Non-Contrast MR Lymphography and Intranodal Dynamic Contrast MR Lymphangiography in Children with Congenital Heart Disease—Imaging Findings as well as Impact on Patient Management and Outcome
Abstract
:1. Introduction
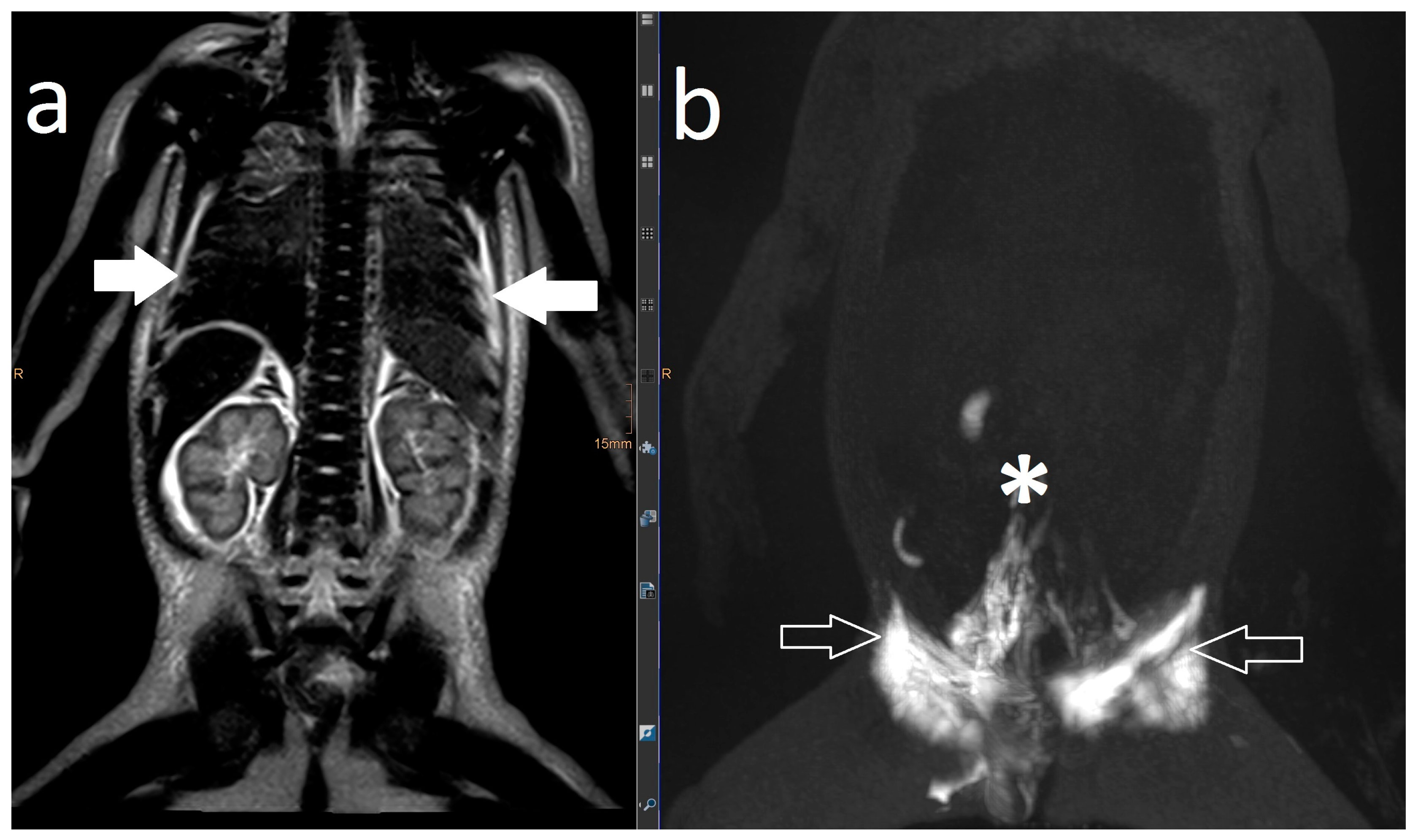
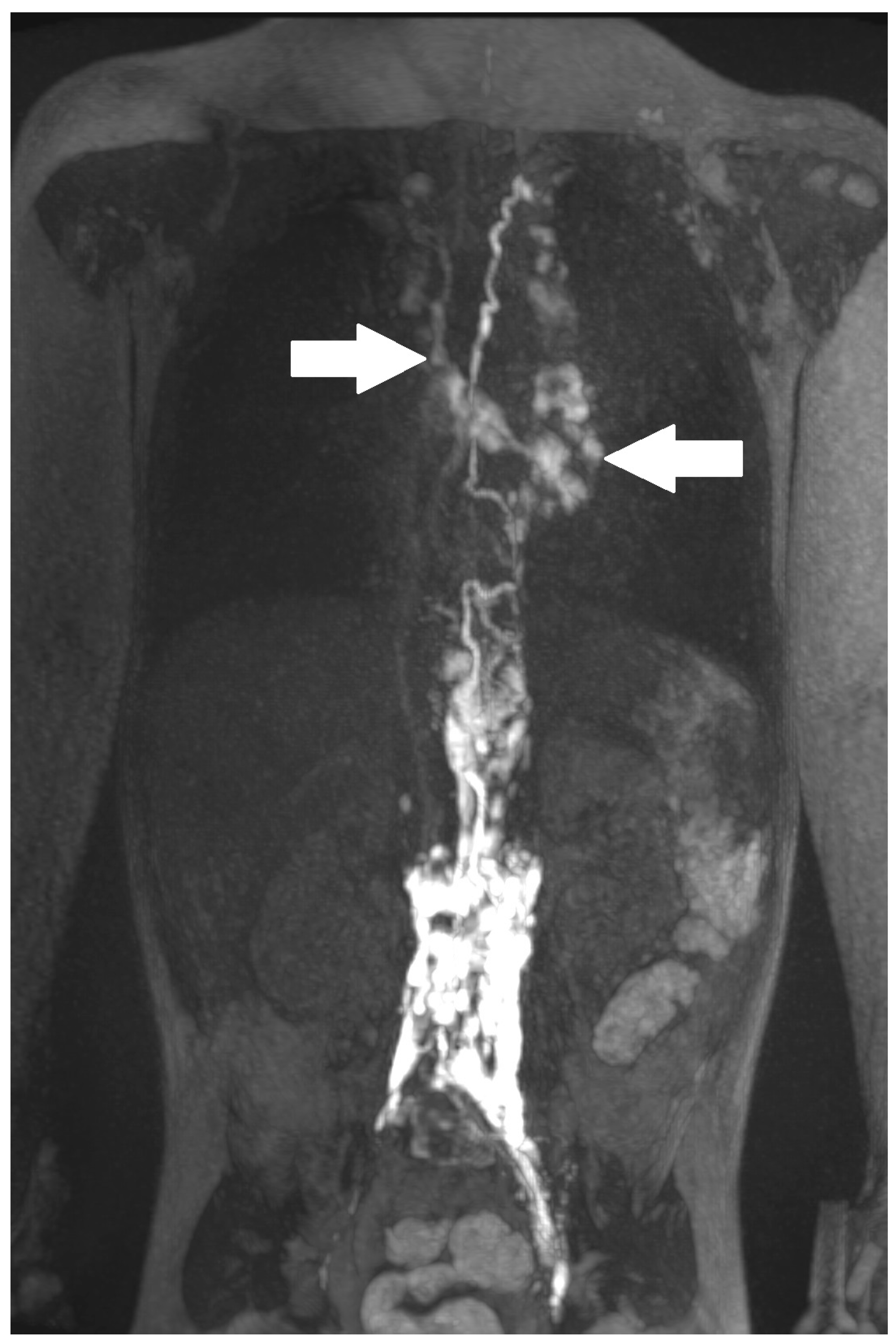
2. Results
3. Discussion
4. Materials and Methods
4.1. Lymphatic Imaging Procedure
4.2. Image Analysis
4.3. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rychik, J.; Atz, A.M.; Celermajer, D.S.; Deal, B.J.; Gatzoulis, M.A.; Gewillig, M.H.; Hsia, T.-Y.; Hsu, D.T.; Kovacs, A.H.; McCrindle, B.W.; et al. Evaluation and Management of the Child and Adult with Fontan Circulation: A Scientific Statement from the American Heart Association. Circulation 2019, 140, e234–e284. [Google Scholar] [CrossRef]
- Maleux, G.; Storme, E.; Cools, B.; Heying, R.; Boshoff, D.; Louw, J.J.; Frerich, S.; Malekzadeh-Milanii, S.; Hubrechts, J.; Brown, S.C.; et al. Percutaneous embolization of lymphatic fistulae as treatment for protein-losing enteropathy and plastic bronchitis in patients with failing Fontan circulation. Catheter. Cardiovasc. Interv. 2019, 94, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Hoffman, T.M.; Dori, Y.; Rome, J.J. Decompression of the thoracic duct: A novel transcatheter approach. Catheter. Cardiovasc. Interv. 2020, 95, E56–E61. [Google Scholar] [CrossRef] [PubMed]
- Itkin, M.; Piccoli, D.A.; Nadolski, G.; Rychik, J.; DeWitt, A.; Pinto, E.; Rome, J.; Dori, Y. Protein-Losing Enteropathy in Patients with Congenital Heart Disease. J. Am. Coll. Cardiol. 2017, 69, 2929–2937. [Google Scholar] [CrossRef] [PubMed]
- Hraška, V. Decompression of thoracic duct: New approach for the treatment of failing Fontan. Ann. Thorac. Surg. 2013, 96, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Dori, Y.; Keller, M.S.; Rome, J.J.; Gillespie, M.J.; Glatz, A.C.; Dodds, K.; Goldberg, D.J.; Goldfarb, S.; Rychik, J.; Itkin, M. Percutaneous Lymphatic Embolization of Abnormal Pulmonary Lymphatic Flow as Treatment of Plastic Bronchitis in Patients with Congenital Heart Disease. Circulation 2016, 133, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Mair, R.; Mair, R.; Tulzer, G. Thoracic duct decompression and jugular vein banding—An effective treatment option for protein-losing enteropathy and plastic bronchitis in severe failing Fontan circulation: A case report. Eur. Heart J. Case Rep. 2020, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- António, M.; Gordo, A.; Pereira, C.; Pinto, F.; Fragata, I.; Fragata, J. Thoracic Duct Decompression for Protein-Losing Enteropathy in Failing Fontan Circulation. Ann. Thorac. Surg. 2016, 101, 2370–2373. [Google Scholar] [CrossRef] [PubMed]
- Savla, J.J.; Itkin, M.; Rossano, J.W.; Dori, Y. Post-Operative Chylothorax in Patients With Congenital Heart Disease. J. Am. Coll. Cardiol. 2017, 69, 2410–2422. [Google Scholar] [CrossRef] [PubMed]
- Rychik, J. The Relentless Effects of the Fontan Paradox. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2016, 19, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Dori, Y.; Smith, C.L.; DeWitt, A.G.; Srinivasan, A.; Krishnamurthy, G.; Escobar, F.A.; Biko, D.M. Intramesenteric dynamic contrast pediatric MR lymphangiography: Initial experience and comparison with intranodal and intrahepatic MR lymphangiography. Eur. Radiol. 2020, 30, 5777–5784. [Google Scholar] [CrossRef] [PubMed]
- Biko, D.M.; Smith, C.L.; Otero, H.J.; Saul, D.; White, A.M.; DeWitt, A.; Glatz, A.C.; Piccoli, D.A.; Mamula, P.; Rome, J.J.; et al. Intrahepatic dynamic contrast MR lymphangiography: Initial experience with a new technique for the assessment of liver lymphatics. Eur. Radiol. 2019, 29, 5190–5196. [Google Scholar] [CrossRef] [PubMed]
- Biko, D.M.; DeWitt, A.G.; Pinto, E.M.; Morrison, R.E.; Johnstone, J.A.; Griffis, H.; O’Byrne, M.L.; Fogel, M.A.; Harris, M.A.; Partington, S.L.; et al. MRI Evaluation of Lymphatic Abnormalities in the Neck and Thorax after Fontan Surgery: Relationship with Outcome. Radiology 2019, 291, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Lemley, B.A.; Biko, D.M.; Dewitt, A.G.; Glatz, A.C.; Goldberg, D.J.; Saravanan, M.; O’Byrne, M.; Pinto, E.; Ravishankar, C.; Rome, J.J.; et al. Intrahepatic Dynamic Contrast-Enhanced Magnetic Resonance Lymphangiography: Potential Imaging Signature for Protein-Losing Enteropathy in Congenital Heart Disease. J. Am. Heart Assoc. 2021, 10, e021542. [Google Scholar] [CrossRef] [PubMed]
- Biko, D.M.; Reisen, B.; Otero, H.J.; Ravishankar, C.; Victoria, T.; Glatz, A.C.; Rome, J.J.; Dori, Y. Imaging of central lymphatic abnormalities in Noonan syndrome. Pediatr. Radiol. 2019, 49, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Dori, Y. Novel Lymphatic Imaging Techniques. Tech. Vasc. Interv. Radiol. 2016, 19, 255–261. [Google Scholar] [CrossRef] [PubMed]
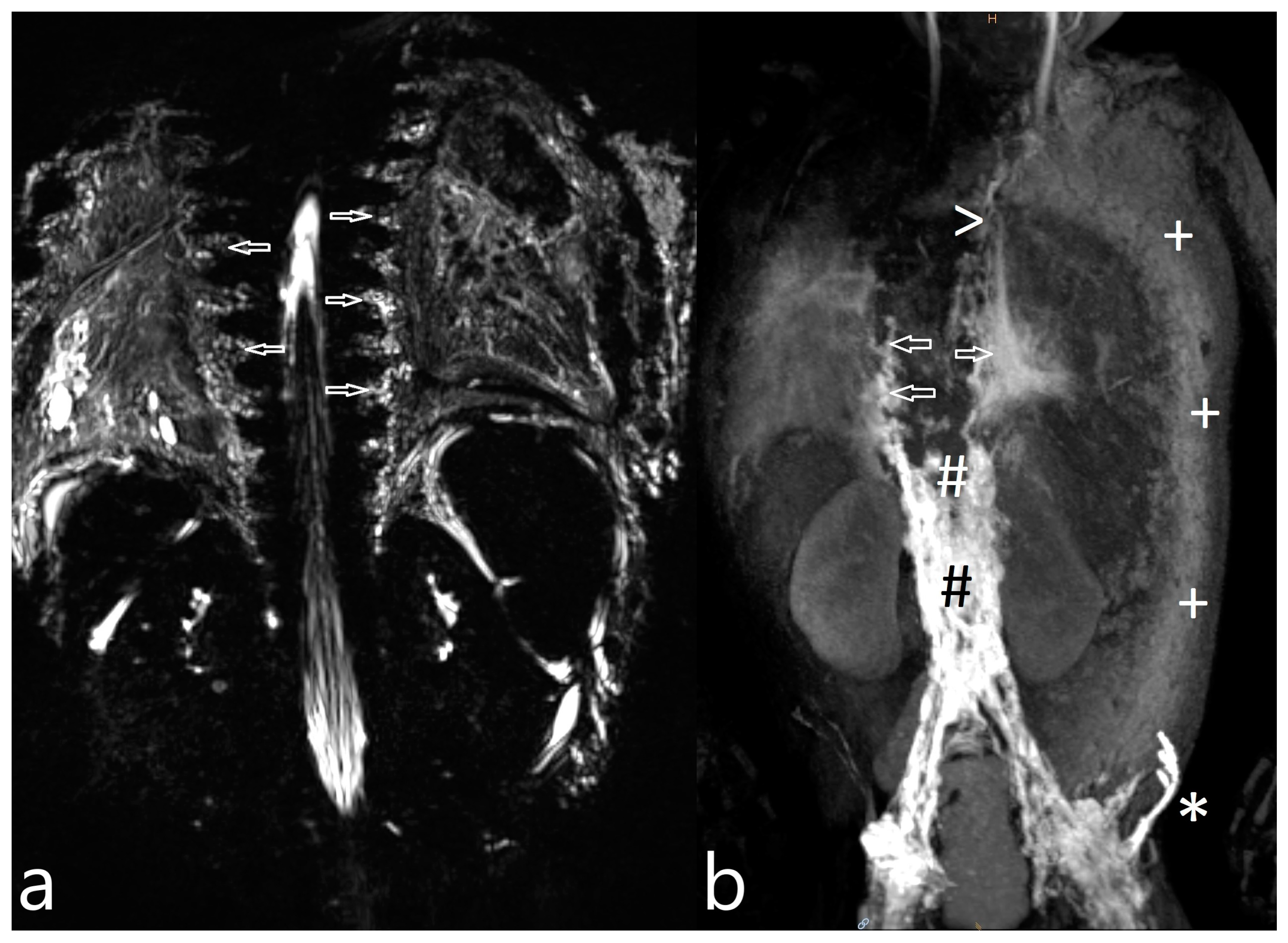
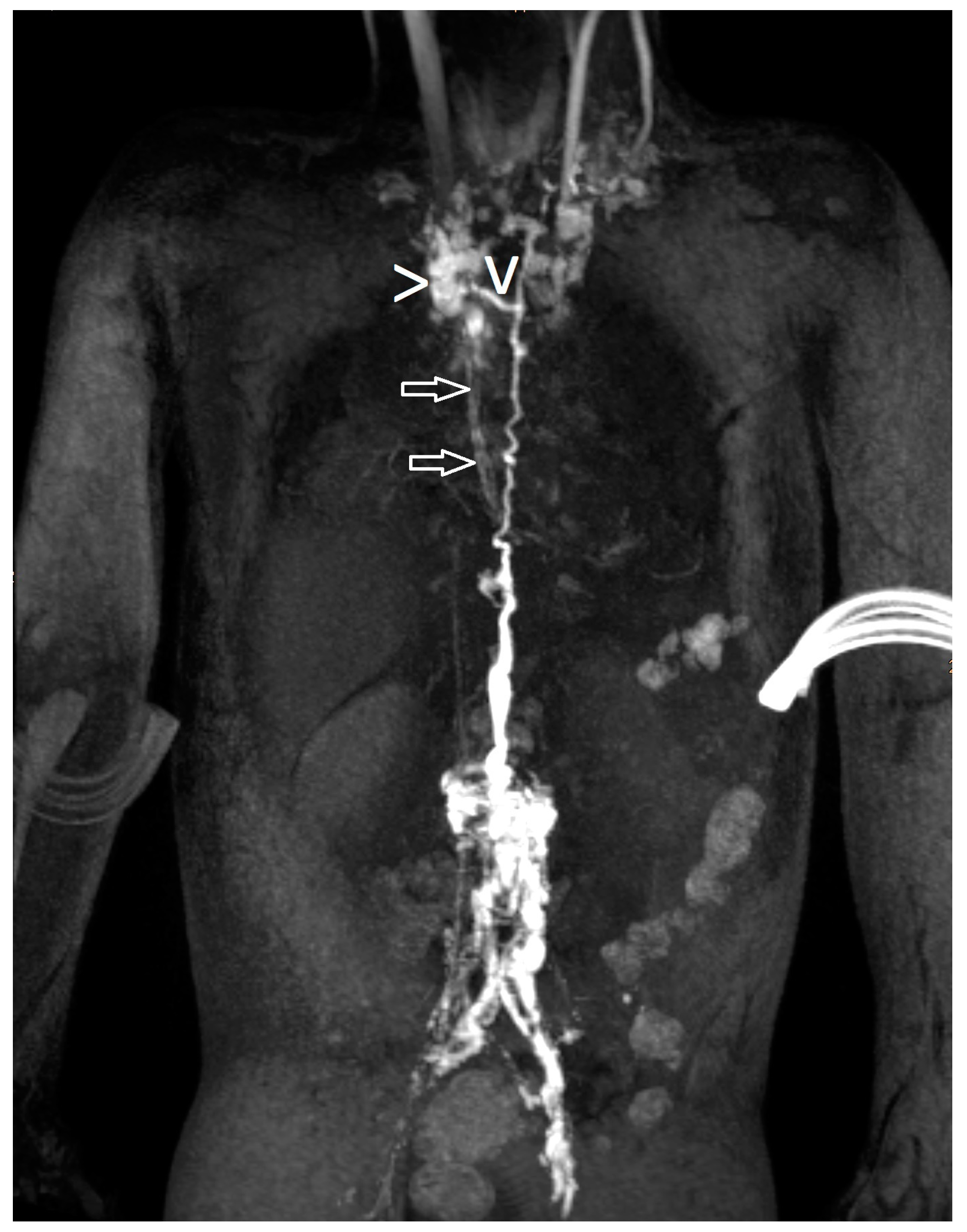
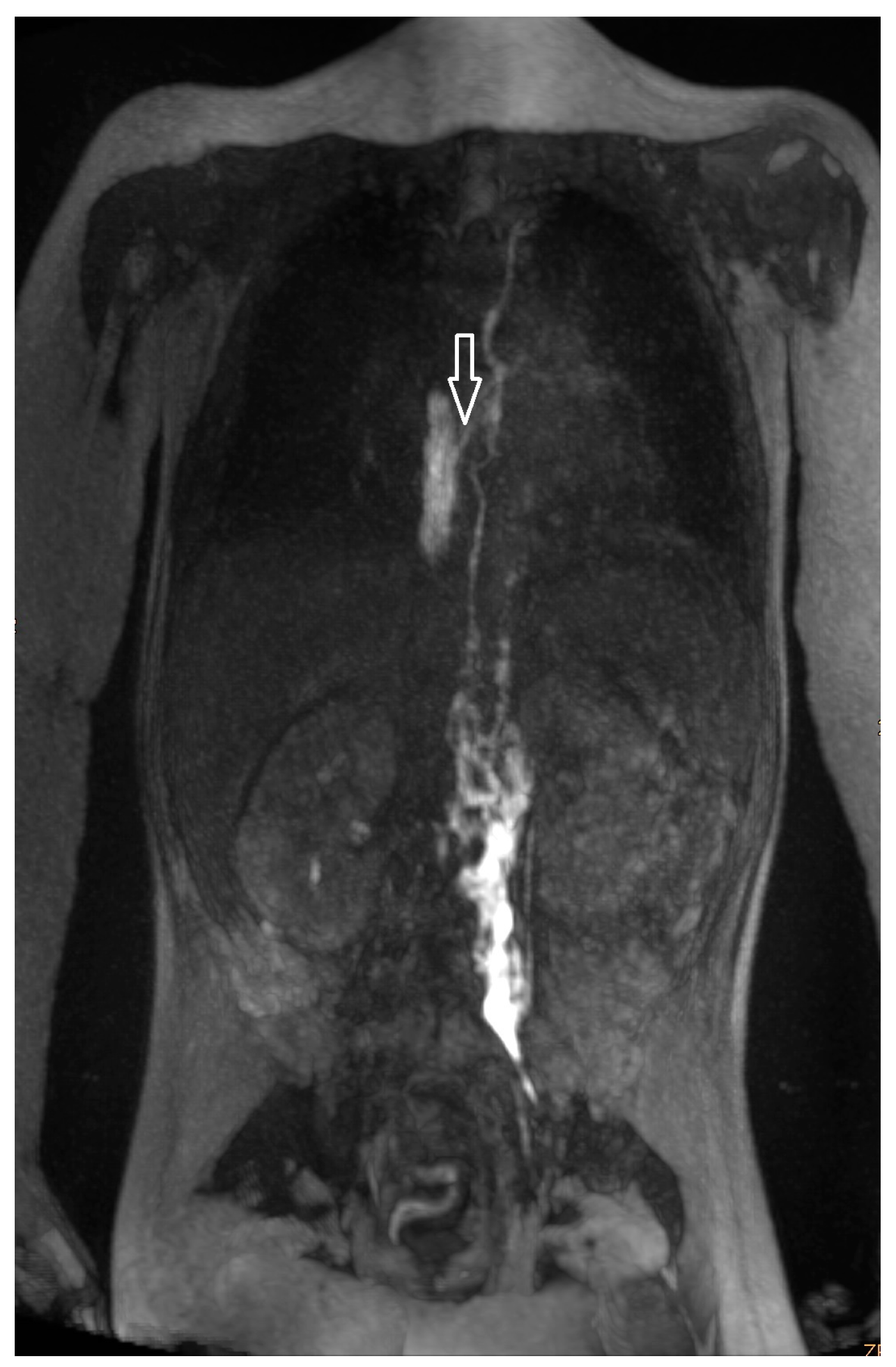
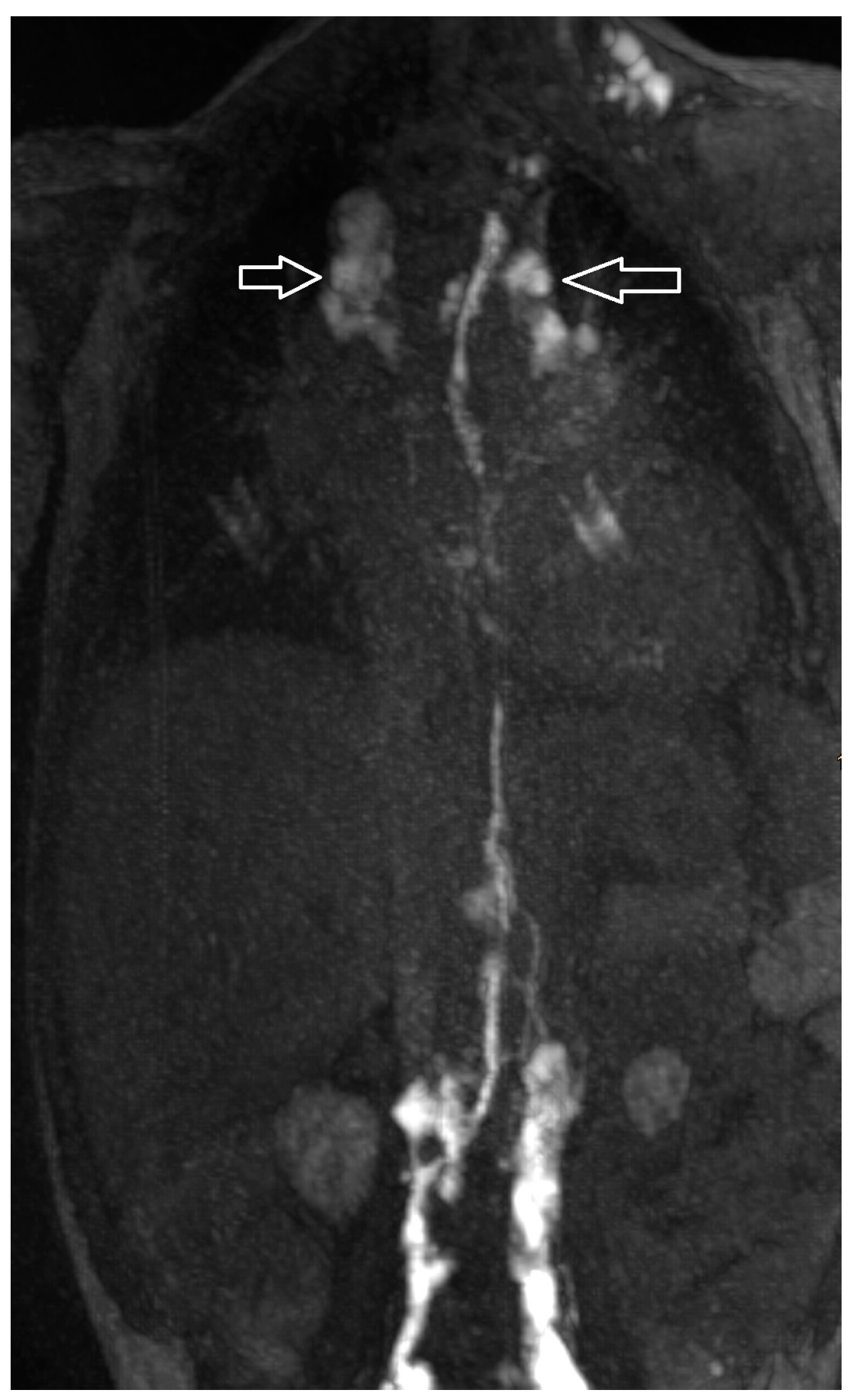
| Patient Number | Gender * | Age ** | Weight (kg) | Clinical Presentation | Congenital Heart Disease | Surgical Repair |
|---|---|---|---|---|---|---|
| Noonan syndrome: | ||||||
| 1 | M | 13 m | 6.6 | persistent congenital chylothorax with respiratory compromise | subvalvular-valvular-supravalvular PS | PV repair |
| 2 | F | 11 m | 7 | pleural effusion with respiratory compromise | ASD II, double aortic arch, valvular PS | ASD closure, ligation of left aortic arch |
| 3 | F | 6 a 10 m | 12.4 | persistent congenital chylothorax, respiratory compromise, ascites | ASD II, subvalvular- valvular- supravalvular PS, dysplastic aortic valve, RPA aLPA hypoplasia | RVOT-plastic, aortic valve repair |
| Chylothorax | ||||||
| 4 | M | 3 a 9 m | 13.7 | HLHS | Fontan *** | |
| 5 | F | 35 d | 3.3 | HLHR, LTGA | Norwood I/Sano shunt | |
| 6 | M | 22 d | 3.4 | HLHS, PAVPR | Norwood I/Sano shunt | |
| 7 | M | 2 a 9 m | 13 | TGA, SV, DILV | Fontan *** | |
| 8 | F | 3 a 3 m | 13.9 | unbalanced CAVC, TGA, TAPVR, right atrial isomerism | Fontan *** | |
| 9 | F | 44 d | 3.7 | TAPVR, Shone complex | TAPVR-Repair, end-to end-Anastomosis | |
| 10 | F | 24 d | 3.4 | hypopl. AA, subaortal stenosis, muscular VSD | AA reconstruction, modified Conno, VSD closure | |
| 11 | M | 3 a 9 m | 12 | Shone complex | combined Glenn + Fontan *** | |
| 12 | F | 9 m | 5.8 | Shone complex | Glenn | |
| 13 | M | 9 m | 7.5 | HLHS | Glenn | |
| 14 | M | 10 a 2 m | 21.4 | HLHS, Kabuki syndrome | Fontan *** | |
| Protein-losing enteropathy | ||||||
| 15 | F | 5 a 11 m | 10.7 | Unb. CAVC, CoAc | Fontan *** | |
| 16 | M | 16 a 8 m | 44 | SV, TGA, hypopl. AA | Fontan *** (fenestration closed) | |
| 17 | M | 11 a 2 m | 28.8 | HLHS, LPA stenosis | Fontan *** (fenestration closed) | |
| 18 | M | 15 a 7 m | 38.4 | HLHS, recoarctation of aortae | Fontan *** (fenestration closed) | |
| 19 | F | 9 a 9 m | 28.2 | HLHS, PAPVR | extr. nonfenestrated Fontan | |
| 20 | F | 17 a 9 m | 35.5 | HLHS | Fontan *** (fenestration closed) | |
| Plastic bronchitis | ||||||
| 21 | M | 3 a 8 m | 14 | HLHS | Fontan *** | |
| 22 | F | 6 a 11 m | 21 | HLHS | Fontan *** (fenestration closed) | |
| 23 | M | 7 a 6 m | 20 | HLHS | Fontan *** | |
| 24 | M | 8 a 1 m | 21.5 | HLHS | Fontan *** (fenestration closed) | |
| 25 | F | 9 a 5 m | 26 | HLHS | Fontan *** (fenestration closed) | |
| 26 | F | 12 a 9 m | 25.8 | unbalanced CAVC, HLHS | extr. nonfenestrated Fontan | |
| Pat. Nr. | MR Lymphography | Dynamic Contrast MR Lymphangiography | Preprocedural Management | Intervention/ Revision | Outcome (Time OP to LI/Time OP to Resolution/Time OP to Discharge)/Time Revision to Resolution | |||
|---|---|---|---|---|---|---|---|---|
| LA-Type 1–4 | T2-Findings (Abnormal Signal) | Lymphatic Flow | Etiology | Lymphatic Fistulas | ||||
| 4 | 1 | increased signal neck le | TD intact, reduced flow, retrograde flow towards pleural space le | T | no fistula visualized | MCT diet, octreotide | revision | resolution (57/73/82/8) |
| 5 | 4 | increased signal neck, axilla, mediastinum, hilum and body wall, pleural effusion le > ri, ascites | TD intact, retrograde flow towards hilum le, dermal backflow in abdominal wall, reduced central flow | CLFD | no fistula visualized | CT revision, furosemide, etacrynic acid, captopril, | MCT diet | resolution (21/44/63) |
| 6 | 4 | increased signal neck, axilla, mediastinum, hilum and body wall, pleural effusion, ascites | TD intact, delayed central lymphatic flow, retrograde flow towards pleural space ri > le, hilum le, dermal backflow in thoracic and abdominal wall | CLFD | no fistula visualized | CT revision | not indicated | died (20/-/-) |
| 7 | 4 | increased signal neck, mediastinum and hilum, bilateral pleural effusion | TD intact, retrograde flow towards mediastinum and lung parenchyma bilateral | PLPS | TD bilateral TV 2 | CT revision, levosimedane | MCT diet, sandostatin, levosimedane | resolution (31/63/75)) |
| 8 | 3 | increased signal neck, axilla le > ri, mediastinum le > ri, pleural effusion | TD intact, retrograde flow to mediastinum, lung ri | PLPS | no fistula visualized | MCT diet | revision | resolution (22/42/50/19) |
| 9 | 4 | increased signal neck, axilla, mediastinum, hilum and body wall, pleural effusion ri > le, ascites | TD discontinued from LV 3 | CLFD | no fistula visualized | CT revision | MCT diet | resolution (24/44/59) |
| 10 | 4 | increased signal neck, mediastinum le > ri and hilum le > ri, pleural effusion le > ri, ascites | non-diagnostic | - | no fistula visualized | MCT diet | no intervention | resolution (16/29/33) |
| 11 | 3 | increased signal neck le > ri, mediastinum and hilum ri > le, pleural effusion | TD intact, retrograde flow towards mediastinum and hilum le | PLPS | no fistula visualized | diet modification | revision | resolution (42/62/71/20) |
| 12 | 4 | increased signal neck le > ri, mediastinum and hilum ri > le, pleural effusion ri > le, ascites | TD intact, retrograde flow towards mediastinum, hilum, lungs ri > le | PLPS | no fistula visualized | MCT diet, somatostatin | Glenn takedown | resolution (84/142/208/37) |
| 13 | 4 | increased signal neck, axilla, mediastinum, hilum and body wall, pleural effusion | retrograde flow towards hilum le, dermal backflow into thoracic wall | CLFD | TV 7–10 to hilum/ mediastinum | MCT diet | not performed yet | recurrent CT |
| 14 | 1 | increased signal neck le. | non diagnostic | - | no fistula visualized | LPA-stent placement | not indicated | resolution of CT |
| Pat. Nr. | MR Lymphography | Dynamic Contrast MR Lymphangiography | Preprocedural Management | Intervention | Outcome * | ||
|---|---|---|---|---|---|---|---|
| LA-Type 1–4 | T2-Findings (Abnormal Signal) | Lymphatic Flow | Lymphatic Fistulas | ||||
| 21 | 4 | increased signal mediastinum, hilum, lung le > ri | retrograde flow from TD with diffuse mediastinal and peribronchial perfusion | thoracic vertebrae 4–10 to left mediastinum | MCT diet, macitentan, alteplase inhalation, salbutamol, diuretics | glue embolization of TD | cast free after intervention (FU 1 a 4 m) tapering off sildenafil |
| 22 | 4 | increased signal (mediastinum), hilum/lung ri | retrograde lymphatic flow to mediastinum and lung parenchyma ri | from hilum to ri lung | fat reduced diet, sildenafil, alteplase/ fluticasone inhalation | selective glue embolization of 2 branches of TD | cast free after intervention (FU 4 a), cessation of fat-reduced diet, |
| 23 | 4 | increased signal mediastinum, hilum, lung ri > le | retrograde lymphatic flow towards lung parenchyma & mediastinum ri > le and peribronchial perfusion | fistula visualized | fat reduced diet, sildenafil, diuretics, alteplase inhalation, azithromycine | selective glue embolization of fistulas | cast free after intervention (FU 1 a 4 m) |
| 24 | 4 | increased signal at the mediastinum and hilum bilateral | retrograde lymphatic flow towards mediastinum and hilum bilaterally | no fistula visualized | diuretics salbutamol inhalation. Budesonide | TD decompression | cast free after OP, albumin normalized (FU 3 a 5 m) |
| 25 | 4 | increased signal neck, axilla, mediastinum, hilum, lung ri, fistula from liver to ri lung | little contrast | no fistula visualized | lisinopril, diuretics, spironolactone, bisoprolol, sildenafil, diuretics, alteplase inhalation. | not performed yet | still casts |
| 26 | 4 | increased signal neck, axilla, mediastinum, hilum, lung | retrograde lymphatic flow towards mediastinum, hilum and lung parenchyma with peribronchial perfusion ri | from hilum to ri lung | spironolactone, sildenafil, diuretics, alteplase inhalation, hydrochlorothiazide | not performed yet | still casts |
| Pat. Nr. | MR Lymphography | Dynamic Contrast MR Lymphangiography | Preprocedural Management | Intervention | Outcome * | ||
|---|---|---|---|---|---|---|---|
| LA-Type 1–4 | T2-Findings (Abnormal Signal) | Lymphatic Flow | Lymphatic Fistulas | ||||
| 15 | 1 | increased signal neck le | TD intact, no retrograde flow | no | budesonide, sildenafil, macitentan, diuretics | no due to comorbidity | no improvement |
| 16 | 1 | increased signal neck le, hilar lymphadenopathy | TD intact, no retrograde flow | no | budesonide, macitentan, diuretics enalapril | glue embolization | albumin improved (FU 2 a 7 m) |
| 17 | 3 | increased signal neck and hilum | TD intact, no retrograde flow | no | budesonide, sildenafil, losartan | TD decompression | scheduled for transplantation |
| 18 | 1 | increased signal neck le | TD intact, no retrograde flow | no | budesonide, sildenafil, spironolactone | no (patient disapproval) | no improvement |
| 19 | 1 | increased signal neck le | TD intact, no retrograde flow | no | enalapril | no | transient improvement |
| 20 | 1 | slightly increased signal neck le | TD intact, no retrograde flow | no | enalapril, aldactone, budesonide | no | no improvement |
| Pat. Nr. | MRL | DCMRL | Preprocedural Management | Intervention | Outcome * | ||
|---|---|---|---|---|---|---|---|
| LA-Type 1–4 | T2-Findings (Abnormal Signal) | Lymphatic Flow | Lymphatic Fistulas | ||||
| 1 | 4 | increased signal neck, mediastinum, hilum, perihilar, interstitial lung parenchyma, pleural effusion | abnormal, perfusion to the lung, intercostal flow, dilated lymphatic networks in neck, mediastinum, hilum and perihilar | in the right apical lung | diuretics, sildenafil, non-invasive ventilatory support | lymphatic intervention | Resolution of CT (FU: 10 m, cessation of ventilatory support. |
| 2 | 2 | increased signal neck | abnormal, prevertebral, lymphovenous collaterals | no | diuretics, sildenafil, milrinone, levosimedan, invasive ventilatory support. | no indication, ASD closure | resolution of pleural effusion |
| 3 | 4 | increased signal neck, mediastinum, hilum, interstitial lung parenchyma, body wall edema, pleural effusion, ascites | abnormal, perfusion to the lung, intercostal flow, dermal backflow, | TV 6–9 bilateral into the lung | diuretics, sildenafil, MCT diet, non-invasive ventilatory support | no | persistent chylothorax, respiratory support |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, C.; Scala, M.; Sekyra, P.; Fellner, F.; Tulzer, G. Non-Contrast MR Lymphography and Intranodal Dynamic Contrast MR Lymphangiography in Children with Congenital Heart Disease—Imaging Findings as well as Impact on Patient Management and Outcome. Int. J. Mol. Sci. 2023, 24, 14827. https://doi.org/10.3390/ijms241914827
Bauer C, Scala M, Sekyra P, Fellner F, Tulzer G. Non-Contrast MR Lymphography and Intranodal Dynamic Contrast MR Lymphangiography in Children with Congenital Heart Disease—Imaging Findings as well as Impact on Patient Management and Outcome. International Journal of Molecular Sciences. 2023; 24(19):14827. https://doi.org/10.3390/ijms241914827
Chicago/Turabian StyleBauer, Christoph, Mario Scala, Pavel Sekyra, Franz Fellner, and Gerald Tulzer. 2023. "Non-Contrast MR Lymphography and Intranodal Dynamic Contrast MR Lymphangiography in Children with Congenital Heart Disease—Imaging Findings as well as Impact on Patient Management and Outcome" International Journal of Molecular Sciences 24, no. 19: 14827. https://doi.org/10.3390/ijms241914827
APA StyleBauer, C., Scala, M., Sekyra, P., Fellner, F., & Tulzer, G. (2023). Non-Contrast MR Lymphography and Intranodal Dynamic Contrast MR Lymphangiography in Children with Congenital Heart Disease—Imaging Findings as well as Impact on Patient Management and Outcome. International Journal of Molecular Sciences, 24(19), 14827. https://doi.org/10.3390/ijms241914827






