PIP5Kγ Mediates PI(4,5)P2/Merlin/LATS1 Signaling Activation and Interplays with Hsc70 in Hippo–YAP Pathway Regulation
Abstract
:1. Introduction
2. Results
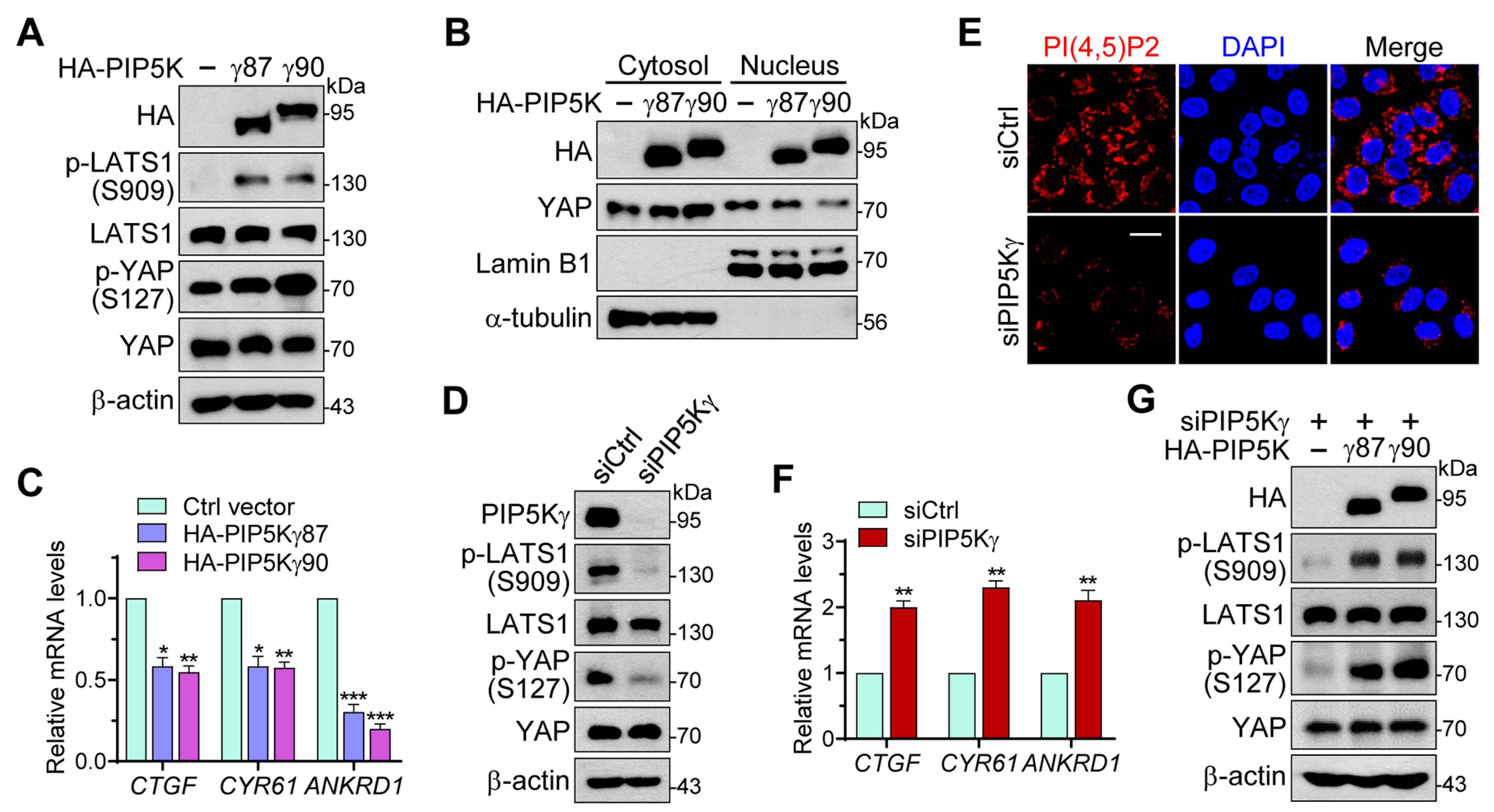
2.1. PIP5Kγ Has a Regulatory Role in the Hippo–YAP Pathway
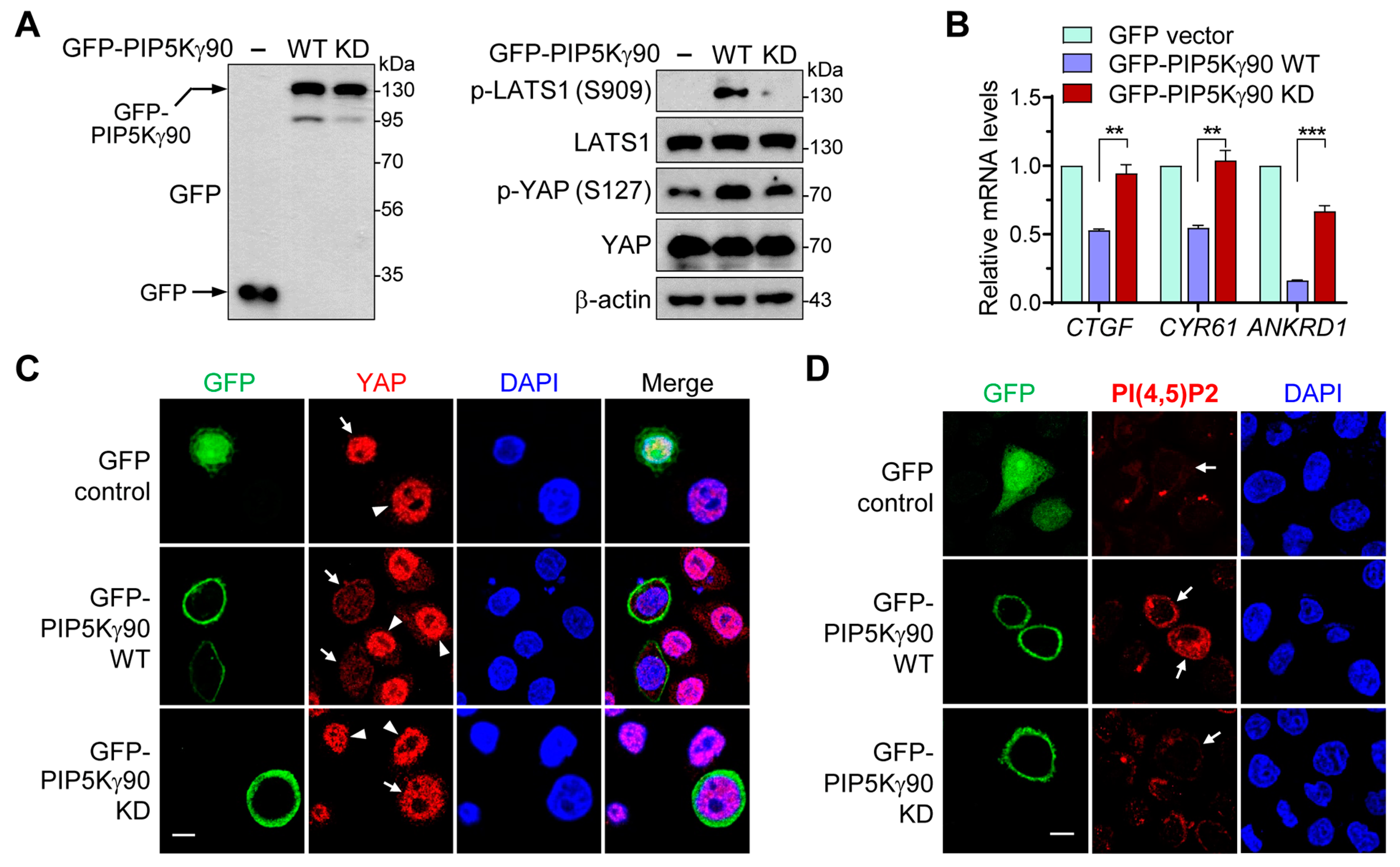
2.2. Regulatory Effects of PIP5Kγ90 on LATS1 and YAP Are Dependent on Its Catalytic Activity
2.3. PIP5Kγ Mediates Restored YAP Phosphorylation in Response to EGF and LPA Stimulation
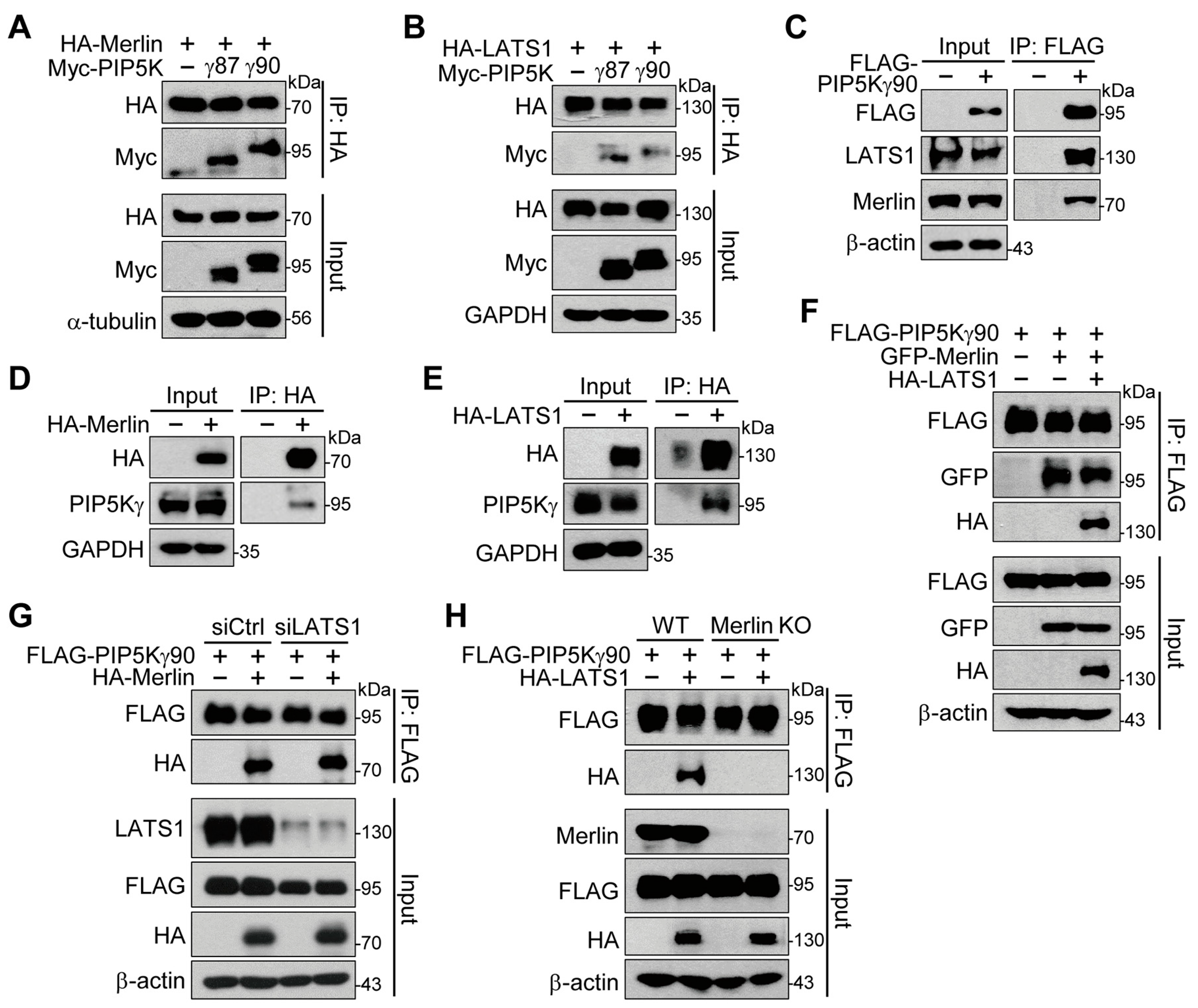
2.4. Merlin Binds to PIP5Kγ and Mediates Interaction of PIP5Kγ90 with LATS1
2.5. PIP5Kγ90 Binds to the FERM Domain of Merlin and Modulates Merlin–LATS1 and YAP–TEAD4 Interactions
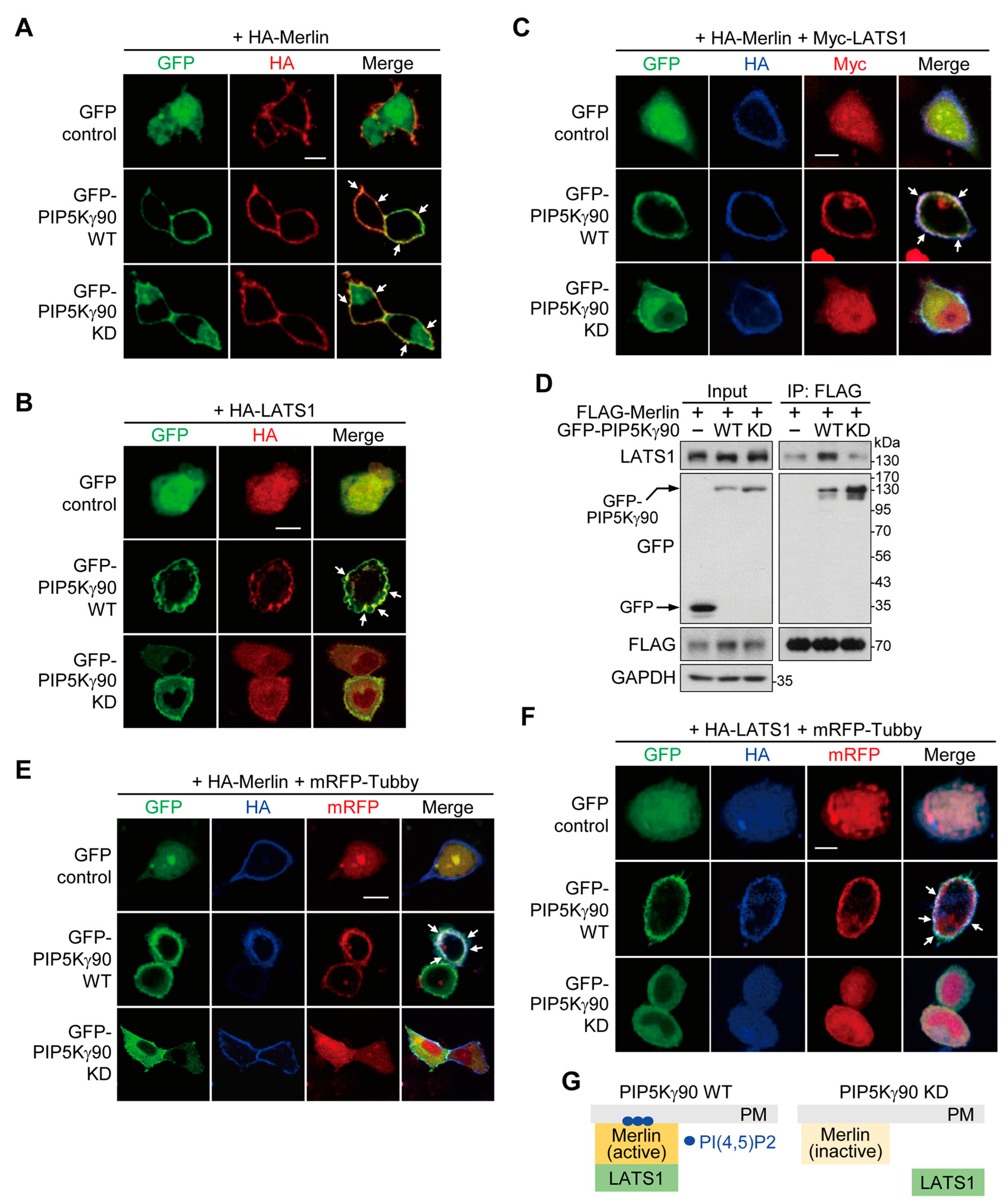
2.6. PI(4,5)P2 Produced by PIP5Kγ90 Induces LATS1 Translocation to Merlin-Rich PM Regions
2.7. Merlin and LATS1 Are Necessary for Hippo–YAP Pathway Regulation by PIP5Kγ
2.8. PIP5Kγ Has the Potential to Suppress YAP-Dependent Cell Proliferation
2.9. Interplay between PIP5Kγ90 and Hsc70 Leads to Activation of the Hippo Pathway
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Expression Constructs
4.3. Cell Culture
4.4. Transfection and Gene Knockdown
4.5. WB and IP Analysis
4.6. qRT-PCR Analysis
4.7. Immunostaining and Cell Imaging
4.8. PI(4,5)P2 Visualization
4.9. Subcellular Fractionation
4.10. Colony Formation and Cell Viability Assays
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aa | amino acids |
| ANKRD1 | ankyrin repeat domain-containing protein 1 |
| CMA | chaperone-mediated autophagy |
| CTGF | connective tissue growth factor |
| CYR61 | cysteine-rich angiogenic inducer 61 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMSO | dimethyl sulfoxide |
| EGF | epidermal growth factor |
| FERM | band 4.1/ezrin/radixin/moesin |
| GFP | green fluorescent protein |
| HEK293 | human embryonic kidney 293 |
| Hsc70 | heat shock cognate 71-kDa protein |
| IP | immunoprecipitation |
| KD | kinase-dead |
| KO | knockout |
| LATS1 | large tumor suppressor kinase 1 |
| LPA | lysophosphatidic acid |
| mRFP | monomeric red fluorescent protein |
| MST1/2 | mammalian Ste20-like kinase 1/2 |
| PH | pleckstrin homology |
| PI(4,5)P2 | phosphatidylinositol 4,5-bisphosphate |
| PIP5Kγ | phosphatidylinositol 4-phosphate 5-kinase γ |
| PLC | phospholipase C |
| PM | plasma membrane |
| qRT-PCR | quantitative real-time reverse transcription polymerase chain reaction |
| siRNA | small interfering RNA |
| TAZ | transcriptional coactivator with PDZ-binding motif |
| WB | Western blotting |
| WT | wild-type |
| YAP | Yes-associated protein |
References
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007, 130, 1120–1133. [Google Scholar] [CrossRef]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef]
- Yu, F.X.; Zhao, B.; Guan, K.L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef] [PubMed]
- Moya, I.M.; Halder, G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 2019, 20, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, L.; Tumaneng, K.; Wang, C.Y.; Guan, K.L. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 2010, 24, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, Y.; Zheng, Y.; Dong, J.; Pan, D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev. Cell 2008, 14, 388–398. [Google Scholar] [CrossRef]
- Li, W.; Cooper, J.; Karajannis, M.A.; Giancotti, F.G. Merlin: A tumour suppressor with functions at the cell cortex and in the nucleus. EMBO Rep. 2012, 13, 204–215. [Google Scholar] [CrossRef]
- Yin, F.; Yu, J.; Zheng, Y.; Chen, Q.; Zhang, N.; Pan, D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell 2013, 154, 1342–1355. [Google Scholar] [CrossRef]
- Zhang, N.; Bai, H.; David, K.K.; Dong, J.; Zheng, Y.; Cai, J.; Giovannini, M.; Liu, P.; Anders, R.A.; Pan, D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell 2010, 19, 27–38. [Google Scholar] [CrossRef]
- Senju, Y.; Hibino, E. Moesin-ezrin-radixin-like protein merlin: Its conserved and distinct functions from those of ERM proteins. Biochim. Biophys. Acta Biomembr. 2023, 1865, 184076. [Google Scholar] [CrossRef]
- Michie, K.A.; Bermeister, A.; Robertson, N.O.; Goodchild, S.C.; Curmi, P.M.G. Two Sides of the Coin: Ezrin/Radixin/Moesin and Merlin Control Membrane Structure and Contact Inhibition. Int. J. Mol. Sci. 2019, 20, 1996. [Google Scholar] [CrossRef]
- Chinthalapudi, K.; Mandati, V.; Zheng, J.; Sharff, A.J.; Bricogne, G.; Griffin, P.R.; Kissil, J.; Izard, T. Lipid binding promotes the open conformation and tumor-suppressive activity of neurofibromin 2. Nat. Commun. 2018, 9, 1338. [Google Scholar] [CrossRef]
- Mani, T.; Hennigan, R.F.; Foster, L.A.; Conrady, D.G.; Herr, A.B.; Ip, W. FERM domain phosphoinositide binding targets merlin to the membrane and is essential for its growth-suppressive function. Mol. Cell. Biol. 2011, 31, 1983–1996. [Google Scholar] [CrossRef] [PubMed]
- Ali Khajeh, J.; Ju, J.H.; Atchiba, M.; Allaire, M.; Stanley, C.; Heller, W.T.; Callaway, D.J.; Bu, Z. Molecular conformation of the full-length tumor suppressor NF2/Merlin--a small-angle neutron scattering study. J. Mol. Biol. 2014, 426, 2755–2768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, B.; Gao, Y.; Long, J.; Zhou, H. The crystal structure of the FERM and C-terminal domain complex of Drosophila Merlin. Biochem. Biophys. Res. Commun. 2021, 553, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Primi, M.C.; Rangarajan, E.S.; Patil, D.N.; Izard, T. Conformational flexibility determines the Nf2/merlin tumor suppressor functions. Matrix Biol. Plus 2021, 12, 100074. [Google Scholar] [CrossRef]
- Wills, R.C.; Hammond, G.R.V. PI(4,5)P2: Signaling the plasma membrane. Biochem. J. 2022, 479, 2311–2325. [Google Scholar] [CrossRef] [PubMed]
- Downes, C.P.; Gray, A.; Lucocq, J.M. Probing phosphoinositide functions in signaling and membrane trafficking. Trends Cell Biol. 2005, 15, 259–268. [Google Scholar] [CrossRef]
- Di Paolo, G.; De Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 2006, 443, 651–657. [Google Scholar] [CrossRef]
- Katan, M.; Cockcroft, S. Phosphatidylinositol(4,5)bisphosphate: Diverse functions at the plasma membrane. Essays Biochem. 2020, 64, 513–531. [Google Scholar] [CrossRef]
- Kwiatkowska, K. One lipid, multiple functions: How various pools of PI(4,5)P(2) are created in the plasma membrane. Cell. Mol. Life Sci. 2010, 67, 3927–3946. [Google Scholar] [CrossRef]
- Ishihara, H.; Shibasaki, Y.; Kizuki, N.; Katagiri, H.; Yazaki, Y.; Asano, T.; Oka, Y. Cloning of cDNAs encoding two isoforms of 68-kDa type I phosphatidylinositol-4-phosphate 5-kinase. J. Biol. Chem. 1996, 271, 23611–23614. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Shibasaki, Y.; Kizuki, N.; Wada, T.; Yazaki, Y.; Asano, T.; Oka, Y. Type I phosphatidylinositol-4-phosphate 5-kinases. Cloning of the third isoform and deletion/substitution analysis of members of this novel lipid kinase family. J. Biol. Chem. 1998, 273, 8741–8748. [Google Scholar] [CrossRef] [PubMed]
- van den Bout, I.; Divecha, N. PIP5K-driven PtdIns(4,5)P2 synthesis: Regulation and cellular functions. J. Cell Sci. 2009, 122, 3837–3850. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, G.; Pellegrini, L.; Letinic, K.; Cestra, G.; Zoncu, R.; Voronov, S.; Chang, S.; Guo, J.; Wenk, M.R.; De Camilli, P. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1 gamma by the FERM domain of talin. Nature 2002, 420, 85–89. [Google Scholar] [CrossRef]
- Hergovich, A.; Schmitz, D.; Hemmings, B.A. The human tumour suppressor LATS1 is activated by human MOB1 at the membrane. Biochem. Biophys. Res. Commun. 2006, 345, 50–58. [Google Scholar] [CrossRef]
- Rausch, V.; Hansen, C.G. The Hippo Pathway, YAP/TAZ, and the Plasma Membrane. Trends Cell Biol. 2020, 30, 32–48. [Google Scholar] [CrossRef]
- Hong, A.W.; Meng, Z.; Plouffe, S.W.; Lin, Z.; Zhang, M.; Guan, K.L. Critical roles of phosphoinositides and NF2 in Hippo pathway regulation. Genes Dev. 2020, 34, 511–525. [Google Scholar] [CrossRef]
- Yu, F.X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; Li, H.; et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012, 150, 780–791. [Google Scholar] [CrossRef]
- Heck, J.N.; Mellman, D.L.; Ling, K.; Sun, Y.; Wagoner, M.P.; Schill, N.J.; Anderson, R.A. A conspicuous connection: Structure defines function for the phosphatidylinositol-phosphate kinase family. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 15–39. [Google Scholar] [CrossRef]
- Kunz, J.; Wilson, M.P.; Kisseleva, M.; Hurley, J.H.; Majerus, P.W.; Anderson, R.A. The activation loop of phosphatidylinositol phosphate kinases determines signaling specificity. Mol. Cell 2000, 5, 1–11. [Google Scholar] [CrossRef]
- Fan, R.; Kim, N.G.; Gumbiner, B.M. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc. Natl. Acad. Sci. USA 2013, 110, 2569–2574. [Google Scholar] [CrossRef]
- Tran, M.H.; Seo, E.; Min, S.; Nguyen, Q.T.; Choi, J.; Lee, U.J.; Hong, S.S.; Kang, H.; Mansukhani, A.; Jou, I.; et al. NEDD4-induced degradative ubiquitination of phosphatidylinositol 4-phosphate 5-kinase alpha and its implication in breast cancer cell proliferation. J. Cell. Mol. Med. 2018, 22, 4117–4129. [Google Scholar] [CrossRef]
- Le, T.P.H.; Nguyen, N.T.T.; Le, D.D.T.; Anwar, M.A.; Lee, S.Y. Lipid kinase PIP5Kalpha contributes to Hippo pathway activation via interaction with Merlin and by mediating plasma membrane targeting of LATS1. Cell Commun. Signal. 2023, 21, 149. [Google Scholar] [CrossRef] [PubMed]
- Fehon, R.G.; McClatchey, A.I.; Bretscher, A. Organizing the cell cortex: The role of ERM proteins. Nat. Rev. Mol. Cell Biol. 2010, 11, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Kim, Y.M.; Kim, T.D.; Le, O.T.; Kim, J.J.; Kang, H.C.; Hasegawa, H.; Kanaho, Y.; Jou, I.; Lee, S.Y. Phosphatidylinositol 4-phosphate 5-kinase alpha facilitates Toll-like receptor 4-mediated microglial inflammation through regulation of the Toll/interleukin-1 receptor domain-containing adaptor protein (TIRAP) location. J. Biol. Chem. 2013, 288, 5645–5659. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjostedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, 2507. [Google Scholar] [CrossRef]
- Nagy, A.; Munkacsy, G.; Gyorffy, B. Pancancer survival analysis of cancer hallmark genes. Sci. Rep. 2021, 11, 6047. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.D.; Loo, L.; Street, S.E.; Ma, A.; Taylor-Blake, B.; Stashko, M.A.; Jin, J.; Janzen, W.P.; Frye, S.V.; Zylka, M.J. The lipid kinase PIP5K1C regulates pain signaling and sensitization. Neuron 2014, 82, 836–847. [Google Scholar] [CrossRef]
- Calses, P.C.; Crawford, J.J.; Lill, J.R.; Dey, A. Hippo Pathway in Cancer: Aberrant Regulation and Therapeutic Opportunities. Trends Cancer 2019, 5, 297–307. [Google Scholar] [CrossRef]
- Kirchner, P.; Bourdenx, M.; Madrigal-Matute, J.; Tiano, S.; Diaz, A.; Bartholdy, B.A.; Will, B.; Cuervo, A.M. Proteome-wide analysis of chaperone-mediated autophagy targeting motifs. PLoS Biol. 2019, 17, e3000301. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Cuervo, A.M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 365–381. [Google Scholar] [CrossRef]
- Hao, Y.; Kacal, M.; Ouchida, A.T.; Zhang, B.; Norberg, E.; Vakifahmetoglu-Norberg, H. Targetome analysis of chaperone-mediated autophagy in cancer cells. Autophagy 2019, 15, 1558–1571. [Google Scholar] [CrossRef]
- Anguiano, J.; Garner, T.P.; Mahalingam, M.; Das, B.C.; Gavathiotis, E.; Cuervo, A.M. Chemical modulation of chaperone-mediated autophagy by retinoic acid derivatives. Nat. Chem. Biol. 2013, 9, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Bandyopadhyay, S.; Tyagi, K.; Roy, A. Recent advances in understanding the molecular role of phosphoinositide-specific phospholipase C gamma 1 as an emerging onco-driver and novel therapeutic target in human carcinogenesis. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188619. [Google Scholar] [CrossRef]
- Kelley, G.G.; Kaproth-Joslin, K.A.; Reks, S.E.; Smrcka, A.V.; Wojcikiewicz, R.J. G-protein-coupled receptor agonists activate endogenous phospholipase Cepsilon and phospholipase Cbeta3 in a temporally distinct manner. J. Biol. Chem. 2006, 281, 2639–2648. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, F.; Ji, L.; Zhang, L.; Xu, Y.J.; Dhalla, N.S. Role of lysophosphatidic acid in vascular smooth muscle cell proliferation. Can. J. Physiol. Pharmacol. 2020, 98, 103–110. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Li, F.; Chan, S.W.; Lin, Z.; Wei, Z.; Yang, Z.; Guo, F.; Lim, C.J.; Xing, W.; et al. Angiomotin binding-induced activation of Merlin/NF2 in the Hippo pathway. Cell Res. 2015, 25, 801–817. [Google Scholar] [CrossRef] [PubMed]
- Balla, T. Phosphoinositides: Tiny lipids with giant impact on cell regulation. Physiol. Rev. 2013, 93, 1019–1137. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Qiu, Y.; Lin, K.C.; Kumar, A.; Placone, J.K.; Fang, C.; Wang, K.C.; Lu, S.; Pan, M.; Hong, A.W.; et al. RAP2 mediates mechanoresponses of the Hippo pathway. Nature 2018, 560, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Thapa, N.; Choi, S.; Tan, X.; Wise, T.; Anderson, R.A. Phosphatidylinositol Phosphate 5-Kinase Igamma and Phosphoinositide 3-Kinase/Akt Signaling Couple to Promote Oncogenic Growth. J. Biol. Chem. 2015, 290, 18843–18854. [Google Scholar] [CrossRef]
- Desideri, E.; Castelli, S.; Dorard, C.; Toifl, S.; Grazi, G.L.; Ciriolo, M.R.; Baccarini, M. Impaired degradation of YAP1 and IL6ST by chaperone-mediated autophagy promotes proliferation and migration of normal and hepatocellular carcinoma cells. Autophagy 2023, 19, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Doughman, R.L.; Firestone, A.J.; Anderson, R.A. Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J. Membr. Biol. 2003, 194, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Voronov, S.; Letinic, K.; Nairn, A.C.; Di Paolo, G.; De Camilli, P. Regulation of the interaction between PIPKI gamma and talin by proline-directed protein kinases. J. Cell Biol. 2005, 168, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Adler, J.J.; Johnson, D.E.; Heller, B.L.; Bringman, L.R.; Ranahan, W.P.; Conwell, M.D.; Sun, Y.; Hudmon, A.; Wells, C.D. Serum deprivation inhibits the transcriptional co-activator YAP and cell growth via phosphorylation of the 130-kDa isoform of Angiomotin by the LATS1/2 protein kinases. Proc. Natl. Acad. Sci. USA 2013, 110, 17368–17373. [Google Scholar] [CrossRef]
- Borreguero-Munoz, N.; Fletcher, G.C.; Aguilar-Aragon, M.; Elbediwy, A.; Vincent-Mistiaen, Z.I.; Thompson, B.J. The Hippo pathway integrates PI3K-Akt signals with mechanical and polarity cues to control tissue growth. PLoS Biol. 2019, 17, e3000509. [Google Scholar] [CrossRef]
- Totaro, A.; Panciera, T.; Piccolo, S. YAP/TAZ upstream signals and downstream responses. Nat. Cell Biol. 2018, 20, 888–899. [Google Scholar] [CrossRef]
- Yu, F.X.; Guan, K.L. The Hippo pathway: Regulators and regulations. Genes Dev. 2013, 27, 355–371. [Google Scholar] [CrossRef]
- Le, O.T.; Cho, O.Y.; Tran, M.H.; Kim, J.A.; Chang, S.; Jou, I.; Lee, S.Y. Phosphorylation of phosphatidylinositol 4-phosphate 5-kinase gamma by Akt regulates its interaction with talin and focal adhesion dynamics. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 2432–2443. [Google Scholar] [CrossRef]






| Genes | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| Human CTGF | CCTGCAGGCTAGAGAAGCAG | TGGAGATTTTGGGAGTACGG |
| Human CYR61 | AAGAAACCCGGATTTGTGAG | GCTGCATTTCTTGCCCTTT |
| Human ANKRD1 | TTTGGCAATTGTGGAGAAGTTA | AAACATCCAGGTTTCCTCCA |
| Human GAPDH | AGGGCTGCTTTTAACTCTGGT | CCCCACTTGATTTTGGAGGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, D.D.T.; Le, T.P.H.; Lee, S.Y. PIP5Kγ Mediates PI(4,5)P2/Merlin/LATS1 Signaling Activation and Interplays with Hsc70 in Hippo–YAP Pathway Regulation. Int. J. Mol. Sci. 2023, 24, 14786. https://doi.org/10.3390/ijms241914786
Le DDT, Le TPH, Lee SY. PIP5Kγ Mediates PI(4,5)P2/Merlin/LATS1 Signaling Activation and Interplays with Hsc70 in Hippo–YAP Pathway Regulation. International Journal of Molecular Sciences. 2023; 24(19):14786. https://doi.org/10.3390/ijms241914786
Chicago/Turabian StyleLe, Duong Duy Thai, Truc Phan Hoang Le, and Sang Yoon Lee. 2023. "PIP5Kγ Mediates PI(4,5)P2/Merlin/LATS1 Signaling Activation and Interplays with Hsc70 in Hippo–YAP Pathway Regulation" International Journal of Molecular Sciences 24, no. 19: 14786. https://doi.org/10.3390/ijms241914786




