Role of Biomaterials in the Development of Epithelial Support in 3D In Vitro Airway Epithelium Development: A Systematic Review
Abstract
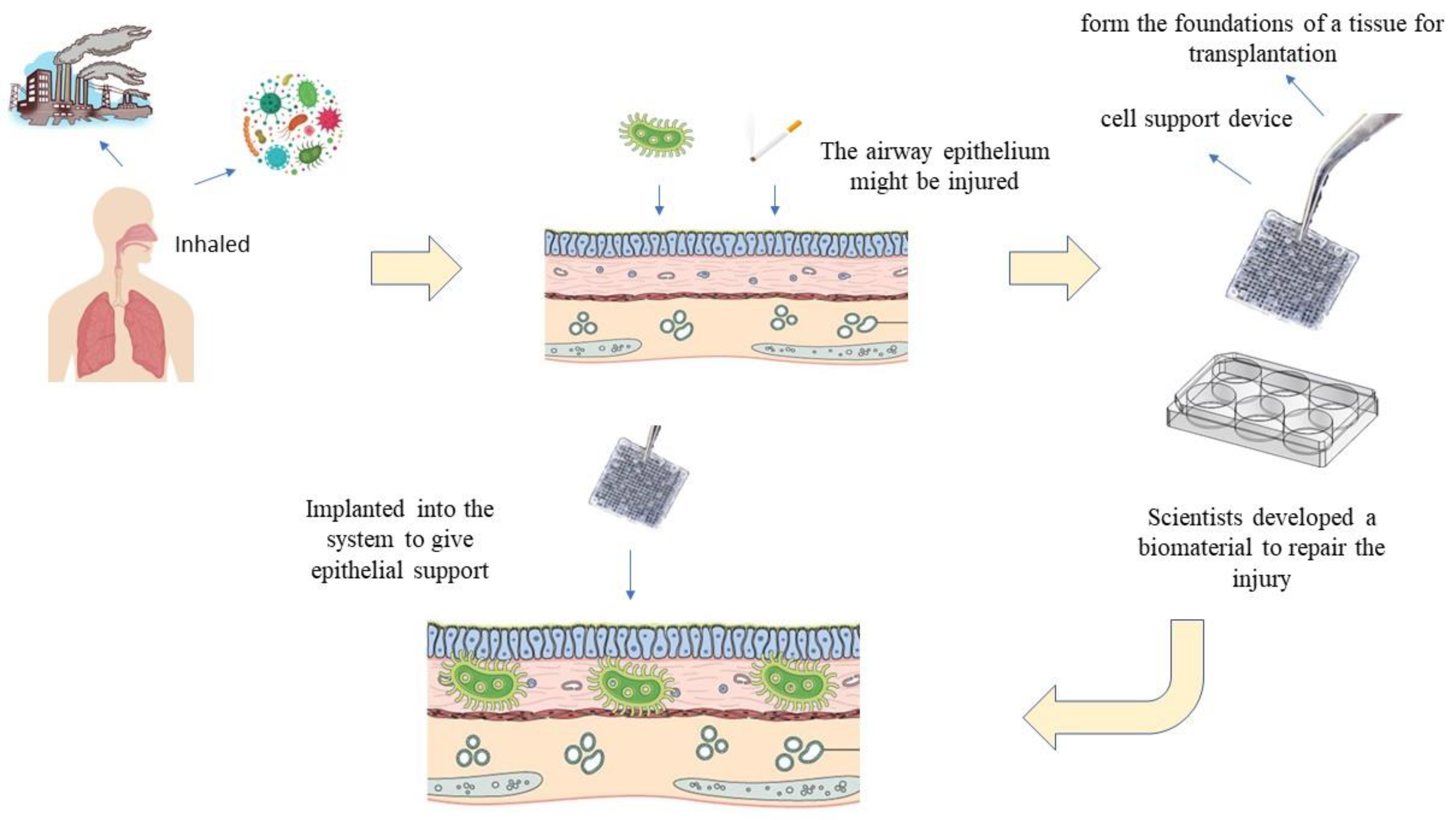
:1. Introduction
2. Materials and Methods
2.1. Search Criteria
2.2. Data Extraction and Analysis
3. Results
3.1. Study Characteristics
3.2. Physicochemical, Mechanical, and Biological Properties
| Type of Biomaterials/Scaffolds | Type of Cells | Outcomes | Conclusion |
|---|---|---|---|
| Microgrooved gelatin hydrogel crosslinked with glutaraldehyde [25] | Human bronchial epithelial cell line (BEAS-2B) | 1. Topographical
|
|
| Non-woven bilayered biodegradable chitosan-gelatin-polylactide (CGP) with hyaluronic acid (HA) immobilization scaffold [22] | Human respiratory epithelium cells (HRECs) | 1. Physicochemical properties
|
|
| Three-dimensionally printed porous structure of a thermoresponsive injectable polyethercarbonate (3D-TIPS) stiffness-softening elastomer nanohybrid impregnated with collagen nanofibrous hydrogel [26] |
| 1. Mechanical properties
|
|
| Electrospun nanofibers of poly(ε-caprolactone)/ depolymerized chitosan (PCL/chitosan) [27] | Porcine tracheobronchial epithelial (PTBE) cells | 1. Mechanical properties
|
|
| Respiratory epithelial cells (RECs) | 1. Contact angle
|
|
| Three-dimensional-printing of silk fibroin/hydroxypropyl methylcellulose (SF/HPMC) thixotropic hydrogel [20] | Normal human bronchial epithelial cell line (BEAS-2B) | 1. Ultrastructure
|
|
| Electrospun polyethylene terephthalate scaffold (PET) [18] | The epithelial cell line (Calu-3) The fibroblast (MRC-5) cell lines | 1. Trans-epithelial electrical resistance (TEER) measurements
|
|
| Novel electrospun biphasic scaffold [21] | MRC5 and CALU3 cell lines | 1. Diameter
|
|
| Collagen IV- and laminin-containing extracellular matrix [28] | Human bronchial epithelial cells (HBECs) | 1. Cell attachment, differentiation, and proliferation
| Collagen IV and laminin, two extracellular matrix proteins, are crucial for respiratory epithelial adhesion and growth in vitro. |
| Fibrin gel [29] | Respiratory epithelial cells | 1. Cell differentiation
|
|
| Porous three-dimensional silk fibroin scaffolds (3D SF) [30] | Human tracheobronchial epithelial cells (HBECs) | 1. Cell viability and proliferation:
|
|
| Calu-3 bronchial epithelial cell line | 1. Biocompatibility:
|
|
| Microfluidic lung airway-on-a-chip with arrayable suspended gels [32] |
| 1. Cell adhesion:
|
|
| Human plasma [33] |
| 1. Histological analysis:
|
|
| Urinary bladder-derived ECM hydrogels [34] | Human bronchial epithelial cells (HBECs) | 1. Cell Proliferation and differentiation
|
|
|
| 1. Cell differentiation and proliferation
|
|
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forum of International Respiratory Societies; Levine, S.; Marciniuk, D.; Aglan, A.; Celedón, J.C.; Fong, K.; Horsburgh, R.; Malhotra, A.; Masekela, R.; Mortimer, K.; et al. The Global Impact of Respiratory Disease, 3rd ed.; European Respiratory Society: Lausanne, Switzerland, 2021; Available online: https://www.firsnet.org/images/publications/FIRS_Master_09202021.pdf (accessed on 22 September 2021).
- Burnett, R.T.; Pope, C.A., III; Ezzati, M.; Olives, C.; Lim, S.S.; Mehta, S.; Shin, H.H.; Singh, G.; Hubbell, B.; Brauer, M.; et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ. Health Perspect. 2014, 122, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Fiordelisi, A.; Piscitelli, P.; Trimarco, B.; Coscioni, E.; Iaccarino, G.; Sorriento, D. The mechanisms of air pollution and particulate matter in cardiovascular diseases. Heart Fail. Rev. 2017, 22, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, P.S.; Grootaers, G.; van der Does, A.M.; Krul, C.A.; Kooter, I.M. Human lung epithelial cell cultures for analysis of inhaled toxicants: Lessons learned and future directions. Toxicol. In Vitro 2018, 47, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Iosifidis, T.; Garratt, L.W.; Coombe, D.R.; Knight, D.A.; Stick, S.M.; Kicic, A. Airway epithelial repair in health and disease: Orchestrator or simply a player? Respirology 2016, 21, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Soleas, J.P.; Paz, A.; Marcus, P.; McGuigan, A.; Waddell, T.K. Engineering Airway Epithelium. J. Biomed. Biotechnol. 2012, 2012, 982971. [Google Scholar] [CrossRef]
- Amatngalim, G.D.; Hiemstra, P.S. Airway Epithelial Cell Function and Respiratory Host Defense in Chronic Obstructive Pulmonary Disease. Chin. Med. J. 2018, 131, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.L.; Goldblatt, D.L.; Evans, S.E.; Tuvim, M.J.; Dickey, B.F. Airway Epithelial Innate Immunity. Front. Physiol. 2021, 12, 749077. [Google Scholar] [CrossRef]
- Davis, J.D.; Wypych, T.P. Cellular and functional heterogeneity of the airway epithelium. Mucosal Immunol. 2021, 14, 978–990. [Google Scholar] [CrossRef]
- Cortez, V.; Schultz-Cherry, S. The role of goblet cells in viral pathogenesis. FEBS J. 2021, 288, 7060–7072. [Google Scholar] [CrossRef]
- Linfield, D.T.; Raduka, A.; Aghapour, M.; Rezaee, F. Airway tight junctions as targets of viral infections: Tight Junctions and Viral Infections. Tissue Barriers 2021, 9, 1883965. [Google Scholar] [CrossRef]
- Holtzman, M.J.; Byers, D.E.; Alexander-Brett, J.; Wang, X. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat. Rev. Immunol. 2014, 14, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Ozgun, A.; Lomboni, D.; Arnott, H.; Staines, W.A.; Woulfe, J.; Variola, F. Biomaterials-based strategies for in vitro neural models. Biomater. Sci. 2022, 10, 1134–1165. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.V.; Lee, J.-H. Alveolar wars: The rise of in vitro models to understand human lung alveolar maintenance, regeneration, and disease. STEM CELLS Transl. Med. 2020, 9, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Jammalamadaka, U.; Tappa, K. Recent advances in biomaterials for 3D printing and tissue engineering. J. Funct. Biomater. 2018, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Fadilah, N.I.M.; Isa, I.L.M.; Zaman, W.S.W.K.; Tabata, Y.; Fauzi, M.B. The Effect of Nanoparticle-Incorporated Natural-Based Biomaterials towards Cells on Activated Pathways: A Systematic Review. Polymers 2022, 14, 476. [Google Scholar] [CrossRef] [PubMed]
- Harrington, H.; Cato, P.; Salazar, F.; Wilkinson, M.; Knox, A.; Haycock, J.W.; Rose, F.; Aylott, J.W.; Ghaemmaghami, A.M. Immunocompetent 3D model of human upper airway for disease modeling and in vitro drug evaluation. Mol. Pharm. 2014, 11, 2082–2091. [Google Scholar] [CrossRef]
- Rabiatul, A.; Lokanathan, Y.; Rohaina, C.; Chowdhury, S.; Aminuddin, B.; Ruszymah, B. Surface modification of electrospun poly(methyl methacrylate) (PMMA) nanofibers for the development of in vitro respiratory epithelium model. J. Biomater. Sci. Polym. Ed. 2015, 26, 1297–1311. [Google Scholar] [CrossRef]
- Zhong, N.; Dong, T.; Chen, Z.; Guo, Y.; Shao, Z.; Zhao, X. A novel 3D-printed silk fibroin-based scaffold facilitates tracheal epithelium proliferation in vitro. J. Biomater. Appl. 2019, 34, 3–11. [Google Scholar] [CrossRef]
- Morris, G.E.; Bridge, J.C.; Brace, L.A.; Knox, A.J.; Aylott, J.W.; Brightling, C.E.; Ghaemmaghami, A.M.; Rose, F.R.A.J. A novel electrospun biphasic scaffold provides optimal three-dimensional topography for in vitro co-culture of airway epithelial and fibroblast cells. Biofabrication 2014, 6, 035014. [Google Scholar] [CrossRef]
- Romanova, O.A.; Tenchurin, T.H.; Demina, T.S.; Sytina, E.V.; Shepelev, A.D.; Rudyak, S.G.; Klein, O.I.; Krasheninnikov, S.V.; Safronova, E.I.; Kamyshinsky, R.A.; et al. Non-woven bilayered biodegradable chitosan-gelatin-polylactide scaffold for bioengineering of tracheal epithelium. Cell Prolif. 2019, 52, e12598. [Google Scholar] [CrossRef] [PubMed]
- Busra, F.M.; Lokanathan, Y.; Nadzir, M.M.; Saim, A.; Idrus, R.B.H.; Chowdhury, S.R. Attachment, proliferation, and morphological properties of human dermal fibroblasts on ovine tendon collagen scaffolds: A comparative study. Malays. J. Med. Sci. 2017, 24, 33–43. [Google Scholar] [CrossRef]
- Varma, R.; Aoki, F.G.; Soon, K.; Karoubi, G.; Waddell, T.K. Optimal biomaterials for tracheal epithelial grafts: An in vitro systematic comparative analysis. Acta Biomater. 2018, 81, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Soleas, J.P.; Waddell, T.K.; McGuigan, A.P. Topographically grooved gel inserts for aligning epithelial cells during air–liquid-interface culture. Biomater. Sci. 2015, 3, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Magaz, A.; Huo, S.; Darbyshire, A.; Loizidou, M.; Emberton, M.; Birchall, M.; Song, W. Human airway-like multilayered tissue on 3D-TIPS printed thermoresponsive elastomer/collagen hybrid scaffolds. Acta Biomater. 2020, 113, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, C.; Conklin, D.; Waterman, J.; Sankar, J.; Bhattarai, N. Electrospun nanofibers of poly(ε-caprolactone)/depolymerized chitosan for respiratory tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2016, 27, 611–625. [Google Scholar] [CrossRef]
- Hamilton, N.J.; Lee, D.D.H.; Gowers, K.H.; Butler, C.R.; Maughan, E.F.; Jevans, B.; Orr, J.C.; McCann, C.J.; Burns, A.J.; MacNeil, S.; et al. Bioengineered airway epithelial grafts with mucociliary function based on collagen IV- and laminin-containing extracellular matrix scaffolds. Eur. Respir. J. 2020, 55, 1901200. [Google Scholar] [CrossRef] [PubMed]
- Albers, S.; Thiebes, A.L.; Gessenich, K.L.; Jockenhoevel, S.; Cornelissen, C.G. Differentiation of respiratory epithelium in a 3-dimensional co-culture with fibroblasts embedded in fibrin gel. Multidiscip. Respir. Med. 2016, 11, 6. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, N.; Wen, J.; Jia, M.; Guo, Y.; Shao, Z.; Zhao, X. Porous Three-Dimensional Silk Fibroin Scaffolds for Tracheal Epithelial Regeneration In Vitro and In Vivo. ACS Biomater. Sci. Eng. 2018, 4, 2977–2985. [Google Scholar] [CrossRef]
- O’Leary, C.; Soriano, L.; Fagan-Murphy, A.; Ivankovic, I.; Cavanagh, B.; O’Brien, F.J.; Cryan, S.-A. The Fabrication and in vitro Evaluation of Retinoic Acid-Loaded Electrospun Composite Biomaterials for Tracheal Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 190. [Google Scholar] [CrossRef]
- Humayun, M.; Chow, C.-W.; Young, E.W.K. Microfluidic lung airway-on-a-chip with arrayable suspended gels for studying epithelial and smooth muscle cell interactions. Lab A Chip 2018, 18, 1298–1309. [Google Scholar] [CrossRef]
- Yunus, M.H.M.; Rashidbenam, Z.; Fauzi, M.B.; Idrus, R.B.H.; Bin Saim, A. Evaluating feasibility of human tissue-engineered respiratory epithelium construct as a potential model for tracheal mucosal reconstruction. Molecules 2021, 26, 6724. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, A.; D’Angelo, W.; Zhang, L.; Reing, J.; Johnson, S.; Myerburg, M.; Badylak, S.F. Human Bronchial Epithelial Cell Growth on Homologous Versus Heterologous Tissue Extracellular Matrix. J. Surg. Res. 2021, 263, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Kreimendahl, F.; Ossenbrink, S.; Köpf, M.; Westhofen, M.; Schmitz-Rode, T.; Fischer, H.; Jockenhoevel, S.; Thiebes, A.L. Combination of vascularization and cilia formation for three-dimensional airway tissue engineering. J. Biomed. Mater. Res. Part A 2019, 107, 2053–2062. [Google Scholar] [CrossRef]
- Murugan, S.S. Mechanical Properties of Materials: Definition, Testing and Application. Int. J. Mod. Stud. Mech. Eng. (IJMSME) 2020, 6, 28–38. [Google Scholar]
- McGowan, K.B.; Stiegman, G. Regulatory Challenges for Cartilage Repair Technologies. Cartilage 2013, 4, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, A.G.G.; Ferreras, L.A.B.; Bennett, K.E.; Casey, S.M.; Brama, P.A.; O’Brien, F.J. Systematic Comparison of Biomaterials-Based Strategies for Osteochondral and Chondral Repair in Large Animal Models. Adv. Health Mater. 2021, 10, e2100878. [Google Scholar] [CrossRef] [PubMed]
- Fang, I.-J.; Trewyn, B.G. Application of Mesoporous Silica Nanoparticles in Intracellular Delivery of Molecules and Proteins. Methods Enzymol. 2012, 508, 41–59. [Google Scholar] [CrossRef]
- Salleh, A.; Mustafa, N.; Teow, Y.H.; Fatimah, M.N.; Khairudin, F.A.; Ahmad, I.; Fauzi, M.B. Dual-Layered Approach of Ovine Collagen-Gelatin/Cellulose Hybrid Biomatrix Containing Graphene Oxide-Silver Nanoparticles for Cutaneous Wound Healing: Fabrication, Physicochemical, Cytotoxicity and Antibacterial Characterisation. Biomedicines 2022, 10, 816. [Google Scholar] [CrossRef]
- Guarino, V.; Raucci, M.; Ronca, A.; Cirillo, V.; Ambrosio, L. Multifunctional scaffolds for bone regeneration. In Bone Substitute Biomaterials; Woodhead Publishing: Sawston, UK, 2014; pp. 95–117. [Google Scholar] [CrossRef]
- Ball, V. Physicochemical properties of biomaterials. In Biomaterials for Organ and Tissue Regeneration: New Technologies and Future Prospects; Elsevier: Amsterdam, The Netherlands, 2020; pp. 19–32. [Google Scholar] [CrossRef]
- Eon Kuek, L.; Lee, R.J. First contact: The role of respiratory cilia in host-pathogen interactions in the airways. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, 603–619. [Google Scholar] [CrossRef]
- Jing, J.C.; Chen, J.J.; Chou, L.; Wong, B.J.F.; Chen, Z. Visualization and Detection of Ciliary Beating Pattern and Frequency in the Upper Airway using Phase Resolved Doppler Optical Coherence Tomography. Sci. Rep. 2017, 7, 8522. [Google Scholar] [CrossRef]
- Hamilton, N.; Bullock, A.J.; MacNeil, S.; Janes, S.M.; Birchall, M. Tissue engineering airway mucosa: A systematic review. Laryngoscope 2014, 124, 961–968. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nashihah, A.K.; Muhammad Firdaus, F.I.; Fauzi, M.B.; Mobarak, N.N.; Lokanathan, Y. Role of Biomaterials in the Development of Epithelial Support in 3D In Vitro Airway Epithelium Development: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 14935. https://doi.org/10.3390/ijms241914935
Nashihah AK, Muhammad Firdaus FI, Fauzi MB, Mobarak NN, Lokanathan Y. Role of Biomaterials in the Development of Epithelial Support in 3D In Vitro Airway Epithelium Development: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(19):14935. https://doi.org/10.3390/ijms241914935
Chicago/Turabian StyleNashihah, Ab Karim, Fairuz Izan Muhammad Firdaus, Mh. Busra Fauzi, Nadhratun Naiim Mobarak, and Yogeswaran Lokanathan. 2023. "Role of Biomaterials in the Development of Epithelial Support in 3D In Vitro Airway Epithelium Development: A Systematic Review" International Journal of Molecular Sciences 24, no. 19: 14935. https://doi.org/10.3390/ijms241914935
APA StyleNashihah, A. K., Muhammad Firdaus, F. I., Fauzi, M. B., Mobarak, N. N., & Lokanathan, Y. (2023). Role of Biomaterials in the Development of Epithelial Support in 3D In Vitro Airway Epithelium Development: A Systematic Review. International Journal of Molecular Sciences, 24(19), 14935. https://doi.org/10.3390/ijms241914935









