Identification of Potential Mechanisms of Rk1 Combination with Rg5 in the Treatment of Type II Diabetes Mellitus by Integrating Network Pharmacology and Experimental Validation
Abstract
:1. Introduction
2. Results
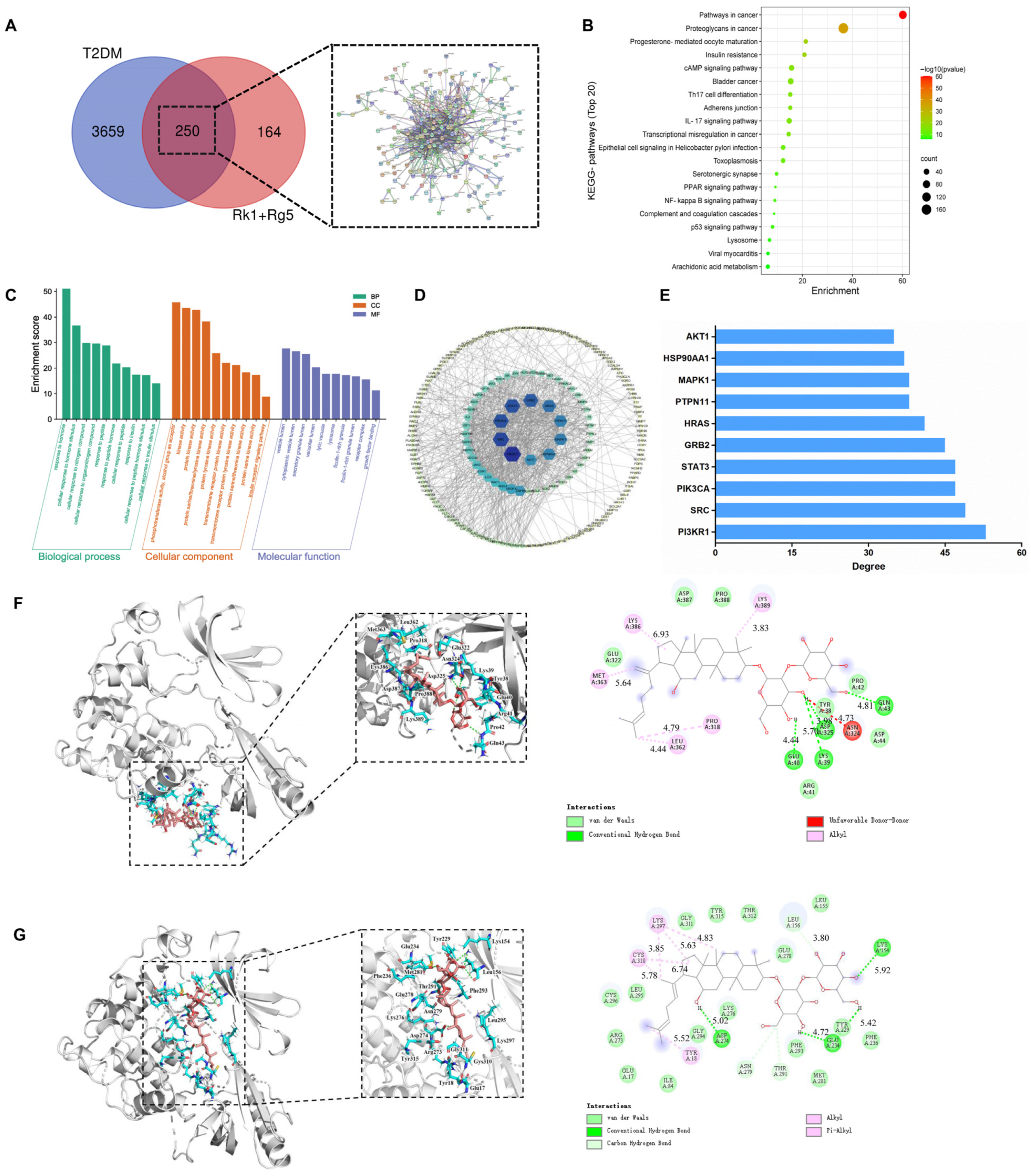
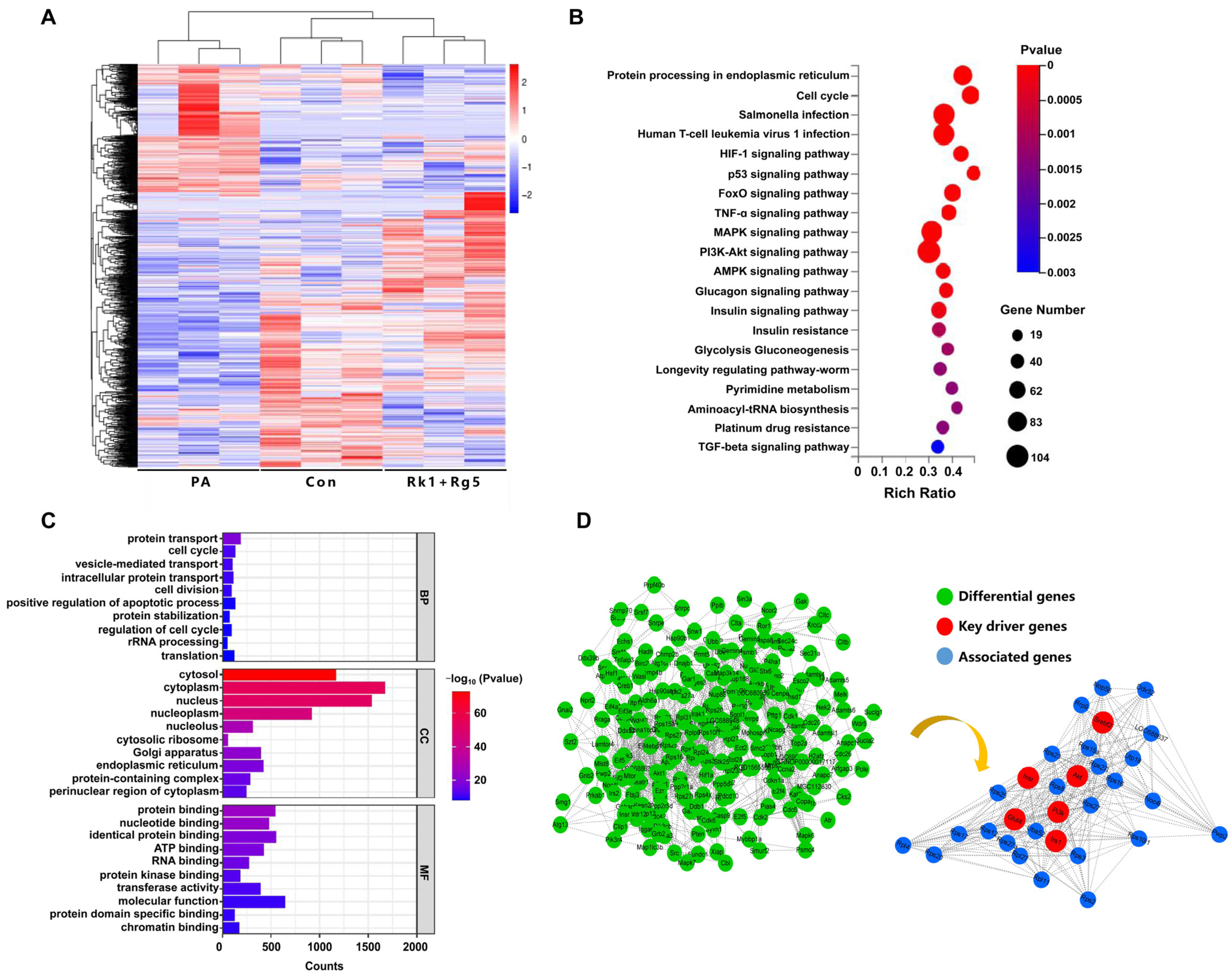
2.1. Network Pharmacology Prediction
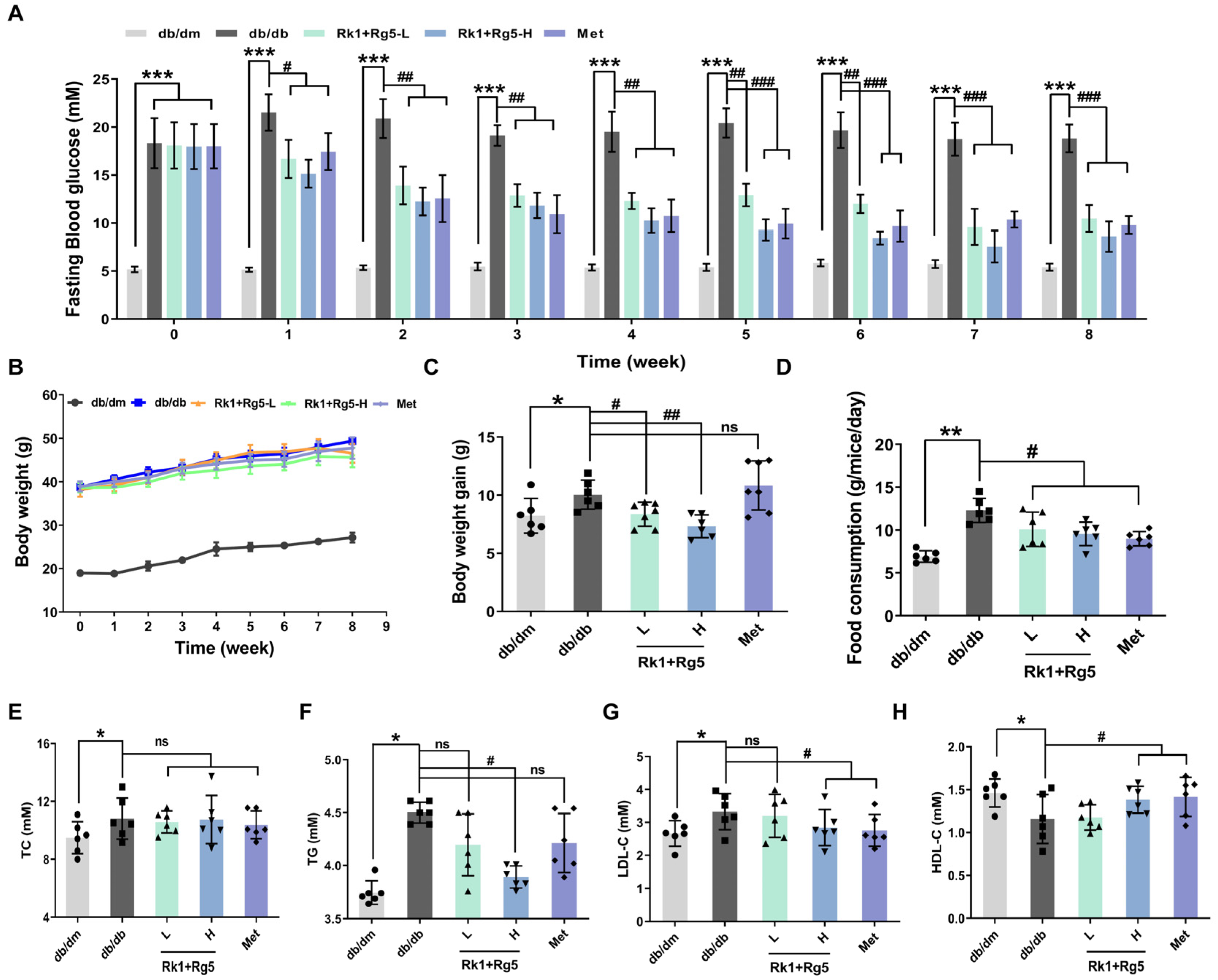
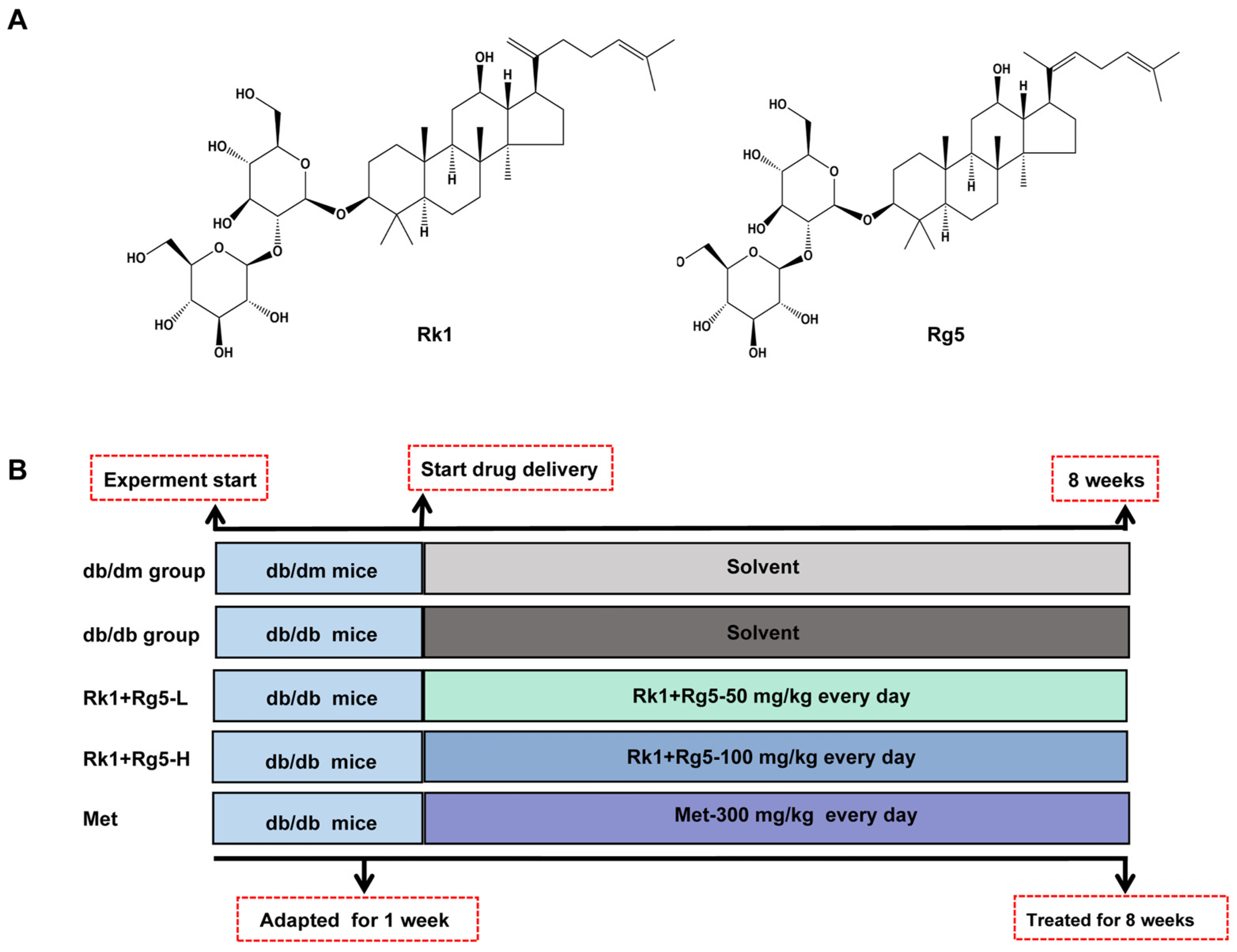
2.2. Effect of Rk1+Rg5 Treatment in db/db Mice
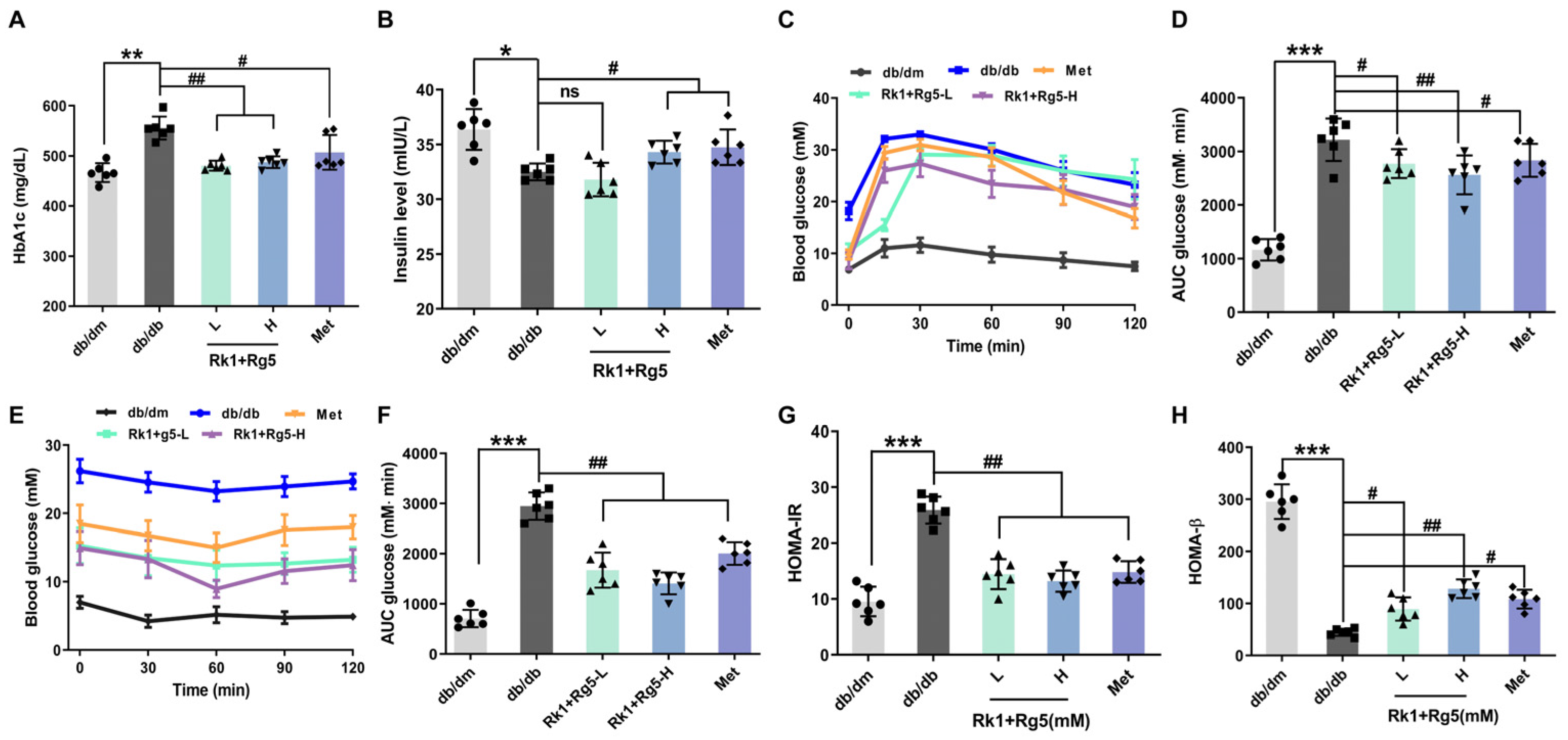
2.3. Effect of Rk1+Rg5 on the Glucose Metabolism in db/db Mice
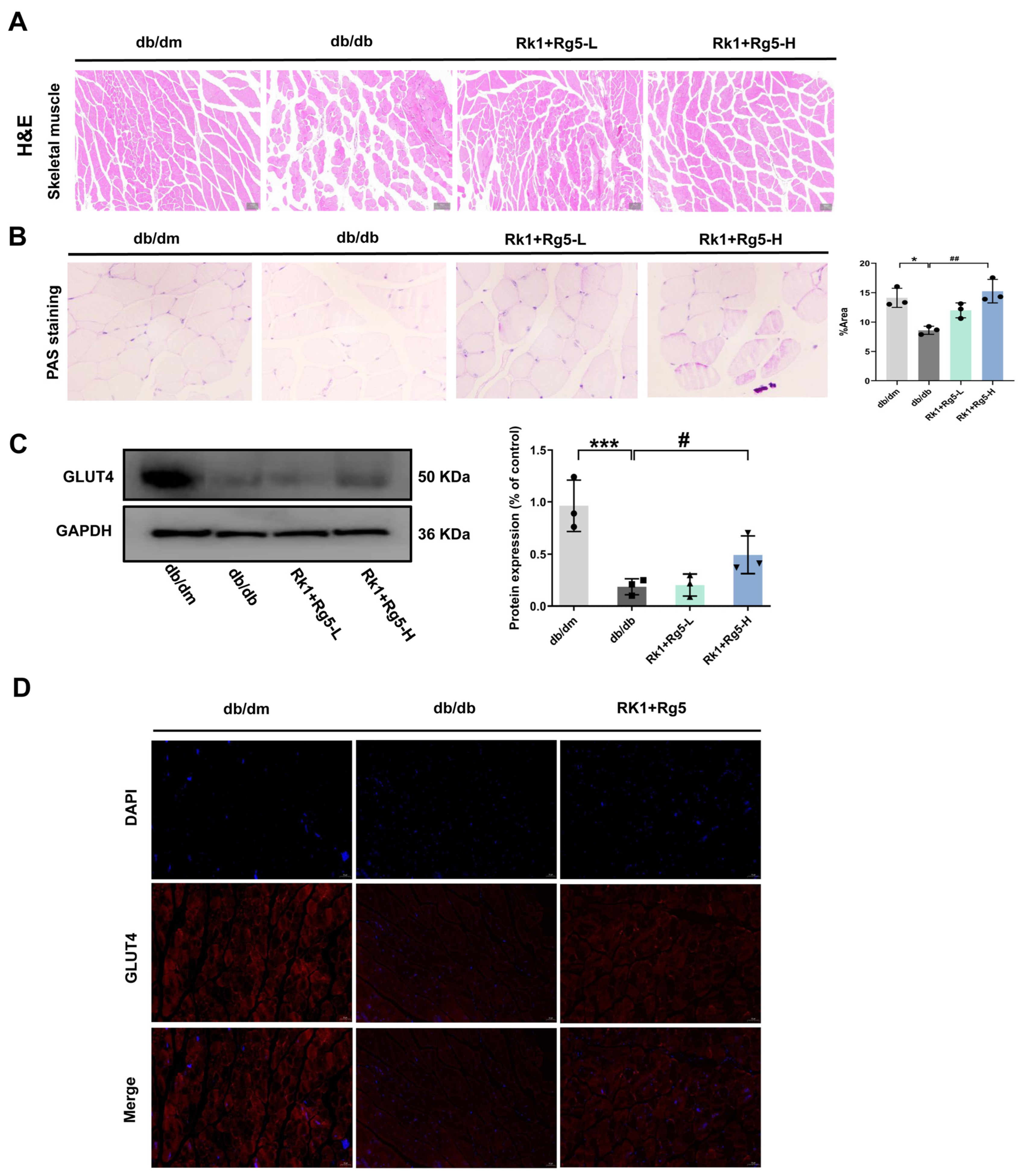
2.4. Effects of Rk1+Rg5 on Glucose Transporter in the Skeletal Muscle of db/db Mice
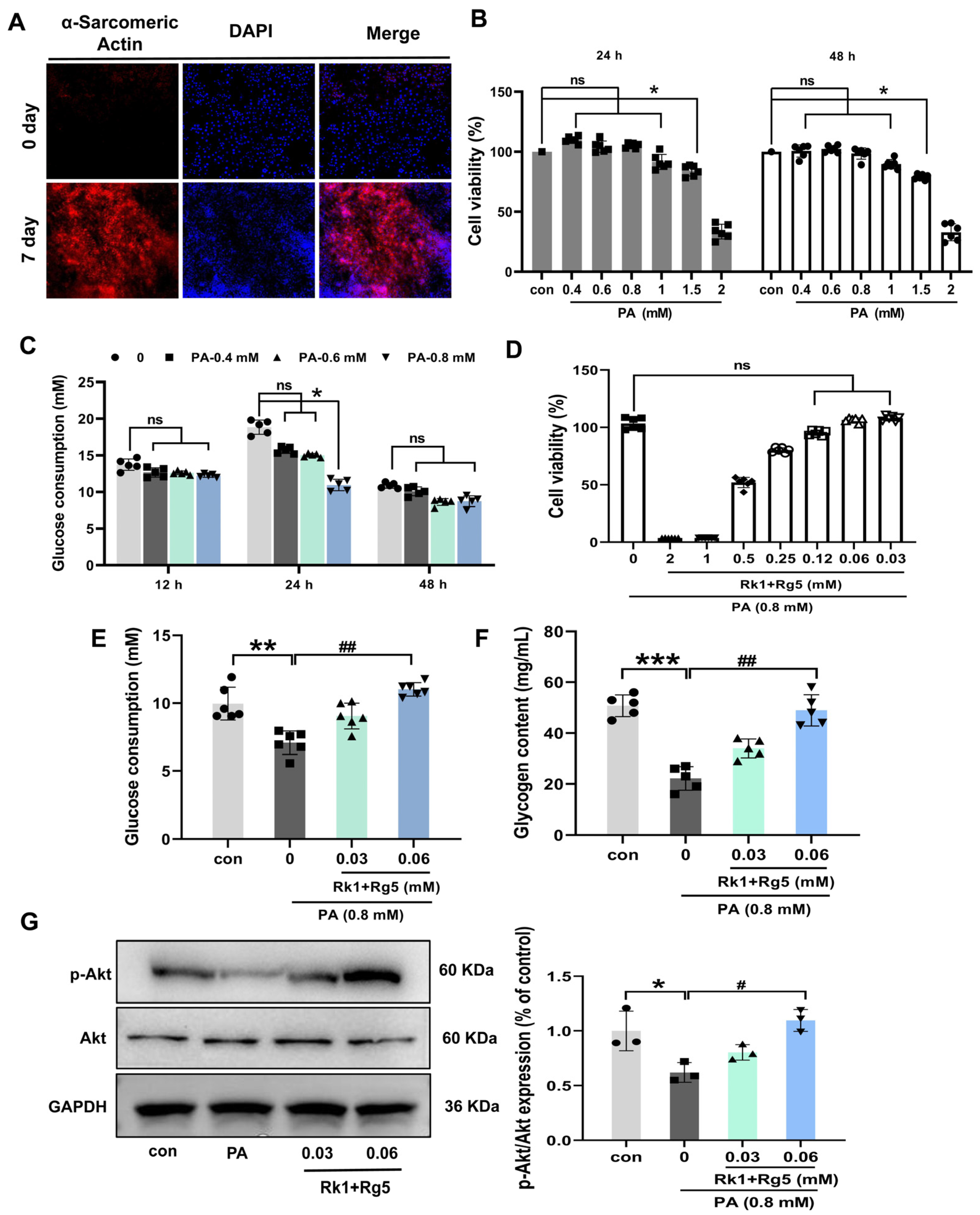
2.5. Effects of Rk1+Rg5 on Glucose Uptake in L6 Cells
2.6. Effect of Rk1+Rg5 on the Potential Mechanism in T2DM
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Potential Target Proteins, PPI, GO and KEGG Pathway Enrichment Analysis
4.3. Molecular Docking
4.4. Animals and Experimental Design
4.5. Analysis of FBG, OGTT, and ITT
4.6. Biochemical Analyses
4.7. HOMA-IR and Index of HOMA-β Analysis
4.8. Histopathological Analysis
4.9. Immunofluorescence of Skeletal Muscle
4.10. Cell Culture and Differentiation
4.11. PA-Induced Insulin Resistance Model in L6 Cells
4.12. Glucose Consumption
4.13. Western Blotting Analysis
4.14. RNA Sequence Analysis
4.15. Data Processing and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baena-Diez, J.M.; Penafiel, J.; Subirana, I.; Ramos, R.; Elosua, R.; Marin-Ibanez, A.; Guembe, M.J.; Rigo, F.; Tormo-Diaz, M.J.; Moreno-Iribas, C.; et al. Risk of Cause-Specific Death in Individuals with Diabetes: A Competing Risks Analysis. Diabetes Care 2016, 39, 1987–1995. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; Nie, S.P.; Xie, M.Y. Antidiabetic Mechanism of Dietary Polysaccharides Based on Their Gastrointestinal Functions. J. Agric. Food Chem. 2018, 66, 4781–4786. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; Fernandes, J.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Park, S.K.; Hyun, S.H.; In, G.; Park, C.K.; Han, C.K. The antioxidant activities of Korean red ginseng (Panax ginseng) and ginsenosides: A systemic review through in vivo and clinical trials. J. Ginseng Res. 2020, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Defronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef]
- Jheng, H.F.; Tsai, P.J.; Guo, S.M.; Kuo, L.H.; Chang, C.S.; Su, I.J.; Chang, C.R.; Tsai, Y.S. Mitochondrial Fission Contributes to Mitochondrial Dysfunction and Insulin Resistance in Skeletal Muscle. Mol. Cell. Biol. 2012, 32, 309–319. [Google Scholar] [CrossRef]
- Guo, X.; Sun, W.; Luo, G.; Wu, L.; Xu, G. Panax notoginseng saponins alleviate skeletal muscle insulin resistance by regulating the IRS1-PI3K-AKT signaling pathway and GLUT4 expression. FEBS Open Bio 2019, 9, 1008–1019. [Google Scholar] [CrossRef]
- Nobuko, H. Genetic Dissection of the Physiological Role of Skeletal Muscle in Metabolic Syndrome. New J. Sci. 2014, 2014, 635146. [Google Scholar]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013, 93, 993. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 22. [Google Scholar] [CrossRef]
- Scheen, A.J. Cardiovascular Effects of New Oral Glucose-Lowering Agents DPP-4 and SGLT-2 Inhibitors. Circ. Res. 2018, 122, 1439–1459. [Google Scholar] [CrossRef]
- Joshi, S.R.; Standl, E.; Tong, N.W.; Shah, P.; Kalra, S.; Rathod, R. Therapeutic potential of alpha-glucosidase inhibitors in type 2 diabetes mellitus: An evidence-based review. Expert Opin. Pharmacother. 2015, 16, 1959–1981. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.L.; Li, H.X.; Chen, C.; Liu, Y.Q.; Liu, H. Baicalin attenuates high fat diet-induced insulin resistance and ectopic fat storage in skeletal muscle, through modulating the protein kinase B/Glycogen synthase kinase 3 beta pathway. Chin. J. Nat. Med. 2016, 14, 48–55. [Google Scholar]
- Zhang, J.D. Resveratrol inhibits insulin responses in a SirT1-independent pathway. Biochem. J. 2006, 397, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Seymour, E.M.; Tanone, I.I.; Urcuyo-Llanes, D.E.; Lewis, S.K.; Kirakosyan, A. Blueberry Intake Alters Skeletal Muscle and Adipose Tissue Peroxisome Proliferator-Activated Receptor Activity and Reduces Insulin Resistance in Obese Rats. J. Med. Food 2011, 14, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.T.; Gao, J.L.; Wei, F.; Zhao, J.; Wang, D.W.; Wei, J.P. Therapeutic Potential of Ginsenosides as an Adjuvant Treatment for Diabetes. Front. Pharmacol. 2018, 9, 14. [Google Scholar] [CrossRef]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, J.N.; Yan, M.H.; Xing, J.J.; Liu, W.C.; Li, W. Caspase-Mediated Anti-Apoptotic Effect of Ginsenoside Rg5, a Main Rare Ginsenoside, on Acetaminophen-Induced Hepatotoxicity in Mice. J. Agric. Food Chem. 2017, 65, 9226–9236. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Liu, Y.; Chen, K.J. Roles and mechanisms of ginsenoside in cardiovascular diseases: Progress and perspectives. Sci. China-Life Sci. 2016, 59, 292–298. [Google Scholar] [CrossRef]
- Shin, B.K.; Kwon, S.W.; Park, J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015, 39, 287–298. [Google Scholar] [CrossRef]
- Deng, J.J.; Liu, Y.; Duan, Z.G.; Zhu, C.H.; Hui, J.F.; Mi, Y.; Ma, P.; Ma, X.X.; Fan, D.D.; Yang, H.X. Protopanaxadiol and Protopanaxatriol-Type Saponins Ameliorate Glucose and Lipid Metabolism in Type 2 Diabetes Mellitus in High-Fat Diet/Streptozocin-Induced Mice. Front. Pharmacol. 2017, 8, 11. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, L.; Yan, Z.; Shen, J.; Chang, Y.; Wang, J. ι-Carrageenan Tetrasaccharide from ι-Carrageenan Inhibits Islet β Cell Apoptosis Via the Upregulation of GLP-1 to Inhibit the Mitochondrial Apoptosis Pathway. J. Agric. Food Chem. 2021, 69, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 2003, 46, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.C.; Oh, D.K. Classification of glycosidases that hydrolyze the specific positions and types of sugar moieties in ginsenosides. Crit. Rev. Biotechnol. 2016, 36, 1036–1049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Liu, H.D.; Liu, J.K. Akt activation: A potential strategy to ameliorate insulin resistance. Diabetes Res. Clin. Pract. 2019, 156, 9. [Google Scholar] [CrossRef]
- Yu, Z.W.; Jin, T.R. New insights into the role of cAMP in the production and function of the incretin hormone glucagon-like peptide-1 (GLP-1). Cell. Signal. 2010, 22, 1–8. [Google Scholar] [CrossRef]
- Vinue, A.; Gonzalez-Navarro, H. Glucose and Insulin Tolerance Tests in the Mouse. Methods Mol Biol. 2015, 1339, 247–254. [Google Scholar]
- Kubota, N.; Terauchi, Y.; Kubota, T.; Kumagai, H.; Itoh, S.; Satoh, H.; Yano, W.; Ogata, H.; Tokuyama, K.; Takamoto, I.; et al. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -independent pathways. J. Biol. Chem. 2006, 281, 8748–8755. [Google Scholar] [CrossRef]
- Fujino, H.; Itoda, S.; Sako, S.; Matsuo, K.; Yokoyama, T. Reliability of HOMA-IR for evaluation of insulin resistance during perioperative period. Jpn. J. Anesthesiol. 2013, 62, 140–146. [Google Scholar]
- Wang, Y.; Yang, L.Z.; Yang, G.; Zhang, Q.Y.; Deng, Z.N.; Wang, K.; Mao, X.J. MiR-21 antagomir improves insulin resistance and lipid metabolism disorder in streptozotocin-induced type 2 diabetes mellitus rats. Ann. Pallliat. Med. 2020, 9, 394–404. [Google Scholar] [CrossRef]
- Kubota, T.; Kubota, N.; Kumagai, H.; Yamaguchi, S.; Kozono, H.; Takahashi, T.; Inoue, M.; Itoh, S.; Takamoto, I.; Sasako, T. Impaired Insulin Signaling in Endothelial Cells Reduces Insulin-Induced Glucose Uptake by Skeletal Muscle. Cell Metab. 2011, 13, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wu, S.; Gong, C.; Chen, L. Neuregulin-1β increases glucose uptake and promotes GLUT4 translocation in palmitate-treated C2C12 myotubes by activating PI3K/AKT signaling pathway. Front. Pharmacol. 2022, 13, 1066279. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A. Is GLUT4 translocation the answer to exercise-stimulated muscle glucose uptake? Am. Physiol. Soc. Rockv. 2021, 320, E240–E243. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Holman, G.D. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E29–E37. [Google Scholar] [CrossRef]
- Guo, S.D. Insulin signaling, resistance, and metabolic syndrome: Insights from mouse models into disease mechanisms. J. Endocrinol. 2014, 220, T1–T23. [Google Scholar] [CrossRef]
- Lukas, C.; Dorn, A.; Cramer, R.; Cramer, R.; Rohde, A. The comparative effect of pioglitazone and metformin on serum osteoprotegerin, adiponectin and intercellular adhesion molecule concentrations in patients with newly diagnosed type 2 diabetes: A randomized clinical trial. Exp. Clin. Endocrinol. Diabetes 2015, 123, 289–295. [Google Scholar]
- Damian, S.; Gable, A.L.; David, L.; Alexander, J.; Stefan, W.; Jaime, H.C.; Milan, S.; Doncheva, N.T.; Morris, J.H.; Peer, B. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2018, 47, D607–D613. [Google Scholar]
- Shashank, T.; Pohl, M.; Yingyao, Z.; Ariel, R.F.; Guojun, W. Meta- and Orthogonal Integration of Influenza “OMICs” Data Defines a Role for UBR4 in Virus Budding. Cell Host Microbe 2020, 18, 723–735. [Google Scholar]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Hsin, K.Y.; Matsuoka, Y.; Asai, Y.; Kamiyoshi, K.; Watanabe, T.; Kawaoka, Y.; Kitano, H. systemsDock: A web server for network pharmacology-based prediction and analysis. Nucleic Acids Res. 2016, 44, W507–W513. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.J.; Fan, D.D. Ginsenoside Rk3 ameliorates high-fat-diet/streptozocin induced type 2 diabetes mellitus in mice via the AMPK/Akt signaling pathway. Food Funct. 2019, 10, 2538–2551. [Google Scholar] [CrossRef] [PubMed]
| Receptor | Binding Interaction Energy (kcal/mol) | VDW Interaction Energy (kcal/mol) | Electrostatic Interaction Energy (kcal/mol) | |||
|---|---|---|---|---|---|---|
| Rk1 | Rg5 | Rk1 | Rg5 | Rk1 | Rg5 | |
| PI3KR1 | −1.64 | −1.06 | −6.89 | PI3KR1 | −1.64 | −1.06 |
| SRC | −4.24 | −5.06 | −9.12 | SRC | −4.24 | −5.06 |
| PIK3CA | −4.15 | −3.65 | −9.28 | PIK3CA | −4.15 | −3.65 |
| STAT3 | −0.99 | −1.71 | −6.29 | STAT3 | −0.99 | −1.71 |
| GRB2 | −5.62 | −5.81 | −10.71 | GRB2 | −5.62 | −5.81 |
| HRAS | −5.13 | −6.19 | −10.11 | HRAS | −5.13 | −6.19 |
| PTPN11 | −4.31 | −5.14 | −9.6 | PTPN11 | −4.31 | −5.14 |
| MAPK1 | −5.42 | −4.52 | −10.49 | MAPK1 | −5.42 | −4.52 |
| HSP90AA1 | −4.86 | −2.84 | −9.96 | HSP90AA1 | −4.86 | −2.84 |
| Akt1 | −4.95 | −5.46 | −9.68 | Akt1 | −4.95 | −5.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, J.; An, C.; Liu, C.; Zhang, Q.; Ding, H.; Ma, S.; Xue, W. Identification of Potential Mechanisms of Rk1 Combination with Rg5 in the Treatment of Type II Diabetes Mellitus by Integrating Network Pharmacology and Experimental Validation. Int. J. Mol. Sci. 2023, 24, 14828. https://doi.org/10.3390/ijms241914828
Liu Y, Zhang J, An C, Liu C, Zhang Q, Ding H, Ma S, Xue W. Identification of Potential Mechanisms of Rk1 Combination with Rg5 in the Treatment of Type II Diabetes Mellitus by Integrating Network Pharmacology and Experimental Validation. International Journal of Molecular Sciences. 2023; 24(19):14828. https://doi.org/10.3390/ijms241914828
Chicago/Turabian StyleLiu, Yao, Jingjing Zhang, Chao An, Chen Liu, Qiwen Zhang, Hao Ding, Saijian Ma, and Wenjiao Xue. 2023. "Identification of Potential Mechanisms of Rk1 Combination with Rg5 in the Treatment of Type II Diabetes Mellitus by Integrating Network Pharmacology and Experimental Validation" International Journal of Molecular Sciences 24, no. 19: 14828. https://doi.org/10.3390/ijms241914828







