Tailoring Properties of Hyaluronate-Based Core–Shell Nanocapsules with Encapsulation of Mixtures of Edible Oils
Abstract
:1. Introduction
2. Results and Discussion
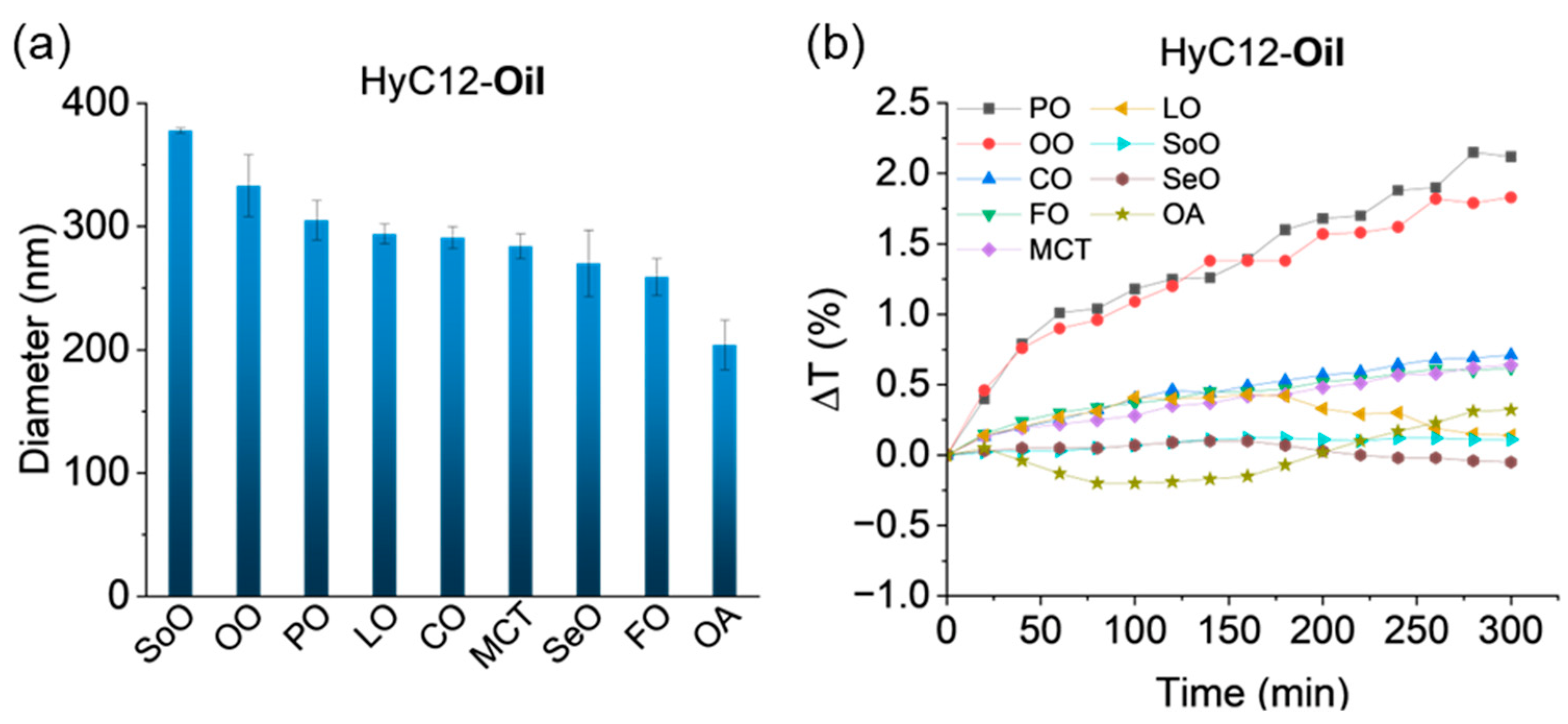
2.1. Capsules Composed of Single Oil Cores
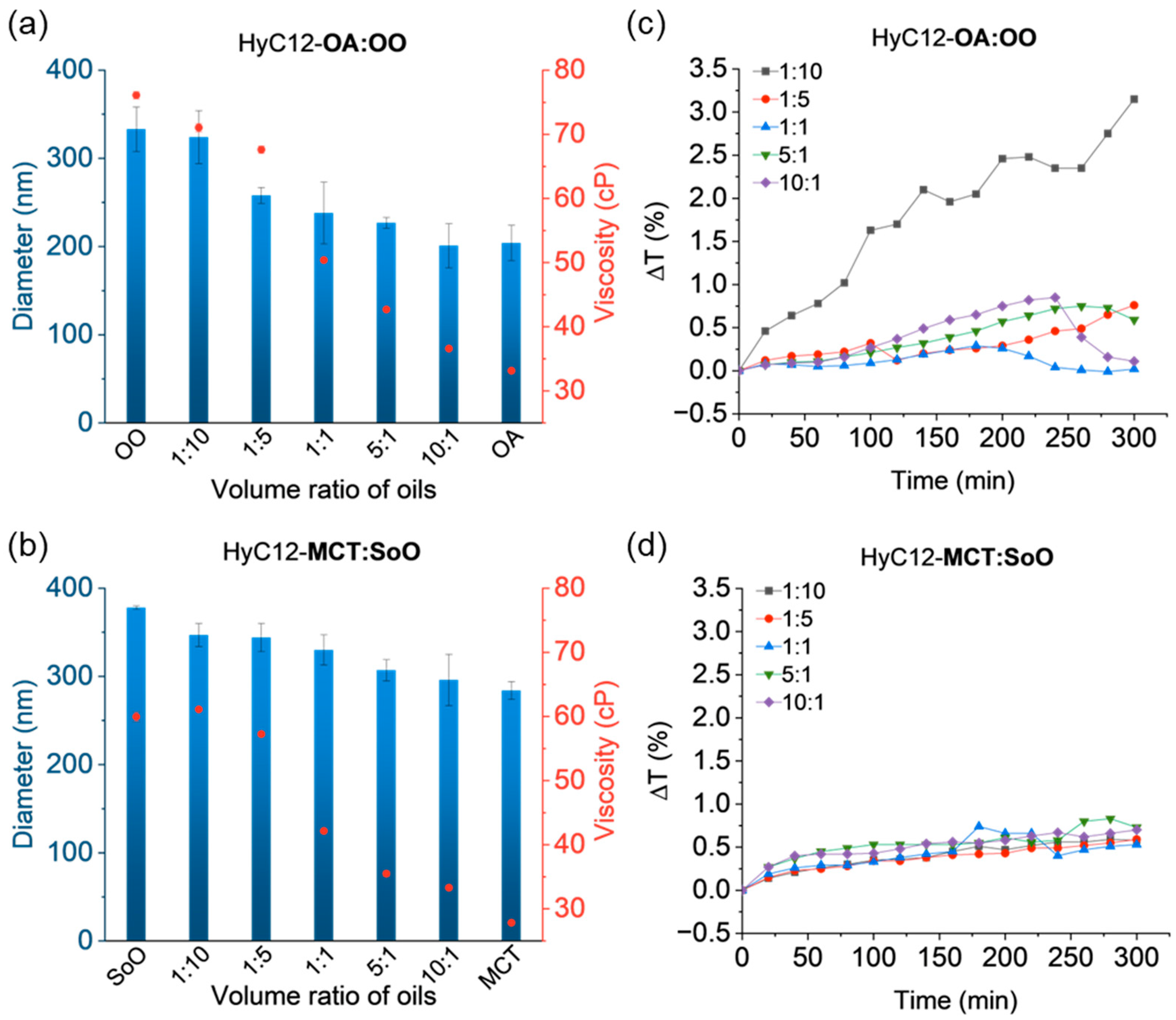
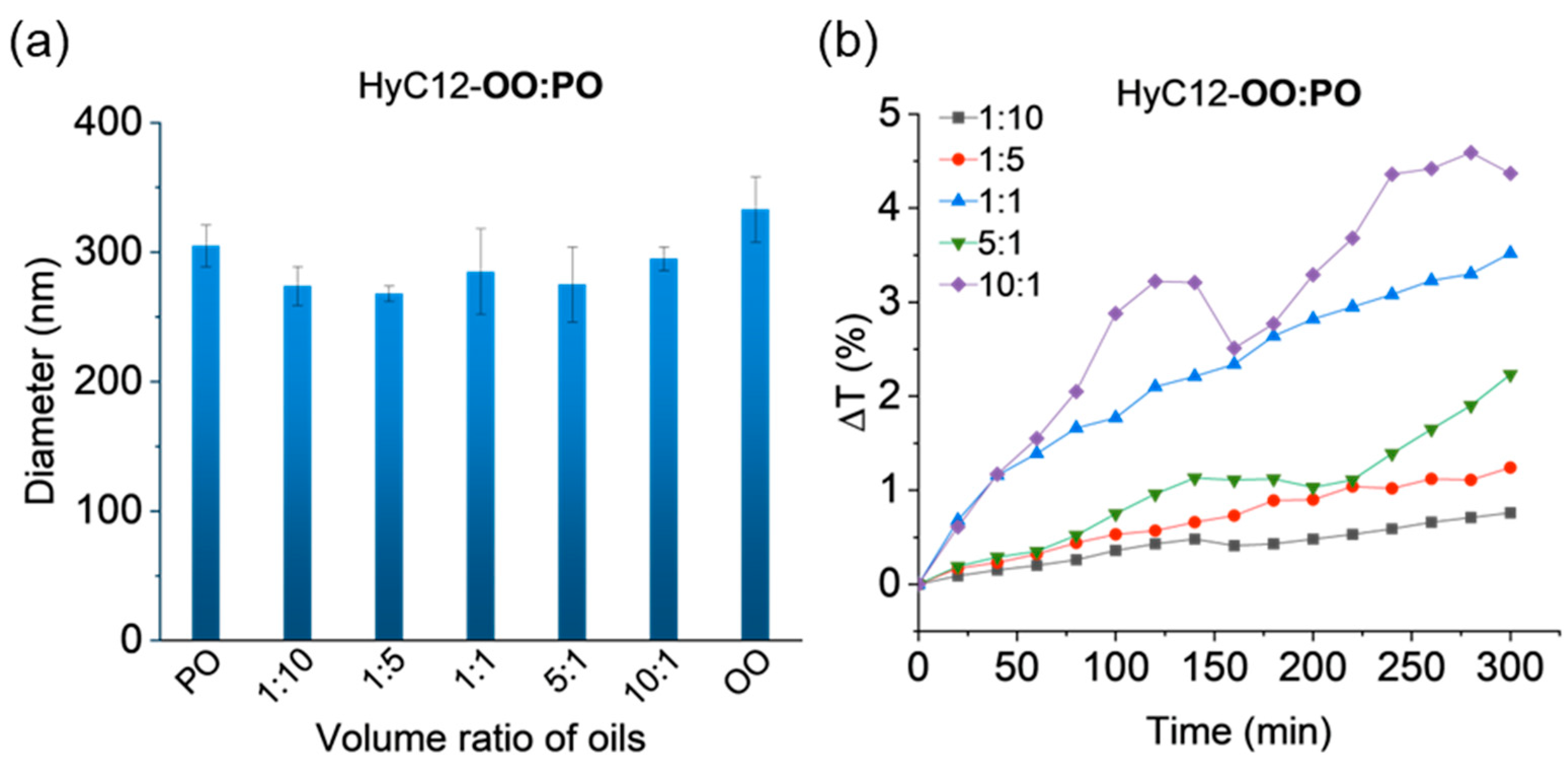
2.2. Capsules Containing Binary Mixtures of Oils—Effects of Density
2.3. Effect of Viscosity
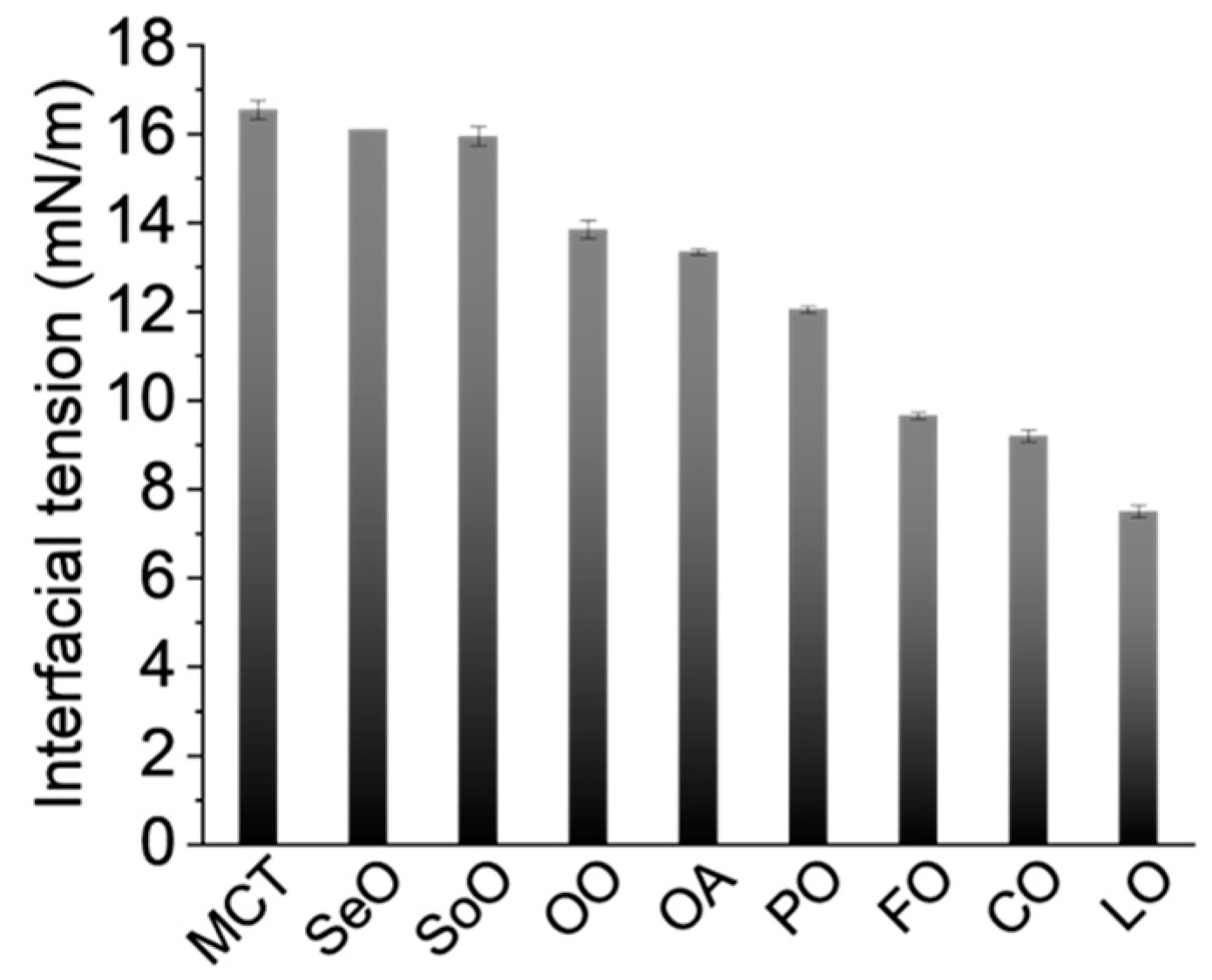
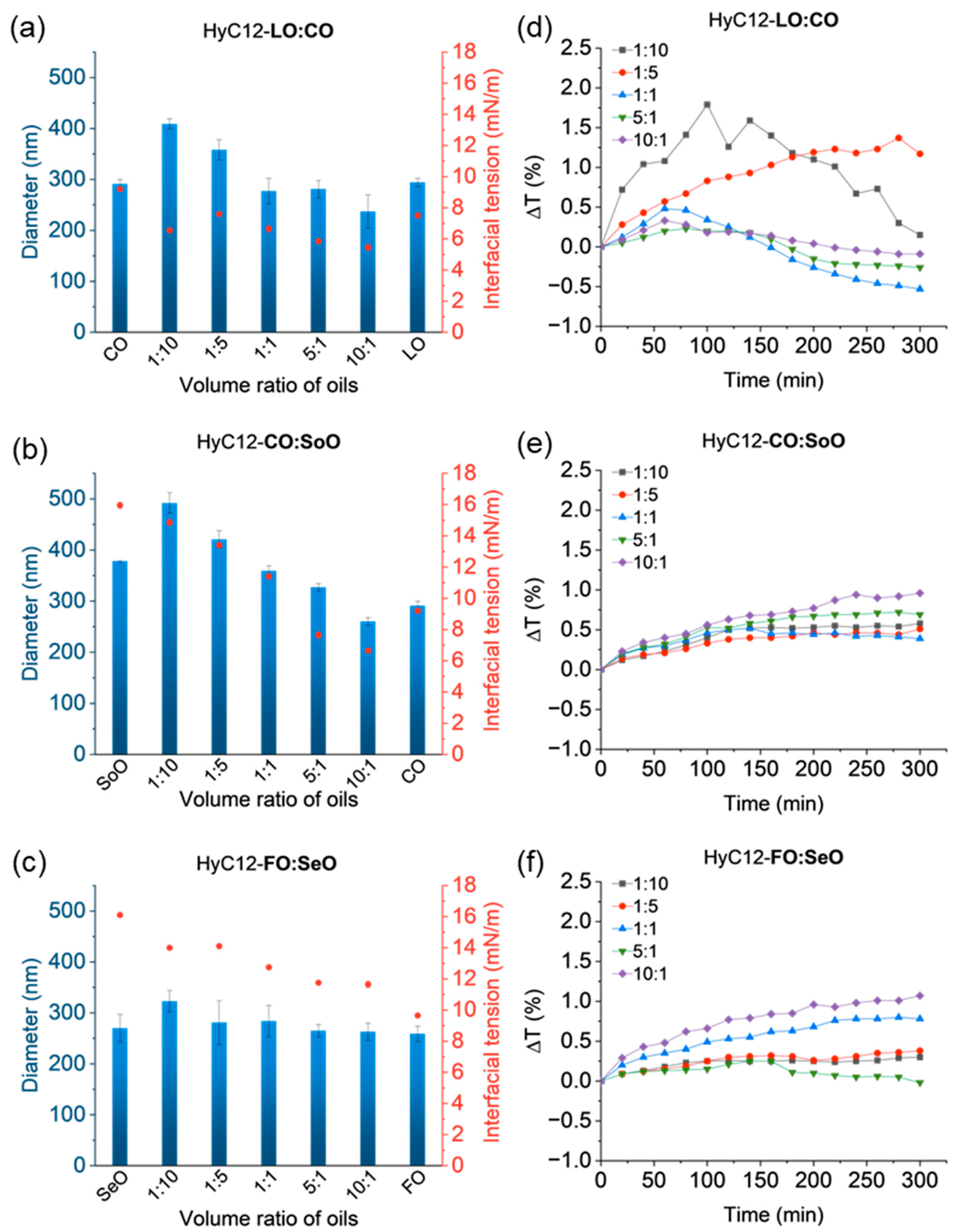
2.4. Effect of Interfacial Tension
2.5. Mixtures of Oils with Similar Bulk Properties
3. Materials and Methods
3.1. Hydrophobic Modification of Hyaluronate
3.2. Capsule Preparation
3.3. Density Measurements
3.4. Viscosity Measurements
3.5. Measurements of Interfacial Tension
3.6. Turbidimetry
3.7. Dynamic Light Scattering
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szafraniec, J.; Błażejczyk, A.; Kus, E.; Janik, M.; Zając, G.; Wietrzyk, J.; Chlopicki, S.; Zapotoczny, S. Robust oil-core nanocapsules with hyaluronate-based shells as promising nanovehicles for lipophilic compounds. Nanoscale 2017, 9, 18867–18880. [Google Scholar] [CrossRef] [PubMed]
- Janik-Hazuka, M.; Szafraniec-Szczęsny, J.; Kamiński, K.; Odrobińska, J.; Zapotoczny, S. Uptake and in vitro anticancer activity of oleic acid delivered in nanocapsules stabilized by amphiphilic derivatives of hyaluronic acid and chitosan. Int. J. Biol. Macromol. 2020, 164, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Janik-Hazuka, M.; Kamiński, K.; Kaczor-Kamińska, M.; Szafraniec-Szczęsny, J.; Kmak, A.; Kassassir, H.; Watała, C.; Wróbel, M.; Zapotoczny, S. Hyaluronic Acid-Based Nanocapsules as Efficient Delivery Systems of Garlic Oil Active Components with Anticancer Activity. Nanomaterials 2021, 11, 1354. [Google Scholar] [CrossRef]
- Czyzynska-Cichon, I.; Janik-Hazuka, M.; Szafraniec-Szczęsny, J.; Jasinski, K.; Węglarz, W.P.; Zapotoczny, S.; Chłopicki, S. Low Dose Curcumin Administered in Hyaluronic Acid-Based Nanocapsules Induces Hypotensive Effect in Hypertensive Rats. Int. J. Nanomed. 2021, 16, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- McClements, D.J. Food hydrocolloids: Application as functional ingredients to control lipid digestion and bioavailability. Food Hydrocoll. 2021, 111, 106404. [Google Scholar] [CrossRef]
- Choi, S.J.; McClements, D.J. Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Sci. Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef]
- Islam, F.; Saeed, F.; Afzaal, M.; Hussain, M.; Ikram, A.; Khalid, M.A. Food grade nanoemulsions: Promising delivery systems for functional ingredients. J. Food Sci. Technol. 2023, 60, 1461–1471. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and Their Potential Applications in Food Industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Fessi, H.; Elaissari, A. Plant oils: From chemical composition to encapsulated form use. Int. J. Pharm. 2021, 601, 120538. [Google Scholar] [CrossRef] [PubMed]
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; Mousadik, A.E.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Vergallo, C. Nutraceutical Vegetable Oil Nanoformulations for Prevention and Management of Diseases. Nanomaterials 2020, 10, 1232. [Google Scholar] [CrossRef]
- Semenova, M.; Antipova, A.; Martirosova, E.; Zelikina, D.; Palmina, N.; Chebotarev, S. Essential contributions of food hydrocolloids and phospholipid liposomes to the formation of carriers for controlled delivery of biologically active substances via the gastrointestinal tract. Food Hydrocoll. 2021, 120, 106890. [Google Scholar] [CrossRef]
- González, C.; Resa, J.M.; Concha, R.G.; Goenaga, J.M. Enthalpies of mixing and heat capacities of mixtures containing acetates and ketones with corn oil at 25°C. J. Food Eng. 2007, 79, 1104–1109. [Google Scholar] [CrossRef]
- Noureddini, H.; Teoh, B.C.; Davis Clements, L. Viscosities of vegetable oils and fatty acids. J. Am. Oil Chem. Soc. 1992, 69, 1189–1191. [Google Scholar] [CrossRef]
- Lizhi, H.; Toyoda, K.; Ihara, I. Dielectric properties of edible oils and fatty acids as a function of frequency, temperature, moisture, and composition. J. Food Eng. 2008, 88, 151–158. [Google Scholar] [CrossRef]
- Yin, H.; Sathivel, S. Physical Properties and Oxidation Rates of Unrefined Menhaden Oil (Brevoortia patronus). J. Food Sci. 2010, 75, E163–E168. [Google Scholar] [CrossRef]
- Calligaris, S.; Mirolo, G.; Da Pieve, S.; Arrighetti, G.; Nicoli, M.C. Effect of Oil Type on Formation, Structure and Thermal Properties of γ-oryzanol and β-sitosterol-Based Organogels. Food Biophys. 2013, 9, 69–75. [Google Scholar] [CrossRef]
- Agrawal, S.; Bhatnagar, D. Dielectric study of binary mixtures of edible unsaturated oils. Indian J. Pure Appl. 2005, 43, 624–629. [Google Scholar]
- Ostertag, F.; Weiss, J.; McClements, D.J. Low-energy formation of edible nanoemulsions: Factors influencing droplet size produced by emulsion phase inversion. J. Colloid Interface Sci. 2012, 388, 95–102. [Google Scholar] [CrossRef]
- Spohner, M. Study of dielectric properties of mineral oils and natural oils and methyl esters of natural oils. In Proceedings of the 2017 IEEE 19th International Conference on Dielectric Liquids (ICDL), Manchester, UK, 25–29 June 2017. [Google Scholar] [CrossRef]
- Diamante, L.; Lan, T. Absolute Viscosities of Vegetable Oils at Different Temperatures and Shear Rate Range of 64.5 to 4835 s−1. J. Food Process. 2014, 2014, 234583. [Google Scholar] [CrossRef]
- Kaibara, K.; Iwata, E.; Eguchi, Y.; Suzuki, M.; Maeda, H. Dispersion behavior of oleic acid in aqueous media: From micelles to emulsions. Colloid Polym. Sci. 1997, 275, 777–783. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, W.; Liu, C.; Huang, F. Composition and Oil-Water Interfacial Tension Studies in Different Vegetable Oils. Food Biophys. 2020, 15, 229–239. [Google Scholar] [CrossRef]
- Kim, H.; Burgess, D.J. Prediction of Interfacial Tension between Oil Mixtures and Water. J. Colloid Interface Sci. 2001, 241, 509–513. [Google Scholar] [CrossRef]
- Alonso, G.; Gamallo, P.; Rincón, C.; Sayós, R. Interfacial behavior of binary, ternary and quaternary oil/water mixtures described from molecular dynamics simulations. J. Mol. Liq. 2021, 324, 114661. [Google Scholar] [CrossRef]

| Oil | d (g/cm3) | η (cP) | ε | References |
|---|---|---|---|---|
| Corn Oil (CO) | 0.916 | 52.3 a | 3.127 | d: [16], η: [17], ε: [18] |
| Fish Oil (FO) | 0.93 | 50 | - | d: safety data sheet, Sigma-Aldrich, η: [19] |
| Linseed Oil (LO) | 0.93 | 53 b | 3.87 c | d: safety data sheet, Sigma-Aldrich, η: [20], ε: [21] |
| Medium-Chain Triglycerides (MCT) | 0.946 (0.950) f | 25.5 (27.9) g | 3.930 d | d: [22], η: [22], ε: [23] |
| Oleic Acid (OA) | 0.89 (0.899) f | 29 a (33.2) g | 2.377 | d: safety data sheet, Sigma-Aldrich, η: [17], ε: [18] |
| Olive Oil (OO) | 0.915 | 56.2 e (76.1) g | 3.062 | d: safety data sheet, Sigma-Aldrich, η: [24], ε: [18] |
| Peanut Oil (PO) | 0.91 | 57.4 e | 3.62 c | d: safety data sheet, Sigma-Aldrich, η: [24], ε: [21] |
| Sesame Oil (SeO) | 0.92 | 52.5 e | 3.110 | d: safety data sheet, Sigma-Aldrich, η: [24], ε: [18] |
| Soybean Oil (SoO) | 0.917 | 54.3 a (60.0) g | 3.115 | d: safety data sheet, Sigma-Aldrich, η: [17], ε: [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednorz, J.; Smela, K.; Zapotoczny, S. Tailoring Properties of Hyaluronate-Based Core–Shell Nanocapsules with Encapsulation of Mixtures of Edible Oils. Int. J. Mol. Sci. 2023, 24, 14995. https://doi.org/10.3390/ijms241914995
Bednorz J, Smela K, Zapotoczny S. Tailoring Properties of Hyaluronate-Based Core–Shell Nanocapsules with Encapsulation of Mixtures of Edible Oils. International Journal of Molecular Sciences. 2023; 24(19):14995. https://doi.org/10.3390/ijms241914995
Chicago/Turabian StyleBednorz, Justyna, Krzysztof Smela, and Szczepan Zapotoczny. 2023. "Tailoring Properties of Hyaluronate-Based Core–Shell Nanocapsules with Encapsulation of Mixtures of Edible Oils" International Journal of Molecular Sciences 24, no. 19: 14995. https://doi.org/10.3390/ijms241914995





