Na+/H+ Exchangers (NHEs) in Mammalian Sperm: Essential Contributors to Male Fertility
Abstract
:1. Introduction
2. SLC9C1 (NHE10/sNHE)
2.1. Molecular Genetics and Expression Patterns
2.2. Sperm Physiology and Fertility
2.3. NHE10 Transport Activity
2.4. NHE10 and Human Fertility
3. SLC9C2 (NHE11)
3.1. Molecular Genetics and Expression Patterns
3.2. Sperm Physiology and Fertility
3.3. NHE11 Transport Activity
3.4. NHE11 and Human Fertility
4. SLC9B1 (NHA1/NHEDC1)
4.1. Molecular Genetics and Expression Patterns
4.2. Sperm Physiology and Fertility
4.3. NHA1 Transport Activity
4.4. NHA1 and Human Fertility
5. SLC9B2 (NHA2/NHEDC2)
5.1. Molecular Genetics and Expression Patterns
5.2. Sperm Physiology and Fertility
5.3. NHA2 Transport Activity
5.4. NHA2 and Human Fertility
6. SLC9A1 (NHE1)
6.1. Molecular Genetics and Expression Patterns
6.2. Sperm Physiology and Fertility
6.3. NHE1 Transport Activity
6.4. NHE1 and Human Fertility
7. SLC9A3 (NHE3)
7.1. Molecular Genetics and Expression Patterns
7.2. Sperm Physiology and Fertility
7.3. NHE3 Transport Activity
7.4. NHE3 and Human Fertility
8. SLC9A5 (NHE5)
8.1. Molecular Genetics and Expression Patterns
8.2. Sperm Physiology and Fertility
8.3. NHE5 Transport Activity
8.4. NHE5 and Human Fertility
9. SLC9A8 (NHE8)
9.1. Molecular Genetics and Expression Patterns
9.2. Sperm Physiology and Fertility
9.3. NHE8 Transport Activity
9.4. NHE8 and Human Fertility
10. Discussion
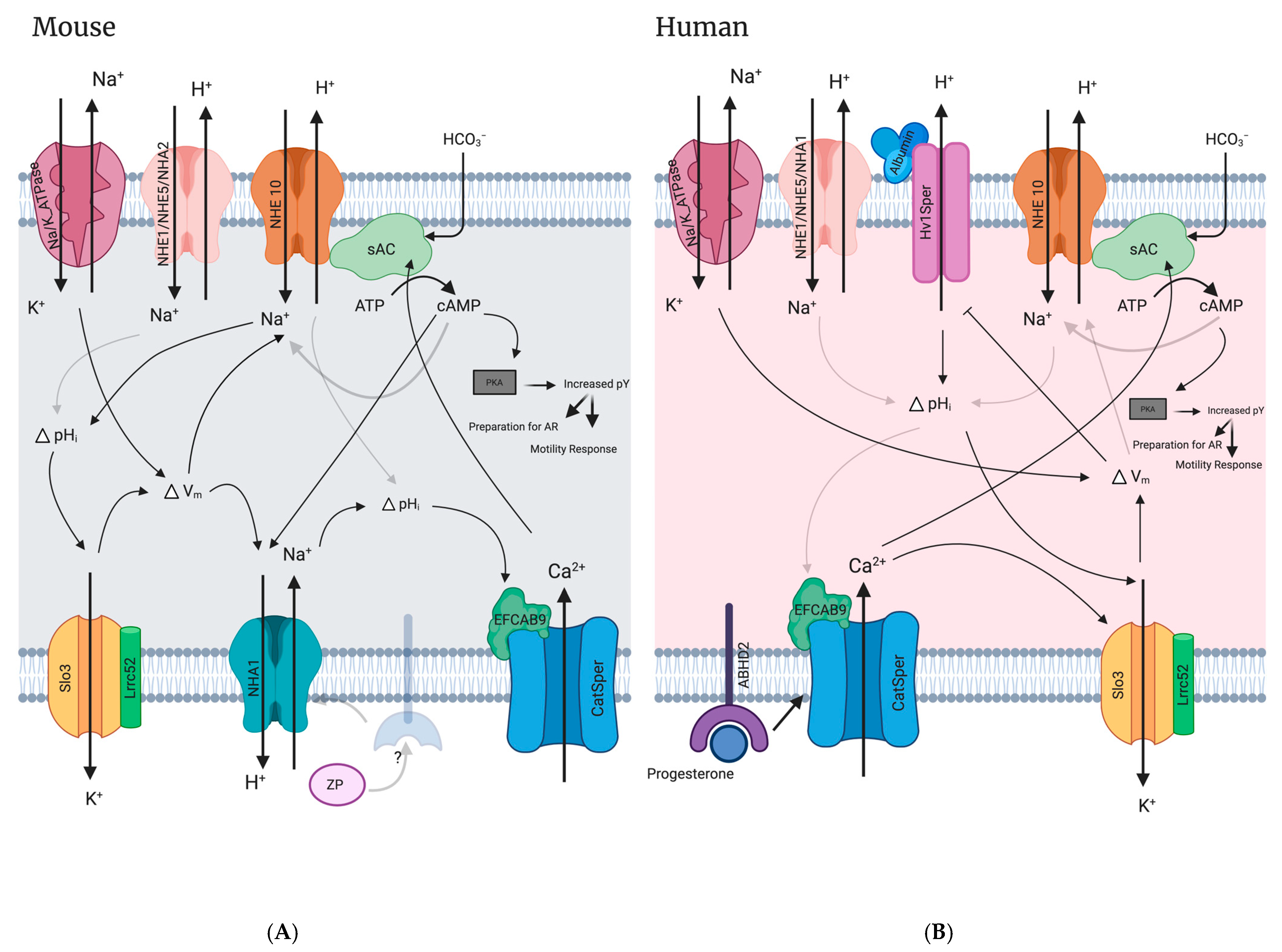
10.1. NHE10/11’s Likely Functional Dependence with SLO1/3-Mediated Hyperpolarization in Mammalian Sperm
10.2. Interaction of NHEs and sAC in Sperm
10.3. Functional Interdependence of CatSper and NHEs
10.4. NHEs versus Hv1 Ion Channel: Determining the Regulator of pHi Human Sperm
10.5. NHEs and the Functional Dependence on Na,K-ATPases
10.6. Importance of the Characterization of NHE Transport Activity in Sperm
10.7. NHEs as an Attractive Target for Male Contraceptive Drugs
10.8. Models for NHE Contribution to Mammalian Sperm Physiology
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orlowski, J.; Grinstein, S. Diversity of the Mammalian Sodium/Proton Exchanger SLC9 Gene Family. Pflug. Arch. Eur. J. Physiol. 2004, 447, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.Y.D.; Lee, W.M.; Tsang, A.Y.F. The Effects of Extracellular Sodium on Acid Release and Motility Initiation in Rat Caudal Epididymal Spermatozoa in Vitro. Exp. Cell Res. 1981, 131, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.H.C.; Tse, C.M.; Nath, S.K.; Levine, S.A.; Brant, S.R.; Donowitz, M. Mammalian Na+/H+ Exchanger Gene Family: Structure and Function Studies. Am. J. Physiol. Gastrointest. Liver Physiol. 1995, 269, G1–G11. [Google Scholar] [CrossRef] [PubMed]
- Counillon, L.; Pouysségur, J. The Expanding Family of Eucaryotic Na+/H+ Exchangers. J. Biol. Chem. 2000, 275, 1–4. [Google Scholar] [CrossRef]
- Donowitz, M.; Ming Tse, C.; Fuster, D. SLC9/NHE Gene Family, a Plasma Membrane and Organellar Family of Na+/H+ Exchangers. Mol. Asp. Med. 2013, 34, 236–251. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Counillon, L. The SLC9A-C Mammalian Na+/H+ Exchanger Family: Molecules, Mechanisms, and Physiology. Physiol. Rev. 2019, 99, 2015–2113. [Google Scholar] [CrossRef]
- Bell, S.M.; Schreiner, C.M.; Schultheis, P.J.; Miller, M.L.; Evans, R.L.; Vorhees, C.V.; Shull, G.E.; Scott, W.J. Targeted Disruption of the Murine Nhe1 Locus Induces Ataxia, Growth Retardation, and Seizures. Am. J. Physiol. Cell Physiol. 1999, 276, C788–C795. [Google Scholar] [CrossRef]
- Wang, D.; King, S.M.; Quill, T.A.; Doolittle, L.K.; Garbers, D.L. A New Sperm-Specific Na+/H+ Exchanger Required for Sperm Motility and Fertility. Nat. Cell Biol. 2003, 5, 1117–1122. [Google Scholar] [CrossRef]
- Oberheide, K.; Puchkov, D.; Jentsch, T.J. Loss of the Na+/H+ Exchanger NHE8 Causes Male Infertility in Mice by Disrupting Acrosome Formation. J. Biol. Chem. 2017, 292, 10845–10854. [Google Scholar] [CrossRef]
- Chen, S.R.; Chen, M.; Deng, S.L.; Hao, X.X.; Wang, X.X.; Liu, Y.X. Sodium–Hydrogen Exchanger NHA1 and NHA2 Control Sperm Motility and Male Fertility. Cell Death Dis. 2016, 7, e2152–e2159. [Google Scholar] [CrossRef]
- Balbach, M.; Hamzeh, H.; Jikeli, J.F.; Brenker, C.; Schiffer, C.; Hansen, J.N.; Neugebauer, P.; Trötschel, C.; Jovine, L.; Han, L.; et al. Molecular Mechanism Underlying the Action of Zona-Pellucida Glycoproteins on Mouse Sperm. Front. Cell Dev. Biol. 2020, 8, 572735. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Wu, H.; Zhang, H.; Zhang, H.; Mao, J.; Liu, D.; Zhao, L.; Lin, H.C.; Tang, W.; et al. Sodium-Hydrogen-Exchanger Expression in Human Sperm and Its Relationship with Semen Parameters. J. Assist. Reprod. Genet. 2017, 34, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Cavarocchi, E.; Whitfield, M.; Chargui, A.; Stouvenel, L.; Lorès, P.; Coutton, C.; Arnoult, C.; Santulli, P.; Patrat, C.; Thierry-Mieg, N.; et al. The Sodium/Proton Exchanger SLC9C1 (SNHE) Is Essential for Human Sperm Motility and Fertility. Clin. Genet. 2021, 99, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.N.; Chen, K.C.; Wu, C.C.; Lin, Y.H.; Chiang, H.S. SLC9A3 Affects Vas Deferens Development and Associates with Taiwanese Congenital Bilateral Absence of the Vas Deferens. BioMed Res. Int. 2019, 2019, 3562719. [Google Scholar] [CrossRef]
- Schultheis, P.J.; Clarke, L.L.; Meneton, P.; Miller, M.L.; Soleimani, M.; Gawenis, L.R.; Riddle, T.M.; Duffy, J.J.; Doetschman, T.; Wang, T.; et al. Renal and Intestinal Absorptive Defects in Mice Lacking the NHE3 Na+/H+ Exchanger. Nat. Genet. 1998, 19, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Tanaka, S.; Teko, Y.; Mitsui, K.; Kanazawa, H. Four Na+/H+ Exchanger Isoforms Are Distributed to Golgi and Post-Golgi Compartments and Are Involved in Organelle PH Regulation. J. Biol. Chem. 2005, 280, 1561–1572. [Google Scholar] [CrossRef]
- Chang, M. Fertilizing Capacity of Spermatozoa Deposited into the Fallopian Tubes. Nature 1951, 168, 697–698. [Google Scholar] [CrossRef]
- Babcock, D.F.; Rufo, G.; Lardy, H.A. Potassium-Dependent Increases in Cytosolic PH Stimulate Metabolism and Motility of Mammalian Sperm. Proc. Natl. Acad. Sci. USA 1983, 80, 1327–1331. [Google Scholar]
- Vijayaraghavan, S.; Critchlow, L.M.; Hoskins, D.D. Evidence for a Role for Cellular Alkalinization in the Cyclic Adenosine 3′,5′-Monophosphate-Mediated Initiation of Motility in Bovine Caput Spermatozoa. Biol. Reprod. 1985, 32, 489–500. [Google Scholar] [CrossRef]
- Carr, D.W.; Acott, T.S. Intracellular PH Regulates Bovine Sperm Motility and Protein Phosphorylation. Biol. Reprod. 1989, 920, 907–920. [Google Scholar] [CrossRef]
- Matamoros-Volante, A.; Trevino, C.L. Capacitation-Associated Alkalization in Human Sperm Is Differentially Controlled at the Subcellular Level. J. Cell Sci. 2020, 133, jcs238816. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Bailey, J.L.; Moore, G.D.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of Mouse Spermatozoa. Development 1995, 121, 1129–1137. [Google Scholar]
- Wertheimer, E.; Krapf, D.; De La Vega-Beltran, J.L.; Sánchez-Cárdenas, C.; Navarrete, F.; Haddad, D.; Escoffier, J.; Salicioni, A.M.; Levin, L.R.; Buck, J.; et al. Compartmentalization of Distinct CAMP Signaling Pathways in Mammalian Sperm. J. Biol. Chem. 2013, 288, 35307–35320. [Google Scholar] [CrossRef]
- Xie, F.; Garcia, M.A.; Carlson, A.E.; Schuh, S.M.; Babcock, D.F.; Jaiswal, B.S.; Gossen, J.A.; Esposito, G.; van Duin, M.; Conti, M. Soluble Adenylyl Cyclase (SAC) Is Indispensable for Sperm Function and Fertilization. Dev. Biol. 2006, 296, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Chávez, J.C.; Ferreira, J.J.; Butler, A.; De La Vega Beltrán, J.L.; Treviño, C.L.; Darszon, A.; Salkoff, L.; Santi, C.M. SLO3 K+ Channels Control Calcium Entry through CATSPER Channels in Sperm. J. Biol. Chem. 2014, 289, 32266–32275. [Google Scholar] [CrossRef] [PubMed]
- Brenker, C.; Zhou, Y.; Müller, A.; Echeverry, F.A.; Trötschel, C.; Poetsch, A.; Xia, X.M.; Bönigk, W.; Lingle, C.J.; Kaupp, U.B.; et al. The Ca2+-Activated K+ Current of Human Sperm Is Mediated by Slo3. eLife 2014, 2014, e01438. [Google Scholar] [CrossRef]
- Mannowetz, N.; Naidoo, N.M.; Choo, S.-A.S.; Smith, J.F.; Lishko, P.V. Slo1 Is the Principal Potassium Channel of Human Spermatozoa. eLife 2013, 2, e01009. [Google Scholar] [CrossRef]
- Molina, L.C.P.; Gunderson, S.; Riley, J.; Lybaert, P.; Borrego-Alvarez, A.; Jungheim, E.S.; Santi, C.M. Membrane Potential Determined by Flow Cytometry Predicts Fertilizing Ability of Human Sperm. Front. Cell Dev. Biol. 2020, 7, 387. [Google Scholar] [CrossRef]
- Baro Graf, C.; Ritagliati, C.; Torres-Monserrat, V.; Stival, C.; Carizza, C.; Buffone, M.G.; Krapf, D. Membrane Potential Assessment by Fluorimetry as a Predictor Tool of Human Sperm Fertilizing Capacity. Front. Cell Dev. Biol. 2020, 7, 383. [Google Scholar] [CrossRef]
- Kirichok, Y.; Navarro, B.; Clapham, D.E. Whole-Cell Patch-Clamp Measurements of Spermatozoa Reveal an Alkaline-Activated Ca2+ Channel. Nature 2006, 439, 737–740. [Google Scholar] [CrossRef]
- Strünker, T.; Goodwin, N.; Brenker, C.; Kashikar, N.D.; Weyand, I.; Seifert, R.; Kaupp, U.B. The CatSper Channel Mediates Progesterone-Induced Ca2+ Influx in Human Sperm. Nature 2011, 471, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Nishigaki, T.; José, O.; González-Cota, A.L.; Romero, F.; Treviño, C.L.; Darszon, A. Intracellular PH in Sperm Physiology. Biochem. Biophys. Res. Commun. 2014, 450, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C. A Membrane Potential-Sensitive Na+-H+ Exchange System in Flagella Isolated from Sea Urchin Spermatozoa. J. Biol. Chem. 1984, 259, 15315–15319. [Google Scholar] [PubMed]
- Hwang, J.Y.; Mannowetz, N.; Zhang, Y.; Everley, R.A.; Gygi, S.P.; Bewersdorf, J.; Lishko, P.V.; Chung, J.J. Dual Sensing of Physiologic PH and Calcium by EFCAB9 Regulates Sperm Motility. Cell 2019, 177, 1480–1494.e19. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Liu, M.; Zhang, W.; Huang, R.Z.; Zhao, N.; Chen, C.; Zeng, X.H. Na+/H+ Exchangers Involve in Regulating the Ph-Sensitive Ion Channels in Mouse Sperm. Int. J. Mol. Sci. 2021, 22, 1612. [Google Scholar] [CrossRef]
- Gunderson, S.J.; Puga Molina, L.C.; Spies, N.; Balestrini, P.A.; Buffone, M.G.; Jungheim, E.S.; Riley, J.; Santi, C.M. Machine-Learning Algorithm Incorporating Capacitated Sperm Intracellular PH Predicts Conventional in Vitro Fertilization Success in Normospermic Patients. Fertil. Steril. 2021, 115, 930–939. [Google Scholar] [CrossRef]
- Keskes, L.; Giroux-Widemann, V.; Serres, C.; Pignot-Paintrand, I.; Jouannet, P.; Feneux, D. The Reactivation of Demembranated Human Spermatozoa Lacking Outer Dynein Arms Is Independent of PH. Mol. Reprod. Dev. 1998, 49, 416–425. [Google Scholar] [CrossRef]
- Hansbrough, J.R.; Garbers, D.L. Sodium-Dependent Activation of Sea Urchin Spermatozoa by Speract and Monensin. J. Biol. Chem. 1981, 256, 2235–2241. [Google Scholar]
- Gatti, J.L.; Christen, R. Regulation of Internal PH of Sea Urchin Sperm. J. Biol. Chem. 1985, 260, 7599–7602. [Google Scholar]
- Garcia, M.A.; Meizel, S. Regulation of Intracellular PH in Capacitated Human Spermatozoa by a Na+/H+ Exchanger. Mol. Reprod. Dev. 1999, 52, 189–195. [Google Scholar] [CrossRef]
- Garcia, M.A.; Meizel, S. Importance of Sodium Ion to the Progesterone-Initiated Acrosome Reaction in Human Sperm. Mol. Reprod. Dev. 1996, 45, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Chiang, H.S.; Cheng, C.Y.; Wu, Y.N.; Lin, Y.C.; Liu, H.C.; Tsai, W.K.; Chen, Y.L.; Lin, Y.H. SLC9A3 Protein Is Critical for Acrosomal Formation in Postmeiotic Male Germ Cells. Int. J. Mol. Sci. 2018, 19, 103. [Google Scholar] [CrossRef]
- Gardner, C.C.; James, P.F. The SLC9C2 Gene Product (Na+/H+ Exchanger Isoform 11; NHE11) Is a Testis-Specific Protein Localized to the Head of Mature Mammalian Sperm. Int. J. Mol. Sci. 2023, 24, 5329. [Google Scholar] [CrossRef]
- Brett, C.L.; Donowitz, M.; Rao, R. Evolutionary Origins of Eukaryotic Sodium/Proton Exchangers. Am. J. Physiol. Cell Physiol. 2005, 288, C223–C239. [Google Scholar] [CrossRef]
- Taglicht, D.; Padan, E.; Schuldiner, S. Proton-Sodium Stoichiometry of NhaA, an Electrogenic Antiporter from Escherichia coli. J. Biol. Chem. 1993, 268, 5382–5387. [Google Scholar]
- Pinner, E.; Padan, E.; Schuldiner, S. Kinetic Properties of NhaB, a Na+/H+ Antiporter from Escherichia coli. J. Biol. Chem. 1994, 269, 26274–26279. [Google Scholar] [PubMed]
- Romero, F.; Nishigaki, T. Comparative Genomic Analysis Suggests That the Sperm-Specific Sodium/Proton Exchanger and Soluble Adenylyl Cyclase Are Key Regulators of CatSper among the Metazoa. Zool. Lett. 2019, 5, 25. [Google Scholar] [CrossRef]
- Masrati, G.; Dwivedi, M.; Rimon, A.; Gluck-Margolin, Y.; Kessel, A.; Ashkenazy, H.; Mayrose, I.; Padan, E.; Ben-Tal, N. Broad Phylogenetic Analysis of Cation/Proton Antiporters Reveals Transport Determinants. Nat. Commun. 2018, 9, 4205. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (ITOL) v4: Recent Updates and New Developments. Nucleic Acids Res. 2019, 47, 256–259. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, Y.; Ilie, A.; Kim, D.S.; Boucher, A.; Li, B.; Zhang, X.C.; Orlowski, J.; Zhao, Y. Structure and Mechanism of the Human NHE1-CHP1 Complex. Nat. Commun. 2021, 12, 3474. [Google Scholar] [CrossRef]
- Woo, A.L.; James, P.F.; Lingrel, J.B. Roles of the Na,K-ATPase A4 Isoform and the Na+/H+ Exchanger in Sperm Motility. Mol. Reprod. Dev. 2002, 62, 348–356. [Google Scholar] [CrossRef]
- Nozawa, K.; Garcia, T.X.; Kent, K.; Leng, M.; Jain, A.; Malovannaya, A.; Yuan, F.; Yu, Z.; Ikawa, M.; Matzuk, M.M. Testis-specific Serine Kinase 3 Is Required for Sperm Morphogenesis and Male Fertility. Andrology 2022, 11, 826–839. [Google Scholar] [CrossRef]
- Izadyar, F.; Wong, J.; Maki, C.; Pacchiarotti, J.; Ramos, T.; Howerton, K.; Yuen, C.; Greilach, S.; Zhao, H.H.; Chow, M.; et al. Identification and Characterization of Repopulating Spermatogonial Stem Cells from the Adult Human Testis. Hum. Reprod. 2011, 26, 1296–1306. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, J.; Haines, C.J.; Feng, H.; Lai, L.; Teng, X.; Han, Y. C-Kit and Its Related Genes in Spermatogonial Differentiation. Spermatogenesis 2011, 1, 186–194. [Google Scholar] [CrossRef]
- Unni, S.K.; Modi, D.N.; Pathak, S.G.; Dhabalia, J.V.; Bhartiya, D. Stage-Specific Localization and Expression of c-Kit in the Adult Human Testis. J. Histochem. Cytochem. 2009, 57, 861–869. [Google Scholar] [CrossRef]
- Helsel, A.R.; Yang, Q.E.; Oatley, M.J.; Lord, T.; Sablitzky, F.; Oatley, J.M. Id4 Levels Dictate the Stem Cell State in Mouse Spermatogonia. Development 2017, 144, 624–634. [Google Scholar] [CrossRef]
- Robertson, M.J.; Kent, K.; Tharp, N.; Nozawa, K.; Dean, L.; Mathew, M.; Grimm, S.L.; Yu, Z.; Légaré, C.; Fujihara, Y.; et al. Large-Scale Discovery of Male Reproductive Tract-Specific Genes through Analysis of RNA-Seq Datasets. BMC Biol. 2020, 18, 103. [Google Scholar] [CrossRef]
- Paris, S.; Pouyssegur, J. Biochemical Characterization of the Amiloride-Sensitive Na+/H+ Antiport in Chinese Hamster Lung Fibroblasts. J. Biol. Chem. 1982, 258, 3503–3508. [Google Scholar]
- Masereel, B.; Pochet, L.; Laeckmann, D. An Overview of Inhibitors of Na+/H+ Exchanger. Eur. J. Med. Chem. 2003, 38, 547–554. [Google Scholar] [CrossRef]
- Bernardazzi, C.; Sheikh, I.A.; Xu, H.; Ghishan, F.K. The Physiological Function and Potential Role of the Ubiquitous Na+/H+ Exchanger Isoform 8 (NHE8): An Overview Data. Int. J. Mol. Sci. 2022, 23, 10857. [Google Scholar] [CrossRef]
- Poet, M.; Doyen, D.; Van Obberghen, E.; Jarretou, G.; Bouret, Y.; Counillon, L. How Does Our Knowledge on the Na+/H+ Exchanger NHE1 Obtained by Biochemical and Molecular Analyses Keep up With Its Recent Structure Determination? Front. Physiol. 2022, 13, 907587. [Google Scholar] [CrossRef] [PubMed]
- Counillon, L.; Scholz, W.; Lang, H.J.; Pouysségur, J. Pharmacological Characterization of Stably Transfected Na/H Antiporter Isoforms Using Amiloride Analogs and a New Inhibitor Properties. Mol. Pharmacol. 1993, 44, 1041–1045. [Google Scholar]
- Nwia, S.M.; Li, X.C.; Leite, A.P.d.O.; Hassan, R.; Zhuo, J.L. The Na+/H+ Exchanger 3 in the Intestines and the Proximal Tubule of the Kidney: Localization, Physiological Function, and Key Roles in Angiotensin II-Induced Hypertension. Front. Physiol. 2022, 13, 861659. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Clarke, L.; Nie, R.; Carnes, K.; Lai, L.W.; Lien, Y.H.H.; Verkman, A.; Lubahn, D.; Fisher, J.S.; Katzenellenbogen, B.S.; et al. Estrogen Action and Male Fertility: Roles of the Sodium/Hydrogen Exchanger-3 and Fluid Reabsorption in Reproductive Tract Function. Proc. Natl. Acad. Sci. USA 2001, 98, 14132–14137. [Google Scholar] [PubMed]
- Wang, Y.Y.; Lin, Y.H.; Wu, Y.N.; Chen, Y.L.; Lin, Y.C.; Cheng, C.Y.; Chiang, H.S. Loss of SLC9A3 Decreases CFTR Protein and Causes Obstructed Azoospermia in Mice. PLoS Genet. 2017, 13, e1006715. [Google Scholar] [CrossRef]
- Orlowski, J. Heterologous Expression and Functional Properties of Amiloride High Affinity (NHE-1) and Low Affinity (NHE-3) Isoforms of the Rat Na/H Exchanger. J. Biol. Chem. 1993, 268, 16369–16377. [Google Scholar] [CrossRef]
- Dong, Y.; Li, H.; Ilie, A.; Gao, Y.; Boucher, A.; Zhang, X.C.; Orlowski, J.; Zhao, Y. Structural Basis of Autoinhibition of the Human NHE3-CHP1 Complex. Sci. Adv. 2022, 8, eabn3925. [Google Scholar] [CrossRef]
- Attaphitaya, S.; Park, K.; Melvin, J.E. Molecular Cloning and Functional Expression of a Rat Na+/H+ Exchanger (NHE5) Highly Expressed in Brain. J. Biol. Chem. 1999, 274, 4383–4388. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Tang, L.; Wang, J.; Shen, C.; Liu, J.; Lu, S.; Zhang, H.; Kuang, Y.; Fei, J.; et al. Nhe5 Deficiency Enhances Learning and Memory via Upregulating Bdnf/TrkB Signaling in Mice. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2017, 174, 828–838. [Google Scholar] [CrossRef]
- Wiebe, S.A.; Plain, A.; Pan, W.; O’neill, D.; Braam, B.; Alexander, R.T. NHE8 Attenuates Ca2+ Influx into NRK Cells and the Proximal Tubule Epithelium. Am. J. Physiol. Ren. Physiol. 2019, 317, F240–F253. [Google Scholar] [CrossRef]
- Xu, H.; Chen, H.; Li, J.; Zhao, Y.; Ghishan, F.K. Disruption of NHE8 Expression Impairs Leydig Cell Function in the Testes. Am. J. Physiol. Cell Physiol. 2015, 308, C330–C338. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Chen, C.; Han, D.; Xiong, X.; Kong, Y.; Wan, B.; Yu, L. Cloning of a Novel Human NHEDC1 (Na+/H+ Exchanger like Domain Containing 1) Gene Expressed Specifically in Testis. Mol. Biol. Rep. 2006, 33, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, M.A.; Gyimesi, G.; Ho, T.M.; Hediger, M.A.; Fuster, D.G. The Less Well-Known Little Brothers: The SLC9B/NHA Sodium Proton Exchanger Subfamily—Structure, Function, Regulation and Potential Drug-Target Approaches. Front. Physiol. 2022, 13, 898508. [Google Scholar] [CrossRef] [PubMed]
- Fuster, D.G.; Zhang, J.; Shi, M.; Alexandru Bobulescu, I.; Andersson, S.; Moe, O.W. Characterization of the Sodium/Hydrogen Exchanger NHA2. J. Am. Soc. Nephrol. 2008, 19, 1547–1556. [Google Scholar] [CrossRef]
- Xiang, M.; Feng, M.; Muend, S.; Rao, R. A Human Na+/H+ Antiporter Sharing Evolutionary Origins with Bacterial NhaA May Be a Candidate Gene for Essential Hypertension. Proc. Natl. Acad. Sci. USA 2007, 104, 18677–18681. [Google Scholar] [CrossRef]
- Kondapalli, K.C.; Kallay, L.M.; Muszelik, M.; Rao, R. Unconventional Chemiosmotic Coupling of NHA2, a Mammalian Na+/H+ Antiporter, to a Plasma Membrane H+ Gradient. J. Biol. Chem. 2012, 287, 36239–36250. [Google Scholar] [CrossRef]
- Matsuoka, R.; Fudim, R.; Jung, S.; Zhang, C.; Bazzone, A.; Chatzikyriakidou, Y.; Robinson, C.V.; Nomura, N.; Iwata, S.; Landreh, M.; et al. Structure, Mechanism and Lipid-Mediated Remodeling of the Mammalian Na+/H+ Exchanger NHA2. Nat. Struct. Mol. Biol. 2022, 29, 108–120. [Google Scholar] [CrossRef]
- Uzdavinys, P.; Coinçon, M.; Nji, E.; Ndi, M.; Winkelmann, I.; Von Ballmoos, C.; Drew, D. Dissecting the Proton Transport Pathway in Electrogenic Na+/H+ Antiporters. Proc. Natl. Acad. Sci. USA 2017, 114, E1101–E1110. [Google Scholar] [CrossRef]
- Landreh, M.; Marklund, E.G.; Uzdavinys, P.; Degiacomi, M.T.; Coincon, M.; Gault, J.; Gupta, K.; Liko, I.; Benesch, J.L.P.; Drew, D.; et al. Integrating Mass Spectrometry with MD Simulations Reveals the Role of Lipids in Na+/H+ Antiporters. Nat. Commun. 2017, 8, 13993. [Google Scholar] [CrossRef]
- Wang, D.; Hu, J.; Bobulescu, I.A.; Quill, T.A.; McLeroy, P.; Moe, O.W.; Garbers, D.L. A Sperm-Specific Na+/H+ Exchanger (SNHE) Is Critical for Expression and in Vivo Bicarbonate Regulation of the Soluble Adenylyl Cyclase (SAC). Proc. Natl. Acad. Sci. USA 2007, 104, 9325–9330. [Google Scholar] [CrossRef]
- Windler, F.; Bönigk, W.; Körschen, H.G.; Grahn, E.; Strünker, T.; Seifert, R.; Kaupp, U.B. The Solute Carrier SLC9C1 Is a Na+/H+-Exchanger Gated by an S4-Type Voltage-Sensor and Cyclic-Nucleotide Binding. Nat. Commun. 2018, 9, 2809. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.C.; Abele, J.A.; Winkler, T.J.; Reckers, C.N.; Anas, S.A.; James, P.F. Common as Well as Unique Methylation-Sensitive DNA Regulatory Elements in Three Mammalian SLC9C1 Genes. bioRxiv 2023. [Google Scholar] [CrossRef]
- Jansen, V.; Alvarez, L.; Balbach, M.; Strünker, T.; Hegemann, P.; Kaupp, U.B.; Wachten, D. Controlling Fertilization and CAMP Signaling in Sperm by Optogenetics. eLife 2015, 4, e05161. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Garduño, S.; Chávez, J.C.; Matamoros-Volante, A.; Sánchez-Guevara, Y.; Torres, P.; Treviño, C.L.; Nishigaki, T. Hyperpolarization Induces Cytosolic Alkalization of Mouse Sperm Flagellum Probably through Sperm Na+/H+ Exchanger. Reproduction 2022, 164, 125–134. [Google Scholar] [CrossRef]
- Lee, H.C.; Garbers, D.L. Modulation of the Voltage-Sensitive Na+/H+ Exchange in Sea Urchin Spermatozoa through Membrane Potential Changes Induced by the Egg Peptide Speract. J. Biol. Chem. 1986, 261, 16026–16032. [Google Scholar] [CrossRef]
- Trötschel, C.; Hamzeh, H.; Alvarez, L.; Pascal, R.; Lavryk, F.; Bönigk, W.; Körschen, H.G.; Müller, A.; Poetsch, A.; Rennhack, A.; et al. Absolute Proteomic Quantification Reveals Design Principles of Sperm Flagellar Chemosensation. EMBO J. 2020, 39, e102723. [Google Scholar] [CrossRef]
- Urizar-Arenaza, I.; Osinalde, N.; Akimov, V.; Puglia, M.; Candenas, L.; Pinto, F.M.; Muñoa-Hoyos, I.; Gianzo, M.; Matorras, R.; Irazusta, J.; et al. Phosphoproteomic and Functional Analyses Reveal Sperm-Specific Protein Changes Downstream of Kappa Opioid Receptor in Human Spermatozoa. Mol. Cell. Proteom. 2019, 18, S118–S131. [Google Scholar] [CrossRef]
- Vandenbrouck, Y.; Lane, L.; Carapito, C.; Duek, P.; Rondel, K.; Bruley, C.; Macron, C.; Gonzalez De Peredo, A.; Couté, Y.; Chaoui, K.; et al. Looking for Missing Proteins in the Proteome of Human Spermatozoa: An Update. J. Proteome Res. 2016, 15, 3998–4019. [Google Scholar] [CrossRef]
- Grahn, E.; Kaufmann, S.V.; Askarova, M.; Ninov, M.; Welp, L.M.; Berger, T.K.; Urlaub, H.; Kaupp, U.B. Control of Intracellular PH and Bicarbonate by CO2 Diffusion into Human Sperm. Nat. Commun. 2023, 14, 5395. [Google Scholar] [CrossRef]
- Holmes, R.S.; Spradling-Reeves, K.D.; Cox, L.A. Evolution of Vertebrate Solute Carrier Family 9B Genes and Proteins (SLC9B): Evidence for a Marsupial Origin for Testis Specific SLC9B1 from an Ancestral Vertebrate SLC9B2 Gene. J. Phylogenet. Evol. Biol. 2016, 4, 167. [Google Scholar] [CrossRef]
- Liu, T.; Huang, J.C.; Zuo, W.L.; Lu, C.L.; Chen, M.; Zhang, X.S.; Li, Y.C.; Cai, H.; Zhou, W.L.; Hu, Z.Y.; et al. A Novel Testis-Specific Na+/H+ Exchanger Is Involved in Sperm Motility and Fertility. Front. Biosci. Elit. 2010, 2, 566–581. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.L.; James, P.F. Identification and Characterization of Methylation-Dependent/ Independent DNA Regulatory Elements in the Human SLC9B1 Gene. Gene 2015, 561, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Huang, J.C.; Lu, C.L.; Yang, J.L.; Hu, Z.Y.; Gao, F.; Liu, Y.X. Immunization with a DNA Vaccine of Testis-Specific Sodium-Hydrogen Exchanger by Oral Feeding or Nasal Instillation Reduces Fertility in Female Mice. Fertil. Steril. 2010, 93, 1556–1566. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Botchkina, I.L.; Kirichok, Y. Progesterone Activates the Principal Ca2+ Channel of Human Sperm. Nature 2011, 471, 387–392. [Google Scholar] [CrossRef]
- Santi, C.M.; Martínez-López, P.; de la Vega-Beltrán, J.L.; Butler, A.; Alisio, A.; Darszon, A.; Salkoff, L. The SLO3 Sperm-Specific Potassium Channel Plays a Vital Role in Male Fertility. FEBS Lett. 2010, 584, 1041–1046. [Google Scholar] [CrossRef]
- Zeng, X.H.; Yang, C.; Xia, X.M.; Liua, M.; Lingle, C.J. SLO3 Auxiliary Subunit LRRC52 Controls Gating of Sperm KSPER Currents and Is Critical for Normal Fertility. Proc. Natl. Acad. Sci. USA 2015, 112, 2599–2604. [Google Scholar] [CrossRef]
- Yang, C.; Zeng, X.H.; Zhou, Y.; Xia, X.M.; Lingle, C.J. LRRC52 (Leucine-Rich-Repeat-Containing Protein 52), a Testis-Specific Auxiliary Subunit of the Alkalization-Activated Slo3 Channel. Proc. Natl. Acad. Sci. USA 2011, 108, 19419–19424. [Google Scholar] [CrossRef]
- Lyon, M.; Li, P.; Ferreira, J.J.; Lazarenko, R.M.; Kharade, S.V.; Kramer, M.; McClenahan, S.J.; Days, E.; Bauer, J.A.; Spitznagel, B.D.; et al. A Selective Inhibitor of the Sperm-Specific Potassium Channel SLO3 Impairs Human Sperm Function. Proc. Natl. Acad. Sci. USA 2023, 120, e2212338120. [Google Scholar] [CrossRef]
- Lv, M.; Liu, C.; Ma, C.; Yu, H.; Shao, Z.; Gao, Y.; Liu, Y.; Wu, H.; Tang, D.; Tan, Q.; et al. Homozygous Mutation in SLO3 Leads to Severe Asthenoteratozoospermia Due to Acrosome Hypoplasia and Mitochondrial Sheath Malformations. Reprod. Biol. Endocrinol. 2022, 20, 5. [Google Scholar] [CrossRef]
- Chintapalli, V.R.; Kato, A.; Henderson, L.; Hirata, T.; Woods, D.J.; Overend, G.; Davies, S.A.; Romero, M.F.; Dow, J.A.T. Transport Proteins NHA1 and NHA2 Are Essential for Survival, but Have Distinct Transport Modalities. Proc. Natl. Acad. Sci. USA 2015, 112, 11720–11725. [Google Scholar] [CrossRef]
- Naseem, M.T.; Beaven, R.; Koyama, T.; Naz, S.; Su, S.; Leader, D.P.; Klaerke, D.A.; Calloe, K.; Denholm, B.; Halberg, K.V. NHA1 Is a Cation/Proton Antiporter Essential for the Water-Conserving Functions of the Rectal Complex in Tribolium castaneum. Proc. Natl. Acad. Sci. USA 2023, 120, 2017. [Google Scholar] [CrossRef]
- Schaschl, H.; Wallner, B. Population-Specific, Recent Positive Directional Selection Suggests Adaptation of Human Male Reproductive Genes to Different Environmental Conditions. BMC Evol. Biol. 2020, 20, 27. [Google Scholar] [CrossRef]
- Deisl, C.; Simonin, A.; Anderegg, M.; Albano, G.; Kovacs, G.; Ackermann, D.; Moch, H.; Dolci, W.; Thorens, B.; Hediger, M.A.; et al. Sodium/Hydrogen Exchanger NHA2 Is Critical for Insulin Secretion in β-Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 10004–10009. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, W.; Siegrist, M.; Simonin, A.; Bonny, O.; Fuster, D.G. Sodium/Hydrogen Exchanger NHA2 in Osteoclasts: Subcellular Localization and Role in Vitro and in Vivo. Bone 2010, 47, 331–340. [Google Scholar] [CrossRef]
- Battaglino, R.A.; Pham, L.; Morse, L.R.; Vokes, M.; Sharma, A.; Odgren, P.R.; Yang, M.; Sasaki, H.; Stashenko, P. NHA-Oc/NHA2: A Mitochondrial Cation-Proton Antiporter Selectively Expressed in Osteoclasts. Bone 2008, 42, 180–192. [Google Scholar] [CrossRef]
- Kondapalli, K.C.; Todd Alexander, R.; Pluznick, J.L.; Rao, R. NHA2 Is Expressed in Distal Nephron and Regulated by Dietary Sodium. J. Physiol. Biochem. 2017, 73, 199–205. [Google Scholar] [CrossRef]
- Anderegg, M.A.; Albano, G.; Hanke, D.; Deisl, C.; Uehlinger, D.E.; Brandt, S.; Bhardwaj, R.; Hediger, M.A.; Fuster, D.G. The Sodium/Proton Exchanger NHA2 Regulates Blood Pressure through a WNK4-NCC Dependent Pathway in the Kidney. Kidney Int. 2021, 99, 350–363. [Google Scholar] [CrossRef]
- Ho, T.M.; Berger, S.; Müller, P.; Simonin, C.; Reymond, J.; Von Ballmoos, C.; Fuster, D.G. Physiological and Molecular Function of the Sodium/Hydrogen Exchanger NHA2 (SLC9B2). Chimia 2022, 76, 1019. [Google Scholar] [CrossRef]
- Huang, X.; Morse, L.R.; Xu, Y.; Zahradka, J.; Sychrová, H.; Stashenko, P.; Fan, F.; Battaglino, R.A. Mutational Analysis of NHAoc/NHA2 in Saccharomyces Cerevisiae. Biochim. Biophys. Acta-Gen. Subj. 2010, 1800, 1241–1247. [Google Scholar] [CrossRef]
- Miller, R.T.; Counillon, L.; Pages, G.; Lifton, R.P.; Sardet, C.; Pouyssegur, J. Structure of the 5′-Flanking Regulatory Region and Gene for the Human Growth Factor-Activatable Na/H Exchanger NHE-1. J. Biol. Chem. 1991, 266, 10813–10819. [Google Scholar] [CrossRef]
- Bullis, B.L.; Li, X.; Rieder, C.V.; Singh, D.N.; Berthiaume, L.G.; Fliegel, L. Properties of the Na+/H+ Exchanger Protein Detergent-Resistant Aggregation and Membrane Microdistribution. Eur. J. Biochem. 2002, 269, 4887–4895. [Google Scholar] [CrossRef] [PubMed]
- Tekpli, X.; Huc, L.; Lacroix, J.; Rissel, M.; Poët, M.; Noël, J.; Dimanche-Boitrel, M.T.; Counillon, L.; Lagadic-Gossmann, D. Regulation of Na+/H+ Exchanger 1 Allosteric Balance by Its Localization in Cholesterol- and Caveolin-Rich Membrane Microdomains. J. Cell. Physiol. 2008, 216, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.; Recuero, S.; Maside, C.; Salas-Huetos, A.; Bonet, S.; Pinart, E. Blocking Nhe Channels Reduces the Ability of in Vitro Capacitated Mammalian Sperm to Respond to Progesterone Stimulus. Int. J. Mol. Sci. 2021, 22, 12646. [Google Scholar] [CrossRef]
- Muzzachi, S.; Guerra, L.; Martino, N.A.; Favia, M.; Punzi, G.; Silvestre, F.; Guaricci, A.C.; Roscino, M.T.; Pierri, C.L.; Elena, M.; et al. Effect of Cariporide on Ram Sperm PH Regulation and Motility: Possible Role of NHE1. Reproduction 2018, 155, 433–445. [Google Scholar]
- Fafournoux, P.; Noël, J.; Pouysségur, J. Evidence That Na+/H+ Exchanger Isoforms NHE1 and NHE3 Exist as Stable Dimers in Membranes with a High Degree of Specificity for Homodimers. J. Biol. Chem. 1994, 269, 2589–2596. [Google Scholar]
- Hisamitsu, T.; Ben Ammar, Y.; Nakamura, T.Y.; Wakabayashi, S. Dimerization Is Crucial for the Function of the Na+/H+ Exchanger NHE1. Biochemistry 2006, 45, 13346–13355. [Google Scholar] [CrossRef]
- Wakabayashi, S.; Fafournoux, P.; Sardet, C.; Pouysségur, J. The Na+/H+ Antiporter Cytoplasmic Domain Mediates Growth Factor Signals and Controls “H+-Sensing”. Proc. Natl. Acad. Sci. USA 1992, 89, 2424–2428. [Google Scholar] [CrossRef]
- Hisamitsu, T.; Pang, T.; Shigekawa, M.; Wakabayashi, S. Dimeric Interaction between the Cytoplasmic Domains of the Na+/H+ Exchanger NHE1 Revealed by Symmetrical Intermolecular Cross-Linking and Selective Co-Immunoprecipitation. Biochemistry 2004, 43, 11135–11143. [Google Scholar] [CrossRef]
- Fuster, D.; Moe, O.W.; Hilgemann, D.W. Steady-State Function of the Ubiquitous Mammalian Na/H Exchanger (NHE1) in Relation to Dimer Coupling Models with 2Na/2H Stoichiometry. J. Gen. Physiol. 2008, 132, 465–480. [Google Scholar] [CrossRef]
- Grinstein, S.; Goetz, J.D.; Rothstein, A. 22Na+ Fluxes in Thymic Lymphocytes: II. Amiloride-Sensitive Na+/H+ Exchange Pathway; Reversibility of Transport and Asymmetry of the Modifier Site. J. Gen. Physiol. 1984, 84, 585–600. [Google Scholar] [CrossRef]
- Sjøgaard-Frich, L.M.; Prestel, A.; Pedersen, E.S.; Severin, M.; Kristensen, K.K.; Olsen, J.G.; Kragelund, B.B.; Pedersen, S.F.; Kay, L.E. Dynamic Na+/H+ Exchanger 1 (NHE1)-Calmodulin Complexes of Varying Stoichiometry and Structure Regulate Ca2+-Dependent NHE1 Activation. eLife 2021, 10, e60889. [Google Scholar] [CrossRef] [PubMed]
- Snabaitis, A.K.; Cuello, F.; Avkiran, M. Protein Kinase B/Akt Phosphorylates and Inhibits the Cardiac Na+/H+ Exchanger NHE1. Circ. Res. 2008, 103, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Meima, M.E.; Webb, B.A.; Witkowska, H.E.; Barber, D.L. The Sodium-Hydrogen Exchanger NHE1 Is an Akt Substrate Necessary for Actin Filament Reorganization by Growth Factors. J. Biol. Chem. 2009, 284, 26666–26675. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, S.; Ikeda, T.; Iwamoto, T.; Pouysségur, J.; Shigekawa, M. Calmodulin-Binding Autoinhibitory Domain Controls “PH-Sensing” in the Na+/H+ Exchanger NHE1 through Sequence-Specific Interaction. Biochemistry 1997, 36, 12854–12861. [Google Scholar] [CrossRef] [PubMed]
- Kleyman, T.R.; Cragoe, E.J. Amiloride and Its Analogs as Tools in the Study of Ion Transport. J. Membr. Biol. 1988, 105, 1–21. [Google Scholar] [CrossRef]
- Mihaila, R.G. A Minireview on NHE1 Inhibitors. A Rediscovered Hope in Oncohematology. Biomed. Pap. 2015, 159, 519–526. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Y.; Zhou, T.; Shi, X.; Yu, J.; Yang, Y.; Wu, Y.; Wang, J.; Liu, M.; Chen, X.; et al. In-Depth Proteomic Analysis of the Human Sperm Reveals Complex Protein Compositions. J. Proteom. 2013, 79, 114–122. [Google Scholar] [CrossRef]
- Guissart, C.; Li, X.; Leheup, B.; Drouot, N.; Montaut-Verient, B.; Raffo, E.; Jonveaux, P.; Roux, A.F.; Claustres, M.; Fliegel, L.; et al. Mutation of SLC9A1, Encoding the Major Na+/H+ Exchanger, Causes Ataxia-Deafness Lichtenstein-Knorr Syndrome. Hum. Mol. Genet. 2015, 24, 463–470. [Google Scholar] [CrossRef]
- Iwama, K.; Osaka, H.; Ikeda, T.; Mitsuhashi, S.; Miyatake, S.; Takata, A.; Miyake, N.; Ito, S.; Mizuguchi, T.; Matsumoto, N. A Novel SLC9A1 Mutation Causes Cerebellar Ataxia. J. Hum. Genet. 2018, 63, 1049–1054. [Google Scholar] [CrossRef]
- Fliegel, L. Role of Genetic Mutations of the Na+/H+ Exchanger Isoform 1, in Human Disease and Protein Targeting and Activity. Mol. Cell. Biochem. 2020, 476, 1221–1232. [Google Scholar] [CrossRef]
- Bagnis, C.; Marsolais, M.; Biemesderfer, D.; Laprade, R.; Breton, S. Na+/H+-exchange activity and immunolocalization of NHE3 in rat epididymis. Renal Physiology. 2001, 280, F426–F436. [Google Scholar] [CrossRef] [PubMed]
- Hihnala, S.; Kujala, M.; Toppari, J.; Kere, J.; Holmberg, C.; Höglund, P. Expression of SLC26A3, CFTR and NHE3 in the Human Male Reproductive Tract: Role in Male Subfertility Caused by Congenital Chloride Diarrhoea. Mol. Hum. Reprod. 2006, 12, 107–111. [Google Scholar] [CrossRef]
- Ahn, W.; Kim, K.H.; Lee, J.A.; Kim, J.Y.; Choi, J.Y.; Moe, O.W.; Milgram, S.L.; Muallem, S.; Lee, M.G. Regulatory Interaction between the Cystic Fibrosis Transmembrane Conductance Regulator and HCO3-Salvage Mechanisms in Model Systems and the Mouse Pancreatic Duct. J. Biol. Chem. 2001, 276, 17236–17243. [Google Scholar] [CrossRef] [PubMed]
- Bagorda, A.; Guerra, L.; Di Sole, F.; Hemle-Kolb, C.; Cardone, R.A.; Fanelli, T.; Reshkin, S.J.; Gisler, S.M.; Murer, H.; Casavola, V. Reciprocal Protein Kinase A Regulatory Interactions between Cystic Fibrosis Transmembrane Conductance Regulator and Na+/H+ Exchanger Isoform 3 in a Renal Polarized Epithelial Cell Model. J. Biol. Chem. 2002, 277, 21480–21488. [Google Scholar] [CrossRef] [PubMed]
- Dorin, J.R.; Dickinson, P.; Alton, E.W.F.W.; Smith, S.N.; Geddes, D.M.; Stevenson, B.J.; Kimber, W.L.; Fleming, S.; Clarke, A.R.; Hooper, M.L.; et al. Cystic Fibrosis in the Mouse by Targeted Insertional Mutagenesis. Nature 1992, 359, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Reynaert, I.; Van Der Schueren, B.; Degeest, G.; Manin, M.; Cuppens, H.; Scholte, B.; Cassiman, J.-J. Morphological Changes in the Vas Deferens and Expression of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) in Control, F508 and Knock-out CFTR Mice during Postnatal Life. Mol. Reprod. Dev. 2000, 55, 125–135. [Google Scholar] [CrossRef]
- Kurashima, K.; Yu, F.H.; Cabado, A.G.; Szabó, E.Z.; Grinstein, S.; Orlowski, J. Identification of Sites Required for Down-Regulation of Na+/H+ Exchanger NHE3 Activity by CAMP-Dependent Protein Kinase: Phosphorylation-Dependent and -Independent Mechanisms. J. Biol. Chem. 1997, 272, 28672–28679. [Google Scholar] [CrossRef]
- Moe, O.W.; Amemiya, M.; Yamaji, Y. Activation of Protein Kinase A Acutely Inhibits and Phosphorylates Na/H Exchanger NHE-3. J. Clin. Investig. 1995, 96, 2187–2194. [Google Scholar] [CrossRef]
- Yun, C.H.C.; Lamprecht, G.; Forster, D.V.; Sidor, A. NHE3 Kinase A Regulatory Protein E3KARP Binds the Epithelial Brush Border Na+/H+ Exchanger NHE3 and the Cytoskeletal Protein Ezrin. J. Biol. Chem. 1998, 273, 25856–25863. [Google Scholar] [CrossRef]
- Donowitz, M.; Li, X. Regulatory Binding Partners and Complexes of NHE3. Physiol. Rev. 2007, 87, 825–872. [Google Scholar] [CrossRef]
- Szászi, K.; Paulsen, A.; Szabó, E.Z.; Numata, M.; Grinstein, S.; Orlowski, J. Clathrin-Mediated Endocytosis and Recycling of the Neuron-Specific Na+/H+ Exchanger NHE5 Isoform: Regulation by Phosphatidylinositol 3′-Kinase and the Actin Cytoskeleton. J. Biol. Chem. 2002, 277, 42623–42632. [Google Scholar] [CrossRef] [PubMed]
- Baird, N.R.; Orlowski, J.; Szabó, E.Z.; Zaun, H.C.; Schultheis, P.J.; Menon, A.G.; Shull, G.E. Molecular Cloning, Genomic Organization, and Functional Expression of Na+/H+ Exchanger Isoform 5 (NHE5) from Human Brain. J. Biol. Chem. 1999, 274, 4377–4382. [Google Scholar] [CrossRef] [PubMed]
- Attaphitaya, S.; Nehrke, K.; Melvin, J.E. Acute Inhibition of Brain-Specific Na+/H+ Exchanger Isoform 5 by Protein Kinases A and C and Cell Shrinkage. Am. J. Physiol. Cell Physiol. 2001, 281, 1146–1157. [Google Scholar] [CrossRef] [PubMed]
- Jinadasa, T.; Szabó, E.Z.; Numata, M.; Orlowski, J. Activation of AMP-Activated Protein Kinase Regulates Hippocampal Neuronal PH by Recruiting Na+/H+ Exchanger NHE5 to the Cell Surface. J. Biol. Chem. 2014, 289, 20879–20897. [Google Scholar] [CrossRef]
- Martin-Hidalgo, D.; de Llera, A.H.; Calle-Guisado, V.; Gonzalez-Fernandez, L.; Garcia-Marin, L.; Bragado, M.J. AMPK Function in Mammalian Spermatozoa. Int. J. Mol. Sci. 2018, 19, 3293. [Google Scholar] [CrossRef]
- Gu, B.; Zhang, J.; Wu, Y.; Zhang, X.; Tan, Z.; Lin, Y.; Huang, X.; Chen, L.; Yao, K.; Zhang, M. Proteomic Analyses Reveal Common Promiscuous Patterns of Cell Surface Proteins on Human Embryonic Stem Cells and Sperms. PLoS ONE 2011, 6, e19386. [Google Scholar] [CrossRef]
- Lawrence, S.P.; Bright, N.A.; Luzio, J.P.; Bowers, K. The Sodium/Proton Exchanger NHE8 Regulates Late Endosomal Morphology and Function. Mol. Biol. Cell 2010, 21, 3540–3551. [Google Scholar] [CrossRef]
- Joseph, C.; Twombley, K.; Gattineni, J.; Zhang, Q.; Dwarakanath, V.; Baum, M. Acid Increases NHE8 Surface Expression and Activity in NRK Cells. Am. J. Physiol. Ren. Physiol. 2012, 302, 495–503. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, B.; Li, J.; Wang, C.; Chen, H.; Ghishan, F.K. Impaired Mucin Synthesis and Bicarbonate Secretion in the Colon of NHE8 Knockout Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, 335–343. [Google Scholar] [CrossRef]
- Zhou, K.; Amiri, M.; Salari, A.; Yu, Y.; Xu, H.; Seidler, U.; Nikolovska, K. Functional Characterization of the Sodium/Hydrogen Exchanger 8 and Its Role in Proliferation of Colonic Epithelial Cells. Am. J. Physiol. Physiol. 2021, 321, C471–C488. [Google Scholar] [CrossRef]
- Berger, T.K.; Fußhöller, D.M.; Goodwin, N.; Bönigk, W.; Müller, A.; Dokani Khesroshahi, N.; Brenker, C.; Wachten, D.; Krause, E.; Kaupp, U.B.; et al. Post-Translational Cleavage of Hv1 in Human Sperm Tunes PH- and Voltage-Dependent Gating. J. Physiol. 2017, 595, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- López-González, I.; Torres-Rodríguez, P.; Sánchez-Carranza, O.; Solís-López, A.; Santi, C.M.; Darszon, A.; Treviño, C.L. Membrane Hyperpolarization during Human Sperm Capacitation. Mol. Hum. Reprod. 2014, 20, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.G.; Publicover, S.J.; Mansell, S.A.; Lishko, P.V.; Williams, H.L.; Ramalingam, M.; Wilson, S.M.; Barratt, C.L.R.; Sutton, K.A.; Da Silva, S.M. Depolarization of Sperm Membrane Potential Is a Common Feature of Men with Subfertility and Is Associated with Low Fertilization Rate at IVF. Hum. Reprod. 2016, 31, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Calzada, L.; Tellez, J. Defective Function of Membrane Potential (Ψ) on Sperm of Infertile Men. Arch. Androl. 1997, 38, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Chávez, J.C.; de la Vega-Beltrán, J.L.; Escoffier, J.; Visconti, P.E.; Treviño, C.L.; Darszon, A.; Salkoff, L.; Santi, C.M. Ion Permeabilities in Mouse Sperm Reveal an External Trigger for SLO3-Dependent Hyperpolarization. PLoS ONE 2013, 8, e60578. [Google Scholar] [CrossRef]
- Geng, Y.; Ferreira, J.J.; Dzikunu, V.; Butler, A.; Lybaert, P.; Yuan, P.; Magleby, K.L.; Salkoff, L.; Santi, C.M. A Genetic Variant of the Sperm-Specific SLO3 K+ Channel Has Altered PH and Ca2+ Sensitivities. J. Biol. Chem. 2017, 292, 8978–8987. [Google Scholar] [CrossRef]
- Sánchez-Carranza, O.; Torres-Rodríguez, P.; Darszon, A.; Treviño, C.L.; López-González, I. Pharmacology of HSlo3 Channels and Their Contribution in the Capacitation-Associated Hyperpolarization of Human Sperm. Biochem. Biophys. Res. Commun. 2015, 466, 554–559. [Google Scholar] [CrossRef]
- Nomura, M.; Vacquier, V.D. Proteins Associated with Soluble Adenylyl Cyclase in Sea Urchin Sperm Flagella. Cell Motil. Cytoskelet. 2006, 63, 582–590. [Google Scholar] [CrossRef]
- Miller, M.R.; Mannowetz, N.; Iavarone, A.T.; Safavi, R.; Gracheva, E.O.; Smith, J.F.; Hill, R.Z.; Bautista, D.M.; Kirichok, Y.; Lishko, P.V. Unconventional Endocannabinoid Signaling Governs Sperm Activation via the Sex Hormone Progesterone. Science 2016, 352, 555–559. [Google Scholar] [CrossRef]
- Qi, H.; Moran, M.M.; Navarro, B.; Chong, J.A.; Krapivinsky, G.; Krapivinsky, L.; Kirichok, Y.; Ramsey, I.S.; Quill, T.A.; Clapham, D.E. All Four CatSper Ion Channel Proteins Are Required for Male Fertility and Sperm Cell Hyperactivated Motility. Proc. Natl. Acad. Sci. USA 2007, 104, 1219–1223. [Google Scholar] [CrossRef]
- Lishko, P.V.; Botchkina, I.L.; Fedorenko, A.; Kirichok, Y. Acid Extrusion from Human Spermatozoa Is Mediated by Flagellar Voltage-Gated Proton Channel. Cell 2010, 140, 327–337. [Google Scholar] [CrossRef]
- Miller, M.R.; Kenny, S.J.; Mannowetz, N.; Mansell, S.A.; Wojcik, M.; Mendoza, S.; Zucker, R.S.; Xu, K.; Lishko, P.V. Asymmetrically Positioned Flagellar Control Units Regulate Human Sperm Rotation. Cell Rep. 2018, 24, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Geragotelis, A.D.; Wood, M.L.; Göddeke, H.; Hong, L.; Webster, P.D.; Wong, E.K.; Freites, J.A.; Tombola, F.; Tobias, D.J. Voltage-Dependent Structural Models of the Human Hv1 Proton Channel from Long-Timescale Molecular Dynamics Simulations. Proc. Natl. Acad. Sci. USA 2020, 117, 13490–13498. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Mansell, S.A.; Meyers, S.A.; Lishko, P.V. Flagellar Ion Channels of Sperm: Similarities and Differences between Species. Cell Calcium 2015, 58, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, I.S.; Ruchti, E.; Kaczmarek, J.S.; Clapham, D.E. Hv1 Proton Channels Are Required for High-Level NADPH Oxidase-Dependent Superoxide Production during the Phagocyte Respiratory Burst. Proc. Natl. Acad. Sci. USA 2009, 106, 7642–7647. [Google Scholar] [CrossRef]
- Zhao, R.; Dai, H.; Arias, R.J.; De Blas, G.A.; Orta, G.; Pavarotti, M.A.; Shen, R.; Perozo, E.; Mayorga, L.S.; Darszon, A.; et al. Direct Activation of the Proton Channel by Albumin Leads to Human Sperm Capacitation and Sustained Release of Inflammatory Mediators by Neutrophils. Nat. Commun. 2021, 12, 3855. [Google Scholar] [CrossRef]
- Zhao, R.; Kennedy, K.; De Blas, G.A.; Orta, G.; Pavarotti, M.A.; Arias, R.J.; de la Vega-Beltrán, J.L.; Li, Q.; Dai, H.; Perozo, E.; et al. Role of Human Hv1 Channels in Sperm Capacitation and White Blood Cell Respiratory Burst Established by a Designed Peptide Inhibitor. Proc. Natl. Acad. Sci. USA 2018, 115, E11847–E11856. [Google Scholar] [CrossRef]
- Florman, H.M.; Ducibella, T. Chapter 2—Fertilization in Mammals. In Knobil and Neill’s Physiology of Reproduction, 3rd ed.; Neill, J.D., Ed.; Academic Press: St. Louis, MO, USA, 2006; pp. 55–112. ISBN 978-0-12515-400-0. [Google Scholar]
- Wagoner, K.; Sanchez, G.; Nguyen, A.N.; Enders, G.C.; Blanco, G. Different Expression and Activity of the A1 and A4 Isoforms of the Na,K-ATPase during Rat Male Germ Cell Ontogeny. Reproduction 2005, 130, 627–641. [Google Scholar] [CrossRef]
- Sanchez, G.; Nguyen, A.N.T.; Timmerberg, B.; Tash, J.S.; Blanco, G. The Na,K-ATPase A4 Isoform from Humans Has Distinct Enzymatic Properties and Is Important for Sperm Motility. Mol. Hum. Reprod. 2006, 12, 565–576. [Google Scholar] [CrossRef]
- Woo, A.L.; James, P.F.; Lingrel, J.B. Sperm Motility Is Dependent on a Unique Isoform of the Na,K-ATPase. J. Biol. Chem. 2000, 275, 20693–20699. [Google Scholar] [CrossRef]
- Hlivko, J.T.; Chakraborty, S.; Hlivko, T.J.; Sengupta, A.; James, P.F. The Human Na,K-ATPase Alpha4 Isoform Is a Ouabain-Sensitive Alpha Isoform That Is Expressed in Sperm. Mol. Reprod. Dev. 2006, 73, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Lestari, S.W.; Miati, D.N.; Seoharso, P.; Sugiyanto, R.; Pujianto, D.A. Sperm Na+, K+-ATPase A4 and Plasma Membrane Ca2+-ATPase (PMCA) 4 Regulation in Asthenozoospermia. Syst. Biol. Reprod. Med. 2017, 63, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, T.; McDermott, J.P.; Sánchez, G.; Blanco, G. Na, K-ATPase A4 Isoform Is Essential for Sperm Fertility. Proc. Natl. Acad. Sci. USA 2011, 108, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, T.; Sánchez, G.; Blanco, G. Activity of the Na,K-ATPase A4 Isoform Is Regulated during Sperm Capacitation to Support Sperm Motility. J. Androl. 2012, 33, 1047–1057. [Google Scholar] [CrossRef]
- Arcos-Hernández, C.; Suárez-Delgado, E.; Islas, L.D.; Romero, F.; López-González, I.; Ai, H.W.; Nishigaki, T. How to Study a Highly Toxic Protein to Bacteria: A Case of Voltage Sensor Domain of Mouse Sperm-Specific Sodium/Proton Exchanger. Protein Expr. Purif. 2023, 201, 106172. [Google Scholar]



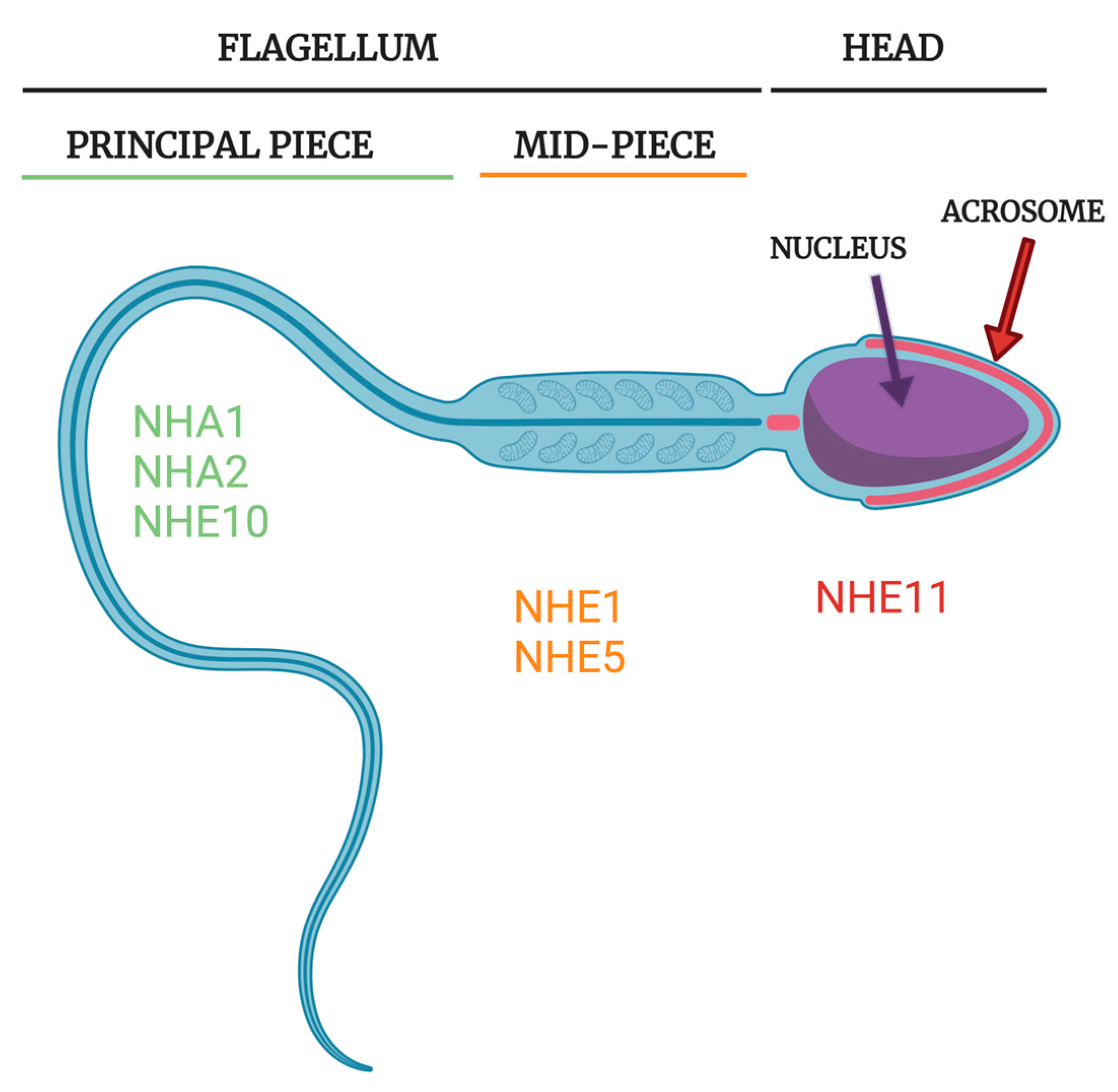
| Gene (Protein) | Tissue Expression | Subcellular Localization | Sperm Localization | Transport Activity | Mouse Male Fertility KO Phenotype | Human Male Fertility Phenotype | Known Inhibitors | References |
|---|---|---|---|---|---|---|---|---|
| SLC9A1 (NHE1) | Near ubiquitous | Plasma membrane | Midpiece | Na+/H+ 1:1 (electroneutral) | Fertile | Unknown | Amiloride, EIPA, HMA, DMA, HOE-694, SM-20550, S-3226, clonidine, cimetidine | [7,50,51,58,59,60,61] |
| SLC9A3 (NHE3) | Highly expressed in digestive tract and kidney; low expression in testis and other | Plasma membrane and endosomal (recycling) | Developing acrosome | Na+/H+ 1:1 (electroneutral) | Sperm lack acrosomes; Vas deferens defects; Infertile | Congenital bilateral absence of the vas deferens (CBAVD); Infertile | Amiloride, tenapanor, HMA, DMA, HOE-694, zoniporide, S-3226, clonidine, cimetidine | [6,15,42,59,62,63,64,65,66,67] |
| SLC9A5 (NHE5) | Brain, kidney, testis, other | Plasma membrane and endosomal (recycling) | Midpiece | Na+/H+ 1:1 (electroneutral) | Fertile | Unknown | Amiloride, EIPA, HMA, HOE-694, cimetidine | [51,59,68,69] |
| SLC9A8 (NHE8) | Ubiquitous | Intracellular organelles | Developing acrosome | Na+/H+ (electroneutral) | Sperm lack acrosomes; Infertile | Unknown | HOE-642, S-3226 | [9,16,60,70,71] |
| SLC9B1 (NHA1) | Testis/Sperm specific | Plasma membrane | Principal piece | (Likely based off of structural homology models) Na+/H+ (electroneutral) | Submotile sperm, subfertile; NHA1/2 dKO completely infertile | Unknown | Unknown | [10,11,72,73] |
| SLC9B2 (NHA2) | Near ubiquitous | Plasma membrane; Intracellular (cell type specific) | Principal piece | Na+(Li+)/H+ (electroneutral) | Submotile sperm, subfertile; NHA1/2 dKO completely infertile | Unknown | Phloretin | [10,74,75,76,77,78,79] |
| SLC9C1 (NHE10) | Testis/Sperm specific | Plasma membrane | Principal piece; Flagellum | Unknown * Na+/H+ Exchanger 1:1 (electroneutral); Voltage sensitive; Cyclic nucleotide gated | Immotile sperm; Infertile | Immotile sperm; Sperm structural defects; Infertile | Unknown | [8,13,80,81] |
| SLC9C2 (NHE11) | Testis/Sperm specific | Likely plasma membrane | Sperm head (likely plasma membrane overlaying acrosome) | Unknown * Na+/H+ Exchanger 1:1 (electroneutral); Voltage sensitive; Cyclic nucleotide gated | Unknown | Unknown | Unknown | [6,43,81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gardner, C.C.; James, P.F. Na+/H+ Exchangers (NHEs) in Mammalian Sperm: Essential Contributors to Male Fertility. Int. J. Mol. Sci. 2023, 24, 14981. https://doi.org/10.3390/ijms241914981
Gardner CC, James PF. Na+/H+ Exchangers (NHEs) in Mammalian Sperm: Essential Contributors to Male Fertility. International Journal of Molecular Sciences. 2023; 24(19):14981. https://doi.org/10.3390/ijms241914981
Chicago/Turabian StyleGardner, Cameron C., and Paul F. James. 2023. "Na+/H+ Exchangers (NHEs) in Mammalian Sperm: Essential Contributors to Male Fertility" International Journal of Molecular Sciences 24, no. 19: 14981. https://doi.org/10.3390/ijms241914981





