Combined or Sequential Treatment with Immune Checkpoint Inhibitors and Car-T Cell Therapies for the Management of Haematological Malignancies: A Systematic Review
Abstract
1. Introduction
2. Material and Methods
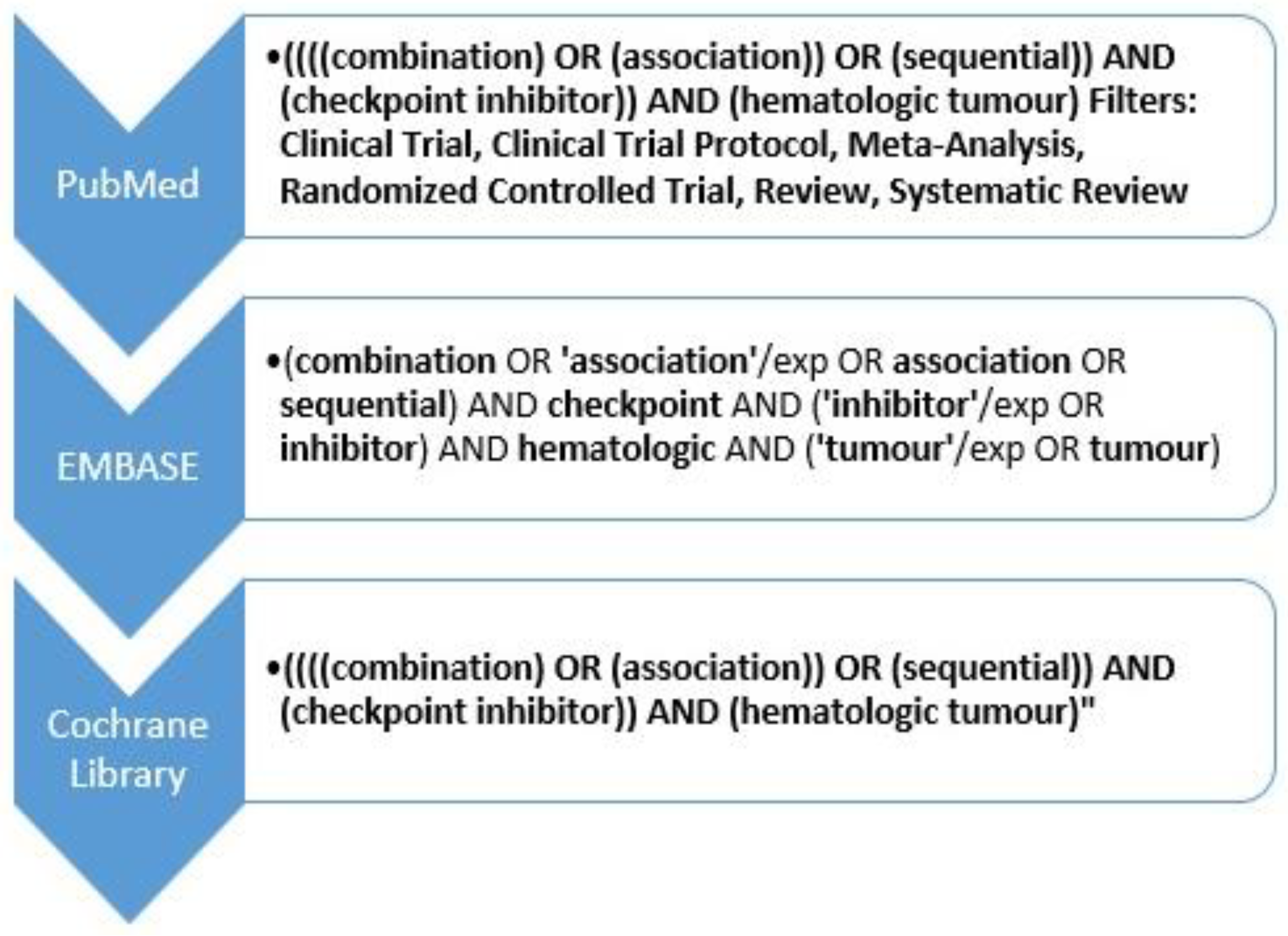
2.1. Literature Search
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Study Selection
2.4. Data Extraction and Quality Assessment
2.5. Study Endpoints
3. Results
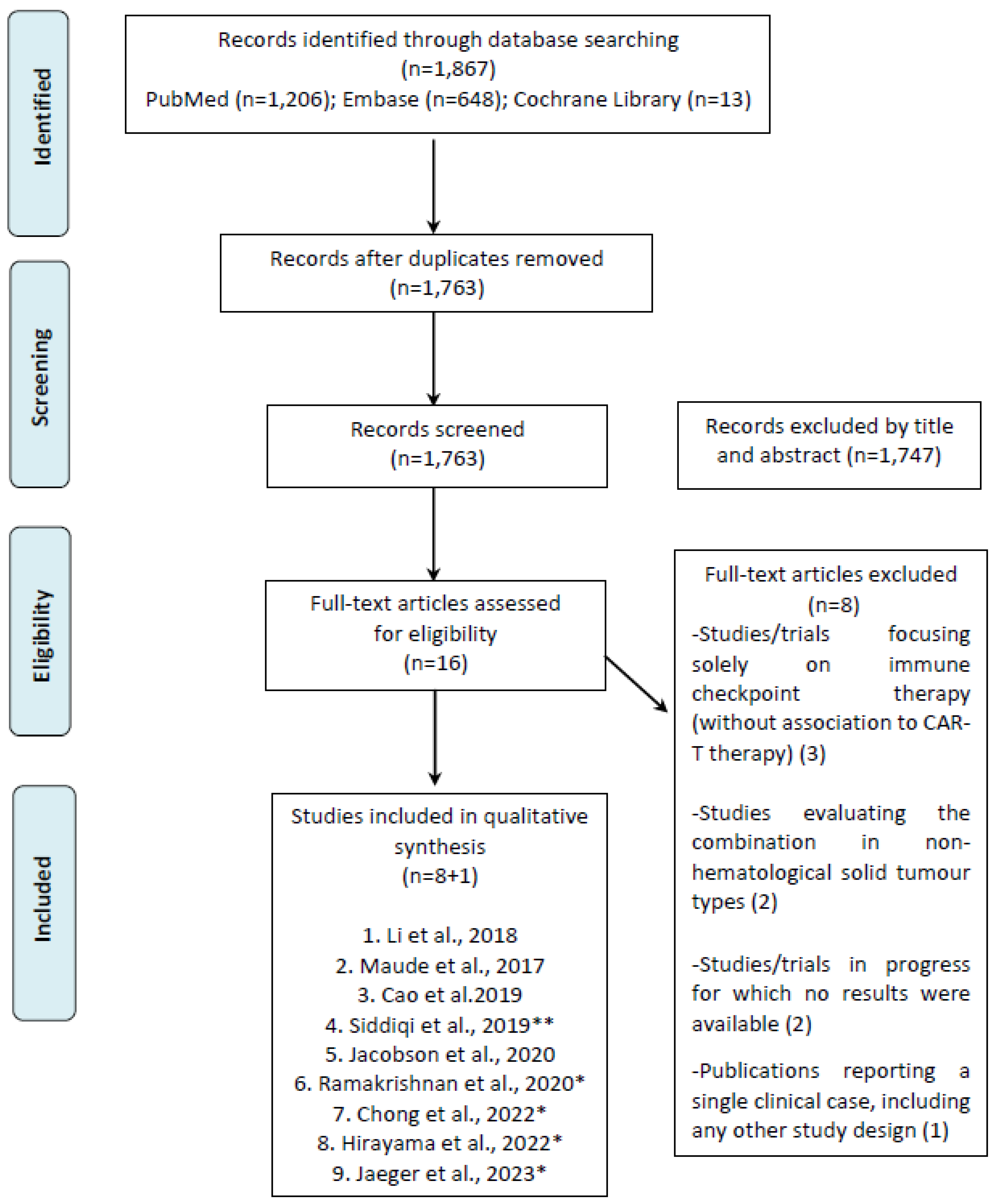
3.1. Literature Search Results
3.2. Clinical Study Features and Efficacy Results
3.3. LLA-B
3.4. B-NHL
| Author, Year | Type of Study | Age (Eligible) | Treatment/Period/Regimen | Patients (N) | Pathology (N) | Disease Status at Treatment (N) |
|---|---|---|---|---|---|---|
| Li et al. [28], 2017 | Single institution Pilot clinical trial | Range: 4–17 years | CD19-directed CAR-T cell therapy in combination with pembrolizumab (n = 13) or nivolumab (n = 1) | 14 | B-ALL (13) B-LL (1) | Relapsed (13) Refractory (1) |
| Maude et al. [29], 2017 | Phase I/pilot trial | Range: 1–24 years | 1–3 doses of pembrolizumab starting 14 d-2 months post Anti-CD19 CAR-T cell infusion | 4 | B-ALL (4) | Relapsed |
| Cao et al. [30], 2019 | Cohort study | Median age: 65 years (range 26–75) | Nivolumab 3 mg/kg in a single dose on the 3rd day after CD19 CAR-T cell infusion | 11 | DLBCL (10) -Stage III (2) -Stage IV (8) BL/IV (1) | Primary refractory (5) Relapsed (6) |
| Siddiqi et al. [31], 2019 (PLATFORM study) | Multiarm, parallel cohort, phase 1/2 study | Range: 53–78 years | Liso-cel at 1 of 2 dose levels (DL): DL1 = 50 × 10e6 or DL2 = 100 × 10e6 CAR- T cells Durvalumab 1500 mg every 4 weeks as an IV infusion from day 29 at a total dose of for up to 12 months | 18 (11 *) | DLBCL (10) FL (1) | Refractory Relapsed |
| Jacobson et al. [33], 2020 (ZUMA-6 trial) | Multi-centre open-label phase I/IIl study | ≥18 years | A single infusion of KTE-C19 CAR-T cells IV followed by four doses of atezolizumab 1200 mg/dose IV every 3 weeks, beginning after 21 days (Phase 1 Cohort 1), 14 days (Phase 1 Cohort 2) or 1 day (Phase 1, Cohort 3, and Phase 2) | 28 | DLBCL (28) | Refractory (28) |
| Ramakrishnan et al. [34], 2020 (ALEXANDER trial) | Single Arm, multi-centre, open-label, phase I/II study | Median age: 59 (range 28–83) | AUTO3 alone, or with three doses of pembrolizumab 200 mg every 3 weeks starting on D14 (regimen A), or with a single dose of pembrolizumab 200 mg on D-1 (regimen B) | 33 (29 *) | DLBCL-NOS (25) t-DLBCL (6) High-grade B-cell lymphoma (2) | Refractory (26) |
| Chong et al. [35], 2022 | Prospective, open-label, single-institution, phase I/IIa study | Median age: 58 years (range 30–78) | After treatment with CART19 Pts received a fixed dose of pembrolizumab 200 mg IV every 3 weeks until the progression of disease, therapy limiting-toxicity, or elective protocol discontinuation | 12 | DLBCL (11) -GCB-THL (3) -T-cell rich DLBCL (1) -tFL (1) FL (1) | Refractory (9) Relapsed (3) |
| Hirayama et al. [36], 2022 | Multi-centre, open-label phase Ib trial. | Median age: 58 years (range 32–69) | (a) Durvalumab (225 mg/750 mg/1500 mg) 21 and 28 days after JCAR014 (b) Durvalumab (7.5 mg/22.5 mg/75 mg, 225 mg/750 mg or 1500 mg) 1 day before JCAR014 infusion and every 4 weeks (until PD or unacceptable toxicity, maximum 10 doses) | 29 (26 *) | B-cell NHL -DLBCL (13) -t-DLBCL (8) -High-grade B-cell lymphoma (6) -Other (2) | Refractory Relapsed |
| Jaeger et al. [37], 2023 (PORTIA trial) | Multi-centre, open-label, phase Ib study | ≥18 years | Single tisagenlecleucel (CTL019) IV on Day 1 and Pembrolizumab 200 mg every 3 weeks, for up to six doses starting on day 15 after (in cohort 1), on day 8 or 22 (in subsequent cohorts) | 15 (12 *) | DLBCL (12) | Refractory Relapsed |
| Author, Year | Pathology | Study Treatment | Patients (N) | Response N (%) | Re-Expansion CAR N (%) | PFS (Months) | OS # (%) | DOR # (%) |
|---|---|---|---|---|---|---|---|---|
| Li et al. [28], 2017 | B-ALL | Anti-CD19 + Nivolumab or Pembrolizumab | 14 | ORR: 6 (42.9%) - CR: 2 (14.3%) - PR: 4 (28.6%) PD: 1 (7.1%) | ||||
| Maude et al. [29], 2017 | B-ALL | Tisa-cel+ Pembrolizumab | 4 | ORR: 2 (50.0%) - CR: 1 (25.0%) - PR: 1 (25.0%) | 4 (100%) | |||
| Cao et al. [30], 2019 | B cell-NHL | Anti-CD19+ Nivolumab | 11 | ORR: 9 (81.8%) - CR: 5 (45.5%) - PR: 4 (36.4%) | 6 (1–14) | |||
| Siddiqi et al. [31], 2019 (PLATFORM study) | B cell-NHL | Liso-cel + Durvalumab | 18 (11 *) | ORR: 10 (90.9%) - CR: 7 (63.6) | 3 (27.3%) | |||
| Jacobson et al. [33], 2020 (ZUMA-6 trial) | B cell-NHL | Axi-cel + Atezolizumab | 28 | ORR: 21 (75.0%) - CR: 13 (46.4%) | 50% # | 71% # | 62% # | |
| Ramakrishnan et al. [34], 2020 (ALEXANDER trial) | B cell-NHL | AUTO3 + Pembrolizumab | 33 (29 *) | ORR: 20 (69.0%) - CR: 15 (51.7%) | ||||
| Chong et al. [35], 2022 | B cell-NHL | Tisa-cel + Pembrolizumab | 12 | ORR: 3 (25.0%) - CR: 1 (8.3%) - PR: 2 (16.7%) PD: 8 (66.7%) SD: 1 (8.3%) | 10 (83.3%) | 2.8 (0.4–35.2) | ||
| Hirayama et al. [36], 2022 | B cell-NHL | Anti-CD19+ Durvalumab | 29 (26 *) | ORR: 9 (34.6%) - RC: 7 (26.9%) | ||||
| Jaeger et al. [37], 2023 (PORTIA trial) | B cell-NHL | Tisa-cel + Pembrolizumab | 12 | ORR: 6 (50.0%) - CR: 4 (33.3%) - PR: 2 (16.7%) PD: 6 (50.0%) | 0% |
3.5. Safety Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministerio de Sanidad Consumo y Binestar Social. Plan Del Sistema de Abordaje de Las Terapias Avanzadas en el Sistema Nacional de Salud: Medicamentos CAR; Ministerio de Sanidad Consumo y Binestar Social: Madrid, Spain, 2018; pp. 1–24.
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- European Medicines Agency. Tercatus: Brexucabtagene Autoleucel. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tecartus (accessed on 26 July 2023).
- European Medicines Agency. Carvykti Ciltacabtagene Autoleucel. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/carvykti (accessed on 26 July 2023).
- Ministerio de Sanidad. Informe de Seguimiento de la Dirección General de Cartera Común de Servicios del Sistema Nacional de Salud (SNS) y Farmacia Sobre el Plan para el Abordaje de las Terapias Avanzadas en el SNS; Ministerio de Sanidad: Madrid, Spain, 2021; pp. 1–38.
- European Medicines Agency. Abecma: Idecabtagen Vicleucel. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/abecma (accessed on 26 July 2023).
- Agencia Española de Medicamentos y Productos Sanitarios. ARI-0001 Dispersión Para Perfusión, Que Contiene 0, 1-1 × 106 células/kg—Hospital Clínic de Barcelona. Available online: https://www.aemps.gob.es/medicamentos-de-uso-humano/terapias-avanzadas/autorizaciones-de-uso-de-medicamentos-de-terapia-avanzada/ (accessed on 26 July 2023).
- European Medicines Agency. Breyanzi. Lisocabtagene Maraleucel. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/breyanzi (accessed on 26 July 2023).
- Makita, S.; Imaizumi, K.; Kurosawa, S.; Tobinai, K. Chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma: Opportunities and challenges. Drugs Context 2019, 8, 212567. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, A.V.; Gauthier, J.; Hay, K.A.; Voutsinas, J.M.; Wu, Q.; Pender, B.S.; Hawkins, R.M.; Vakil, A.; Steinmetz, R.N.; Riddell, S.R.; et al. High rate of durable complete remission in follicular lymphoma after CD19 CAR-T cell immunotherapy. Blood 2019, 134, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Yang, J.F.; Deng, B.P.; Zhao, X.J.; Zhang, X.; Lin, Y.H.; Wu, Y.N.; Deng, Z.L.; Zhang, Y.L.; Liu, S.H.; et al. High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia 2017, 31, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Huang, S.; Xiao, X.; Sun, Q.; Liang, X.; Chen, S.; Zhao, Z.; Huo, Z.; Tu, S.; Li, Y. Challenges and Clinical Strategies of CAR T-Cell Therapy for Acute Lymphoblastic Leukemia: Overview and Developments. Front. Immunol. 2020, 11, 569117. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, L.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef]
- Moreno-Martínez, M.E.; Vinent-Genestar, J.; Muñoz-Sánchez, C.; Carreras-Soler, M.J. Hospital pharmacist’s roles and responsibilities with CAR-T medicines. Farm. Hosp. 2020, 44, 26–31. [Google Scholar]
- Schmitz, F.; Wolf, D.; Holderried, T.A.W. The Role of Immune Checkpoints after Cellular Therapy. Int. J. Mol. Sci. 2020, 21, 3650. [Google Scholar] [CrossRef]
- Cerrano, M.; Ruella, M.; Perales, M.A.; Vitale, C.; Faraci, D.G.; Giaccone, L.; Coscia, M.; Maloy, M.; Sanchez-Escamilla, M.; Elsabah, H.; et al. The Advent of CAR T-Cell Therapy for Lymphoproliferative Neoplasms: Integrating Research into Clinical Practice. Front. Immunol. 2020, 11, 888. [Google Scholar] [CrossRef] [PubMed]
- John, L.B.; Devaud, C.; Duong, C.P.; Yong, C.S.; Beavis, P.A.; Haynes, N.M.; Chow, M.T.; Smyth, M.J.; Kershaw, M.H.; Darcy, P.K. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin. Cancer Res. 2013, 19, 5636–5646. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.M.; Maher, J. Novel approaches to promote CAR T-cell function in solid tumors. Expert Opin. Biol. Ther. 2019, 19, 789–799. [Google Scholar] [CrossRef]
- Galon, J.; Rossi, J.; Turcan, S.; Danan, C.; Locke, F.L.; Neelapu, S.S.; Miklos, D.B.; Bartlett, N.L.; Jacobson, C.A.; Braunschweig, I.; et al. Characterization of anti-CD19 chimeric antigen receptor (CAR) T cell-mediated tumor microenvironment immune gene profile in a multicenter trial (ZUMA-1) with axicabtagene ciloleucel (axi-cel, KTE-C19). J. Clin. Oncol. 2017, 35 (Suppl. 15), 3025. [Google Scholar] [CrossRef]
- Chong, E.A.; Svoboda, J.; Nasta, S.D.; Landsburg, D.J.; Winchell, N.; Napier, E.; Mato, A.R.; Melenhorst, J.J.; Ruella, M.; Lacey, S.F.; et al. Sequential Anti-CD19 Directed Chimeric Antigen Receptor Modified T-Cell Therapy (CART19) and PD-1 Blockade with Pembrolizumab in Patients with Relapsed or Refractory B-Cell Non-Hodgkin Lymphomas. Blood 2018, 132 (Suppl. 1), 4198. [Google Scholar] [CrossRef]
- Hill, B.T.; Roberts, Z.J.; Xue, A.; Rossi, J.M.; Smith, M.R. Rapid tumor regression from PD-1 inhibition after anti-CD19 chimeric antigen receptor T-cell therapy in refractory diffuse large B-cell lymphoma. Bone Marrow Transplant. 2020, 55, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.Y.; Sun, L.; Wen, S.P.; Song, Z.R.; Xing, L.; Wang, Y.; Li, J.Q.; Zhang, X.J.; Wang, F.X. Programmed cell death protein-1 inhibitor combined with chimeric antigen receptor T cells in the treatment of relapsed refractory non-Hodgkin lymphoma: A case report. World J. Clin. Cases 2021, 9, 2394–2399. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.H.; Osborn, M.J.; Tolar, J.; Kim, C.J. Incorporation of Immune Checkpoint Blockade into Chimeric Antigen Receptor T Cells (CAR-Ts): Combination or Built-In CAR-T. Int. J. Mol. Sci. 2018, 19, 340. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Network SIG. Methodology. Checklist. Available online: http://www.sign.ac.uk (accessed on 26 July 2023).
- Li, A.M.; Hucks, G.E.; Dinofia, A.M.; Seif, A.E.; Teachey, D.T.; Baniewicz, D.; Callahan, C.; Fasano, C.; McBride, B.; Gonzalez, V.; et al. Checkpoint Inhibitors Augment CD19-Directed Chimeric Antigen Receptor (CAR) T Cell Therapy in Relapsed B-Cell Acute Lymphoblastic Leukemia. Blood 2018, 132 (Suppl. 1), 556. [Google Scholar] [CrossRef]
- Maude, S.L.; Hucks, G.E.; Seif, A.E.; Talekar, M.K.; Teachey, D.T.; Baniewicz, D.; Callahan, C.; Gonzalez, V.; Nazimuddin, F.; Gupta, M.; et al. The effect of pembrolizumab in combination with CD19-targeted chimeric antigen receptor (CAR) T cells in relapsed acute lymphoblastic leukemia (ALL). J. Clin. Oncol. 2017, 35, 103. [Google Scholar] [CrossRef]
- Cao, Y.; Lu, W.; Sun, R.; Jin, X.; Cheng, L.; He, X.; Wang, L.; Yuan, T.; Lyu, C.; Zhao, M.; et al. Anti-CD19 Chimeric Antigen Receptor T Cells in Combination with Nivolumab Are Safe and Effective against Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma. Front. Oncol. 2019, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, T.; Abramson, J.S.; Lee, H.J.; Schuster, S.; Hasskarl, J.; Montheard, S.; Aringa, J.D.; Thompson, E.; Ananthakrishnan, R.; Lunning, M. Safety of lisocabtagene maraleucel given with durvalumab in patients with relapsed/refractory aggressive B-Cell Non-Hodgkin Lymphoma: First results from the platform study. Hematol. Oncol. 2019, 37 (Suppl. S2), 171–172. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, C.A.; Westin, J.R.; Miklos, D.B.; Herrera, A.F.; Lee, J.; Seng, J.; Rossi, J.M.; Sun, J.; Dong, J.; Roberts, Z.J.; et al. Abstract CT055: Phase 1/2 primary analysis of ZUMA-6: Axicabtagene ciloleucel (Axi-Cel) in combination with atezolizumab (Atezo) for the treatment of patients (Pts) with refractory diffuse large B cell lymphoma (DLBCL). Cancer Res. 2020, 80 (Suppl. 16), CT055. [Google Scholar] [CrossRef]
- Ramakrishnan, A.; Ardeshna, K.M.; Batlevi, C.L.; Marzolini, M.A.V.; Wendy Osborne, M.; Eleni Tholouli, M.; Bachier, C.; McSweeney, P.A.; Budde, E.; Bartlett, M.D.; et al. Phase 1 Alexander Study of AUTO3, the First CD19/22 Dual Targeting CAR T Cell Therapy, with Pembrolizumab in Patients with Relapsed/Refractory (r/r) DLBCL. In Proceedings of the 62nd ASH Annual Meeting and Exposition, Washington, DC, USA, 7 December 2020; Aso, H., Ed.; [Google Scholar]
- Chong, E.A.; Alanio, C.; Svoboda, J.; Nasta, S.D.; Landsburg, D.J.; Lacey, S.F.; Ruella, M.; Bhattacharyya, S.; Wherry, E.J.; Schuster, S.J. Pembrolizumab for B-cell lymphomas relapsing after or refractory to CD19-directed CAR T-cell therapy. Blood 2022, 139, 1026–1038. [Google Scholar] [CrossRef] [PubMed]
- Vinaud Hirayama, A.; Fiorenza, S.; Gauthier, J.; Voutsinas, J.M.; Wu, Q.V.; Kimble, E.L.; Pender, B.S.; Kirchmeier, D.R.; Di, H.A.; Cassaday, R.D.; et al. Timing of PD-L1 Blockade with Durvalumab May Affect Outcomes of CD19 CAR-T Cell Therapy for Relapsed/Refractory Large B-Cell Lymphoma. Blood 2022, 140 (Suppl. 1), 7447–7449. [Google Scholar] [CrossRef]
- Jaeger, U.; Worel, N.; McGuirk, J.P.; Riedell, P.A.; Fleury, I.; Du, Y.; Han, X.; Pearson, D.; Redondo, S.; Waller, E.K.; et al. Safety and efficacy of tisagenlecleucel plus pembrolizumab in patients with r/r DLBCL: Phase 1b PORTIA study results. Blood Adv. 2023, 7, 2283–2286. [Google Scholar] [CrossRef]
- Armengol, M.; Santos, J.C.; Fernández-Serrano, M.; Profitós-Pelejà, N.; Ribeiro, M.L.; Roué, G. Immune-Checkpoint Inhibitors in B-Cell Lymphoma. Cancers 2021, 13, 214. [Google Scholar] [CrossRef]
- Wang, H.; Kaur, G.; Sankin, A.I.; Chen, F.; Guan, F.; Zang, X. Immune checkpoint blockade and CAR-T cell therapy in hematologic malignancies. J. Hematol. Oncol. 2019, 12, 59. [Google Scholar] [CrossRef]
- Spiegel, J.Y.; Dahiya, S.; Jain, M.D.; Tamaresis, J.; Nastoupil, L.J.; Jacobs, M.T.; Ghobadi, A.; Lin, Y.; Lunning, M.; Lekakis, L.; et al. Outcomes of patients with large B-cell lymphoma progressing after axicabtagene ciloleucel therapy. Blood 2021, 137, 1832–1835. [Google Scholar]
| Author, Year | N | TRAEs | Any Grade N (%) | Grade 3–4 N (%) |
|---|---|---|---|---|
| Li et al. [28], 2017 | 14 | Cytopenia | 4 (28.6%) | 4 (28.6%) |
| CRS | 3 (21.4%) | - | ||
| Fever | 3 (21.4%) | - | ||
| Acute pancreatitis | 1 (7.1%) | - | ||
| Hypothyroidism | 1 (7.1%) | - | ||
| Arthralgias | 1 (7.1%) | - | ||
| Urticaria | 1 (7.1%) | - | ||
| Maude et al. [29], 2017 | 4 | Fever (without CRS) | 2 (50.0%) | - |
| Cao et al. [30], 2019 | 11 | CRS (82) | 9 (81.8%) | - |
| Neurotoxicity (9) | 1 (9.1%) | - | ||
| Siddiqi et al. [31], 2019 (PLATFORM study) | 11 | Fever | NR | - |
| CRS | - | |||
| Fatigue | - | |||
| Cytopenia | - | |||
| Haemolytic anaemia | - | |||
| Rash | - | |||
| Neurotoxicity | - | |||
| Jacobson et al. [33], 2020 (ZUMA-6 trial) | 28 | Neurotoxicity | 8 (28.6%) | 8 (28.6%) |
| CRS | 1 (3.6%) | 1 (3.6%) | ||
| Ramakrishnan et al. [34], 2020 (ALEXANDER trial) | 33 | Neutropenia | 24 (72.7%) | 24 (72.7%) |
| Thrombocytopenia | 21 (63.6%) | 16 (48.5%) | ||
| Anaemia | 20 (60.6%) | 16 (48.5%) | ||
| CRS | 11 (33.3%) | - | ||
| Pyrexia | 10 (30.3%) | - | ||
| Constipation | 9 (27.3%) | - | ||
| Fatigue | 8 (24.2%) | |||
| Chong et al. [35], 2022 | 12 | Neutropenia | 4 (33.3%) | 3 (25.0%) |
| Fever (without CRS) | 3 (25.0%) | - | ||
| Hirayama et al. [36], 2022 | 29 | CRS | 12 (41.4%) | 2 (6.9%) |
| Neutropenia | 6 (20.7%) | - | ||
| Neurotoxicity | 5 (17.2%) | - | ||
| Hypogammaglobulinemia | 5 (17.2%) | - | ||
| Jaeger et al. [37], 2023 (PORTIA trial) | 12 | CRS | 7 (58.3%) | 1 (8.3%) |
| Neutropenia | 2 (16.7%) | 2 (16.7%) | ||
| Anaemia | 2 (16.7%) | 1 (8.3%) | ||
| Lymphopenia | 2 (16.7%) | 1 (8.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Moreno, M.A.; Ciudad-Gutiérrez, P.; Jaramillo-Ruiz, D.; Reguera-Ortega, J.L.; Abdel-kader Martín, L.; Flores-Moreno, S. Combined or Sequential Treatment with Immune Checkpoint Inhibitors and Car-T Cell Therapies for the Management of Haematological Malignancies: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 14780. https://doi.org/10.3390/ijms241914780
Pérez-Moreno MA, Ciudad-Gutiérrez P, Jaramillo-Ruiz D, Reguera-Ortega JL, Abdel-kader Martín L, Flores-Moreno S. Combined or Sequential Treatment with Immune Checkpoint Inhibitors and Car-T Cell Therapies for the Management of Haematological Malignancies: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(19):14780. https://doi.org/10.3390/ijms241914780
Chicago/Turabian StylePérez-Moreno, María Antonia, Pablo Ciudad-Gutiérrez, Didiana Jaramillo-Ruiz, Juan Luis Reguera-Ortega, Laila Abdel-kader Martín, and Sandra Flores-Moreno. 2023. "Combined or Sequential Treatment with Immune Checkpoint Inhibitors and Car-T Cell Therapies for the Management of Haematological Malignancies: A Systematic Review" International Journal of Molecular Sciences 24, no. 19: 14780. https://doi.org/10.3390/ijms241914780
APA StylePérez-Moreno, M. A., Ciudad-Gutiérrez, P., Jaramillo-Ruiz, D., Reguera-Ortega, J. L., Abdel-kader Martín, L., & Flores-Moreno, S. (2023). Combined or Sequential Treatment with Immune Checkpoint Inhibitors and Car-T Cell Therapies for the Management of Haematological Malignancies: A Systematic Review. International Journal of Molecular Sciences, 24(19), 14780. https://doi.org/10.3390/ijms241914780






