OGR1 (GPR68) and TDAG8 (GPR65) Have Antagonistic Effects in Models of Colonic Inflammation
Abstract
1. Introduction
2. Results
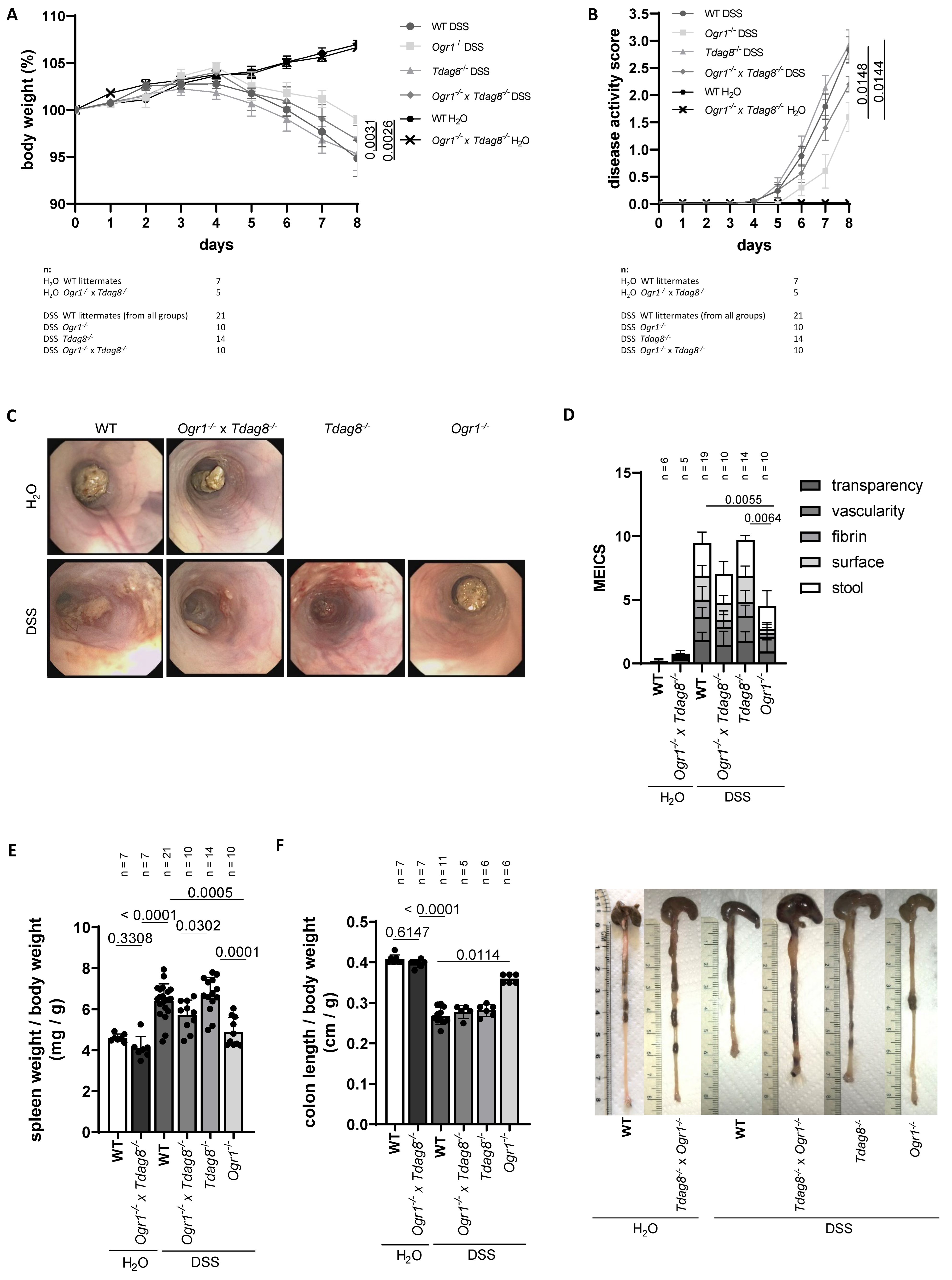
2.1. Ogr1 Deficiency Reduces Clinical Severity in the DSS-Induced Acute Colitis Model, Both in a WT and Tdag8−/− Background
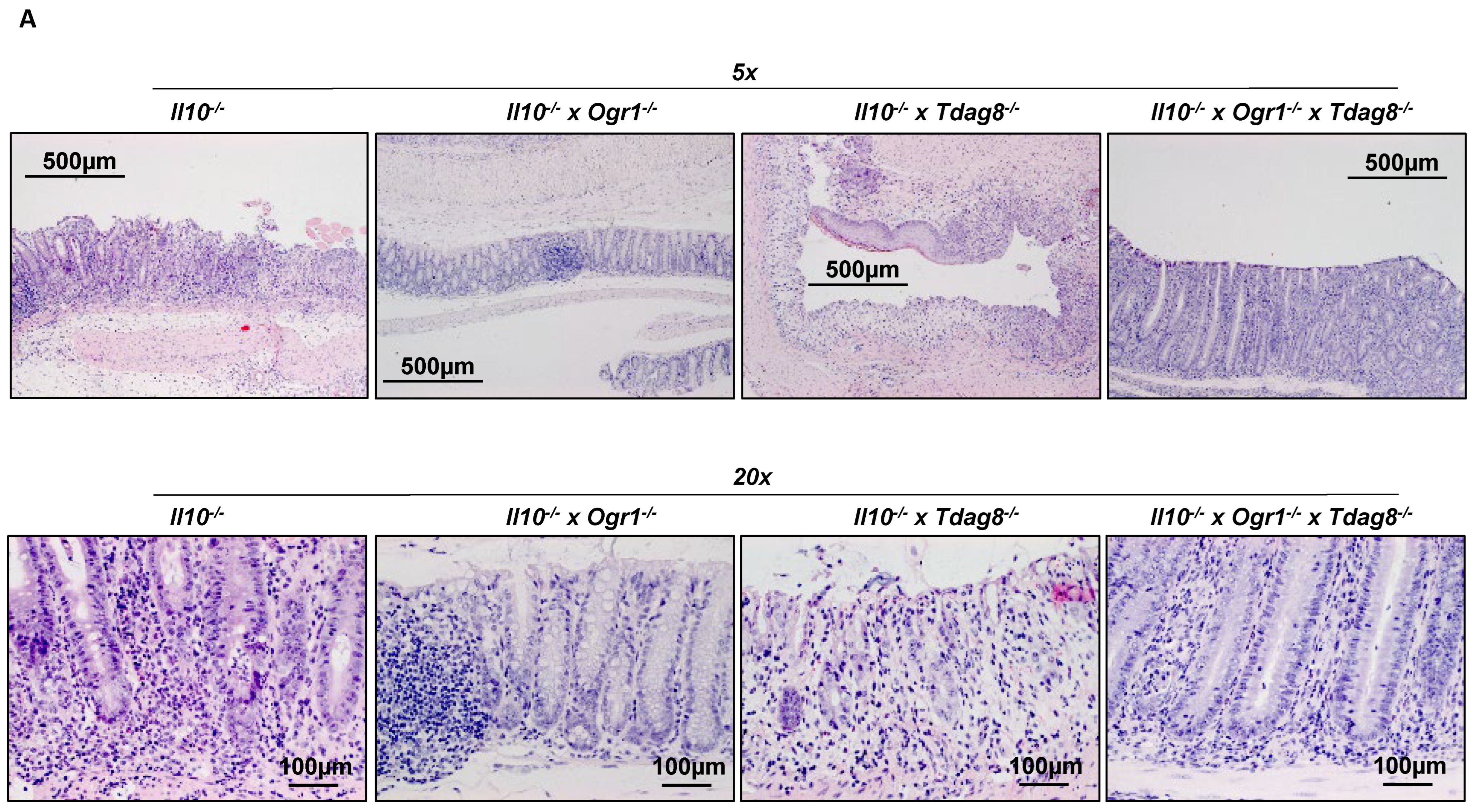
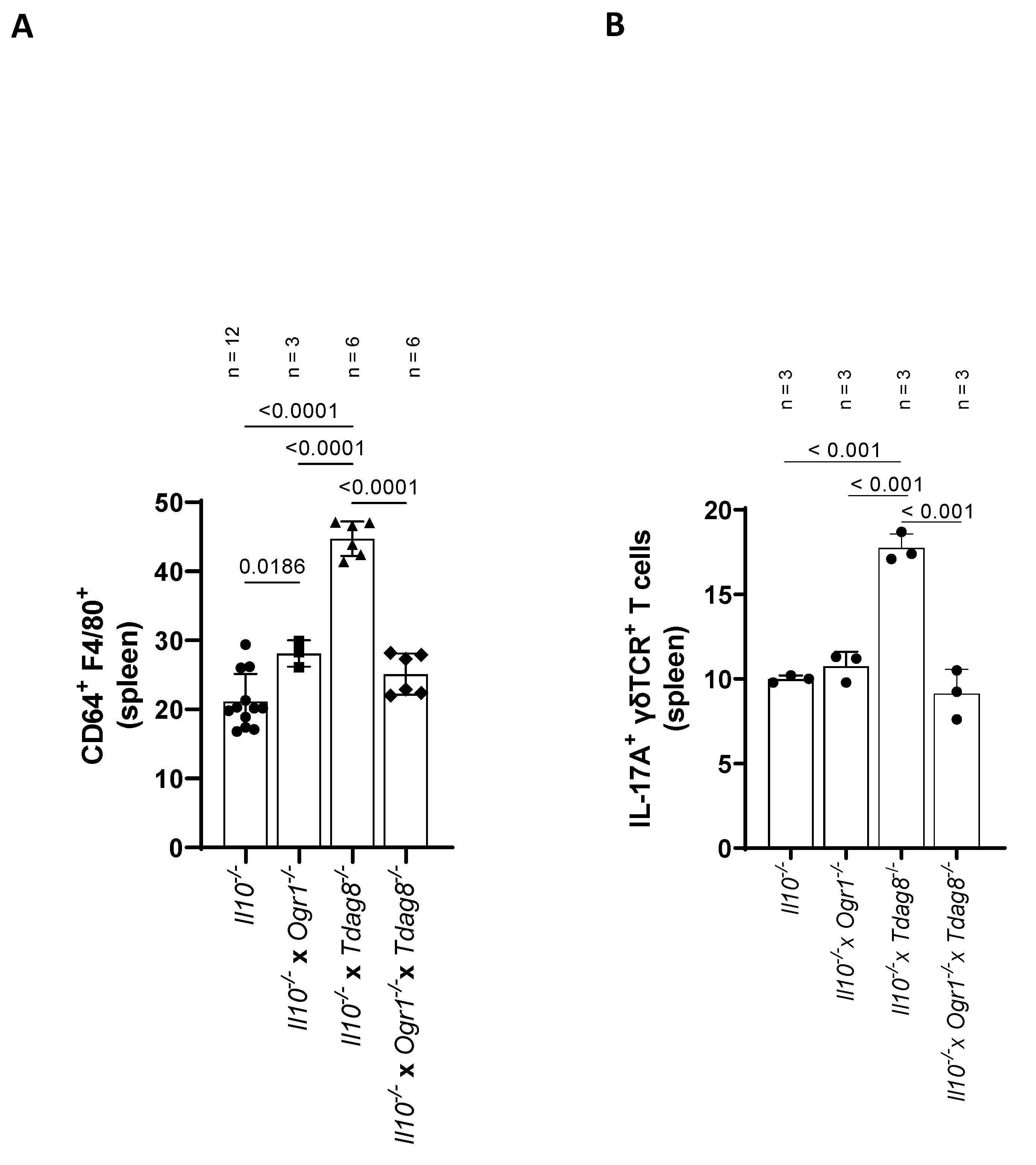
2.2. Il-10 Deficiency: The Absence of Tdag8 Exacerbates Colitis, While the Absence of Ogr1 Is Protective
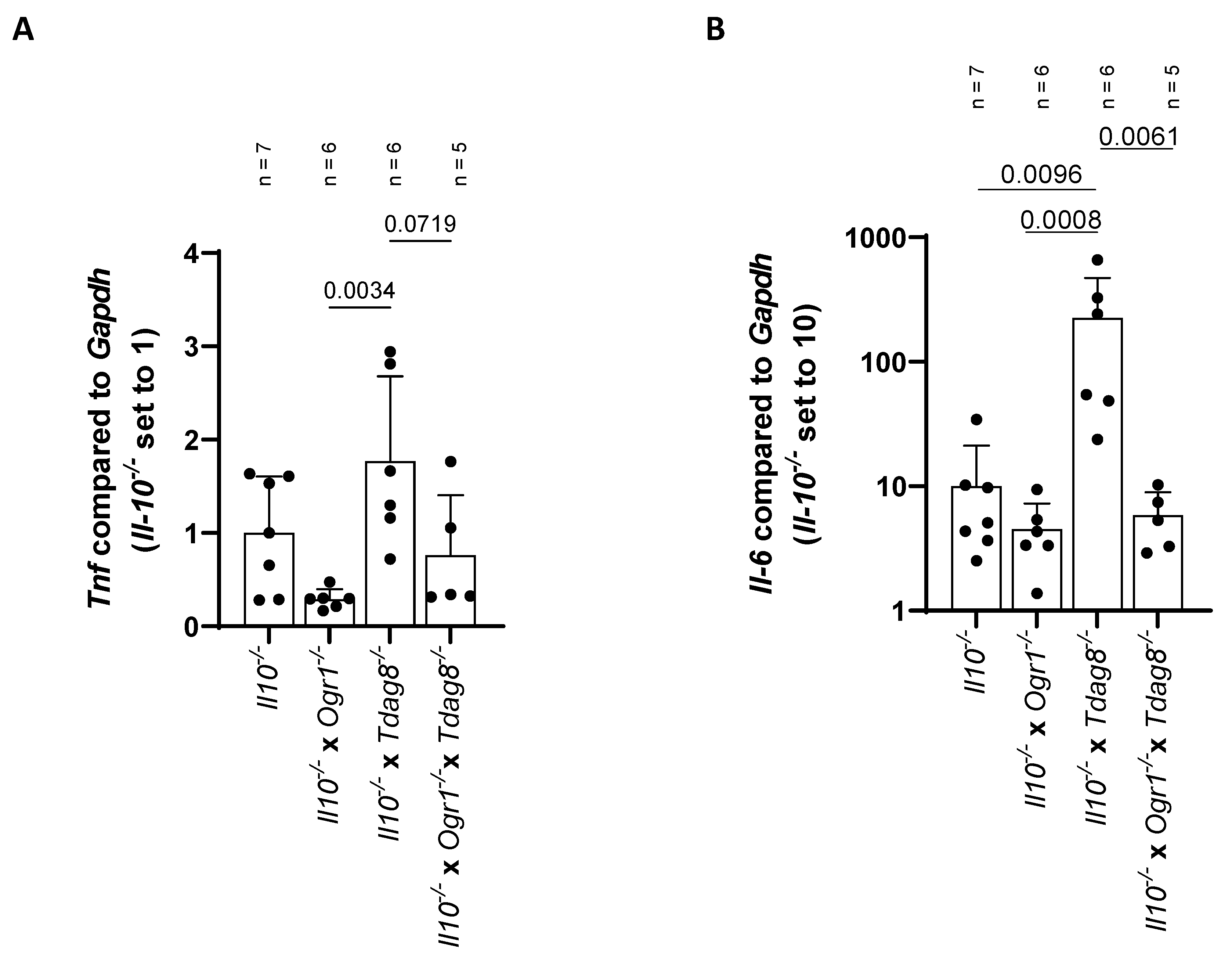
2.3. The Absence of Ogr1 Reduces Pro-Inflammatory Cytokine Expression in Tdag8-Deficient mice in the Model of Spontaneous Colitis
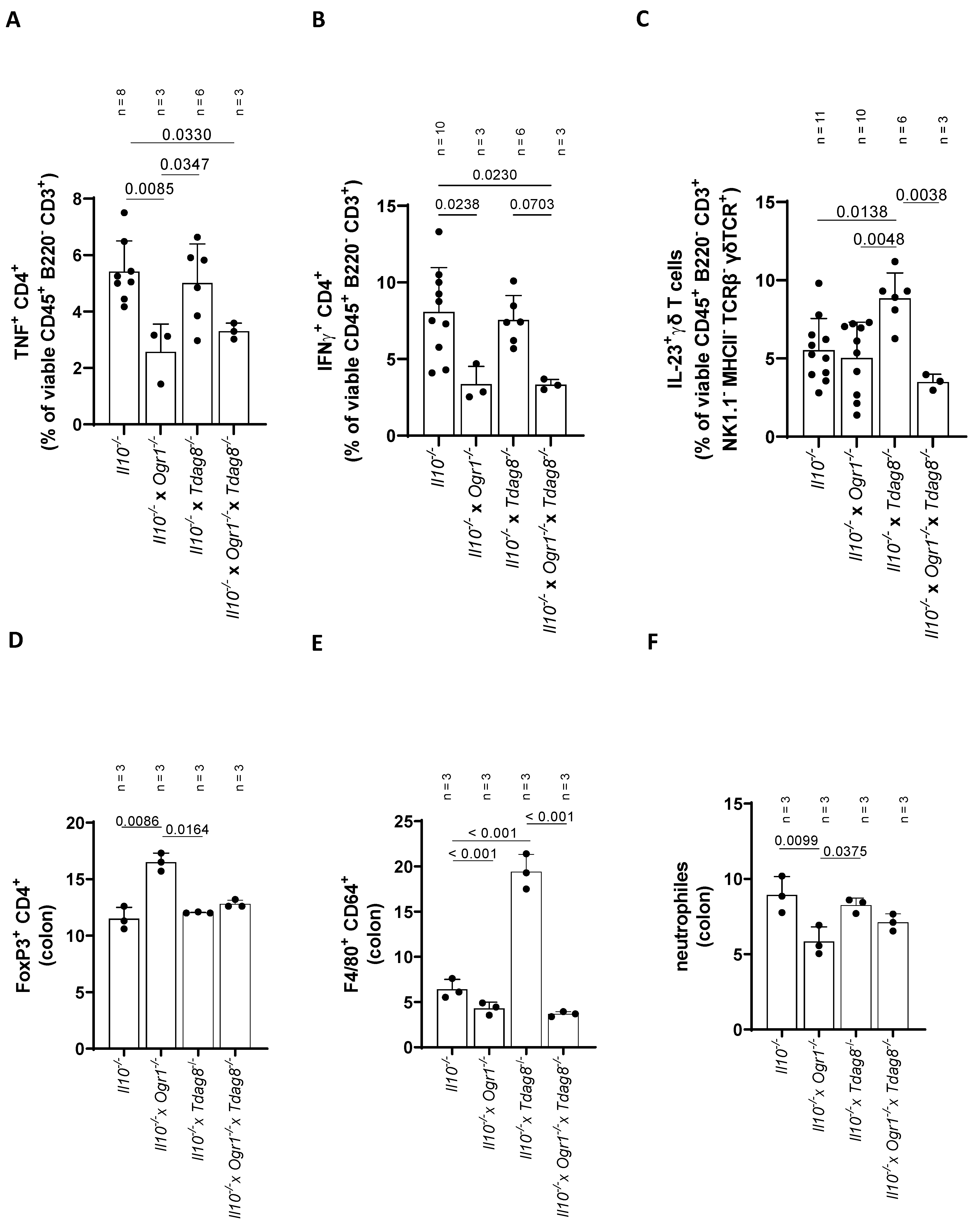
2.4. Ogr1-Deficient Mice Present Decreased Pro-Inflammatory Cell Populations in the Colon Even in the Absence of Tdag8
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. DSS-Induced Acute Colitis
4.3. Spontaneous Il-10 Deficient Colitis Model
4.4. Assessment of Colonoscopy and Histological Score in mice
4.5. Ribonucleic Acid (RNA) Isolation, Complementary DNA (cDNA) Synthesis, and qPCR
4.6. FACS
4.7. Lamina Propria Lymphocytes Isolation
4.8. Splenocyte Isolation
4.9. Compensation Controls
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| cAMP | Cyclic adenosine monophosphate |
| CD | Crohn’s disease |
| cDNA | Complementary DNA |
| DSS | Dextran sodium sulphate |
| FSC | Forward scatter |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase |
| GPRs | G-protein coupled receptors |
| GPR4 | G-protein coupled receptor 4 |
| HBSS | Hanks’ balanced salt solution |
| HE | Hematoxylin and eosin |
| IBD | Inflammatory bowel disease |
| IFNγ | Interferon gamma |
| IL | Interleukin |
| KO | Knockout |
| MEICS | Murine endoscopic index of colitis severity |
| OGR1 | Ovarian cancer GPR 1 (GPR68) |
| PBS | Phosphate-buffered solution |
| qPCR | Real-time quantitative polymerase chain reaction |
| SSC | Side scatter |
| TDAG8 | T-cell death-associated gene 8 (GPR65) |
| TNF | Tumor necrosis factor |
| UC | Ulcerative colitis |
| WT | Wildtype |
References
- Ludwig, M.G.; Vanek, M.; Guerini, D.; Gasser, J.A.; Jones, C.E.; Junker, U.; Hofstetter, H.; Wolf, R.M.; Seuwen, K. Proton-sensing G-protein-coupled receptors. Nature 2003, 425, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Ichimonji, I.; Tomura, H.; Mogi, C.; Sato, K.; Aoki, H.; Hisada, T.; Dobashi, K.; Ishizuka, T.; Mori, M.; Okajima, F. Extracellular acidification stimulates IL-6 production and Ca(2+) mobilization through proton-sensing OGR1 receptors in human airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 299, L567–L577. [Google Scholar] [CrossRef] [PubMed]
- Mogi, C.; Tomura, H.; Tobo, M.; Wang, J.Q.; Damirin, A.; Kon, J.; Komachi, M.; Hashimoto, K.; Sato, K.; Okajima, F. Sphingosylphosphorylcholine antagonizes proton-sensing ovarian cancer G-protein-coupled receptor 1 (OGR1)-mediated inositol phosphate production and cAMP accumulation. J. Pharmacol. Sci. 2005, 99, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Kihara, Y.; Shimizu, T. Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J. Biol. Chem. 2005, 280, 9083–9087. [Google Scholar] [CrossRef] [PubMed]
- Mogi, C.; Tobo, M.; Tomura, H.; Murata, N.; He, X.D.; Sato, K.; Kimura, T.; Ishizuka, T.; Sasaki, T.; Sato, T.; et al. Involvement of proton-sensing TDAG8 in extracellular acidification-induced inhibition of proinflammatory cytokine production in peritoneal macrophages. J. Immunol. 2009, 182, 3243–3251. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Kon, J.; Mogi, C.; Tobo, M.; Damirin, A.; Sato, K.; Komachi, M.; Malchinkhuu, E.; Murata, N.; Kimura, T.; et al. TDAG8 is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J. Biol. Chem. 2004, 279, 45626–45633. [Google Scholar] [CrossRef] [PubMed]
- Imenez Silva, P.H.; Wagner, C.A. Physiological relevance of proton-activated GPCRs. Pflugers Arch. 2022, 474, 487–504. [Google Scholar] [CrossRef]
- Tomura, H.; Mogi, C.; Sato, K.; Okajima, F. Proton-sensing and lysolipid-sensitive G-protein-coupled receptors: A novel type of multi-functional receptors. Cell Signal. 2005, 17, 1466–1476. [Google Scholar] [CrossRef]
- Kottyan, L.C.; Collier, A.R.; Cao, K.H.; Niese, K.A.; Hedgebeth, M.; Radu, C.G.; Witte, O.N.; Khurana Hershey, G.K.; Rothenberg, M.E.; Zimmermann, N. Eosinophil viability is increased by acidic pH in a cAMP- and GPR65-dependent manner. Blood 2009, 114, 2774–2782. [Google Scholar] [CrossRef]
- Tan, J.K.; McKenzie, C.; Marino, E.; Macia, L.; Mackay, C.R. Metabolite-Sensing G Protein-Coupled Receptors-Facilitators of Diet-Related Immune Regulation. Annu. Rev. Immunol. 2017, 35, 371–402. [Google Scholar] [CrossRef]
- Zhu, X.; Mose, E.; Hogan, S.P.; Zimmermann, N. Differential eosinophil and mast cell regulation: Mast cell viability and accumulation in inflammatory tissue are independent of proton-sensing receptor GPR65. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G974–G982. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marie, M.A.; Sanderlin, E.J.; Satturwar, S.; Hong, H.; Lertpiriyapong, K.; Donthi, D.; Yang, L.V. GPR65 (TDAG8) inhibits intestinal inflammation and colitis-associated colorectal cancer development in experimental mouse models. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166288. [Google Scholar] [CrossRef] [PubMed]
- Tcymbarevich, I.; Richards, S.M.; Russo, G.; Kuhn-Georgijevic, J.; Cosin-Roger, J.; Baebler, K.; Lang, S.; Bengs, S.; Atrott, K.; Bettoni, C.; et al. Lack of the pH-sensing Receptor TDAG8 [GPR65] in Macrophages Plays a Detrimental Role in Murine Models of Inflammatory Bowel Disease. J. Crohns Colitis 2019, 13, 245–258. [Google Scholar] [CrossRef]
- Jin, Y.; Sato, K.; Tobo, A.; Mogi, C.; Tobo, M.; Murata, N.; Ishii, S.; Im, D.S.; Okajima, F. Inhibition of interleukin-1beta production by extracellular acidification through the TDAG8/cAMP pathway in mouse microglia. J. Neurochem. 2014, 129, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Hikiji, H.; Endo, D.; Horie, K.; Harayama, T.; Akahoshi, N.; Igarashi, H.; Kihara, Y.; Yanagida, K.; Takeda, J.; Koji, T.; et al. TDAG8 activation inhibits osteoclastic bone resorption. FASEB J. 2014, 28, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Onozawa, Y.; Komai, T.; Oda, T. Activation of T cell death-associated gene 8 attenuates inflammation by negatively regulating the function of inflammatory cells. Eur. J. Pharmacol. 2011, 654, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.; McGovern, D.P.; Barrett, J.C.; Wang, K.; Radford-Smith, G.L.; Ahmad, T.; Lees, C.W.; Balschun, T.; Lee, J.; Roberts, R.; et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat. Genet. 2010, 42, 1118–1125. [Google Scholar] [CrossRef]
- Hardin, M.; Cho, M.; McDonald, M.L.; Beaty, T.; Ramsdell, J.; Bhatt, S.; van Beek, E.J.; Make, B.J.; Crapo, J.D.; Silverman, E.K.; et al. The clinical and genetic features of COPD-asthma overlap syndrome. Eur. Respir. J. 2014, 44, 341–350. [Google Scholar] [CrossRef]
- Hussman, J.P.; Beecham, A.H.; Schmidt, M.; Martin, E.R.; McCauley, J.L.; Vance, J.M.; Haines, J.L.; Pericak-Vance, M.A. GWAS analysis implicates NF-kappaB-mediated induction of inflammatory T cells in multiple sclerosis. Genes Immun. 2016, 17, 305–312. [Google Scholar] [CrossRef]
- International Genetics of Ankylosing Spondylitis Consortium; Cortes, A.; Hadler, J.; Pointon, J.P.; Robinson, P.C.; Karaderi, T.; Leo, P.; Cremin, K.; Pryce, K.; Harris, J.; et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat. Genet. 2013, 45, 730–738. [Google Scholar] [CrossRef]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef] [PubMed]
- de Valliere, C.; Cosin-Roger, J.; Baebler, K.; Schoepflin, A.; Mamie, C.; Mollet, M.; Schuler, C.; Bengs, S.; Lang, S.; Scharl, M.; et al. pH-Sensing G Protein-Coupled Receptor OGR1 (GPR68) Expression and Activation Increases in Intestinal Inflammation and Fibrosis. Int. J. Mol. Sci. 2022, 23, 1419. [Google Scholar] [CrossRef] [PubMed]
- de Valliere, C.; Wang, Y.; Eloranta, J.J.; Vidal, S.; Clay, I.; Spalinger, M.R.; Tcymbarevich, I.; Terhalle, A.; Ludwig, M.G.; Suply, T.; et al. G Protein-coupled pH-sensing Receptor OGR1 Is a Regulator of Intestinal Inflammation. Inflamm. Bowel Dis. 2015, 21, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- de Valliere, C.; Babler, K.; Busenhart, P.; Schwarzfischer, M.; Maeyashiki, C.; Schuler, C.; Atrott, K.; Lang, S.; Spalinger, M.R.; Scharl, M.; et al. A Novel OGR1 (GPR68) Inhibitor Attenuates Inflammation in Murine Models of Colitis. Inflamm. Intest. Dis. 2021, 6, 140–153. [Google Scholar] [CrossRef]
- Li, J.; Guo, B.; Wang, J.; Cheng, X.; Xu, Y.; Sang, J. Ovarian cancer G protein coupled receptor 1 suppresses cell migration of MCF7 breast cancer cells via a Galpha12/13-Rho-Rac1 pathway. J. Mol. Signal. 2013, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.C.; Bianchi, F.; Wang, Y.K.; Tang, M.J.; Ye, H.; Glitsch, M.D. Coincidence Detection of Membrane Stretch and Extracellular pH by the Proton-Sensing Receptor OGR1 (GPR68). Curr. Biol. 2018, 28, 3815–3823.e4. [Google Scholar] [CrossRef]
- Wiley, S.Z.; Sriram, K.; Liang, W.; Chang, S.E.; French, R.; McCann, T.; Sicklick, J.; Nishihara, H.; Lowy, A.M.; Insel, P.A. GPR68, a proton-sensing GPCR, mediates interaction of cancer-associated fibroblasts and cancer cells. FASEB J. 2018, 32, 1170–1183. [Google Scholar] [CrossRef]
- Hutter, S.; van Haaften, W.T.; Hunerwadel, A.; Baebler, K.; Herfarth, N.; Raselli, T.; Mamie, C.; Misselwitz, B.; Rogler, G.; Weder, B.; et al. Intestinal Activation of pH-Sensing Receptor OGR1 [GPR68] Contributes to Fibrogenesis. J. Crohns Colitis 2018, 12, 1348–1358. [Google Scholar] [CrossRef]
- de Valliere, C.; Cosin-Roger, J.; Simmen, S.; Atrott, K.; Melhem, H.; Zeitz, J.; Madanchi, M.; Tcymbarevich, I.; Fried, M.; Kullak-Ublick, G.A.; et al. Hypoxia Positively Regulates the Expression of pH-Sensing G-Protein-Coupled Receptor OGR1 (GPR68). Cell Mol. Gastroenterol. Hepatol. 2016, 2, 796–810. [Google Scholar] [CrossRef]
- Maeyashiki, C.; Melhem, H.; Hering, L.; Baebler, K.; Cosin-Roger, J.; Schefer, F.; Weder, B.; Hausmann, M.; Scharl, M.; Rogler, G.; et al. Activation of pH-Sensing Receptor OGR1 (GPR68) Induces ER Stress Via the IRE1alpha/JNK Pathway in an Intestinal Epithelial Cell Model. Sci. Rep. 2020, 10, 1438. [Google Scholar] [CrossRef]
- Seuwen, K.; Ludwig, M.G.; Wolf, R.M. Receptors for protons or lipid messengers or both? J. Recept. Signal. Transduct. Res. 2006, 26, 599–610. [Google Scholar] [CrossRef] [PubMed]
- de Valliere, C.; Vidal, S.; Clay, I.; Jurisic, G.; Tcymbarevich, I.; Lang, S.; Ludwig, M.G.; Okoniewski, M.; Eloranta, J.J.; Kullak-Ublick, G.A.; et al. The pH-sensing receptor OGR1 improves barrier function of epithelial cells and inhibits migration in an acidic environment. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G475–G490. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.I.B.D. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome-Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef]
- Nakase, H. Treatment of inflammatory bowel disease from the immunological perspective. Immunol. Med. 2020, 43, 79–86. [Google Scholar] [CrossRef]
- Fallingborg, J.; Christensen, L.A.; Jacobsen, B.A.; Rasmussen, S.N. Very low intraluminal colonic pH in patients with active ulcerative colitis. Dig. Dis. Sci. 1993, 38, 1989–1993. [Google Scholar] [CrossRef]
- Nugent, S.G.; Kumar, D.; Rampton, D.S.; Evans, D.F. Intestinal luminal pH in inflammatory bowel disease: Possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 2001, 48, 571–577. [Google Scholar] [CrossRef]
- Vernia, P.; Caprilli, R.; Latella, G.; Barbetti, F.; Magliocca, F.M.; Cittadini, M. Fecal lactate and ulcerative colitis. Gastroenterology 1988, 95, 1564–1568. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef]
- Shouval, D.S.; Biswas, A.; Goettel, J.A.; McCann, K.; Conaway, E.; Redhu, N.S.; Mascanfroni, I.D.; Al Adham, Z.; Lavoie, S.; Ibourk, M.; et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity 2014, 40, 706–719. [Google Scholar] [CrossRef]
- Redhu, N.S.; Bakthavatchalu, V.; Conaway, E.A.; Shouval, D.S.; Tsou, A.; Goettel, J.A.; Biswas, A.; Wang, C.; Field, M.; Muller, W.; et al. Macrophage dysfunction initiates colitis during weaning of infant mice lacking the interleukin-10 receptor. Elife 2017, 6, e27652. [Google Scholar] [CrossRef] [PubMed]
- Patik, I.; Redhu, N.S.; Eran, A.; Bao, B.; Nandy, A.; Tang, Y.; El Sayed, S.; Shen, Z.; Glickman, J.; Fox, J.G.; et al. The IL-10 receptor inhibits cell extrinsic signals necessary for STAT1-dependent macrophage accumulation during colitis. Mucosal. Immunol. 2023, 16, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhang, J.; Liu, H.; Li, S.; Wang, Q. The Role of Tissue-Resident Macrophages in the Development and Treatment of Inflammatory Bowel Disease. Front. Cell Dev. Biol. 2022, 10, 896591. [Google Scholar] [CrossRef] [PubMed]
- McGovern, D.; Powrie, F. The IL23 axis plays a key role in the pathogenesis of IBD. Gut 2007, 56, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Shime, H.; Yabu, M.; Akazawa, T.; Kodama, K.; Matsumoto, M.; Seya, T.; Inoue, N. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J. Immunol. 2008, 180, 7175–7183. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, A.; Mogi, C.; Ono, H.; Nishi, T.; Horii, Y.; Ohba, Y.; Sato, K.; Nakaya, M.; Okajima, F.; Kurose, H. The proton-sensing G protein-coupled receptor T-cell death-associated gene 8 (TDAG8) shows cardioprotective effects against myocardial infarction. Sci. Rep. 2017, 7, 7812. [Google Scholar] [CrossRef]
- Gaublomme, J.T.; Yosef, N.; Lee, Y.; Gertner, R.S.; Yang, L.V.; Wu, C.; Pandolfi, P.P.; Mak, T.; Satija, R.; Shalek, A.K.; et al. Single-Cell Genomics Unveils Critical Regulators of Th17 Cell Pathogenicity. Cell 2015, 163, 1400–1412. [Google Scholar] [CrossRef]
- Lassen, K.G.; McKenzie, C.I.; Mari, M.; Murano, T.; Begun, J.; Baxt, L.A.; Goel, G.; Villablanca, E.J.; Kuo, S.Y.; Huang, H.; et al. Genetic Coding Variant in GPR65 Alters Lysosomal pH and Links Lysosomal Dysfunction with Colitis Risk. Immunity 2016, 44, 1392–1405. [Google Scholar] [CrossRef]
- Chen, X.; Jaiswal, A.; Costliow, Z.; Herbst, P.; Creasey, E.A.; Oshiro-Rapley, N.; Daly, M.J.; Carey, K.L.; Graham, D.B.; Xavier, R.J. pH sensing controls tissue inflammation by modulating cellular metabolism and endo-lysosomal function of immune cells. Nat. Immunol. 2022, 23, 1063–1075. [Google Scholar] [CrossRef]
- D’Souza, C.A.; Zhao, F.L.; Li, X.; Xu, Y.; Dunn, S.E.; Zhang, L. OGR1/GPR68 Modulates the Severity of Experimental Autoimmune Encephalomyelitis and Regulates Nitric Oxide Production by Macrophages. PLoS ONE 2016, 11, e0148439. [Google Scholar] [CrossRef]
- McAleer, J.P.; Fan, J.; Roar, B.; Primerano, D.A.; Denvir, J. Cytokine Regulation in Human CD4 T Cells by the Aryl Hydrocarbon Receptor and Gq-Coupled Receptors. Sci. Rep. 2018, 8, 10954. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, S.; Patik, I.; Redhu, N.S.; Glickman, J.N.; Karagiannis, K.; El Naenaeey, E.S.Y.; Elmowalid, G.A.; Abd El Wahab, A.M.; Snapper, S.B.; Horwitz, B.H. CCR2 promotes monocyte recruitment and intestinal inflammation in mice lacking the interleukin-10 receptor. Sci. Rep. 2022, 12, 452. [Google Scholar] [CrossRef] [PubMed]
- Onozawa, Y.; Fujita, Y.; Kuwabara, H.; Nagasaki, M.; Komai, T.; Oda, T. Activation of T cell death-associated gene 8 regulates the cytokine production of T cells and macrophages in vitro. Eur. J. Pharmacol. 2012, 683, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Fantini, M.C.; Wirtz, S.; Nikolaev, A.; Kiesslich, R.; Lehr, H.A.; Galle, P.R.; Neurath, M.F. In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut 2005, 54, 950–954. [Google Scholar] [CrossRef]
- Obermeier, F.; Kojouharoff, G.; Hans, W.; Scholmerich, J.; Gross, V.; Falk, W. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin. Exp. Immunol. 1999, 116, 238–245. [Google Scholar] [CrossRef]
- Steidler, L.; Hans, W.; Schotte, L.; Neirynck, S.; Obermeier, F.; Falk, W.; Fiers, W.; Remaut, E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 2000, 289, 1352–1355. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perren, L.; Busch, M.; Schuler, C.; Ruiz, P.A.; Foti, F.; Weibel, N.; de Vallière, C.; Morsy, Y.; Seuwen, K.; Hausmann, M.; et al. OGR1 (GPR68) and TDAG8 (GPR65) Have Antagonistic Effects in Models of Colonic Inflammation. Int. J. Mol. Sci. 2023, 24, 14855. https://doi.org/10.3390/ijms241914855
Perren L, Busch M, Schuler C, Ruiz PA, Foti F, Weibel N, de Vallière C, Morsy Y, Seuwen K, Hausmann M, et al. OGR1 (GPR68) and TDAG8 (GPR65) Have Antagonistic Effects in Models of Colonic Inflammation. International Journal of Molecular Sciences. 2023; 24(19):14855. https://doi.org/10.3390/ijms241914855
Chicago/Turabian StylePerren, Leonie, Moana Busch, Cordelia Schuler, Pedro A. Ruiz, Federica Foti, Nathalie Weibel, Cheryl de Vallière, Yasser Morsy, Klaus Seuwen, Martin Hausmann, and et al. 2023. "OGR1 (GPR68) and TDAG8 (GPR65) Have Antagonistic Effects in Models of Colonic Inflammation" International Journal of Molecular Sciences 24, no. 19: 14855. https://doi.org/10.3390/ijms241914855
APA StylePerren, L., Busch, M., Schuler, C., Ruiz, P. A., Foti, F., Weibel, N., de Vallière, C., Morsy, Y., Seuwen, K., Hausmann, M., & Rogler, G. (2023). OGR1 (GPR68) and TDAG8 (GPR65) Have Antagonistic Effects in Models of Colonic Inflammation. International Journal of Molecular Sciences, 24(19), 14855. https://doi.org/10.3390/ijms241914855





