Following Natural Autoantibodies: Further Immunoserological Evidence Regarding Their Silent Plasticity and Engagement in Immune Activation
Abstract
1. Introduction
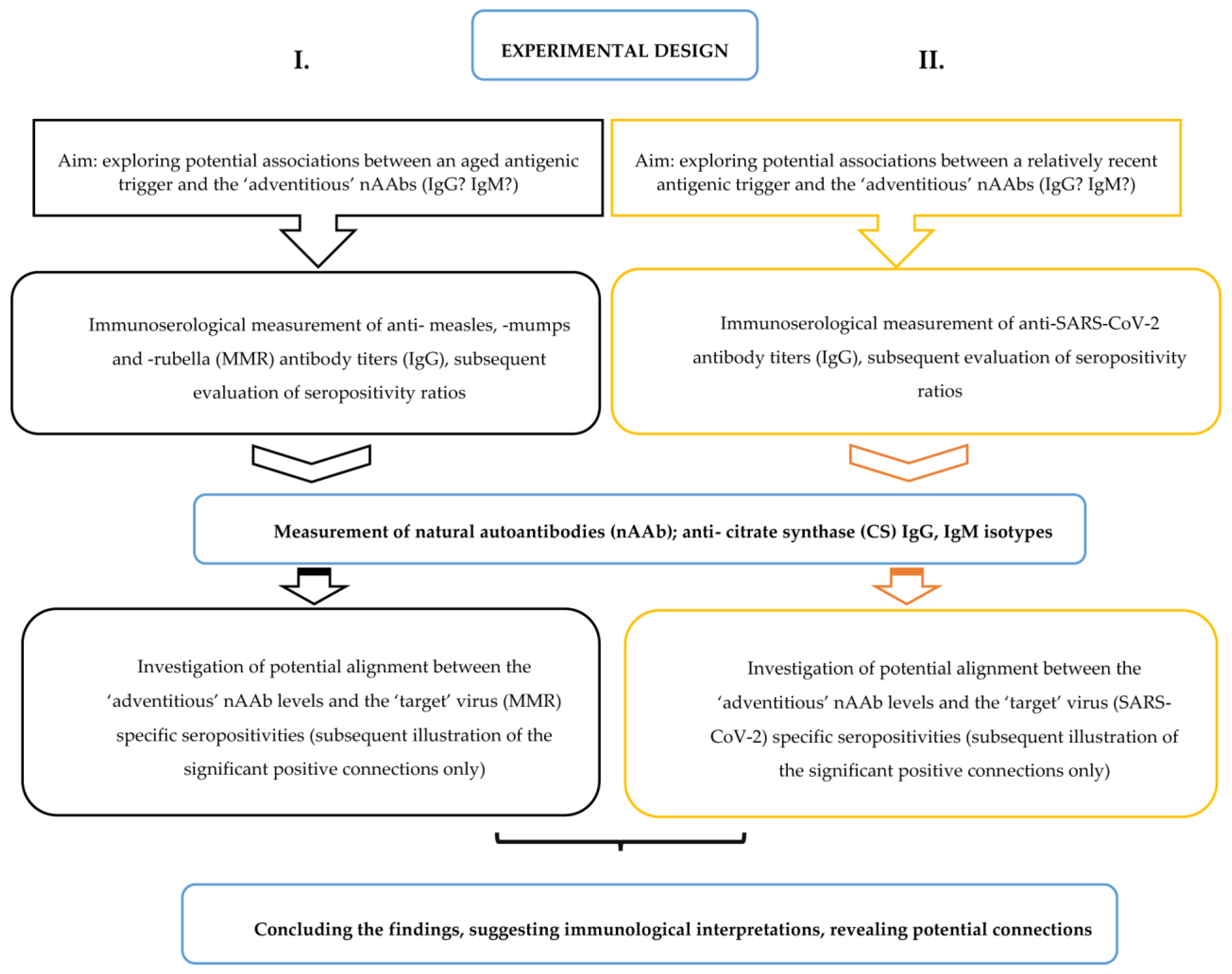
- I.
- Is there an association between the aged, aforetime elicited anti-viral (measles, mumps, and rubella childhood vaccinations or natural infections) antibody levels and the nAAbs?In order to answer this question, firstly, we aimed to evaluate IgG antibody titers elicited by the historical measles, mumps, and rubella (MMR) vaccines (or the relevant viral pathogens). Similarly to our former seroepidemiological reports [33,52,53], we also intended to delineate potential gaps of humoral immunity. Secondly, we compared the specific MMR antigen-induced seropositivity results to nAAb (anti-citrate synthase: anti-CS) titers.
- II.
- Is there an association between the relatively recent anti-SARS-CoV-2 IgG antigen-induced antibodies and the nAAbs?In order to answer this question, firstly, we aimed to evaluate IgG antibody titers elicited by the contemporary SARS-CoV-2 vaccines. Subsequently, our goal was to contrast the specific SARS-CoV-2 antigen-induced seropositivity results with the nAAb (anti-citrate synthase: anti-CS) titers.
2. Results
2.1. Relative Differences in Anti-MMR Seropositivity Ratios by Age Groups
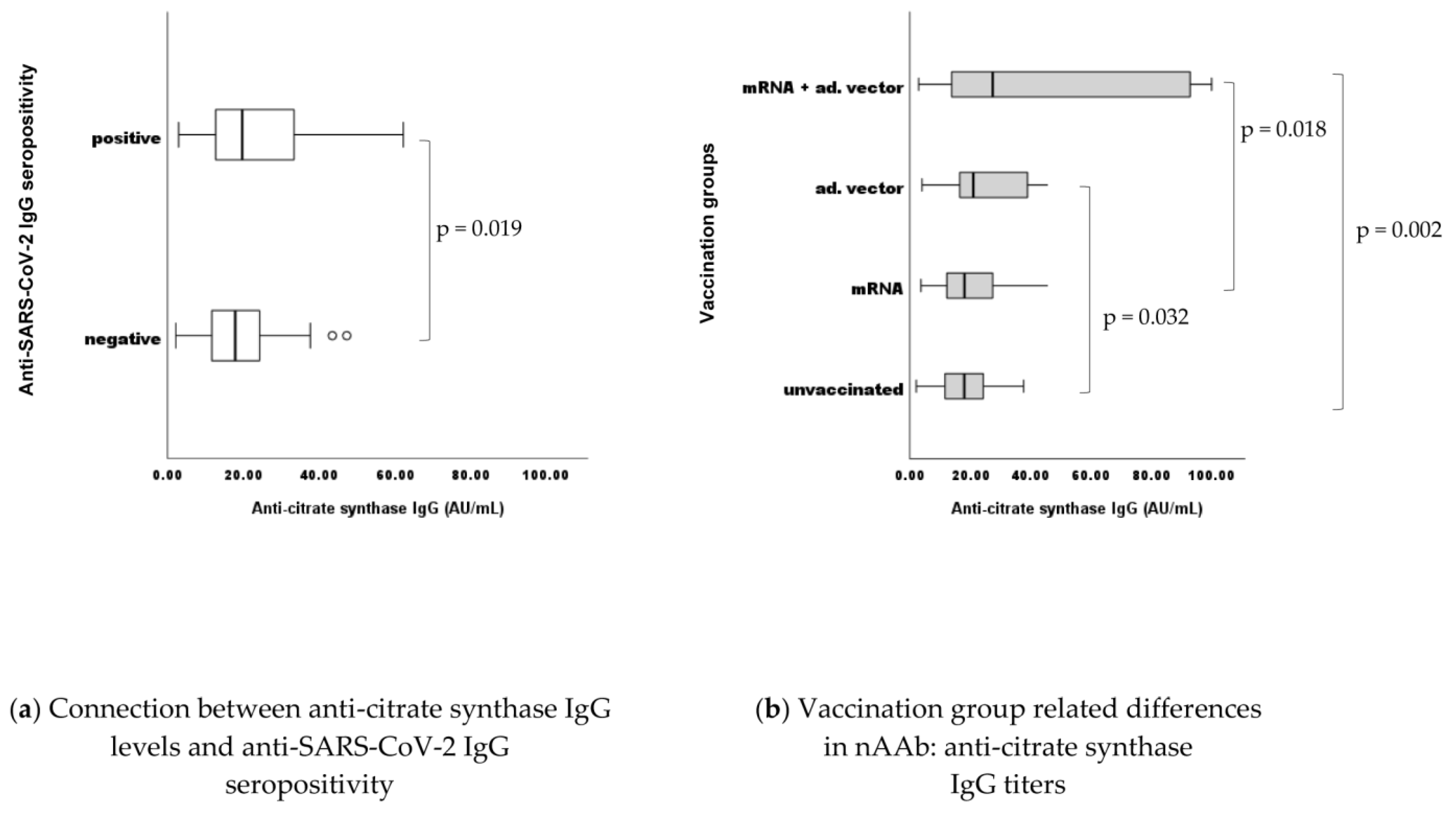
2.2. Connection between nAAb (Anti-Citrate Synthase; Anti-CS) IgM Levels and Anti-Viral (MMR) Humoral IgG Levels
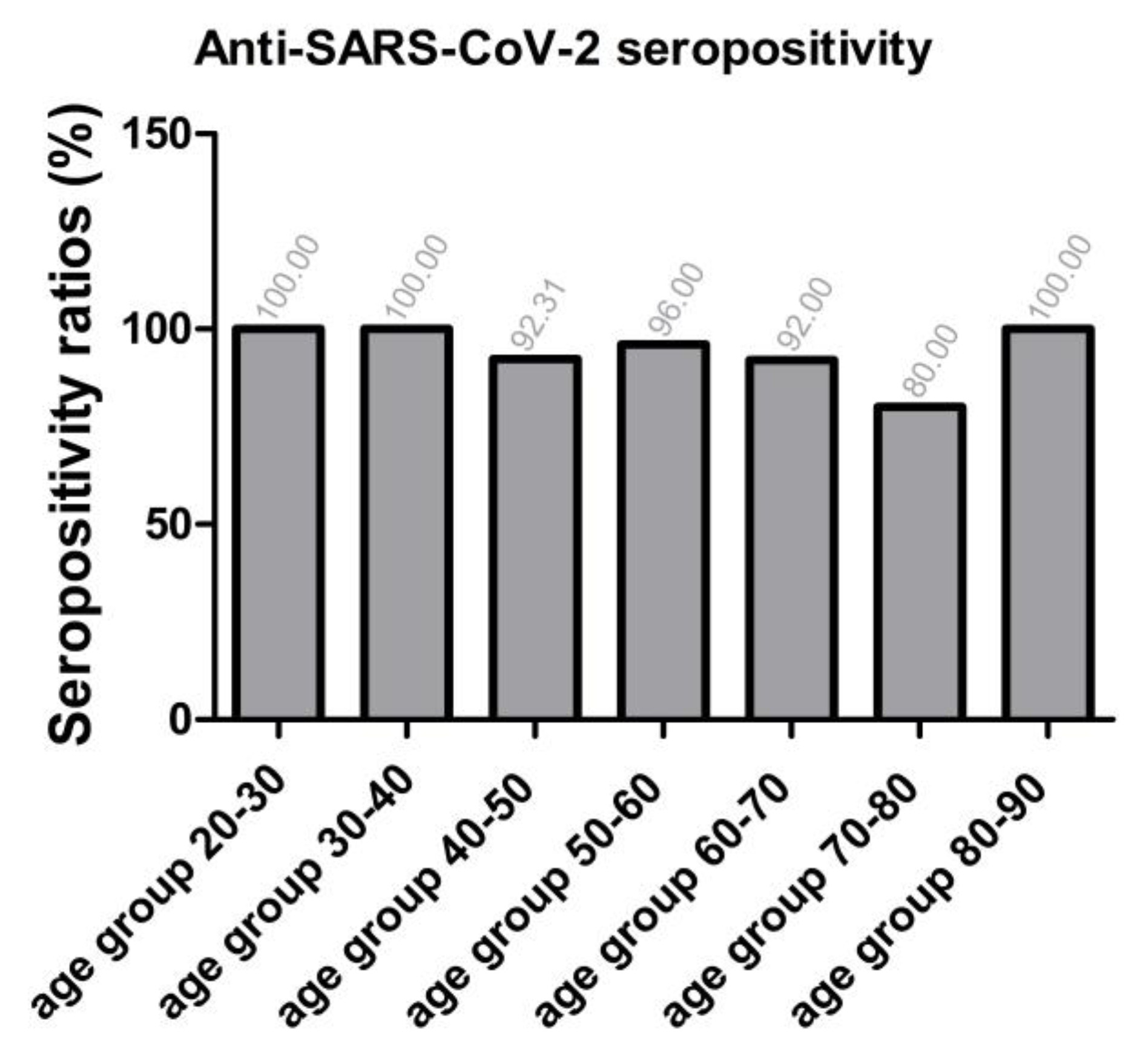
2.3. Relative Differences in Anti-SARS-CoV-2 Specific Seropositivity Ratios by Age Groups
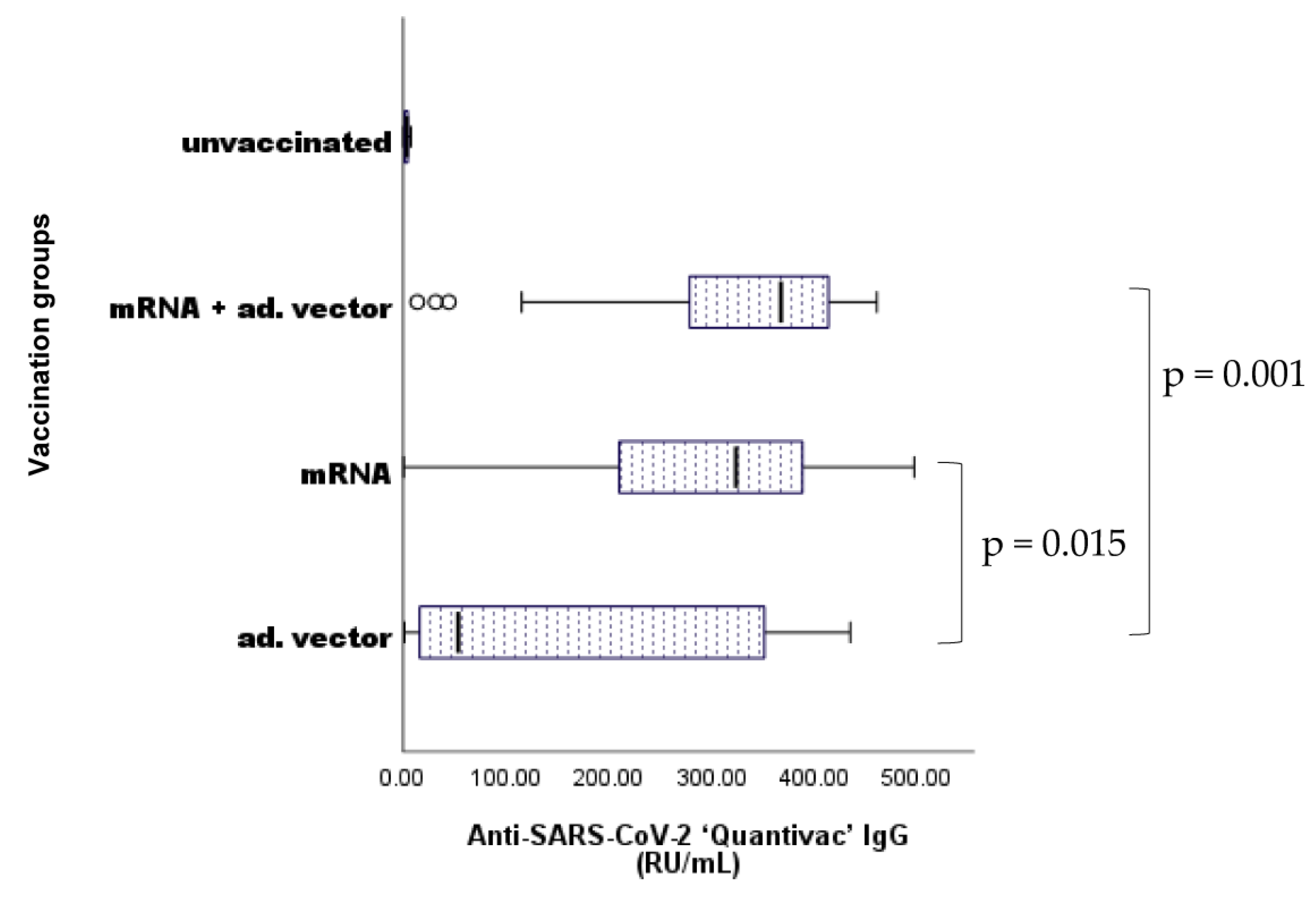
2.4. Differences in Vaccine Response by Anti-SARS-CoV-2 Vaccines
2.5. Differences in nAAb (anti-CS) IgG Levels between Vaccination Groups
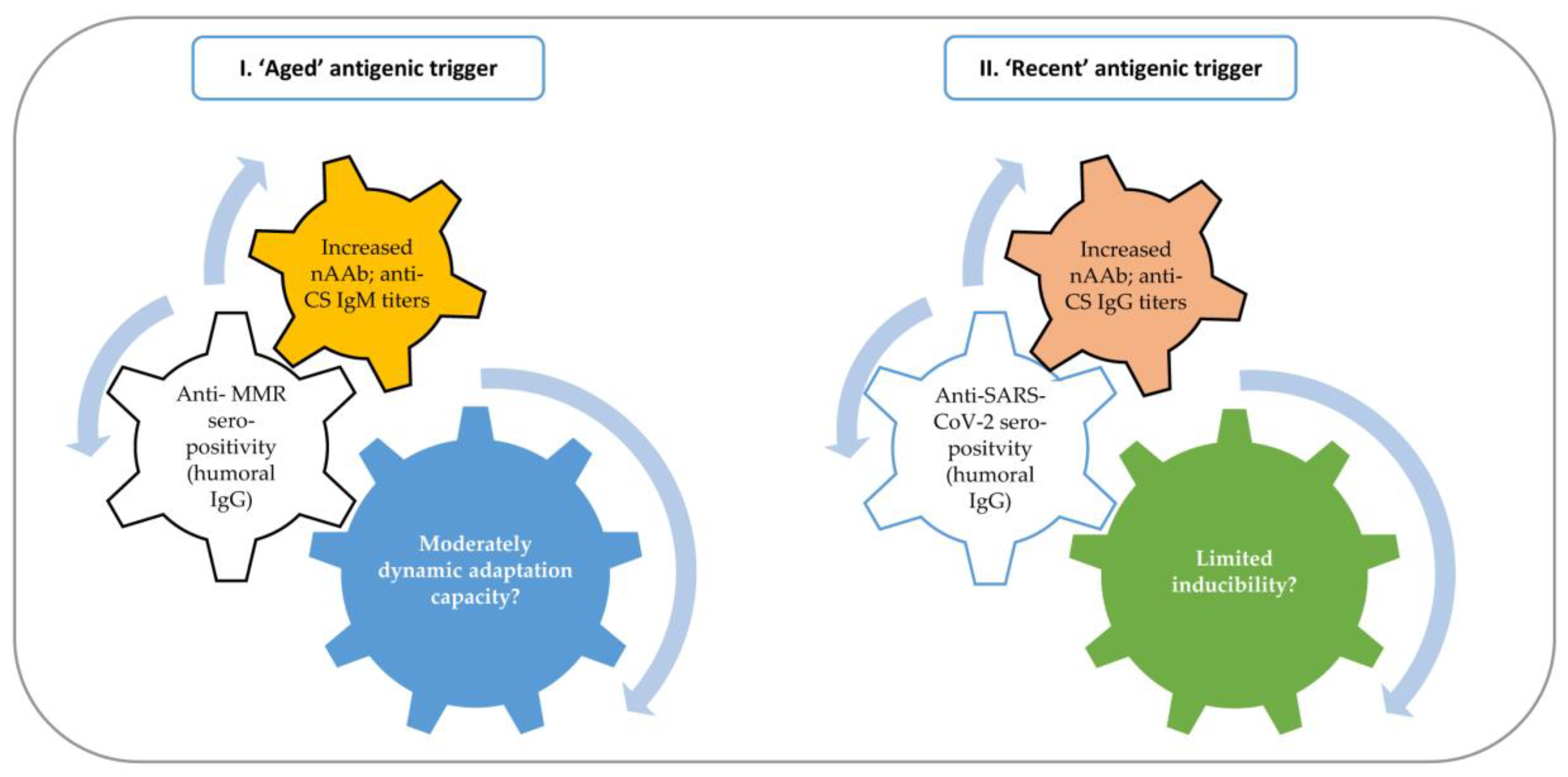
2.6. Global Summary of the Most Important Findings
3. Discussion
4. Materials and Methods
4.1. Human Serum Samples
4.2. Citrate Synthase (CS) IgG and IgM in-House ELISA Assays
4.3. Anti-SARS-CoV-2 Quantivac ELISA (IgG)
4.4. Anti-Measles, Mumps, and Rubella (MMR) IgG in-House ELISA Assays
4.5. Anti-Measles, Mumps, and Rubella Commercial ELISA Assays
4.5.1. Anti-Measles Virus ELISA (IgG) (EI 2610-9601 G)
4.5.2. Anti-Mumps Virus ELISA (IgG) (EI 2630-9601 G)
4.5.3. Anti-Rubella Virus ELISA (IgG) (EI 2590-9601 G)
4.6. Statistical Evaluation
4.7. Experimental Design
5. Conclusions
6. Implications of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAb | Autoantibody |
| AU | arbitrary unit (in-house ELISAs) |
| CS | citrate synthase |
| ELISA | Enzyme-linked immunosorbent assay |
| HIT | Herd immunity threshold |
| IgG | Immunoglobulin, G isotype |
| IgM | Immunoglobulin, M isotype |
| IU | international units |
| MMR | measles, mumps, rubella |
| mRNA | messenger ribonucleic acid |
| n | number of samples |
| nAAb | Natural autoantibody |
| OD | Optical density |
| PVA | polyvinyl alchol |
| RBD | Receptor Binding Domain |
| RT | room temperature |
| RU | relative unit (Euroimmun ELISAs); quantitative measurement entity in linear correlation with the “First WHO International Standard for SARS-CoV-2” |
| S1 | S1 Subunit of the SARS-CoV-2 Spike Protein |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| WB | washing buffer |
| y | years of age |
References
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.M.; Shuai, Z.W.; Ye, D.Q.; Pan, H.F. New-Onset Autoimmune Phenomena Post-COVID-19 Vaccination. Immunology 2022, 165, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Li, X.; Yang, D.; Chan, S.C.W.; Zhou, J.; Wan, E.Y.F.; Chui, C.S.L.; Lai, F.T.T.; Wong, C.K.H.; Chan, E.W.Y.; et al. Risk of Autoimmune Diseases Following COVID-19 and the Potential Protective Effect from Vaccination: A Population-Based Cohort Study. eClinicalMedicine 2023, 63, 102154. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, M.; Pelazza, C.; Bertolotti, M.; Agatea, L.; De Gaspari, P.; Tamiazzo, S.; Ielo, D.; Stobbione, P.; Grappiolo, M.; Bolgeo, T.; et al. The Onset of de Novo Autoantibodies in Healthcare Workers after MRNA Based Anti-SARS-CoV-2 Vaccines: A Single Centre Prospective Follow-up Study. Autoimmunity 2023, 56, 2229072. [Google Scholar] [CrossRef] [PubMed]
- Thurm, C.; Reinhold, A.; Borucki, K.; Kahlfuss, S.; Feist, E.; Schreiber, J.; Reinhold, D.; Schraven, B. Homologous and Heterologous Anti-COVID-19 Vaccination Does Not Induce New-Onset Formation of Autoantibodies Typically Accompanying Lupus Erythematodes, Rheumatoid Arthritis, Celiac Disease and Antiphospholipid Syndrome. Vaccines 2022, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolska, K.; Zarębska-Michaluk, D.; Poniedziałek, B.; Jaroszewicz, J.; Flisiak, R.; Rzymski, P. Overview of Autoantibodies in COVID-19 Convalescents. J. Med. Virol. 2023, 95, e28864. [Google Scholar] [CrossRef] [PubMed]
- Taeschler, P.; Cervia, C.; Zurbuchen, Y.; Hasler, S.; Pou, C.; Tan, Z.; Adamo, S.; Raeber, M.E.; Bächli, E.; Rudiger, A.; et al. Autoantibodies in COVID-19 Correlate with Antiviral Humoral Responses and Distinct Immune Signatures. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 2415–2430. [Google Scholar] [CrossRef] [PubMed]
- Lutz, H.U.; Binder, C.J.; Kaveri, S. Naturally Occurring Auto-Antibodies in Homeostasis and Disease. Trends Immunol. 2009, 30, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Avrameas, S.; Ternynck, T.; Tsonis, I.A.; Lymberi, P. Naturally Occurring B-Cell Autoreactivity: A Critical Overview. J. Autoimmun. 2007, 29, 213–218. [Google Scholar] [CrossRef]
- Reyneveld, G.I.; Savelkoul, H.F.J.; Parmentier, H.K. Current Understanding of Natural Antibodies and Exploring the Possibilities of Modulation Using Veterinary Models. A Review. Front. Immunol. 2020, 11, 2139. [Google Scholar] [CrossRef]
- Grönwall, C.; Silverman, G.J. Natural IgM: Beneficial Autoantibodies for the Control of Inflammatory and Autoimmune Disease. J. Clin. Immunol. 2014, 34, 12–21. [Google Scholar] [CrossRef]
- Lobo, P.I. Role of Natural Autoantibodies and Natural IgM Anti-Leucocyte Autoantibodies in Health and Disease. Front. Immunol. 2016, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Tsujino, K.; Futami, S.; Kida, H. Natural Autoantibodies in Chronic Pulmonary Diseases. Int. J. Mol. Sci. 2020, 21, 1138. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Amelia, A.; Ashdown, G.W.; Mueller, I.; Coussens, A.K.; Eriksson, E.M. Risk Surveillance and Mitigation: Autoantibodies as Triggers and Inhibitors of Severe Reactions to SARS-CoV-2 Infection. Mol. Med. 2021, 27. [Google Scholar] [CrossRef] [PubMed]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against Type I IFNs in Patients with Life-Threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Liu, F.; Zheng, N.S.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse Functional Autoantibodies in Patients with COVID-19. medRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Jeannet, R.; Descazeaud, A.; Daix, T.; Pauthier, H.; Pascal, V.; Hantz, S.; Cam, S.L.; Francois, B.; Feuillard, J.; Lafarge, X. De Novo Natural Anti-M Alloantibody Emergence in Severe Coronavirus Disease 2019. J. Infect. Public Health 2022, 15, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- Holodick, N.E.; Rodríguez-Zhurbenko, N.; Hernández, A.M. Defining Natural Antibodies. Front. Immunol. 2017, 8, 872. [Google Scholar] [CrossRef] [PubMed]
- Baumgarth, N.; Herman, O.C.; Jager, G.C.; Brown, L.E.; Herzenberg, L.A.; Chen, J. B-1 and b-2 Cell-Derived Immunoglobulin m Antibodies Are Nonredundant Components of the Protective Response to Influenza Virus Infection. J. Exp. Med. 2000, 192, 271–280. [Google Scholar] [CrossRef]
- Grönwall, C.; Vas, J.; Silverman, G.J. Protective Roles of Natural IgM Antibodies. Front. Immunol. 2012, 3, 66. [Google Scholar] [CrossRef]
- Cunningham, A.F.; Flores-Langarica, A.; Bobat, S.; Medina, C.C.D.; Cook, C.N.L.; Ross, E.A.; Lopez-Macias, C.; Henderson, I.R. B1b Cells Recognize Protective Antigens after Natural Infection and Vaccination. Front. Immunol. 2014, 5, 535. [Google Scholar] [CrossRef]
- Czömpöly, T.; Olasz, K.; Simon, D.; Nyárády, Z.; Pálinkás, L.; Czirják, L.; Berki, T.; Németh, P. A Possible New Bridge between Innate and Adaptive Immunity: Are the Anti-Mitochondrial Citrate Synthase Autoantibodies Components of the Natural Antibody Network? Mol. Immunol. 2006, 43, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Baumgarth, N. B-1 Cell Heterogeneity and the Regulation of Natural and Antigen-Induced IgM Production. Front. Immunol. 2016, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Zhang, J.; Tan, N.S.; Ho, B.; Ding, J.L. Natural IgG Antibodies Provide Innate Protection against Ficolin-Opsonized Bacteria. EMBO J. 2013, 32, 2905–2919. [Google Scholar] [CrossRef] [PubMed]
- Lacroix-Desmazes, S.; Mouthon, L.; Coutinho, A.; Kazatchkine, M.D. Analysis of the Natural Human IgG Antibody Repertoire: Life-Long Stability of Reactivities towards Self Antigens Contrasts with Age-Dependent Diversification of Reactivities against Bacterial Antigens. Eur. J. Immunol. 1995, 25, 2598–2604. [Google Scholar] [CrossRef] [PubMed]
- Boyden, S.V. Natural Antibodies and the Immune Response. Adv. Immunol. 1966, 5, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Kaveri, S.V.; Silverman, G.J.; Bayry, J. Natural IgM in Immune Equilibrium and Harnessing Their Therapeutic Potential. J. Immunol. 2012, 188, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Merbl, Y.; Zucker-Toledano, M.; Quintana, F.J.; Cohen, I.R. Newborn Humans Manifest Autoantibodies to Defined Self Molecules Detected by Antigen Microarray Informatics. J. Clin. Investig. 2007, 117, 712–718. [Google Scholar] [CrossRef]
- Maddur, M.S.; Lacroix-Desmazes, S.; Dimitrov, J.D.; Kazatchkine, M.D.; Bayry, J.; Kaveri, S.V. Natural Antibodies: From First-Line Defense Against Pathogens to Perpetual Immune Homeostasis. Clin. Rev. Allergy Immunol. 2020, 58, 213–228. [Google Scholar] [CrossRef]
- Amital, H.; Shoenfeld, Y. Natural autoantibodies, heralding, protecting and inducing autoimmunity. In Autoantibodies; Elsevier: Amsterdam, The Netherlands, 2007; pp. 7–12. [Google Scholar] [CrossRef]
- Kearney, J.F.; Patel, P.; Stefanov, E.K.; King, R.G. Natural Antibody Repertoires: Development and Functional Role in Inhibiting Allergic Airway Disease. Annu. Rev. Immunol. 2015, 33, 475–504. [Google Scholar] [CrossRef]
- Avrameas, S.; Alexopoulos, H.; Moutsopoulos, H.M. Natural Autoantibodies: An Undersugn Hero of the Immune System and Autoimmune Disorders-A Point of View. Front. Immunol. 2018, 9, 1320. [Google Scholar] [CrossRef]
- Böröcz, K.; Simon, D.; Erdő-Bonyár, S.; Kovács, K.T.; Tuba, É.; Czirják, L.; Németh, P.; Berki, T. Relationship between Natural and Infection-Induced Antibodies in Systemic Autoimmune Diseases (SAD): SLE, SSc and RA. Clin. Exp. Immunol. 2020, 203, 32–40. [Google Scholar] [CrossRef]
- Böröcz, K.; Kinyó, Á.; Simon, D.; Erdő-Bonyár, S.; Németh, P.; Berki, T. Complexity of the Immune Response Elicited by Different COVID-19 Vaccines, in the Light of Natural Autoantibodies and Immunomodulatory Therapies. Int. J. Mol. Sci. 2023, 24, 6439. [Google Scholar] [CrossRef]
- Simon, D.; Balogh, P.; Erdő-Bonyár, S.; Böröcz, K.; Minier, T.; Czirják, L.; Berki, T. Increased Frequency of Activated Switched Memory B Cells and Its Association With the Presence of Pulmonary Fibrosis in Diffuse Cutaneous Systemic Sclerosis Patients. Front. Immunol. 2021, 12, 686483. [Google Scholar] [CrossRef] [PubMed]
- Hayden, Z.; Erdő-Bonyár, S.; Bóné, B.; Balázs, N.; Bodó, K.; Illes, Z.; Berki, T.; Simon, D. Toll-Like Receptor Homolog CD180 Expression Is Diminished on Natural Autoantibody-Producing B Cells of Patients with Autoimmune CNS Disorders. J. Immunol. Res. 2021, 2021, 9953317. [Google Scholar] [CrossRef] [PubMed]
- Hau, L.; Tényi, T.; László, N.; Kovács, M.Á.; Erdö-Bonyár, S.; Csizmadia, Z.; Berki, T.; Simon, D.; Csábi, G. Anti-Neuronal Autoantibodies (Cell Surface and Onconeural) and Their Association With Natural Autoantibodies in Synthetic Cannabinoid-Induced Psychosis. Front. Psychiatry 2022, 13, 850955. [Google Scholar] [CrossRef]
- Simon, D.; Gilicze, O.; Farkas, N.; Najbauer, J. Correlation of Natural Autoantibodies and Cardiovascular Disease-Related Anti-Bacterial Antibodies in Pericardial Fluid of Cardiac Surgery Patients. Clin. Exp. Immunol. 2018, 193, 55–63. [Google Scholar] [CrossRef]
- Muramatsu, M.; Sankaranand, V.S.; Anant, S.; Sugai, M.; Kinoshita, K.; Davidson, N.O.; Honjo, T. Specific Expression of Activation-Induced Cytidine Deaminase (AID), a Novel Member of the RNA-Editing Deaminase Family in Germinal Center B Cells. J. Biol. Chem. 1999, 274, 18470–18476. [Google Scholar] [CrossRef] [PubMed]
- Irrgang, P.; Gerling, J.; Kocher, K.; Lapuente, D.; Steininger, P.; Habenicht, K.; Wytopil, M.; Beileke, S.; Schäfer, S.; Zhong, J.; et al. Class Switch toward Noninflammatory, Spike-Specific IgG4 Antibodies after Repeated SARS-CoV-2 MRNA Vaccination. Sci. Immunol. 2023, 8, eade279. [Google Scholar] [CrossRef]
- Avrameas, S.; Ternynck, T. The Natural Autoantibodies System: Between Hypotheses and Facts. Mol. Immunol. 1993, 30, 1133–1142. [Google Scholar] [CrossRef]
- Fereidan-Esfahani, M.; Nayfeh, T.; Warrington, A.; Howe, C.L.; Rodriguez, M. IgM Natural Autoantibodies in Physiology and the Treatment of Disease. In Human Monoclonal Antibodies; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019; Volume 1904, pp. 53–81. [Google Scholar] [CrossRef]
- Grönwall, C.; Akhter, E.; Oh, C.; Burlingame, R.W.; Petri, M.; Silverman, G.J. IgM Autoantibodies to Distinct Apoptosis-Associated Antigens Correlate with Protection from Cardiovascular Events and Renal Disease in Patients with SLE. Clin. Immunol. 2012, 142, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Ehrenstein, M.R.; Notley, C.A. The Importance of Natural IgM: Scavenger, Protector and Regulator. Nat. Rev. Immunol. 2010, 10, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Czömpöly, T.; Olasz, K.; Nyárády, Z.; Simon, D.; Bovári, J.; Németh, P. Detailed Analyses of Antibodies Recognizing Mitochondrial Antigens Suggest Similar or Identical Mechanism for Production of Natural Antibodies and Natural Autoantibodies. Autoimmun. Rev. 2008, 7, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Czömpöly, T.; Berki, T.; Minier, T.; Peti, A.; Tóth, E.; Czirják, L.; Németh, P. Naturally Occurring and Disease-Associated Auto-Antibodies against Topoisomerase I: A Fine Epitope Mapping Study in Systemic Sclerosis and Systemic Lupus Erythematosus. Int. Immunol. 2009, 21, 415–422. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nyárády, Z.; Czömpöly, T.; Bosze, S.; Nagy, G.; Petrohai, Á.; Pál, J.; Hudecz, F.; Berki, T.; Németh, P. Validation of in Silico Prediction by in Vitro Immunoserological Results of Fine Epitope Mapping on Citrate Synthase Specific Autoantibodies. Mol. Immunol. 2006, 43, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Nagele, E.P.; Han, M.; Acharya, N.K.; DeMarshall, C.; Kosciuk, M.C.; Nagele, R.G. Natural IgG Autoantibodies Are Abundant and Ubiquitous in Human Sera, and Their Number Is Influenced By Age, Gender, and Disease. PLoS ONE 2013, 8, e60726. [Google Scholar] [CrossRef] [PubMed]
- Devalapalli, A.P.; Lesher, A.; Shieh, K.; Solow, J.S.; Everett, M.L.; Edala, A.S.; Whitt, P.; Long, R.R.; Newton, N.; Parker, W. Increased Levels of IgE and Autoreactive, Polyreactive IgG in Wild Rodents: Implications for the Hygiene Hypothesis. Scand. J. Immunol. 2006, 64, 125–136. [Google Scholar] [CrossRef]
- Beinart, D.; Ren, D.; Pi, C.; Poulton, S.; Holzknecht, Z.E.; Swanson, C.; Parker, W. Immunization Enhances the Natural Antibody Repertoire. EXCLI J. 2017, 16, 1018–1030. [Google Scholar] [CrossRef]
- Pi, C.; Allott, E.H.; Ren, D.; Poulton, S.; Ryan Lee, S.Y.; Perkins, S.; Everett, M.L.; Holzknecht, Z.E.; Lin, S.S.; Parker, W. Increased Biodiversity in the Environment Improves the Humoral Response of Rats. PLoS ONE 2015, 10, e120255. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, C.; Walker, P.G.; Dong, Y. Formulation and Delivery Technologies for MRNA Vaccines. Curr. Top. Microbiol. Immunol. 2022, 440, 71. [Google Scholar] [CrossRef]
- Böröcz, K.; Markovics, Á.; Zsuzsanna, C.; Joseph, N.; Timea, B.; Németh, P. Imported Infections versus Herd Immunity Gaps; a Didactic Demonstration of Compartment Models through the Example of a Minor Measles Outbreak in. Southeast. Eur. Med. J. 2021, 5, 1–16. [Google Scholar]
- Böröcz, K.; Samardžić, S.; Drenjančević, I.; Markovics, Á.; Berki, T.; Németh, P. Dynamic Features of Herd Immunity: Similarities in Age-Specific Anti-Measles Seroprevalence Data between Two Countries of Different Epidemiological History. J. Clin. Med. 2022, 11, 1145. [Google Scholar] [CrossRef] [PubMed]
- Borčić, B.; Mažuran, R.M.; Kaić, B.K. Immunity to Measles in the Croatian Population. Eur. J. Epidemiol. 2003, 18, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Drenjančević, I.; Samardžić, S.; Stupin, A.; Borocz, K.; Nemeth, P.; Berki, T. Measles Vaccination and Outbreaks in Croatia from 2001 to 2019; A Comparative Study to Other European Countries. Int. J. Environ. Res. Public Health 2022, 19, 4140. [Google Scholar] [CrossRef] [PubMed]
- Brzovic, M.; Juretic, K.B.; Jurcev-Savicevic, A.; Mihojevic, L.; Nonkovic, D.; Rizvan, P.; Petrovic, M.V.; Tonkic, M.; Kaic, B.; Babic-Erceg, A.; et al. Measles Cases in Split-Dalmatia County (a Croatian Tourist Region), in May–July 2019: Outbreak Report and Lessons Learnt. Eur. J. Public Health 2022, 32, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Plans-Rubió, P. Low Percentages of Measles Vaccination Coverage with Two Doses of Vaccine and Low Herd Immunity Levels Explain Measles Incidence and Persistence of Measles in the European Union in 2017–2018. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Holt, E. Experts Warn over Potential for Measles in Ukraine. Lancet 2023, 401, 719. [Google Scholar] [CrossRef]
- Plans-Rubió, P. Are the Objectives Proposed by the Who for Routine Measles Vaccination Coverage and Population Measles Immunity Sufficient to Achieve Measles Elimination from Europe? Vaccines 2020, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Angelo, K.M.; Gastañaduy, P.A.; Walker, A.T.; Patel, M.; Reef, S.; Lee, C.V.; Nemhauser, J. Spread of Measles in Europe and Implications for US Travelers. Pediatrics 2019, 144, e20190414. [Google Scholar] [CrossRef]
- Orosz, L.; Gáspár, G.; Rózsa, Á.; Rákos, N.; Sziveri, S.; Bosnyákovits, T. Epidemiological Situation of Measles in Romania, Italy, and Hungary: On What Threats Should We Focus Nowadays? Acta Microbiol. Immunol. Hung. 2018, 65, 127–134. [Google Scholar] [CrossRef]
- Wadman, M. Measles Epidemic in Ukraine Drove Troubling European Year. Science 2019, 363, 677–678. [Google Scholar] [CrossRef] [PubMed]
- Torner, N.; Anton, A.; Barrabeig, I.; Lafuente, S.; Parron, I.; Arias, C.; Camps, N.; Costa, J.; Martínez, A.; Torra, R.; et al. Epidemiology of Two Large Measles Virus Outbreaks in Catalonia: What a Difference the Month of Administration of the First Dose of Vaccine Makes. Hum. Vaccin. Immunother. 2013, 9, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Dascalu, S. Measles Epidemics in Romania: Lessons for Public Health and Future Policy. Front. Public Health 2019, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Habersaat, K.B.; Pistol, A.; Stanescu, A.; Hewitt, C.; Grbic, M.; Butu, C.; Jackson, C. Measles Outbreak in Romania: Understanding Factors Related to Suboptimal Vaccination Uptake. Eur. J. Public Health 2020, 30, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Grenfell, B.T.; Mina, M.J. Waning Immunity and Re-Emergence of Measles and Mumps in the Vaccine Era. Curr. Opin. Virol. 2020, 40, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A.; Gilbert, P.B. Nomenclature for Immune Correlates of Protection after Vaccination. Clin. Infect. Dis. 2012, 54, 1615–1617. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Correlates of Protection Induced by Vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Correlates of Vaccine-Induced Protection: Methods and Implications Immunization, Vaccines and Biologicals; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Plotkin, S.A. Complex Correlates of Protection after Vaccination. Clin. Infect. Dis. 2013, 56, 1458–1465. [Google Scholar] [CrossRef]
- Haralambieva, I.H.; Kennedy, R.B.; Ovsyannikova, I.G.; Schaid, D.J.; Poland, G.A. Current Perspectives in Assessing Humoral Immunity after Measles Vaccination. Expert Rev. Vaccines 2019, 18, 75–87. [Google Scholar] [CrossRef]
- Pandey, A.; Galvani, A.P. Exacerbation of Measles Mortality by Vaccine Hesitancy Worldwide. Lancet Glob. Health 2023, 11, e478–e479. [Google Scholar] [CrossRef]
- Plans-Rubió, P. Why Does Measles Persist in Europe? Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1899–1906. [Google Scholar] [CrossRef]
- Bolotin, S.; Wilson, S.; Murti, M. Achieving and Sustaining Herd Immunity to SARS-CoV-2. Cmaj 2021, 193, E1089. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Smiyan, O.; Popov, S.; Petrashenko, V.; Zaitsev, I.; Redko, O.; Zahorodnii, M.; Kasyan, S. Child Health Care System in Ukraine. Turk. Arch. Pediatr. 2020, 55, S98–S104. [Google Scholar] [CrossRef]
- Suryawanshi, Y.N.; Biswas, D.A. Herd Immunity to Fight against COVID-19: A Narrative Review. Cureus 2023, 15, e33575. [Google Scholar] [CrossRef] [PubMed]
- Soleimanian, S.; Alyasin, S.; Sepahi, N.; Ghahramani, Z.; Kanannejad, Z.; Yaghobi, R.; Karimi, M.H. An Update on Protective Effectiveness of Immune Responses after Recovery From COVID-19. Front. Immunol. 2022, 13, 884879. [Google Scholar] [CrossRef] [PubMed]
- Kwok, K.O.; Lai, F.; Wei, W.I.; Wong, S.Y.S.; Tang, J.W.T. Herd Immunity—Estimating the Level Required to Halt the COVID-19 Epidemics in Affected Countries. J. Infect. 2020, 80, e32–e33. [Google Scholar] [CrossRef] [PubMed]
- Benn, C.S.; Netea, M.G.; Selin, L.K.; Aaby, P. A Small Jab—A Big Effect: Nonspecific Immunomodulation by Vaccines. Trends Immunol. 2013, 34, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Samb, B.; Simondon, F.; Seck, A.M.C.; Knudsen, K.; Whittle, H. Non-Specific Beneficial Effect of Measles Immunisation: Analysis of Mortality Studies from Developing Countries. BMJ 1995, 311, 481–485. [Google Scholar] [CrossRef]
- Aaby, P.; Benn, C.S. Developing the Concept of Beneficial Non-Specific Effect of Live Vaccines with Epidemiological Studies. Clin. Microbiol. Infect. 2019, 25, 1459–1467. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Soares-Weiser, K.; López-López, J.A.; Kakourou, A.; Chaplin, K.; Christensen, H.; Martin, N.K.; Sterne, J.A.C.; Reingold, A.L. Association of BCG, DTP, and Measles Containing Vaccines with Childhood Mortality: Systematic Review. BMJ 2016, 355, 5170. [Google Scholar] [CrossRef]
- Eigentler, A.; Draxl, A.; Wiethüchter, A. Laboratory Protocol: Citrate Synthase a Mitochondrial Marker Enzyme. Mitochondrial Physiol. Netw. 2012, 11, 1–11. [Google Scholar]
- Leek, B.T.; Mudaliar, S.R.D.; Henry, R.; Mathieu-Costello, O.; Richardson, R.S. Effect of Acute Exercise on Citrate Synthase Activity in Untrained and Trained Human Skeletal Muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Petrohai, Á.; Nagy, G.; Bősze, S.; Hudecz, F.; Zsiros, E.; Paragh, G.; Nyárády, Z.; Németh, P.; Berki, T. Detection of Citrate Synthase-Reacting Autoantibodies after Heart Transplantation: An Epitope Mapping Study. Transpl. Int. 2005, 17, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Lu, Y.P.; Yang, L.; Luo, G.H.; Song, J.; Li, Y.P. Detection of Citrate Synthase Autoantibodies in Rats with Chronic Allograft Nephropathy. Transplant. Proc. 2009, 41, 4366–4368. [Google Scholar] [CrossRef] [PubMed]
- EUROIMMUN. Anti-SARS-CoV-2 ELISA IgG/Anti-SARS-CoV-2 QuantiVac ELISA (IgG). Products 2020, 31–32. [Google Scholar]
- Böröcz, K.; Csizmadia, Z.; Markovics; Farkas, N.; Najbauer, J.; Berki, T.; Németh, P. Application of a Fast and Cost-Effective “three-in-One” MMR ELISA as a Tool for Surveying Anti-MMR Humoral Immunity: The Hungarian Experience. Epidemiol. Infect. 2020, 148, e17. [Google Scholar] [CrossRef] [PubMed]
- Böröcz, K.; Csizmadia, Z.; Markovics, Á.; Mészáros, V.; Farkas, K.; Telek, V.; Varga, V.; Maloba, G.O.; Bodó, K.; Najbauer, J.; et al. Development of a Robust and Standardized Immunoserological Assay for Detection of Anti-Measles IgG Antibodies in Human Sera. J. Immunol. Methods 2019, 464, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Qin, C.; Liu, M.; Liu, J. Effectiveness and Safety of SARS-CoV-2 Vaccine in Real-World Studies: A Systematic Review and Meta-Analysis. Infect. Dis. Poverty 2021, 10, 132. [Google Scholar] [CrossRef]



| Age Group | Total Number of Samples/Age Group |
|---|---|
| 20–30 y | 143 |
| 31–40 y | 279 |
| 41–50 y | 359 |
| 51–60 y | 307 |
| 61–70 y | 291 |
| 71–80 y | 253 |
| 81–90 y | 107 |
| TOTAL | 1739 |
| Age Group | Number of Vaccinated Samples | Total Number of Vaccinated Samples (Vaccinated + Unvaccinated) |
|---|---|---|
| 11–20 y | 8 | 8 |
| 21–30 y | 21 | 21 |
| 31–40 y | 30 | 50 |
| 41–50 y | 26 | 47 |
| 51–60 y | 50 | 67 |
| 61–70 y | 50 | 61 |
| 71–80 y | 30 | 49 |
| 81–90 y | 22 | 27 |
| TOTAL | 237 | 330 |
| Vaccination | Number of Samples | Ratio of All Vaccinated Individuals |
|---|---|---|
| mRNA | 170 | 72% |
| mRNA + adenoviral vector | 42 | 18% |
| Adenoviral vector | 25 | 10% |
| Unvaccinated (control) | 93 | - |
| Vaccinated TOTAL | 237 | 100% |
| TOTAL | 330 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szinger, D.; Berki, T.; Németh, P.; Erdo-Bonyar, S.; Simon, D.; Drenjančević, I.; Samardzic, S.; Zelić, M.; Sikora, M.; Požgain, A.; et al. Following Natural Autoantibodies: Further Immunoserological Evidence Regarding Their Silent Plasticity and Engagement in Immune Activation. Int. J. Mol. Sci. 2023, 24, 14961. https://doi.org/10.3390/ijms241914961
Szinger D, Berki T, Németh P, Erdo-Bonyar S, Simon D, Drenjančević I, Samardzic S, Zelić M, Sikora M, Požgain A, et al. Following Natural Autoantibodies: Further Immunoserological Evidence Regarding Their Silent Plasticity and Engagement in Immune Activation. International Journal of Molecular Sciences. 2023; 24(19):14961. https://doi.org/10.3390/ijms241914961
Chicago/Turabian StyleSzinger, David, Timea Berki, Péter Németh, Szabina Erdo-Bonyar, Diana Simon, Ines Drenjančević, Senka Samardzic, Marija Zelić, Magdalena Sikora, Arlen Požgain, and et al. 2023. "Following Natural Autoantibodies: Further Immunoserological Evidence Regarding Their Silent Plasticity and Engagement in Immune Activation" International Journal of Molecular Sciences 24, no. 19: 14961. https://doi.org/10.3390/ijms241914961
APA StyleSzinger, D., Berki, T., Németh, P., Erdo-Bonyar, S., Simon, D., Drenjančević, I., Samardzic, S., Zelić, M., Sikora, M., Požgain, A., & Böröcz, K. (2023). Following Natural Autoantibodies: Further Immunoserological Evidence Regarding Their Silent Plasticity and Engagement in Immune Activation. International Journal of Molecular Sciences, 24(19), 14961. https://doi.org/10.3390/ijms241914961






