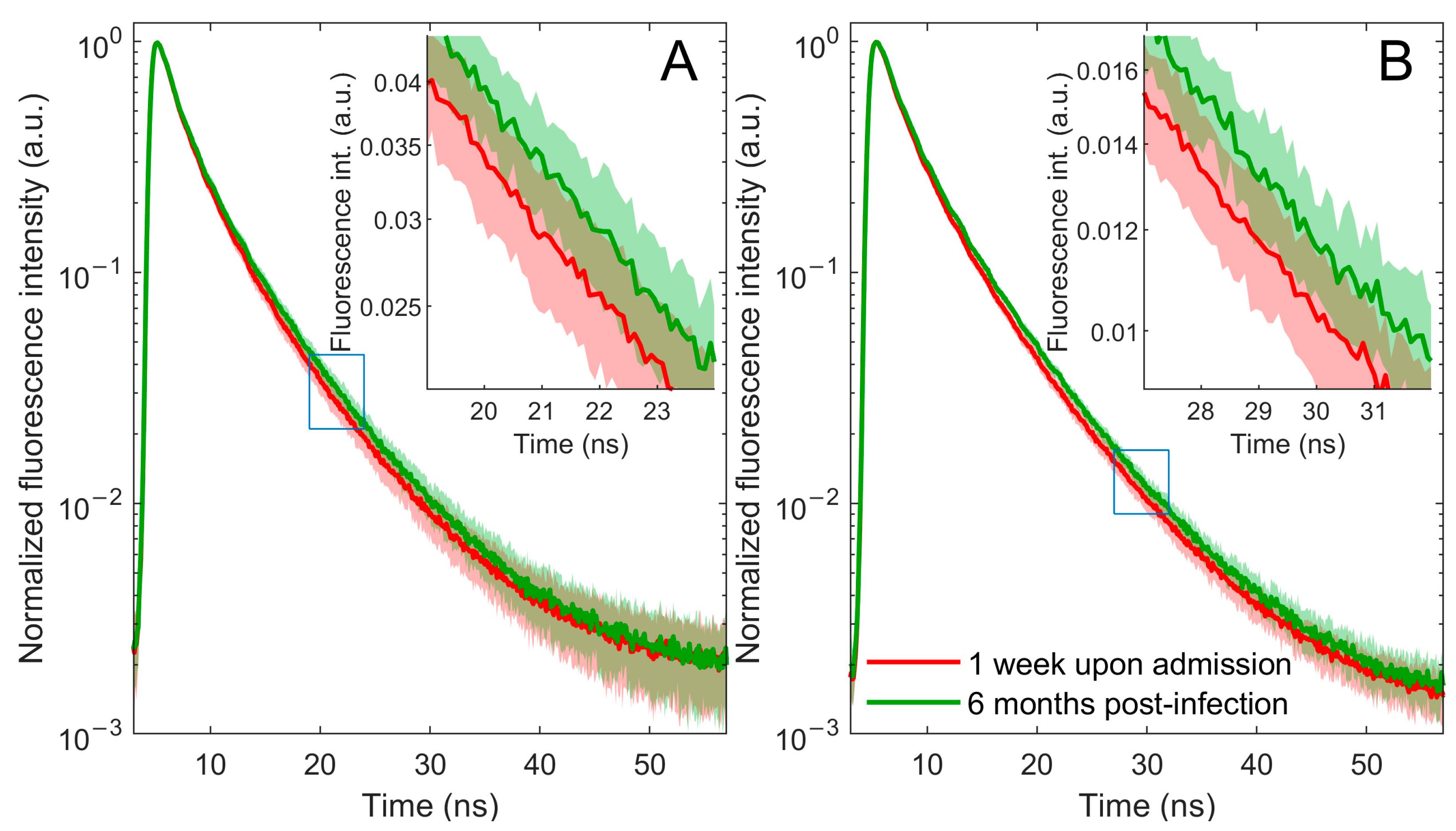
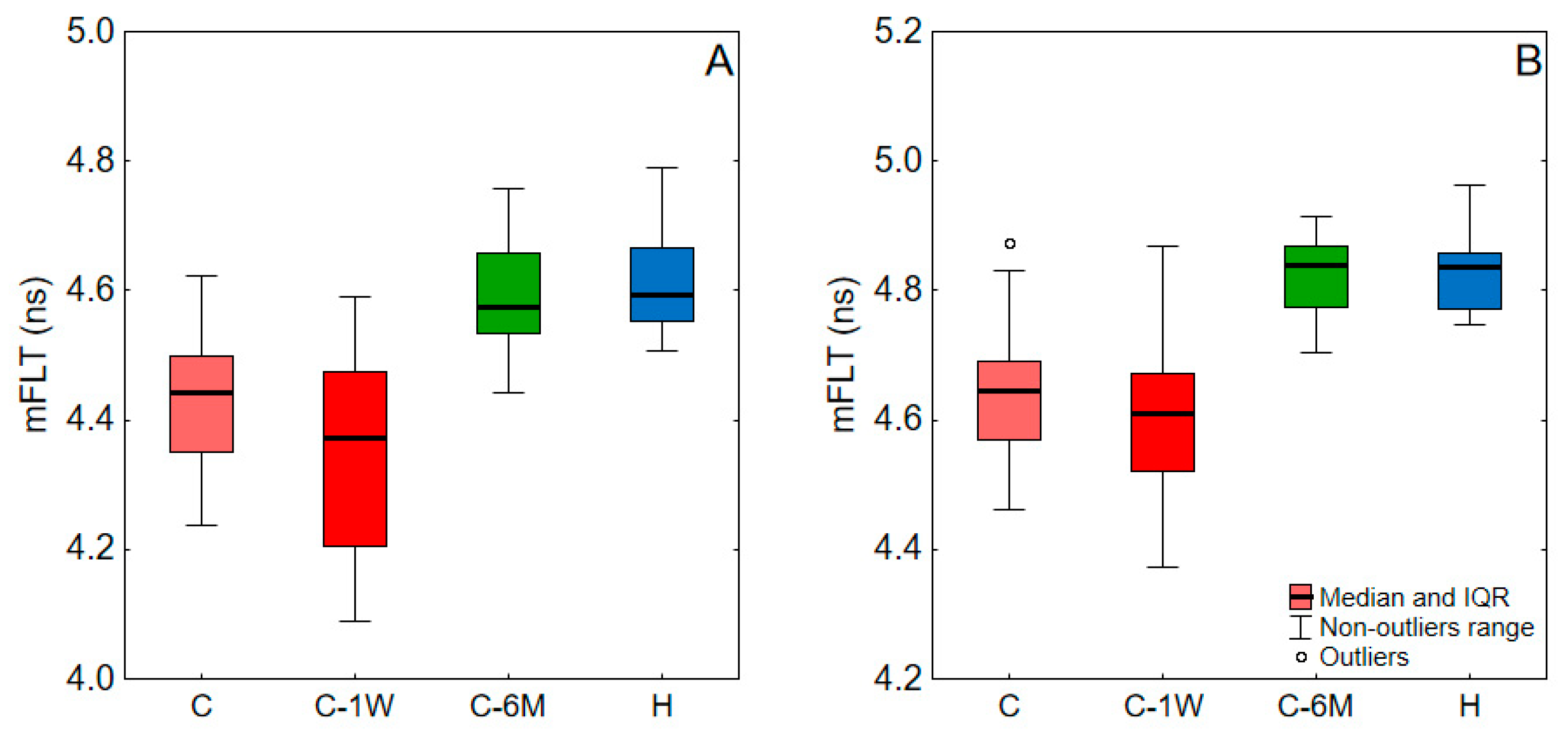
| C | C-1W | C-6M | H | ||
|---|---|---|---|---|---|
| blood | C | - | <0.001 | <0.001 | <0.001 |
| C-1W | <0.001 | - | 0.003 | <0.001 | |
| C-6M | <0.001 | 0.003 | - | 0.478 | |
| plasma | C | - | 0.199 | <0.001 | <0.001 |
| C-1W | 0.199 | - | 0.001 | <0.001 | |
| C-6M | <0.001 | 0.001 | - | 0.937 |
| Parameters (Units) | Median | Interquartile Range | Reference Ranges | Correlation with mFLT (r) | |
|---|---|---|---|---|---|
| Blood | Plasma | ||||
| Age (years) | 65 | 52–72 | −0.173 | −0.229 | |
| Symptoms (days) | 7 | 5–10 | 0.024 | 0.062 | |
| WBC (103/µL) | 6.25 | 5.05–9.1 | 4–10 | −0.266 | −0.183 |
| Neutrophils (103/µL) | 4.6 | 3.5–7.3 | 2.5–5 | −0.195 | −0.097 |
| Lymphocytes (103/µL) | 0.9 | 0.7–1.3 | 1.5–3.5 | −0.159 | −0.042 |
| RBC (106/µL) | 4.5 | 4.2–4.8 | 4.5–5.5 | 0.249 | 0.294 |
| Hgb (g/dL) | 13.8 | 12.8–14.5 | 14–18 | 0.136 | 0.175 |
| PLT (103/µL) | 204.5 | 168.5–290.5 | 130–350 | −0.212 | −0.185 |
| CRP (mg/L) | 81.5 | 41–138 | <5 | −0.383 | −0.216 |
| Procalcitonin (ng/mL) | 0.08 | 0.05–0.15 | <0.05 | −0.417 | −0.208 |
| LDH (U/L) | 639.5 | 525.5–778 | 225–450 | −0.405 | −0.143 |
| D-Dimers (ng/mL) | 900 | 708–1546 | <500 | −0.524 | −0.464 |
| Troponin (ng/L) | 10.1 | 6–18 | <19 | −0.401 | −0.316 |
| Creatinine (mg/dL) | 0.955 | 0.855–1.11 | 0.8–1.3 | 0.067 | 0.017 |
| CPK (U/L) | 128 | 75.5–209 | 25–200 | 0.180 | 0.099 |
| AST (U/L) | 50.5 | 35.5–65 | <37 | −0.390 | −0.306 |
| ALT (U/L) | 42 | 30–61 | <40 | −0.192 | −0.079 |
| IL-6 (pg/mL) | 13.1 | 4.9–35 | <7 | −0.023 | 0.016 |
| HRCT score | 0.25 | 0.15–0.42 | −0.340 | −0.199 | |
| Albumin (g/L) | 34 | 31–37 | 39–51 | 0.407 | 0.434 |
| AOPPs (µM) | 13.7 | 11.4–16.4 | −0.361 | −0.268 | |
| AGR | 1.25 | 1.07–1.40 | 0.355 | 0.588 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wybranowski, T.; Ziomkowska, B.; Cyrankiewicz, M.; Pyskir, J.; Bosek, M.; Napiórkowska, M.; Pilaczyńska-Cemel, M.; Przybylski, G.; Kruszewski, S. Time-Resolved Fluorescence Spectroscopy of Blood, Plasma and Albumin as a Potential Diagnostic Tool for Acute Inflammation in COVID-19 Pneumonia Patients. Int. J. Mol. Sci. 2023, 24, 14703. https://doi.org/10.3390/ijms241914703
Wybranowski T, Ziomkowska B, Cyrankiewicz M, Pyskir J, Bosek M, Napiórkowska M, Pilaczyńska-Cemel M, Przybylski G, Kruszewski S. Time-Resolved Fluorescence Spectroscopy of Blood, Plasma and Albumin as a Potential Diagnostic Tool for Acute Inflammation in COVID-19 Pneumonia Patients. International Journal of Molecular Sciences. 2023; 24(19):14703. https://doi.org/10.3390/ijms241914703
Chicago/Turabian StyleWybranowski, Tomasz, Blanka Ziomkowska, Michał Cyrankiewicz, Jerzy Pyskir, Maciej Bosek, Marta Napiórkowska, Marta Pilaczyńska-Cemel, Grzegorz Przybylski, and Stefan Kruszewski. 2023. "Time-Resolved Fluorescence Spectroscopy of Blood, Plasma and Albumin as a Potential Diagnostic Tool for Acute Inflammation in COVID-19 Pneumonia Patients" International Journal of Molecular Sciences 24, no. 19: 14703. https://doi.org/10.3390/ijms241914703
APA StyleWybranowski, T., Ziomkowska, B., Cyrankiewicz, M., Pyskir, J., Bosek, M., Napiórkowska, M., Pilaczyńska-Cemel, M., Przybylski, G., & Kruszewski, S. (2023). Time-Resolved Fluorescence Spectroscopy of Blood, Plasma and Albumin as a Potential Diagnostic Tool for Acute Inflammation in COVID-19 Pneumonia Patients. International Journal of Molecular Sciences, 24(19), 14703. https://doi.org/10.3390/ijms241914703





