A Metabolite Perspective on the Involvement of the Gut Microbiota in Type 2 Diabetes
Abstract
:1. Introduction
| Metabolite | Microbial Agent | Effect | References |
|---|---|---|---|
| Propionate | Lactobacillus | Promotes GLP-1 secretion, improves blood glucose, lipid levels, and intestinal barrier function and increases beneficial bacteria. | [19] |
| Butyrate | Anaerostipes hadrus | Reduces glycated haemoglobin levels, improves mood, sleep, and blood sugar levels and increases probiotic abundance. | [20] |
| Isovaleric, lactic acids | Prevotella copri | Improves insulin secretion and promotes glucose homeostasis. | [21] |
| BA | Bacteroides fragilis | Affects blood sugar levels | [22] |
| MAM (microbial anti-inflammatory molecule) | Faecalibacterium prausnitzii | Regulation of tight junction protein expression, restoration of intestinal barrier function and resistance to inflammation. | [23] |
| Acetate | Akkermansia | Inhibits intestinal inflammation and promotes intestinal epithelial integrity. | [24] |
| SCFAs and branched-chain fatty acids | Acidaminococcus spp., Clostridia spp. | Promotes healthy metabolism and amino acid fermentation. | [25,26] |
| Tryptamine | Ruminococcus gnavus | Increases bile acid levels and secretion of tryptamine. | [27,28] |
| Histamine | Morganella morganii | Decarboxylation of histidine (histidine decarboxylase (HDC)) | [29,30] |
| Imidazole propionate (ImP) | Adlercreutziae equolifaciens Anaerococcus prevotii | Impairment of glucose tolerance and insulin signalling. | [31] |
| Dopamine | Enterococcus faecalis | Regulates glucose uptake, insulin sensitivity and lipid metabolism. | [32,33] |
2. Method
3. Gut Microbiota in Type 2 Diabetics
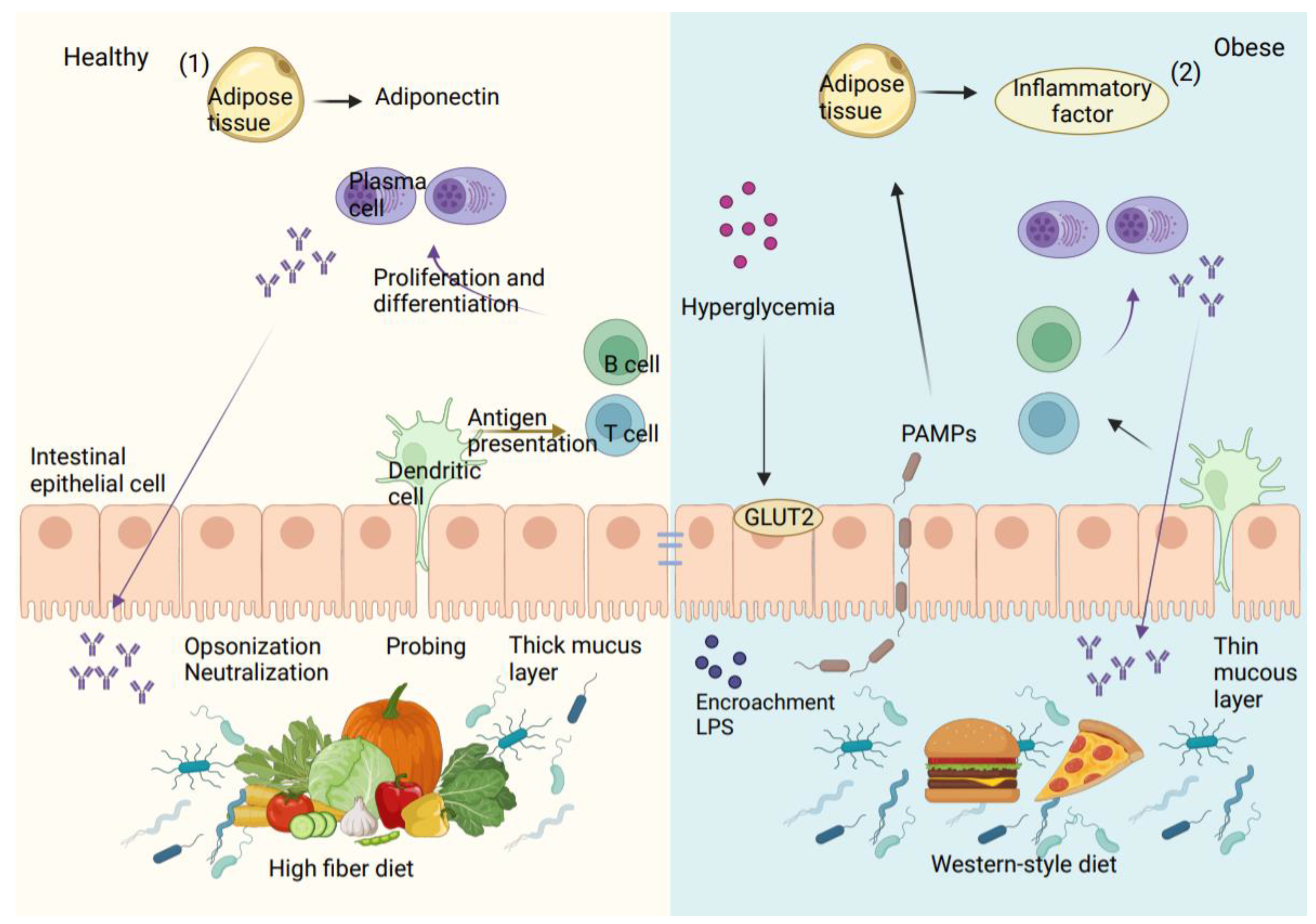
4. Epithelial Barrier Dysfunction in Diabetic Patients
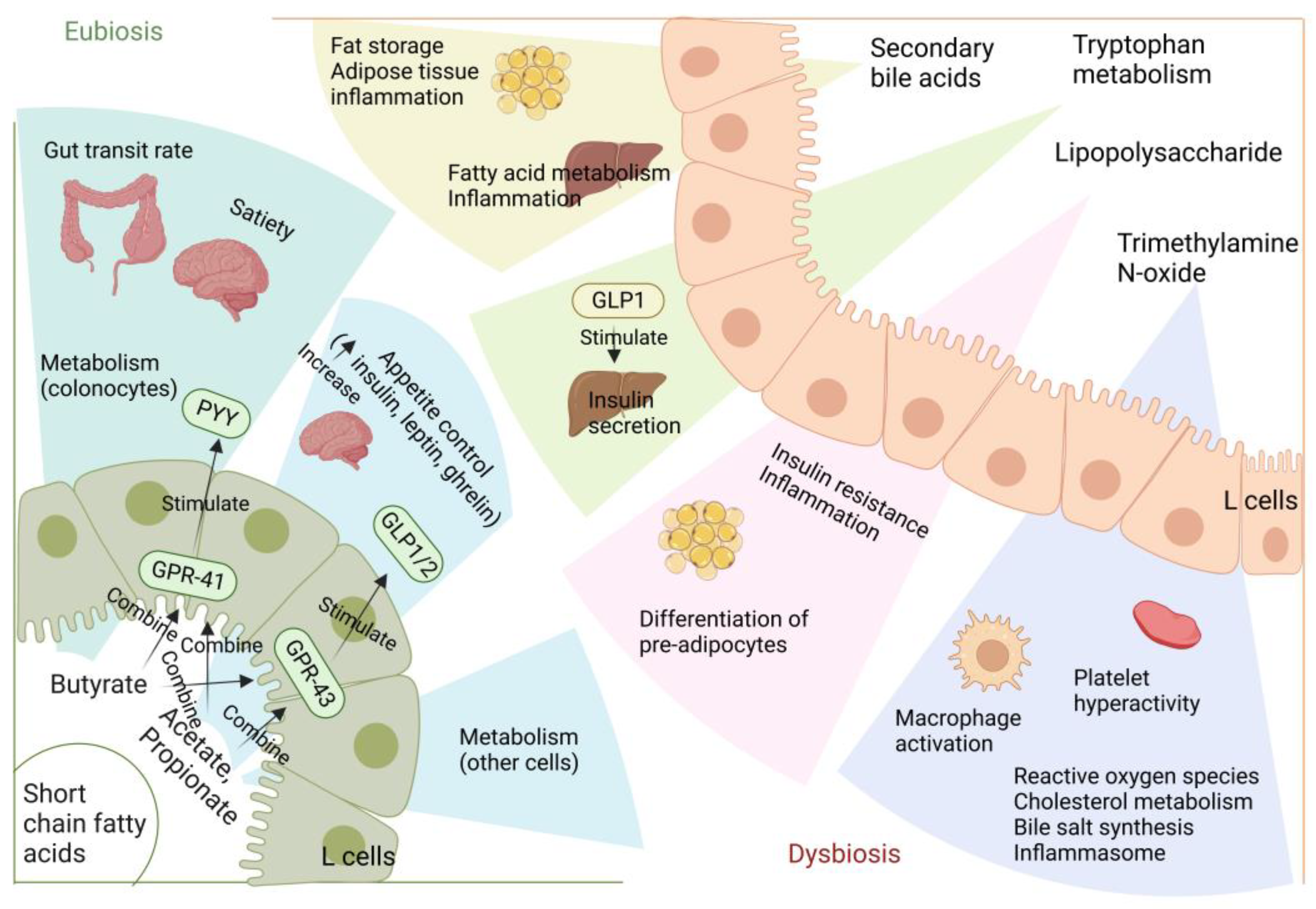
5. Effect of Gut Microbial Metabolites on Type 2 Diabetes
5.1. Short-Chain Fatty Acids
5.2. Lipopolysaccharide
5.3. Bile Acids
5.4. Branched-Chain Amino Acid (BCAAs)
5.5. Trimethylamine N-Oxide (TMAO)
6. The Influence of Gut Microbes on the Progression of Diabetes
6.1. Animal Studies
6.2. Human Studies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Li, W.Z.; Stirling, K.; Yang, J.J.; Zhang, L. Gut microbiota and diabetes: From correlation to causality and mechanism. World J. Diabetes 2020, 11, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.N.; Kim, M.; Bennett, B.J. Modulating the Microbiota as a Therapeutic Intervention for Type 2 Diabetes. Front. Endocrinol. 2021, 12, 632335. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Wu, L.; Guo, J.; Yu, X.; Yao, H.; Han, R.; Ma, T.; Zheng, Y.; Gao, Q.; et al. Effects of Exercise Intervention on Type 2 Diabetes Patients With Abdominal Obesity and Low Thigh Circumference (EXTEND): Study Protocol for a Randomized Controlled Trial. Front. Endocrinol. 2022, 13, 937264. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Zhai, L.; Wu, J.; Lam, Y.Y.; Kwan, H.Y.; Bian, Z.X.; Wong, H.L.X. Gut-Microbial Metabolites, Probiotics and Their Roles in Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 12846. [Google Scholar] [CrossRef]
- Yehualashet, A.S.; Yikna, B.B. Microbial Ecosystem in Diabetes Mellitus: Consideration of the Gastrointestinal System. Diabetes Metab. Syndr. Obes.-Targets Ther. 2021, 14, 1841–1854. [Google Scholar] [CrossRef] [PubMed]
- Croci, S.; D’Apolito, L.I.; Gasperi, V.; Catani, M.V.; Savini, I. Dietary Strategies for Management of Metabolic Syndrome: Role of Gut Microbiota Metabolites. Nutrients 2021, 13, 1389. [Google Scholar] [CrossRef]
- White, P.J.; McGarrah, R.W.; Herman, M.A.; Bain, J.R.; Shah, S.H.; Newgard, C.B. Insulin action, type 2 diabetes, and branched-chain amino acids: A two-way street. Mol. Metab. 2021, 52, 101261. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, R.; Zheng, W.; Guo, H.; Yang, Y.; Zhao, R.; Yao, W. High ambient temperature exposure during late gestation disrupts glycolipid metabolism and hepatic mitochondrial function tightly related to gut microbial dysbiosis in pregnant mice. Microb. Biotechnol. 2021, 14, 2116–2129. [Google Scholar] [CrossRef] [PubMed]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.R.; Lee, H.Y. Mechanisms linking gut microbial metabolites to insulin resistance. World J. Diabetes 2021, 12, 730–744. [Google Scholar] [CrossRef]
- Alsharairi, N.A. Exploring the Diet-Gut Microbiota-Epigenetics Crosstalk Relevant to Neonatal Diabetes. Genes 2023, 14, 1017. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Qin, S.; Yang, H. Composition of Maternal Circulating Short-Chain Fatty Acids in Gestational Diabetes Mellitus and Their Associations with Placental Metabolism. Nutrients 2022, 14, 3727. [Google Scholar] [CrossRef]
- Harbison, J.E.; Thomson, R.L.; Wentworth, J.M.; Louise, J.; Roth-Schulze, A.; Battersby, R.J.; Ngui, K.M.; Penno, M.A.S.; Colman, P.G.; Craig, M.E.; et al. Associations between diet, the gut microbiome and short chain fatty acids in youth with islet autoimmunity and type 1 diabetes. Pediatr. Diabetes 2021, 22, 425–433. [Google Scholar] [CrossRef]
- Hu, J.; Ding, J.; Li, X.; Li, J.; Zheng, T.; Xie, L.; Li, C.; Tang, Y.; Guo, K.; Huang, J.; et al. Distinct signatures of gut microbiota and metabolites in different types of diabetes: A population-based cross-sectional study. EClinicalMedicine 2023, 62, 102132. [Google Scholar] [CrossRef]
- Wang, Y.; Dilidaxi, D.; Wu, Y.; Sailike, J.; Sun, X.; Nabi, X.H. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 125, 109914. [Google Scholar] [CrossRef]
- Frias, J.P.; Lee, M.L.; Carter, M.M.; Ebel, E.R.; Lai, R.H.; Rikse, L.; Washington, M.E.; Sonnenburg, J.L.; Damman, C.J. A microbiome-targeting fibre-enriched nutritional formula is well tolerated and improves quality of life and haemoglobin A1c in type 2 diabetes: A double-blind, randomized, placebo-controlled trial. Diabetes Obes. Metab. 2023, 25, 1203–1212. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, L.; Sun, L.; Ye, X.; Ma, M.; Dou, M.; Shi, L. Association Between Intestinal Prevotella copri Abundance and Glycemic Fluctuation in Patients with Brittle Diabetes. Diabetes Metab. Syndr. Obes. Targets Ther. 2023, 16, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Mastropaolo, M.D.; Evans, N.P.; Byrnes, M.K.; Stevens, A.M.; Robertson, J.L.; Melville, S.B. Synergy in polymicrobial infections in a mouse model of type 2 diabetes. Infect. Immun. 2005, 73, 6055–6063. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liang, R.; Zhang, W.; Tian, K.; Li, J.; Chen, X.; Yu, T.; Chen, Q. Faecalibacterium prausnitzii-derived microbial anti-inflammatory molecule regulates intestinal integrity in diabetes mellitus mice via modulating tight junction protein expression. J. Diabetes 2020, 12, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, J.H.; Yu, T.; Chen, Q.K. Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 118, 109131. [Google Scholar] [CrossRef]
- Deehan, E.C.; Yang, C.; Perez-Muñoz, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.; Bakal, J.A.; Walter, J. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe 2020, 27, 389–404.e386. [Google Scholar] [CrossRef]
- Smith, E.A.; Macfarlane, G.T. Dissimilatory amino Acid metabolism in human colonic bacteria. Anaerobe 1997, 3, 327–337. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, X.; Ding, D.; Lu, Y. Characteristics of the intestinal flora in patients with peripheral neuropathy associated with type 2 diabetes. J. Int. Med. Res. 2020, 48, 300060520936806. [Google Scholar] [CrossRef]
- Williams, B.B.; Van Benschoten, A.H.; Cimermancic, P.; Donia, M.S.; Zimmermann, M.; Taketani, M.; Ishihara, A.; Kashyap, P.C.; Fraser, J.S.; Fischbach, M.A. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe 2014, 16, 495–503. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Barcik, W.; Wawrzyniak, M.; Akdis, C.A.; O’Mahony, L. Immune regulation by histamine and histamine-secreting bacteria. Curr. Opin. Immunol. 2017, 48, 108–113. [Google Scholar] [CrossRef]
- Koh, A.; Molinaro, A.; Ståhlman, M.; Khan, M.T.; Schmidt, C.; Mannerås-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E.; et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell 2018, 175, 947–961.e917. [Google Scholar] [CrossRef] [PubMed]
- Tavares, G.; Marques, D.; Barra, C.; Rosendo-Silva, D.; Costa, A.; Rodrigues, T.; Gasparini, P.; Melo, B.F.; Sacramento, J.F.; Seiça, R.; et al. Dopamine D2 receptor agonist, bromocriptine, remodels adipose tissue dopaminergic signalling and upregulates catabolic pathways, improving metabolic profile in type 2 diabetes. Mol. Metab. 2021, 51, 101241. [Google Scholar] [CrossRef] [PubMed]
- Maini Rekdal, V.; Bess, E.N.; Bisanz, J.E.; Turnbaugh, P.J.; Balskus, E.P. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science 2019, 364, eaau6323. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Sobarzo-Sanchez, E.; Bungau, S. Unravelling the involvement of gut microbiota in type 2 diabetes mellitus. Life Sci. 2021, 273, 119311. [Google Scholar]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Luca, M.; Di Mauro, M.; Di Mauro, M.; Luca, A. Gut Microbiota in Alzheimer’s Disease, Depression, and Type 2 Diabetes Mellitus: The Role of Oxidative Stress. Oxid. Med. Cell Longev. 2019, 2019, 4730539. [Google Scholar] [CrossRef]
- Caesar, R. Pharmacologic and Nonpharmacologic Therapies for the Gut Microbiota in Type 2 Diabetes. Can. J. Diabetes 2019, 43, 224–231. [Google Scholar] [CrossRef]
- Deng, J.; Zeng, L.; Lai, X.; Li, J.; Liu, L.; Lin, Q.; Chen, Y. Metformin protects against intestinal barrier dysfunction via AMPKα1-dependent inhibition of JNK signalling activation. J. Cell Mol. Med. 2018, 22, 546–557. [Google Scholar] [CrossRef]
- Sato, J.; Kanazawa, A.; Ikeda, F.; Yoshihara, T.; Goto, H.; Abe, H.; Komiya, K.; Kawaguchi, M.; Shimizu, T.; Ogihara, T.; et al. Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care 2014, 37, 2343–2350. [Google Scholar] [CrossRef]
- Yang, G.; Wei, J.; Liu, P.; Zhang, Q.; Tian, Y.; Hou, G.; Meng, L.; Xin, Y.; Jiang, X. Role of the gut microbiota in type 2 diabetes and related diseases. Metab. Clin. Exp. 2021, 117, 154712. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef] [PubMed]
- Sittipo, P.; Lobionda, S.; Lee, Y.K.; Maynard, C.L. Intestinal microbiota and the immune system in metabolic diseases. J. Microbiol. 2018, 56, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Namazi, N.; Anjom-Shoae, J.; Najafi, F.; Ayati, M.H.; Darbandi, M.; Pasdar, Y. Pro-inflammatory diet, cardio-metabolic risk factors and risk of type 2 diabetes: A cross-sectional analysis using data from RaNCD cohort study. BMC Cardiovasc. Disord. 2023, 23, 5. [Google Scholar] [CrossRef]
- Demirer, B.; Yardımcı, H.; Erem Basmaz, S. Inflammation level in type 2 diabetes is associated with dietary advanced glycation end products, Mediterranean diet adherence and oxidative balance score: A pathway analysis. J. Diabetes Complicat. 2023, 37, 108354. [Google Scholar] [CrossRef]
- Johansson, M.E.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef]
- Olivares-Villagómez, D.; Van Kaer, L. Intestinal Intraepithelial Lymphocytes: Sentinels of the Mucosal Barrier. Trends Immunol. 2018, 39, 264–275. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Caesar, R.; Tremaroli, V.; Kovatcheva-Datchary, P.; Cani, P.D.; Bäckhed, F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015, 22, 658–668. [Google Scholar] [CrossRef]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.X.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F.; et al. Diet and the Human Gut Microbiome: An International Review. Dig. Dis. Sci. 2020, 65, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef]
- Zhao, R.; Li, N.; Liu, W.; Liu, Q.; Zhang, L.; Peng, X.; Zhao, R.; Hu, H. Low glycemic index potato biscuits alleviate physio-histological damage and gut dysbiosis in rats with type-2 diabetes mellitus induced by high-sugar and high-fat diet and streptozotocin. J. Nutr. Biochem. 2023, 119, 109401. [Google Scholar] [CrossRef]
- Zhen, Q.; Liang, Q.; Wang, H.; Zheng, Y.; Lu, Z.; Bian, C.; Zhao, X.; Guo, X. Theabrownin ameliorates liver inflammation, oxidative stress, and fibrosis in MCD diet-fed C57BL/6J mice. Front. Endocrinol. 2023, 14, 1118925. [Google Scholar] [CrossRef]
- Delghingaro-Augusto, V.; Hosaka, A.; Estaphan, S.; Richardson, A.; Dahlstrom, J.E.; Nolan, C.J. High Dietary Iron in Western Diet-Fed Male Rats Causes Pancreatic Islet Injury and Acute Pancreatitis. J. Nutr. 2023, 153, 723–732. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Xiong, Y.; Miyamoto, N.; Shibata, K.; Valasek, M.A.; Motoike, T.; Kedzierski, R.M.; Yanagisawa, M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc. Natl. Acad. Sci. USA 2004, 101, 1045–1050. [Google Scholar] [CrossRef]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Jiang, M.; Yang, T.; Zhao, G.; Zhan, K. Overexpression of GPR41 attenuated glucose production in propionate-induced bovine hepatocytes. Front. Vet. Sci. 2022, 9, 981640. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fu, X.; Lin, D.; Li, T.; Zhang, N.; Huo, Y.; Zhu, P.; Guo, F.; Huang, F. Conjugated linoleic acid alleviates glycolipid metabolic disorders by modulating intestinal microbiota and short-chain fatty acids in obese rats. Food Funct. 2023, 14, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- McBride, D.A.; Dorn, N.C.; Yao, M.; Johnson, W.T.; Wang, W.; Bottini, N.; Shah, N.J. Short-chain fatty acid-mediated epigenetic modulation of inflammatory T cells in vitro. Drug Deliv. Transl. Res. 2023, 13, 1912–1924. [Google Scholar] [CrossRef]
- Serino, M. Molecular Paths Linking Metabolic Diseases, Gut Microbiota Dysbiosis and Enterobacteria Infections. J. Mol. Biol. 2018, 430, 581–590. [Google Scholar] [CrossRef]
- Wang, G.; Si, Q.; Yang, S.; Jiao, T.; Zhu, H.; Tian, P.; Wang, L.; Li, X.; Gong, L.; Zhao, J.; et al. Lactic acid bacteria reduce diabetes symptoms in mice by alleviating gut microbiota dysbiosis and inflammation in different manners. Food Funct. 2020, 11, 5898–5914. [Google Scholar] [CrossRef]
- Mattace Raso, G.; Simeoli, R.; Russo, R.; Iacono, A.; Santoro, A.; Paciello, O.; Ferrante, M.C.; Canani, R.B.; Calignano, A.; Meli, R. Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PLoS ONE 2013, 8, e68626. [Google Scholar] [CrossRef]
- Matsumoto, K.; Yoshitomi, T.; Ishimoto, Y.; Tanaka, N.; Takahashi, K.; Watanabe, A.; Chiba, K. DS-8500a, an Orally Available G Protein-Coupled Receptor 119 Agonist, Upregulates Glucagon-Like Peptide-1 and Enhances Glucose-Dependent Insulin Secretion and Improves Glucose Homeostasis in Type 2 Diabetic Rats. J. Pharmacol. Exp. Ther. 2018, 367, 509–517. [Google Scholar] [CrossRef]
- Salamone, D.; Costabile, G.; Corrado, A.; Della Pepa, G.; Vitale, M.; Giacco, R.; Luongo, D.; Testa, R.; Rivellese, A.A.; Annuzzi, G.; et al. Circulating short-chain fatty acids in type 2 diabetic patients and overweight/obese individuals. Acta Diabetol. 2022, 59, 1653–1656. [Google Scholar] [CrossRef]
- Teixeira, T.F.; Grześkowiak, Ł.; Franceschini, S.C.; Bressan, J.; Ferreira, C.L.; Peluzio, M.C. Higher level of faecal SCFA in women correlates with metabolic syndrome risk factors. Br. J. Nutr. 2013, 109, 914–919. [Google Scholar] [CrossRef]
- Zhang, Y.; Xi, Y.; Yang, C.; Gong, W.; Wang, C.; Wu, L.; Wang, D. Short-Chain Fatty Acids Attenuate 5-Fluorouracil-Induced THP-1 Cell Inflammation through Inhibiting NF-κB/NLRP3 Signaling via Glycerolphospholipid and Sphingolipid Metabolism. Molecules 2023, 28, 494. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Y.; Lin, F.; Han, K.; Wang, X. Omentin-1 attenuates lipopolysaccharide (LPS)-induced U937 macrophages activation by inhibiting the TLR4/MyD88/NF-κB signaling. Arch. Biochem. Biophys. 2020, 679, 108187. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, B.; Bibin, Y.S.; Prabhu, D.; Shanthirani, C.S.; Gokulakrishnan, K.; Lakshmi, B.S.; Mohan, V.; Balasubramanyam, M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol. Cell Biochem. 2014, 388, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; Zhang, P.; Bowden, D.W.; Devereaux, B.; Davoren, P.M.; Cripps, A.W.; West, N.P. Increased intestinal permeability as a risk factor for type 2 diabetes. Diabetes Metab. 2017, 43, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Jimenez-Lucena, R.; Alcala-Diaz, J.F.; Rangel-Zuñiga, O.A.; Garcia-Carpintero, S.; Lopez-Moreno, J.; Blanco-Rojo, R.; Delgado-Lista, J.; Perez-Martinez, P.; van Ommen, B.; et al. Postprandial endotoxemia may influence the development of type 2 diabetes mellitus: From the CORDIOPREV study. Clin. Nutr. 2019, 38, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Mendes de Oliveira, E.; Silva, J.C.; Ascar, T.P.; Sandri, S.; Marchi, A.F.; Migliorini, S.; Nakaya, H.T.I.; Fock, R.A.; Campa, A. Acute Inflammation Is a Predisposing Factor for Weight Gain and Insulin Resistance. Pharmaceutics 2022, 14, 623. [Google Scholar] [CrossRef] [PubMed]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Speakman, J.R.; Hu, S.; Song, Y.; Qin, S. The gut microbiota and its products: Establishing causal relationships with obesity related outcomes. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2021, 22, e13341. [Google Scholar] [CrossRef]
- Sun, L.; Xie, C.; Wang, G.; Wu, Y.; Wu, Q.; Wang, X.; Liu, J.; Deng, Y.; Xia, J.; Chen, B.; et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med. 2018, 24, 1919–1929. [Google Scholar] [CrossRef]
- Tian, F.; Huang, S.; Xu, W.; Chen, L.; Su, J.; Ni, H.; Feng, X.; Chen, J.; Wang, X.; Huang, Q. Compound K attenuates hyperglycemia by enhancing glucagon-like peptide-1 secretion through activating TGR5 via the remodeling of gut microbiota and bile acid metabolism. J. Ginseng Res. 2022, 46, 780–789. [Google Scholar] [CrossRef]
- Xi, Y.; Li, H. Role of farnesoid X receptor in hepatic steatosis in nonalcoholic fatty liver disease. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 121, 109609. [Google Scholar] [CrossRef]
- Chávez-Talavera, O.; Tailleux, A.; Lefebvre, P.; Staels, B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 1679–1694.e1673. [Google Scholar] [CrossRef] [PubMed]
- Andersson-Hall, U.; Gustavsson, C.; Pedersen, A.; Malmodin, D.; Joelsson, L.; Holmäng, A. Higher Concentrations of BCAAs and 3-HIB Are Associated with Insulin Resistance in the Transition from Gestational Diabetes to Type 2 Diabetes. J. Diabetes Res. 2018, 2018, 4207067. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, L.; Lou, P.; Zhao, M.; Wang, Y.; Tang, M.; Gong, M.; Liao, G.; Yuan, Y.; Li, L.; et al. Elevated branched-chain α-keto acids exacerbate macrophage oxidative stress and chronic inflammatory damage in type 2 diabetes mellitus. Free Radic. Biol. Med. 2021, 175, 141–154. [Google Scholar] [CrossRef]
- Chorell, E.; Otten, J.; Stomby, A.; Ryberg, M.; Waling, M.; Hauksson, J.; Svensson, M.; Olsson, T. Improved Peripheral and Hepatic Insulin Sensitivity after Lifestyle Interventions in Type 2 Diabetes Is Associated with Specific Metabolomic and Lipidomic Signatures in Skeletal Muscle and Plasma. Metabolites 2021, 11, 834. [Google Scholar] [CrossRef]
- Tekwe, C.D.; Yao, K.; Lei, J.; Li, X.; Gupta, A.; Luan, Y.; Meininger, C.J.; Bazer, F.W.; Wu, G. Oral administration of α-ketoglutarate enhances nitric oxide synthesis by endothelial cells and whole-body insulin sensitivity in diet-induced obese rats. Exp. Biol. Med. 2019, 244, 1081–1088. [Google Scholar] [CrossRef]
- Liu, M.; Huang, Y.; Zhang, H.; Aitken, D.; Nevitt, M.C.; Rockel, J.S.; Pelletier, J.P.; Lewis, C.E.; Torner, J.; Rampersaud, Y.R.; et al. Restricting Branched-Chain Amino Acids within a High-Fat Diet Prevents Obesity. Metabolites 2022, 12, 334. [Google Scholar] [CrossRef]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Li, D.Y.; Hazen, S.L. Dietary metabolism, the gut microbiome, and heart failure. Nat. Rev. Cardiol. 2019, 16, 137–154. [Google Scholar] [CrossRef]
- Leustean, A.M.; Ciocoiu, M.; Sava, A.; Costea, C.F.; Floria, M.; Tarniceriu, C.C.; Tanase, D.M. Implications of the Intestinal Microbiota in Diagnosing the Progression of Diabetes and the Presence of Cardiovascular Complications. J. Diabetes Res. 2018, 2018, 5205126. [Google Scholar] [CrossRef]
- Schugar, R.C.; Shih, D.M.; Warrier, M.; Helsley, R.N.; Burrows, A.; Ferguson, D.; Brown, A.L.; Gromovsky, A.D.; Heine, M.; Chatterjee, A.; et al. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017, 19, 2451–2461. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaide, M.A.I.; Singh, R.; Datta, P.; Rewers-Felkins, K.A.; Salguero, M.V.; Al-Obaidi, I.; Kottapalli, K.R.; Vasylyeva, T.L. Gut Microbiota-Dependent Trimethylamine-N-oxide and Serum Biomarkers in Patients with T2DM and Advanced CKD. J. Clin. Med. 2017, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Chen, S.; Lu, X.T.; Fang, A.P.; Chen, Y.M.; Huang, R.Z.; Lin, X.L.; Huang, Z.H.; Ma, J.F.; Huang, B.X.; et al. Serum trimethylamine-N-oxide is associated with incident type 2 diabetes in middle-aged and older adults: A prospective cohort study. J. Transl. Med. 2022, 20, 374. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.Y.; Huo, J.Y.; Wang, S.C.; Cheng, Y.D.; Lyu, Y.T.; Jiang, Z.X.; Shan, Q.J. Trimethylamine N-oxide facilitates the progression of atrial fibrillation in rats with type 2 diabetes by aggravating cardiac inflammation and connexin remodeling. J. Physiol. Biochem. 2022, 78, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Li, X.; Yang, Y.; Du, Y.; Zhang, X.; Wang, L.; Hong, B. Metformin alleviates choline diet-induced TMAO elevation in C57BL/6J mice by influencing gut-microbiota composition and functionality. Nutr. Diabetes 2021, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- León-Mimila, P.; Villamil-Ramírez, H.; Li, X.S.; Shih, D.M.; Hui, S.T.; Ocampo-Medina, E.; López-Contreras, B.; Morán-Ramos, S.; Olivares-Arevalo, M.; Grandini-Rosales, P.; et al. Trimethylamine N-oxide levels are associated with NASH in obese subjects with type 2 diabetes. Diabetes Metab. 2021, 47, 101183. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ye, C.; Hu, B.; Xia, H.; Bian, Q.; Liu, Y.; Kong, M.; Zhou, S.; Liu, H. Regulation of gut microbiota and intestinal metabolites by Poria cocos oligosaccharides improves glycolipid metabolism disturbance in high-fat diet-fed mice. J. Nutr. Biochem. 2022, 107, 109019. [Google Scholar] [CrossRef]
- Di, S.; Wang, Y.; Han, L.; Bao, Q.; Gao, Z.; Wang, Q.; Yang, Y.; Zhao, L.; Tong, X. The Intervention Effect of Traditional Chinese Medicine on the Intestinal Flora and Its Metabolites in Glycolipid Metabolic Disorders. Evid.-Based Complement. Altern. Med. Ecam 2019, 2019, 2958920. [Google Scholar] [CrossRef]
- Zhang, A.; Jiang, X.; Ge, Y.; Xu, Q.; Li, Z.; Tang, H.; Cao, D.; Zhang, D. The Effects of GABA-Rich Adzuki Beans on Glycolipid Metabolism, as Well as Intestinal Flora, in Type 2 Diabetic Mice. Front. Nutr. 2022, 9, 849529. [Google Scholar] [CrossRef]
- Kim, Y.A.; Keogh, J.B.; Clifton, P.M. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr. Res. Rev. 2018, 31, 35–51. [Google Scholar] [CrossRef]
- Sehgal, R.; de Mello, V.D.; Männistö, V.; Lindström, J.; Tuomilehto, J.; Pihlajamäki, J.; Uusitupa, M. Indolepropionic Acid, a Gut Bacteria-Produced Tryptophan Metabolite and the Risk of Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease. Nutrients 2022, 14, 4695. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Miller, M.; Rhyne, J.; Wang, Z.; Hazen, S.L. Differential effect of short-term popular diets on TMAO and other cardio-metabolic risk markers. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Tian, Y.; Randell, E.; Zhou, H.; Sun, G. Unfavorable Associations Between Serum Trimethylamine N-Oxide and L-Carnitine Levels With Components of Metabolic Syndrome in the Newfoundland Population. Front. Endocrinol. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Q.; Zhao, T.T.; Gui, D.K.; Gao, C.L.; Gu, J.L.; Gan, W.J.; Huang, W.; Xu, Y.; Zhou, H.; Chen, W.N.; et al. Sodium Butyrate Improves Liver Glycogen Metabolism in Type 2 Diabetes Mellitus. J. Agric. Food Chem. 2019, 67, 7694–7705. [Google Scholar] [CrossRef]
- Wu, S.; Zuo, J.; Cheng, Y.; Zhang, Y.; Zhang, Z.; Wu, M.; Yang, Y.; Tong, H. Ethanol extract of Sargarsum fusiforme alleviates HFD/STZ-induced hyperglycemia in association with modulation of gut microbiota and intestinal metabolites in type 2 diabetic mice. Food Res. Int. 2021, 147, 110550. [Google Scholar] [CrossRef]
- Stenman, L.K.; Waget, A.; Garret, C.; Briand, F.; Burcelin, R.; Sulpice, T.; Lahtinen, S. Probiotic B420 and prebiotic polydextrose improve efficacy of antidiabetic drugs in mice. Diabetol. Metab. Syndr. 2015, 7, 75. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhou, D.D.; Gan, R.Y.; Huang, S.Y.; Zhao, C.N.; Shang, A.; Xu, X.Y.; Li, H.B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef]
- Andreasen, A.S.; Larsen, N.; Pedersen-Skovsgaard, T.; Berg, R.M.; Møller, K.; Svendsen, K.D.; Jakobsen, M.; Pedersen, B.K. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br. J. Nutr. 2010, 104, 1831–1838. [Google Scholar] [CrossRef]
- Li, Y.; Xia, D.; Chen, J.; Zhang, X.; Wang, H.; Huang, L.; Shen, J.; Wang, S.; Feng, Y.; He, D.; et al. Dietary fibers with different viscosity regulate lipid metabolism via ampk pathway: Roles of gut microbiota and short-chain fatty acid. Poult. Sci. 2022, 101, 101742. [Google Scholar] [CrossRef]
- Rivas, M.Á.; Benito, M.J.; Ruíz-Moyano, S.; Martín, A.; Córdoba, M.G.; Merchán, A.V.; Casquete, R. Improving the Viability and Metabolism of Intestinal Probiotic Bacteria Using Fibre Obtained from Vegetable By-Products. Foods 2021, 10, 2113. [Google Scholar] [CrossRef]
- Verhoog, S.; Taneri, P.E.; Diaz, Z.M.R.; Marques-Vidal, P.; Troup, J.P.; Bally, L.; Franco, O.H.; Glisic, M.; Muka, T. Dietary Factors and Modulation of Bacteria Strains of Akkermansia muciniphila and Faecalibacterium prausnitzii: A Systematic Review. Nutrients 2019, 11, 1565. [Google Scholar] [CrossRef] [PubMed]
- Supruniuk, E.; Żebrowska, E.; Chabowski, A. Branched chain amino acids-friend or foe in the control of energy substrate turnover and insulin sensitivity? Crit. Rev. Food Sci. Nutr. 2023, 63, 2559–2597. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, K.T.; Ning, H.; Allen, N.B.; Carnethon, M.R.; Wallia, A.; Otvos, J.D.; Ben-Sahra, I.; McNally, E.M.; Snell-Bergeon, J.K.; Wilkins, J.T. Longitudinal trajectories of branched chain amino acids through young adulthood and diabetes in later life. JCI Insight 2023, 8, e166956. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Goodarzi, M.O. Metabolites Linking the Gut Microbiome with Risk for Type 2 Diabetes. Curr. Nutr. Rep. 2020, 9, 83–93. [Google Scholar] [CrossRef]
- Wang, M.; Li, L.; Chen, Y.; Lian, G.; Wang, J.; Zhang, J.; Shan, K.; Shang, L.; Tian, F.; Jing, C. Role of Gut Microbiome and Microbial Metabolites in Alleviating Insulin Resistance After Bariatric Surgery. Obes. Surg. 2021, 31, 327–336. [Google Scholar] [CrossRef]
- de Mello, V.D.; Paananen, J.; Lindström, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamäki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O.; et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017, 7, 46337. [Google Scholar] [CrossRef]
- Poblete-Aro, C.; Russell-Guzmán, J.; Parra, P.; Soto-Muñoz, M.; Villegas-González, B.; Cofré-Bolados, C.; Herrera-Valenzuela, T. Exercise and oxidative stress in type 2 diabetes mellitus. Rev. Med. Chil. 2018, 146, 362–372. [Google Scholar] [CrossRef]
- Carbone, S.; Del Buono, M.G.; Ozemek, C.; Lavie, C.J. Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog. Cardiovasc. Dis. 2019, 62, 327–333. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef]



| Exogenous Substances Affecting Metabolites | Regulated Substance | Symptoms of Improvement | References |
|---|---|---|---|
| Poria oligosaccharides | BAs | Improves glucose intolerance and IR, lowers blood glucose levels, reduces intestinal damage and restores normal microbiota balance. | [97] |
| Berberine and metformin | LPS | Reduces food intake, body weight, blood glucose levels, restores SCFA levels, reduces intestinal inflammation and restores intestinal barrier function. | [24] |
| Legumes diet | Gut microorganisms | Reduces body weight, lowers triglyceride and total cholesterol levels, increases probiotic abundance and regulates glucolipid metabolism. | [98,99] |
| Sodium butyrate | Glycogen | Upregulation of GPR43 and GLUT2 expression promotes hepatocyte glycogen metabolism and maintenance of blood glucose homeostasis. | [104] |
| Extract of Sargarsum fusiforme | BCAAs | Reducing food and water intake lowers fasting blood glucose levels while improving glucose tolerance and hepatic oxidative stress. | [105] |
| Probiotics | Intestinal proinsulin | Improves blood glucose and lipid levels, enhances insulin secretion, upregulates claudin-1 and mucin-2 expression, and improves intestinal barrier function. | [19] |
| Probiotics and prebiotics | Gut microbial metabolites | Reduces intestinal endotoxin concentrations, energy gain, and pro-inflammatory factor levels while increasing insulin sensitivity. | [100] |
| Exogenous Substances Affecting Metabolites | Regulated Metabolites | Impact on T2D | References |
|---|---|---|---|
| Dietary fiber | SCFAs | Improve the growth, survival, and metabolic environment of probiotics and promote sugar metabolism. | [110] |
| Fruits and grains | SCFAs | Reduced food intake, lower levels of inflammation and maintenance of intestinal mucosal homeostasis. | [111] |
| Excessive intake of protein | BCAAs | Affects glucose tolerance, interferes with metabolism and increases the risk of IR. | [112,113] |
| Red meat foods | TMAO | Increases the risk of T2D, interferes with hepatic insulin signalling pathways, induces adipose tissue inflammation and impairs glucose tolerance. | [114] |
| Diet | Indolepropionic acid | Maintains normal β cell function, reduces risk of T2D and lowers inflammation levels. | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Li, S.; Xiao, Y.; Liu, G.; Fang, J. A Metabolite Perspective on the Involvement of the Gut Microbiota in Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 14991. https://doi.org/10.3390/ijms241914991
Fu Y, Li S, Xiao Y, Liu G, Fang J. A Metabolite Perspective on the Involvement of the Gut Microbiota in Type 2 Diabetes. International Journal of Molecular Sciences. 2023; 24(19):14991. https://doi.org/10.3390/ijms241914991
Chicago/Turabian StyleFu, Yifeng, Siying Li, Yunhua Xiao, Gang Liu, and Jun Fang. 2023. "A Metabolite Perspective on the Involvement of the Gut Microbiota in Type 2 Diabetes" International Journal of Molecular Sciences 24, no. 19: 14991. https://doi.org/10.3390/ijms241914991
APA StyleFu, Y., Li, S., Xiao, Y., Liu, G., & Fang, J. (2023). A Metabolite Perspective on the Involvement of the Gut Microbiota in Type 2 Diabetes. International Journal of Molecular Sciences, 24(19), 14991. https://doi.org/10.3390/ijms241914991






