An Intrabody against B-Cell Receptor-Associated Protein 31 (BAP31) Suppresses the Glycosylation of the Epithelial Cell-Adhesion Molecule (EpCAM) via Affecting the Formation of the Sec61-Translocon-Associated Protein (TRAP) Complex
Abstract
:1. Introduction
2. Results
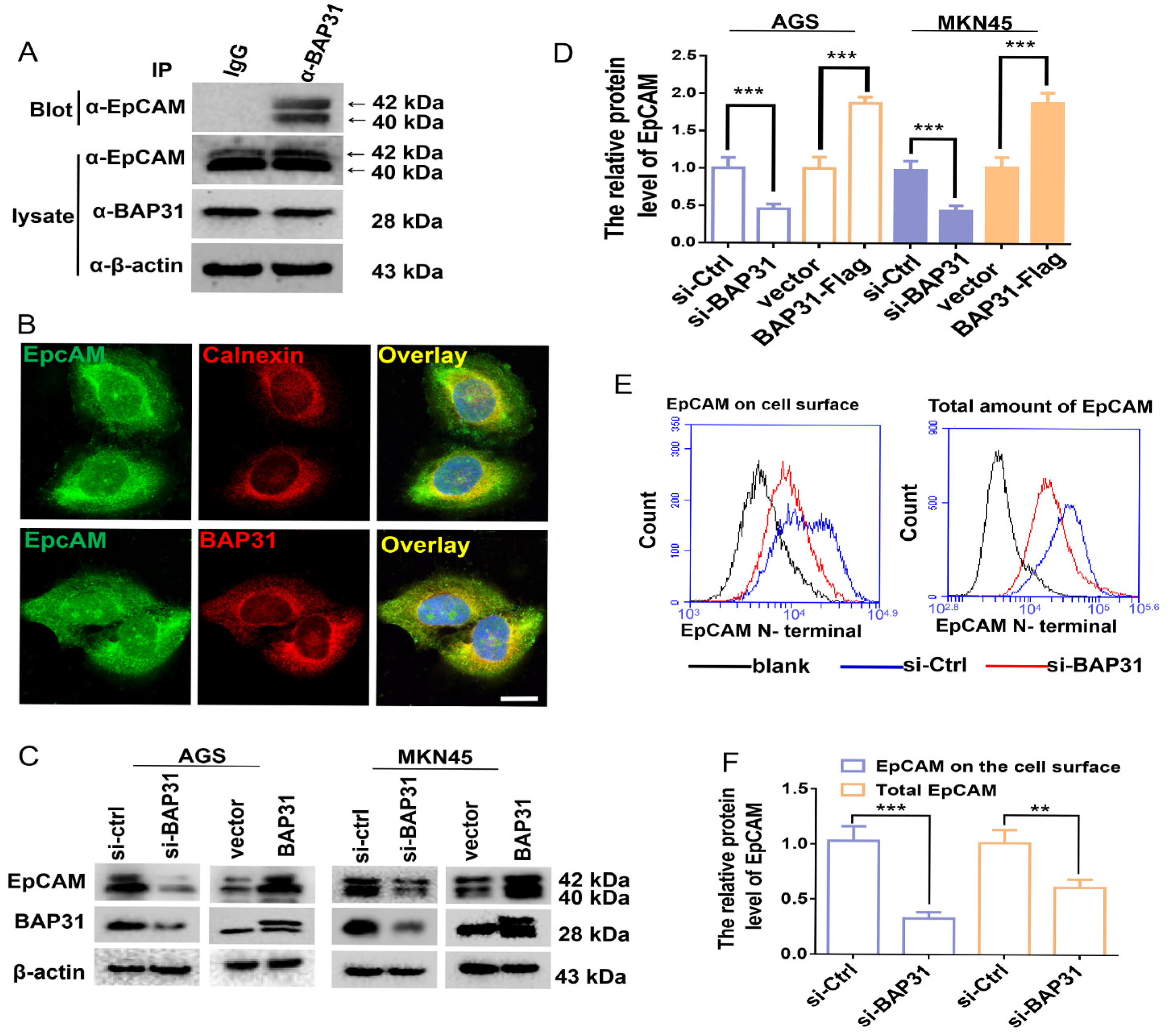
2.1. BAP31 Has an Effect on the Protein Level of EpCAM
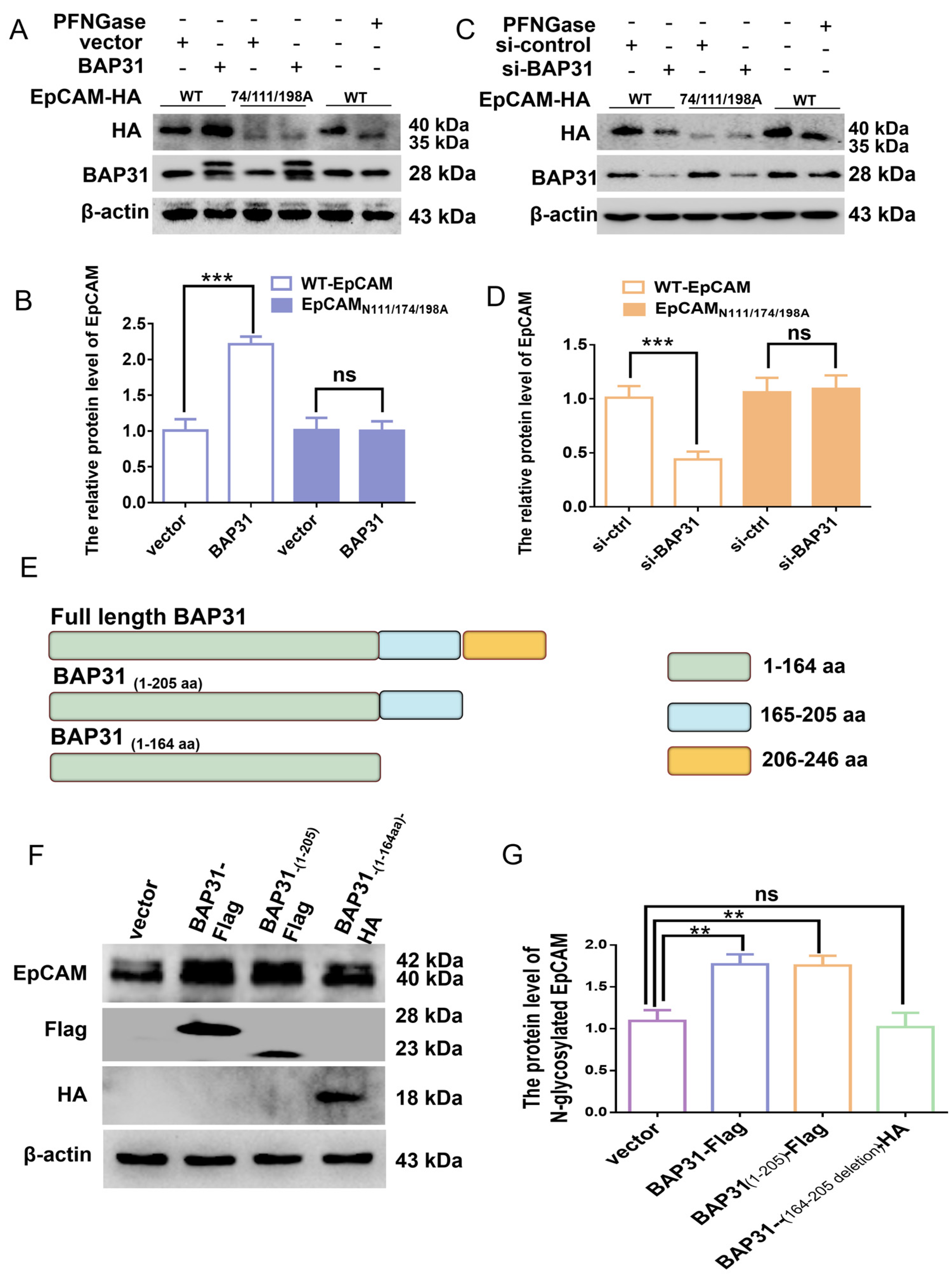
2.2. The Effect of BAP31 on N-Glycosylated EpCAM
2.3. Screening an Anti-BAP31 Human Single-Domain Antibody and Identifying Its Effect on Glycosylated EpCAM
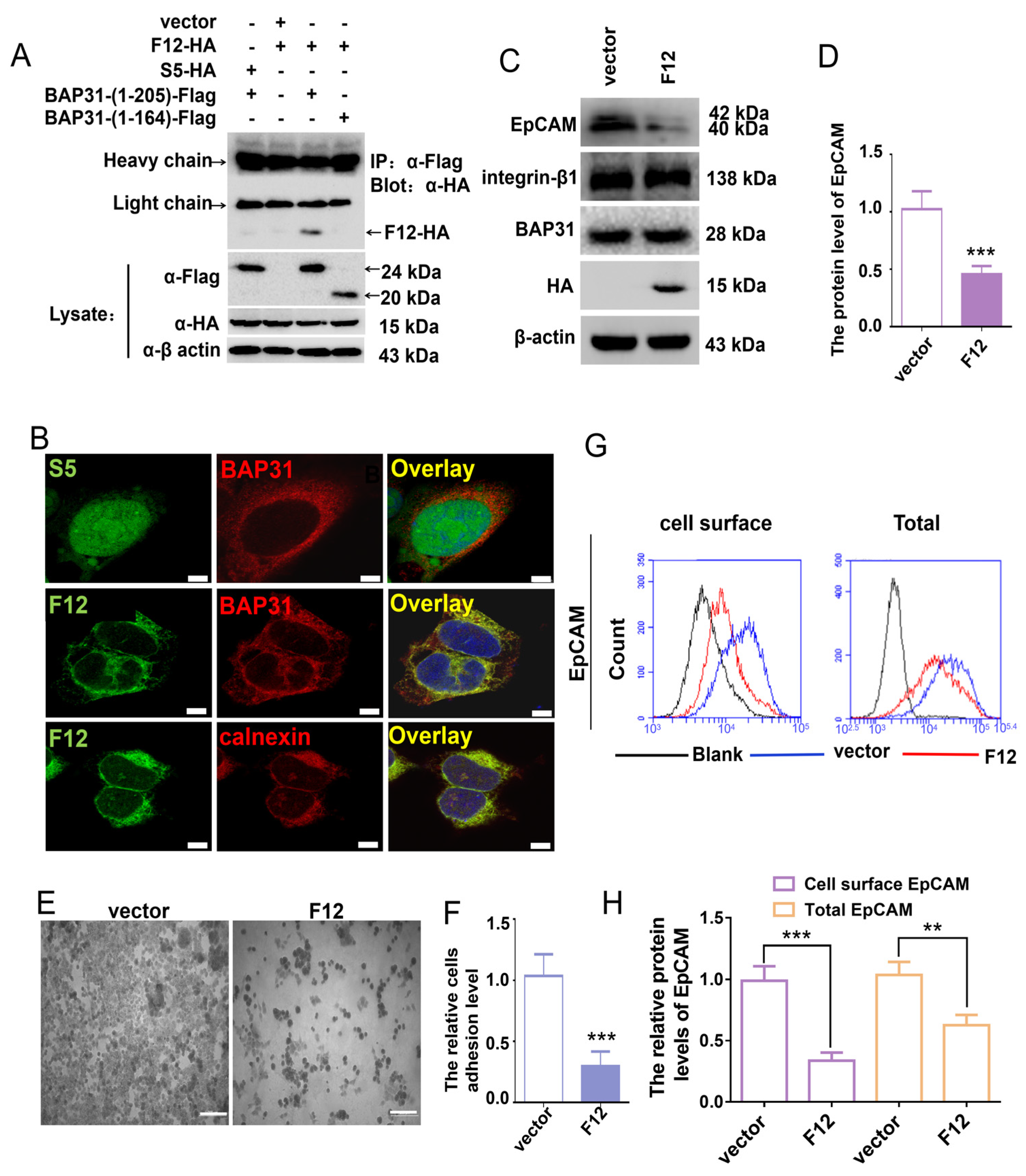
2.4. VH-F12 Colocalizes with BAP31 in the ER and Suppresses N-Glycosylated EpCAM
2.5. VH-F12 Intrabody Promotes MKN-45 Cell Death In Vitro and In Vivo
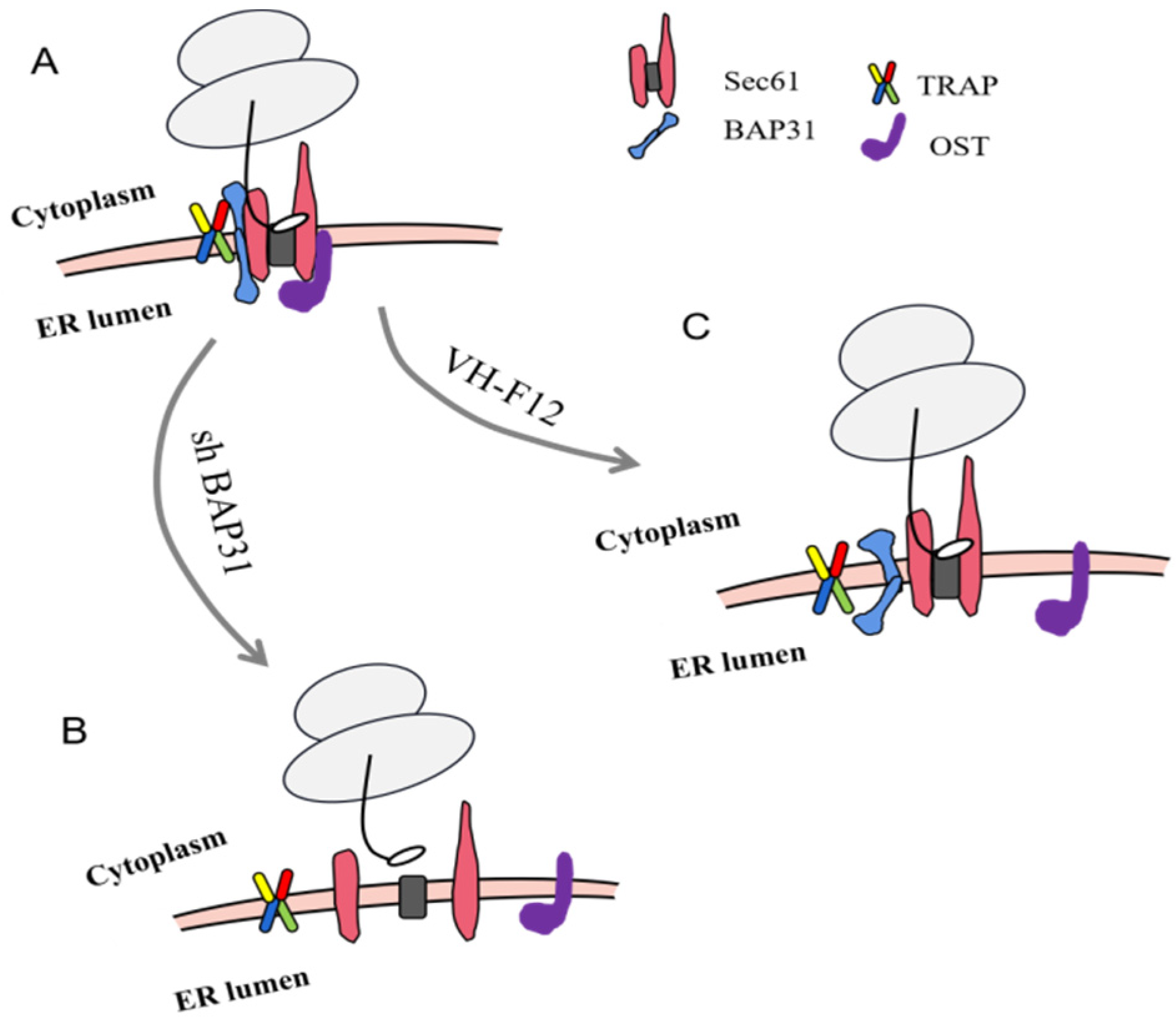
2.6. BAP31 Is Essential for the Formation of Sec61 and TRAP Complex
3. Discussion
4. Material and Methods
4.1. Cell Lines and Animals
4.2. Glycosidase Treatment
4.3. Screening for Anti-BAP31 Human VH Single-Domain Antibody
4.4. Construction of the Plasmid
4.5. Lentiviral Transduction of the GC Cell Line
4.6. Plasmid or RNA Interference Transfection
4.7. Analysis of BAP31/Intrabody Colocalization by Immunofluorescence
4.8. Immunoprecipitation (IP) and Immunoblots
4.9. Apoptosis Analysis
4.10. In Vivo Anti-Tumor Assay
4.11. Blue Native Gel Electrophoresis
4.12. Animal Management
4.13. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics. CA Cancer J. Clin. 2012, 65, 87–108. [Google Scholar] [CrossRef]
- McLean, M.H.; El-Omar, E.M. Genetics of gastric cancer. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Durães, C.; Almeida, G.M.; Seruca, R.; Oliveira, C.; Carneiro, F. Biomarkers for gastric cancer: Prognostic, predictive or targets of therapy? Virchows Arch. 2014, 464, 367–378. [Google Scholar] [CrossRef]
- Went, P.; Vasei, M.; Bubendorf, L.; Terracciano, L.; Tornillo, L.; Riede, U.; Kononen, J.; Simon, R.; Sauter, G.; Baeuerle, P.A. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br. J. Cancer 2006, 94, 128–135. [Google Scholar] [CrossRef]
- Du, W.; Ji, H.; Cao, S.; Wang, L.; Bai, F.; Liu, J.; Fan, D. Retraction Note: EpCAM: A Potential Antimetastatic Target for Gastric Cancer. Dig. Dis. Sci. 2013, 58, 1811. [Google Scholar] [CrossRef]
- Chong, J.M.; Speicher, D.W. Determination of disulfide bond assignments and N-glycosylation sites of the human gastrointestinal carcinoma antigen GA733-2 (CO17-1A, EGP, KS1-4, KSA, and Ep-CAM). J. Biol. Chem. 2001, 276, 5804–5813. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Yuan, F.; Fu, C.; Shen, G.; Hu, S.; Shen, G. Relationship between epithelial cell adhesion molecule (EpCAM) overexpression and gastric cancer patients: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0175357. [Google Scholar] [CrossRef]
- Schnell, U.; Cirulli, V.; Giepmans, B.N. EpCAM: Structure and function in health and disease. Biochim. Biophys. Acta 2013, 1828, 1989–2001. [Google Scholar] [CrossRef]
- Pauli, C.; Münz, M.; Kieu, C.; Mack, B.; Breinl, P.; Wollenberg, B.; Lang, S.; Zeidler, R.; Gires, O. Tumor-specific glycosylation of the carcinoma-associated epithelial cell adhesion molecule EpCAM in head and neck carcinomas. Cancer Lett. 2003, 193, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Munz, M.; Fellinger, K.; Hofmann, T.; Schmitt, B.; Gires, O. Glycosylation is crucial for stability of tumour and cancer stem cell antigen EpCAM. Front. Biosci. 2008, 13, 5195–5201. [Google Scholar] [CrossRef] [PubMed]
- Conti, B.J.; Devaraneni, P.K.; Yang, Z.; David, L.L.; Skach, W.R. Cotranslational stabilization of Sec62/63 within the ER Sec61 translocon is controlled by distinct substrate-driven translocation events. Mol. Cell 2015, 58, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Li, H.; Li, H.; Lennarz, W.J. Oligosaccharyltransferase directly binds to ribosome at a location near the translocon-binding site. Proc. Natl. Acad. Sci. USA 2009, 106, 6945–6949. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Heath-Engel, H.; Zhang, D.; Nguyen, N.; Thomas, D.Y.; Hanrahan, J.W.; Shore, G.C. BAP31 interacts with Sec61 translocons and promotes retrotranslocation of CFTRDeltaF508 via the derlin-1 complex. Cell 2008, 133, 1080–1092. [Google Scholar] [CrossRef]
- Kim, K.M.; Adachi, T.; Nielsen, P.J.; Terashima, M.; Lamers, M.C.; Köhler, G.; Reth, M. Two new proteins preferentially associated with membrane immunoglobulin D. EMBO J. 1994, 15, 3793–3800. [Google Scholar] [CrossRef] [PubMed]
- Spiliotis, E.T.; Manley, H.; Osorio, M.; Zúñiga, M.C.; Edidin, M. Selective export of MHC class I molecules from the ER after their dissociation from TAP. Immunity 2000, 13, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Paquet, M.E.; Cohen-Doyle, M.; Shore, G.C.; Williams, D.B. Bap29/31 influences the intracellular traffic of MHC class I molecules. J. Immunol. 2004, 172, 7548–7555. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.; Becker, B.; Schreiber, R.; Boucherot, A.; Reth, M.; Kunzelmann, K. Control of cystic fibrosis transmembrane conductance regulator expression by BAP31. J. Biol. Chem. 2001, 8, 20340–20345. [Google Scholar] [CrossRef]
- Bartee, E.; Eyster, C.A.; Viswanathan, K.; Mansouri, M.; Donaldson, J.G.; Früh, K. Membrane-Associated RING-CH proteins associate with Bap31 and target CD81 and CD44 to lysosomes. PLoS ONE 2010, 5, e15132. [Google Scholar] [CrossRef]
- Niu, K.; Xu, J.; Cao, Y.; Hou, Y.; Shan, M.; Wang, Y.; Xu, Y.; Sun, M.; Wang, B. BAP31 is involved in T cell activation through TCR signal pathways. Sci. Rep. 2017, 7, 44809. [Google Scholar] [CrossRef]
- Wang, T.; Chen, J.; Hou, Y.; Yu, Y.; Wang, B. BAP31 deficiency contributes to the formation of amyloid-β plaques in Alzheimer’s disease by reducing the stability of RTN3. FASEB J. 2019, 33, 4936–4946. [Google Scholar] [CrossRef]
- Xu, J.L.; Li, L.Y.; Wang, Y.Q.; Li, Y.Q.; Shan, M.; Sun, S.Z.; Yu, Y.; Wang, B. Hepatocyte-specific deletion of BAP31 promotes SREBP1C activation, promotes hepatic lipid accumulation, and worsens IR in mice. J. Lipid Res. 2018, 59, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.C.; Du, J.; Liu, X.; Jiang, R.; Huang, Y.; Wang, T.; Hou, Y.; Wang, B. B-Cell Receptor-Associated Protein 31 Regulates the Expression of Valosin-Containing Protein through Elf2. Cell Physiol. Biochem. 2018, 51, 1799–1814. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.E.; Blagoev, B.; Kratchmarova, I.; Kristensen, D.B.; Steen, H.; Pandey, A.; Mann, M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteom. 2002, 1, 376–386. [Google Scholar] [CrossRef]
- Harkness, L.; Christiansen, H.; Nehlin, J.; Barington, T.; Andersen, J.S.; Kassem, M. Identification of a membrane proteomic signature for human embryonic stem cells independent of culture conditions. Stem Cell Res. 2008, 1, 219–227. [Google Scholar] [CrossRef]
- Dormeyer, W.; van Hoof, D.; Braam, S.R.; Heck, A.J.; Mummery, C.L.; Krijgsveld, J. Plasma membrane proteomics of human embryonic stem cells and human embryonal carcinoma cells. J. Proteome Res. 2008, 7, 2936–2951. [Google Scholar] [CrossRef] [PubMed]
- Dang, E.; Yang, S.; Song, C.; Jiang, D.; Li, Z.; Fan, W.; Sun, Y.; Tao, L.; Wang, J.; Liu, T.; et al. BAP31, a newly defined cancer/testis antigen, regulates proliferation, migration, and invasion to promote cervical cancer progression. Cell Death Dis. 2018, 9, 791. [Google Scholar] [CrossRef]
- Yu, S.; Wang, F.; Fan, L.; Wei, Y.; Li, H.; Sun, Y.; Yang, A.; Jin, B.; Song, C.; Yang, K. BAP31, a promising target for the immunotherapy of malignant melanomas. J. Exp. Clin. Cancer Res. 2015, 34, 36. [Google Scholar] [CrossRef]
- Ma, C.; Jin, R.M.; Chen, K.J.; Hao, T.; Li, B.S.; Zhao, D.H.; Jiang, H. Low expression of B-Cell-Associated protein 31 is associated with unfavorable prognosis in human colorectal cancer. Pathol. Res. Pract. 2018, 214, 661–666. [Google Scholar] [CrossRef]
- Chen, J.; Guo, H.; Jiang, H.; Namusamba, M.; Wang, C.; Lan, T.; Wang, T.; Wang, B. A BAP31 intrabody induces gastric cancer cell death by inhibiting p27kip1 proteasome degradation. Int. J. Cancer 2019, 144, 2051–2062. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Q.; Zhao, Q.; Yang, F.; Liu, T.; Huang, X.; Yan, Q.; Yang, X. Deglycosylated EpCAM regulates proliferation by enhancing autophagy of breast cancer cells via PI3K/Akt/mTOR pathway. Aging 2022, 14, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Stutz, R.; Schorr, S.; Lang, S.; Pfeffer, S.; Freeze, H.H.; Förster, F.; Helms, V.; Dudek, J.; Zimmermann, R. Proteomics reveals signal peptide features determining the client specificity in human TRAP-dependent ER protein import. Nat. Commun. 2018, 9, 3765. [Google Scholar] [CrossRef] [PubMed]
- Dejgaard, K.; Theberge, J.F.; Heath-Engel, H.; Chevet, E.; Tremblay, M.L.; Thomas, D.Y. Organization of the Sec61 translocon, studied by high resolution native electrophoresis. J. Proteome Res. 2010, 9, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Shibatani, T.; David, L.L.; McCormack, A.L.; Frueh, K.; Skach, W.R. Proteomic analysis of mammalian oligosaccharyltransferase reveals multiple subcomplexes that contain Sec61, TRAP, and two potential new subunits. Biochemistry 2005, 44, 5982–5992. [Google Scholar] [CrossRef] [PubMed]
- Auclair, S.M.; Bhanu, M.K.; Kendall, D.A. Signal peptidase I: Cleaving the way to mature proteins. Protein Sci. 2012, 21, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, D.J.; Gilmore, R. An evolving view of the eukaryotic oligosaccharyltransferase. Glycobiology 2006, 16, 47R–62R. [Google Scholar] [CrossRef]
- Fons, R.D.; Bogert, B.A.; Hegde, R.S. Substrate-specific function of the translocon-associated protein complex during translocation across the ER membrane. J. Cell Biol. 2003, 17, 529–539. [Google Scholar] [CrossRef]
- Sommer, N.; Junne, T.; Kalies, K.U.; Spiess, M.; Hartmann, E. TRAP assists membrane protein topogenesis at the mammalian ER membrane. Biochim. Biophys. Acta 2013, 1833, 3104–3111. [Google Scholar] [CrossRef]
- LoPiccolo, J.; Blumenthal, G.M.; Bernstein, W.B.; Dennis, P.A. Targeting the PI3K/Akt/mTOR pathway: Effective combinations and clinical considerations. Drug Resis. Updates 2008, 11, 32–50. [Google Scholar] [CrossRef]
- Gao, N.; Flynn, D.C.; Zhang, Z.; Zhong, X.S.; Walker, V.; Liu, K.J.; Shi, X.; Jiang, B.H. G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am. J. Physiol. Cell Physiol. 2004, 287, C281–C291. [Google Scholar] [CrossRef]
- Riquelme, I.; Tapia, O.; Leal, P.; Sandoval, A.; Varga, M.G.; Letelier, P.; Buchegger, K.; Bizama, C.; Espinoza, J.A.; Peek, R.M.; et al. miR-101-2, miR-125b-2 and miR-451a act as potential tumor suppressors in gastric cancer through regulation of the PI3K/AKT/mTOR pathway. Cell Oncol. 2016, 39, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Shrimal, S.; Gilmore, R. Reduced expression of the oligosaccharyltransferase exacerbates protein hypoglycosylation in cells lacking the fully assembled oligosaccharide donor. Glycobiology 2015, 25, 774–783. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Wang, C.; Wang, J.; Wang, B. An Intrabody against B-Cell Receptor-Associated Protein 31 (BAP31) Suppresses the Glycosylation of the Epithelial Cell-Adhesion Molecule (EpCAM) via Affecting the Formation of the Sec61-Translocon-Associated Protein (TRAP) Complex. Int. J. Mol. Sci. 2023, 24, 14787. https://doi.org/10.3390/ijms241914787
Wang T, Wang C, Wang J, Wang B. An Intrabody against B-Cell Receptor-Associated Protein 31 (BAP31) Suppresses the Glycosylation of the Epithelial Cell-Adhesion Molecule (EpCAM) via Affecting the Formation of the Sec61-Translocon-Associated Protein (TRAP) Complex. International Journal of Molecular Sciences. 2023; 24(19):14787. https://doi.org/10.3390/ijms241914787
Chicago/Turabian StyleWang, Tianyi, Changli Wang, Jiyu Wang, and Bing Wang. 2023. "An Intrabody against B-Cell Receptor-Associated Protein 31 (BAP31) Suppresses the Glycosylation of the Epithelial Cell-Adhesion Molecule (EpCAM) via Affecting the Formation of the Sec61-Translocon-Associated Protein (TRAP) Complex" International Journal of Molecular Sciences 24, no. 19: 14787. https://doi.org/10.3390/ijms241914787





