High-Efficient Flame-Retardant Finishing of Cotton Fabrics Based on Phytic Acid
Abstract
:1. Introduction
2. Results and Discussion
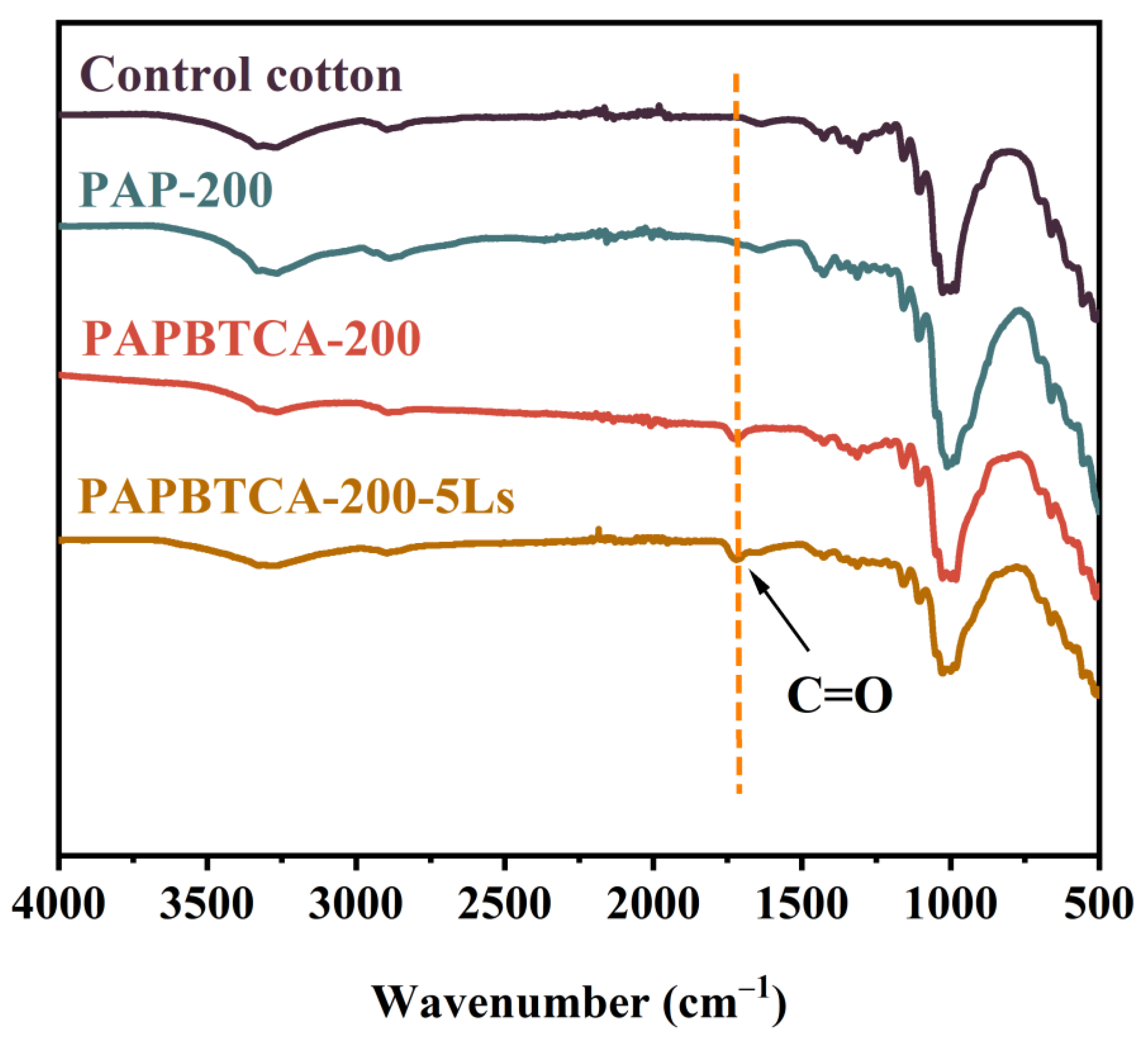
2.1. FTIR of the Control, PAP-200, and PAPBTCA-200
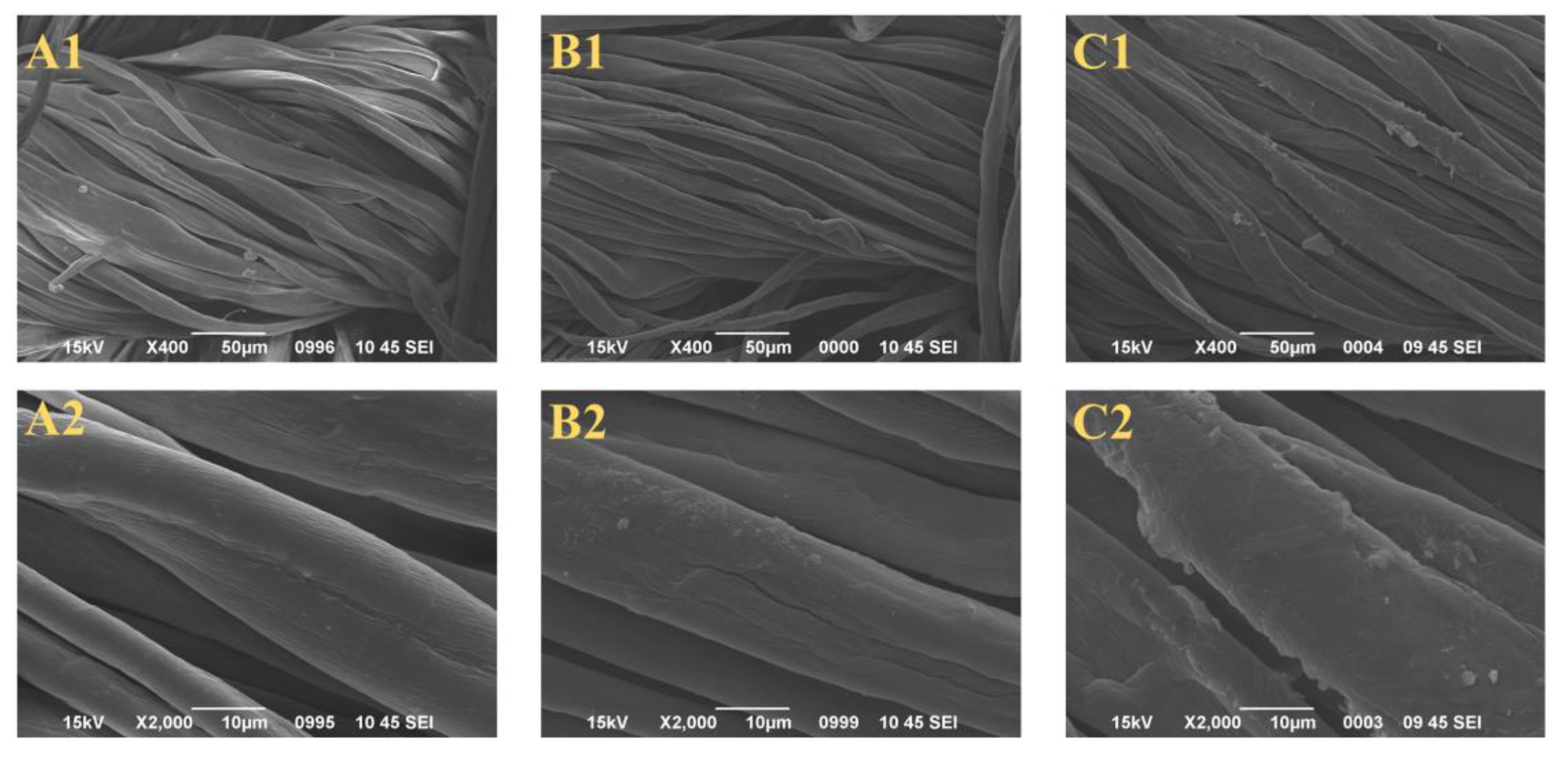
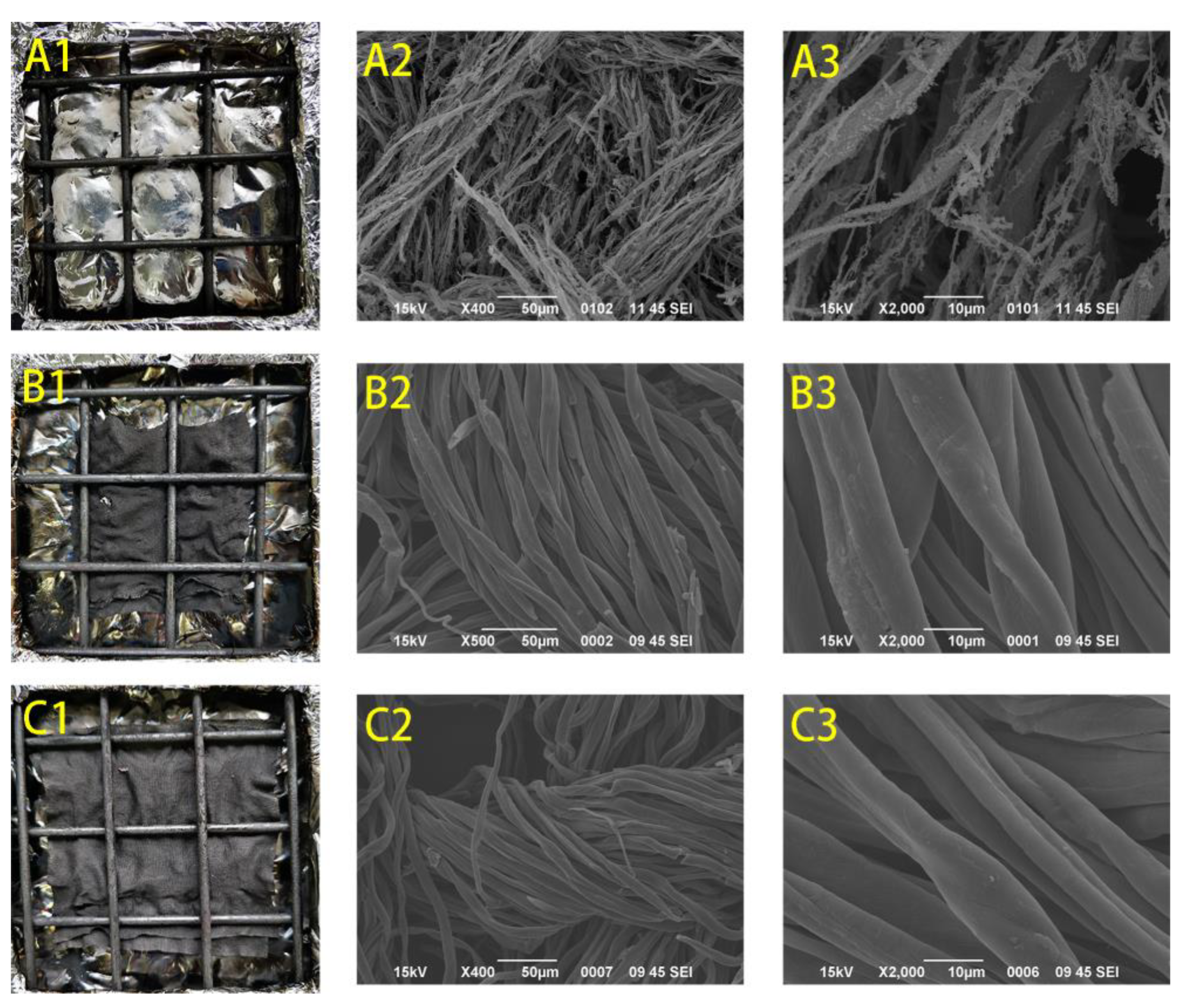
2.2. Surface Morphology of the Control, PAP-200, and PAPBTCA-200
2.3. Flame Retardancy
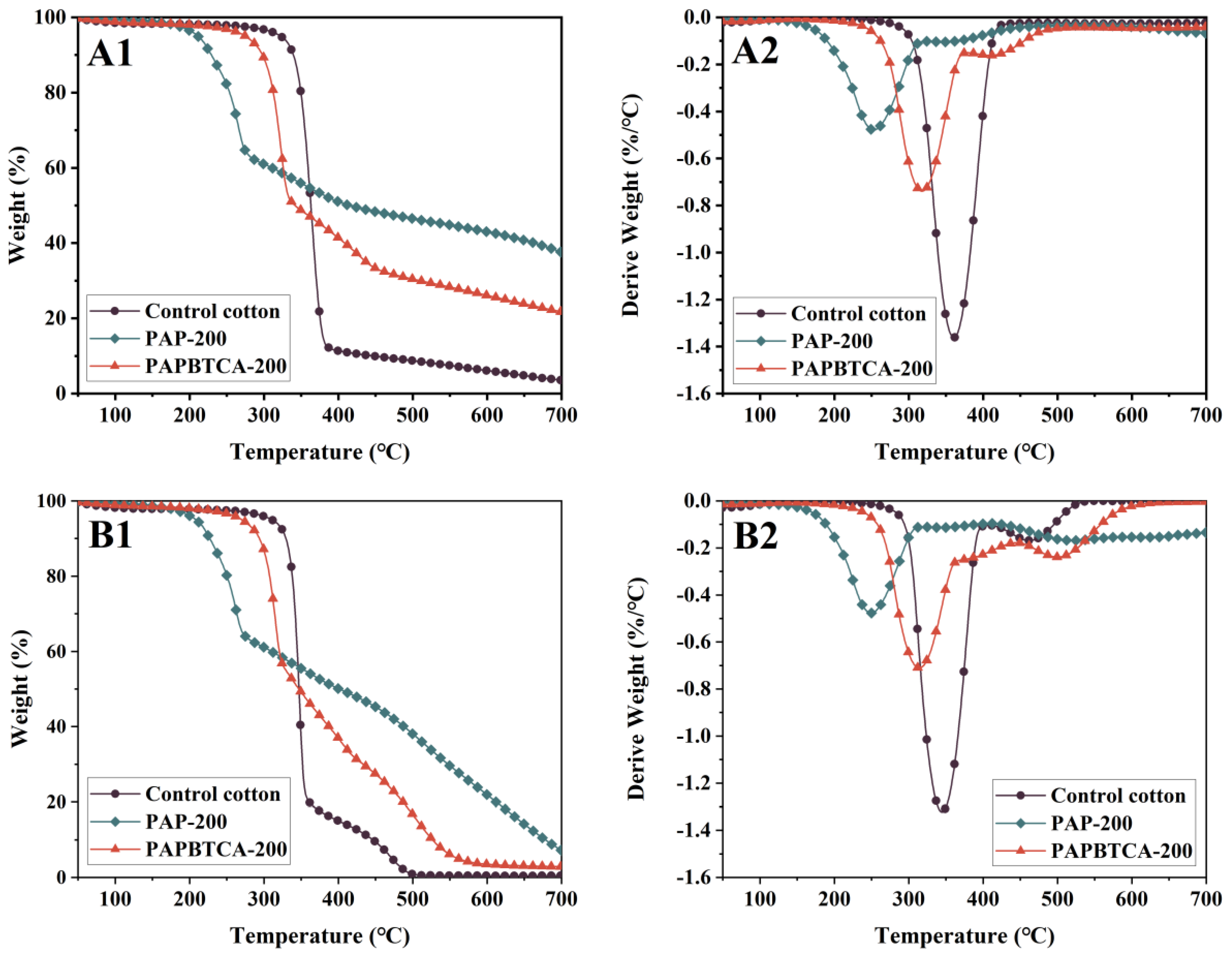
2.4. Thermal Stabilities
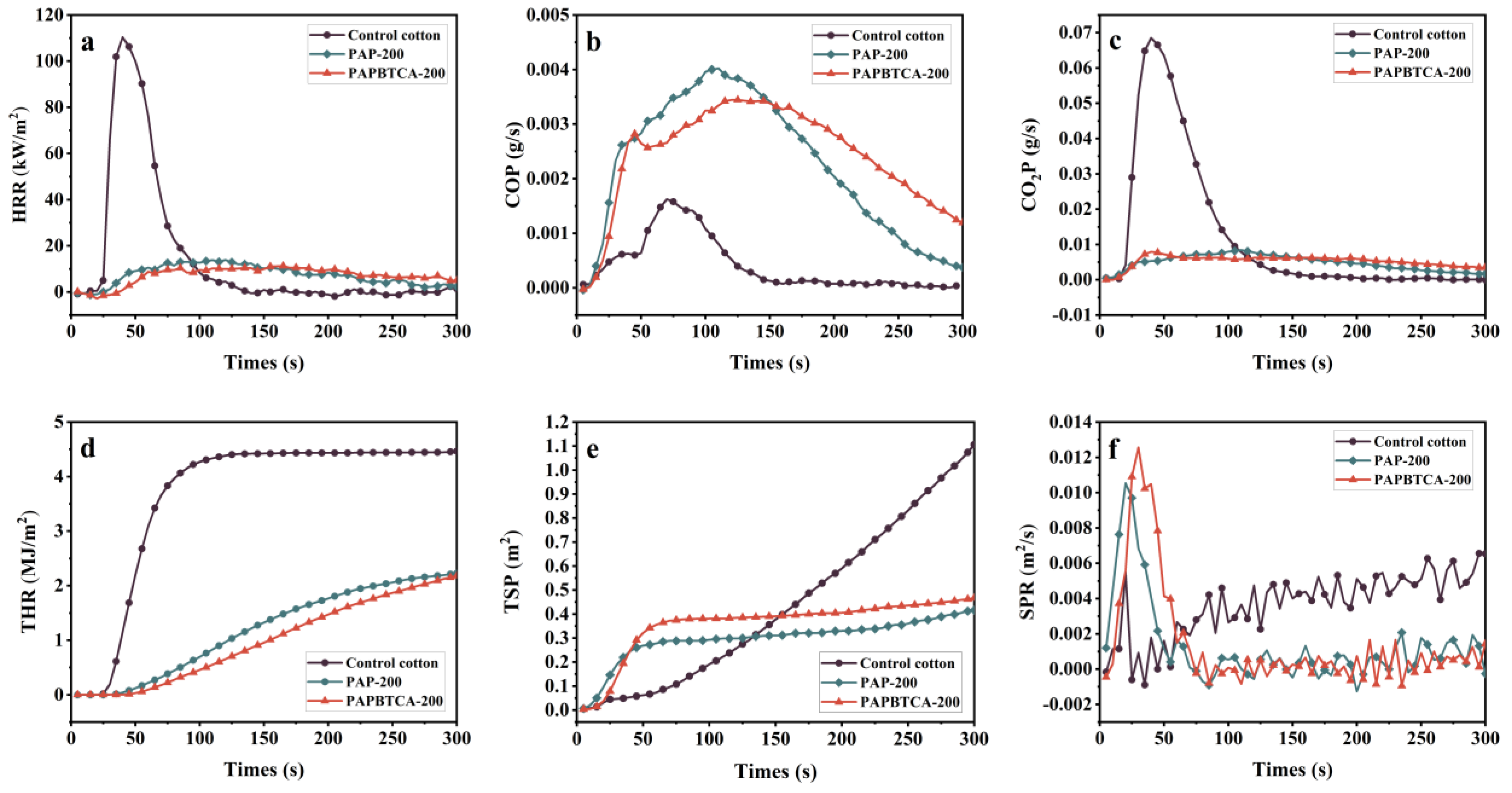
2.5. Burning Behaviors
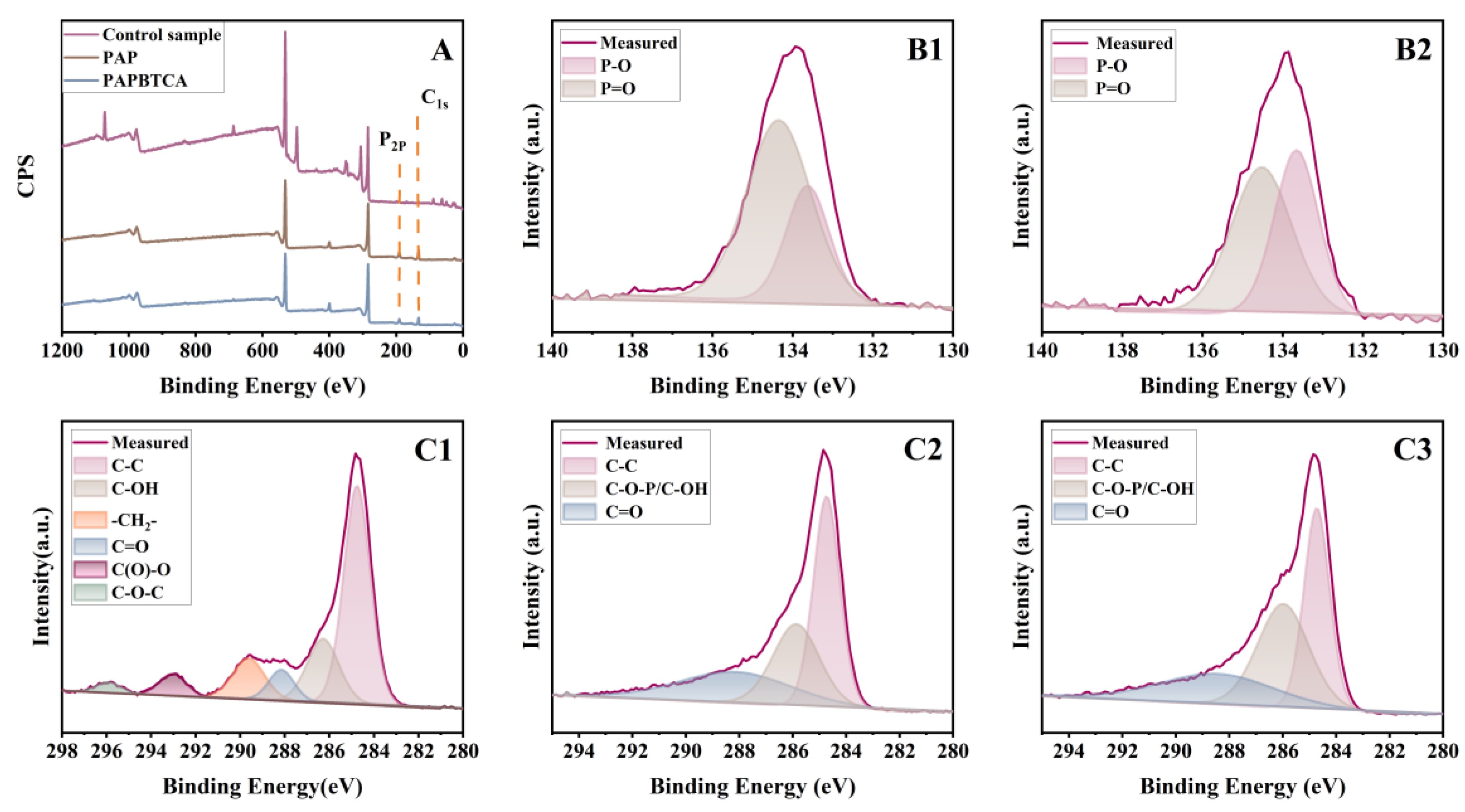
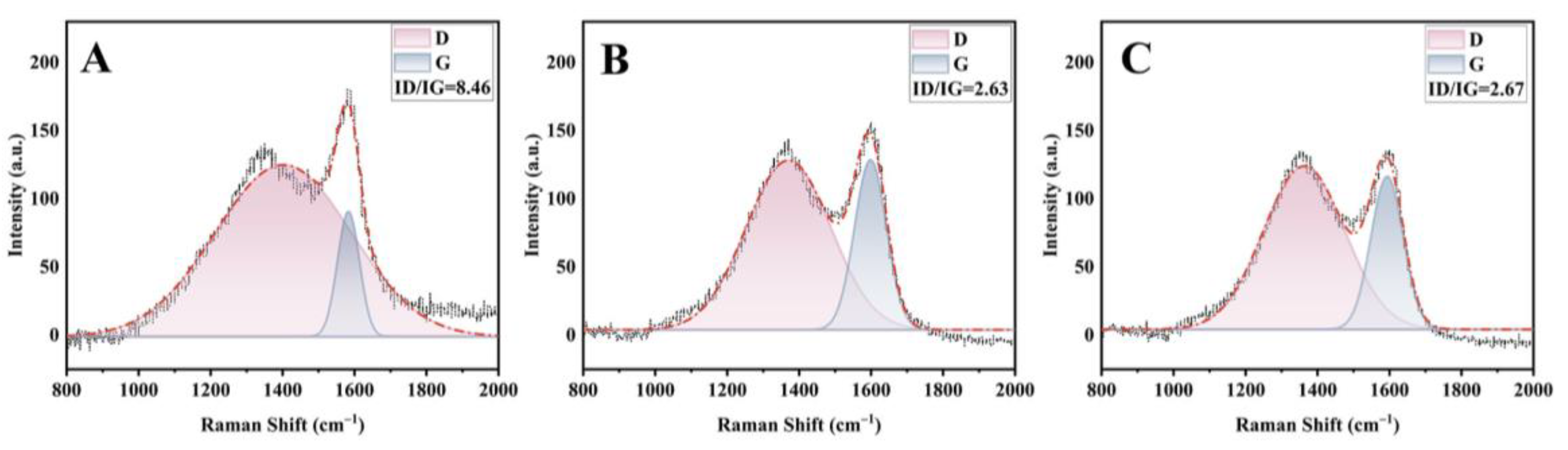
2.6. Flame-Retardant Mechanism
2.6.1. Analysis of the Structure of Char Residues
2.6.2. TG-FTIR
2.7. Anti-Wrinkle Performance
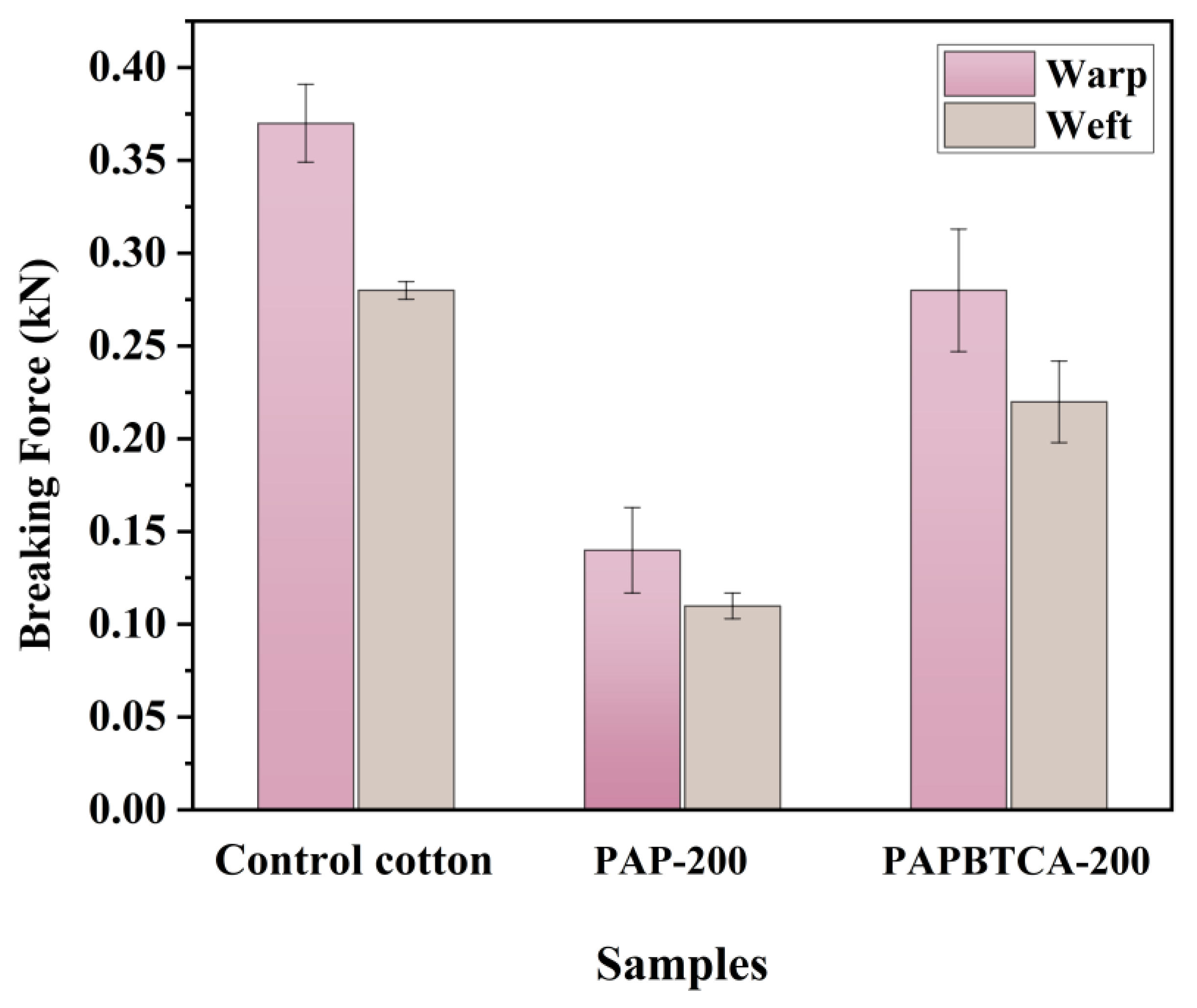
2.8. Breaking Force
3. Materials and Methods
3.1. Materials
3.2. Preparation
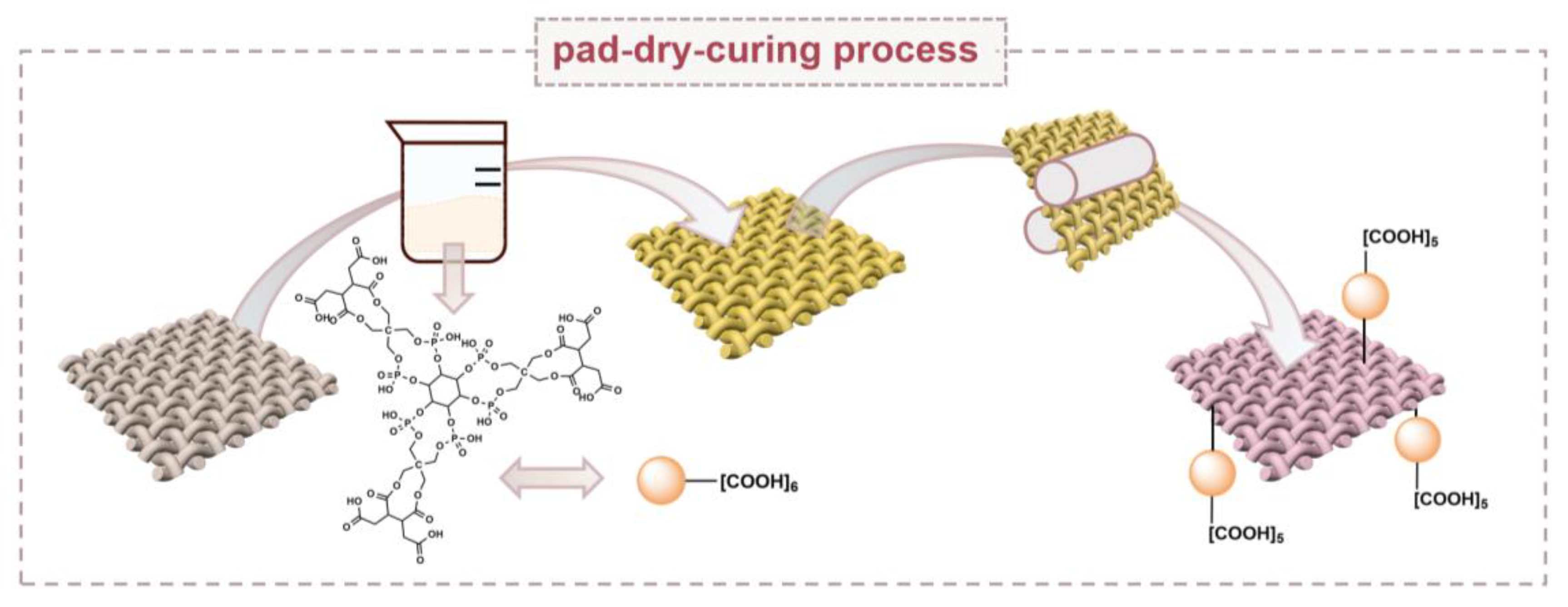
3.2.1. Synthesis of PAP and PAPBTCA
3.2.2. Preparation of Flame-Retardant Cotton Fabrics
3.3. Characterizations
3.3.1. FTIR
3.3.2. NMR
3.3.3. ICP-OES
3.3.4. SEM
3.3.5. Vertical Flame Test
3.3.6. LOI
3.3.7. Washing Durability Test
3.3.8. TG
3.3.9. CCT
3.3.10. XPS
3.3.11. Raman Spectra
3.3.12. TG-FTIR
3.3.13. Anti-Wrinkle Performance Test
3.3.14. Breaking Force
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, C.; Lu, Z.; Zhang, F.; Zhu, P.; Zhang, L.; Sui, S. Preparation and Properties of Cotton Fabrics Treated with a Novel Polysiloxane Water Repellent and Flame Retardant. Mater. Lett. 2015, 152, 276–279. [Google Scholar] [CrossRef]
- El-Shafei, A.; Al-Shemy, M.; Abou-Okeil, A. Eco-Friendly Finishing Agent for Cotton Fabrics to Improve Flame Retardant and Antibacterial Properties. Carbohydr. Polym. 2015, 118, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Lu, Z.; Zhang, F.; Zhu, P.; Wang, P.; Che, Y.; Sui, S. Combustion Behaviors of Cotton Fabrics Treated by a Novel Nitrogen- and Phosphorus-Containing Polysiloxane Flame Retardant. J. Therm. Anal. Calorim. 2016, 123, 535–544. [Google Scholar] [CrossRef]
- Gao, W.-W.; Zhang, G.-X.; Zhang, F.-X. Enhancement of Flame Retardancy of Cotton Fabrics by Grafting a Novel Organic Phosphorous-Based Flame Retardant. Cellulose 2015, 22, 2787–2796. [Google Scholar] [CrossRef]
- Liu, W.; Chen, L.; Wang, Y.-Z. A Novel Phosphorus-Containing Flame Retardant for the Formaldehyde-Free Treatment of Cotton Fabrics. Polym. Degrad. Stab. 2012, 97, 2487–2491. [Google Scholar] [CrossRef]
- Zhu, W.; Hao, S.; Yang, M.; Cheng, B.; Zhang, J. A Synergistic Flame Retardant of Glycosyl Cross-Linking Boron Acid and Ammonium Salt of Phytic Acid to Enhance Durable Flame Retardancy of Cotton Fabrics. Cellulose 2020, 27, 9699–9710. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Liu, B.-W.; Zeng, F.-R.; Lin, X.-C.; Zhang, J.-Y.; Wang, X.-L.; Wang, Y.-Z.; Zhao, H.-B. Fully Recyclable Multifunctional Adhesive with High Durability, Transparency, Flame Retardancy, and Harsh-Environment Resistance. Sci. Adv. 2022, 8, eadd8527. [Google Scholar] [CrossRef]
- Birnbaum, L.S.; Staskal, D.F. Brominated Flame Retardants: Cause for Concern? Environ. Health Perspect. 2004, 112, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Horrocks, A.R.; Kandola, B.K.; Davies, P.J.; Zhang, S.; Padbury, S.A. Developments in Flame Retardant Textiles—A Review. Polym. Degrad. Stab. 2005, 88, 3–12. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Gao, M.; Xia, Y.; Chai, W.; Su, X.; Zheng, Z.; Liu, Y. Bio-Inspired Construction of Super-Hydrophobic, Eco-Friendly Multifunctional and Bio-Based Cotton Fabrics via Impregnation Method. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 651, 129647. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, X.; Chen, Y.; Xiong, Y.; Weijian, X.W. Synthesis and Characterization of Phosphorus-Containing Epoxy Resin for Flame Retardance. Chem. Ind. Eng. Prog. 2007, 26, 56–59. [Google Scholar]
- Liao, Y.; Chen, Y.; Zhang, F. A Biological Reactive Flame Retardant for Flame Retardant Modification of Cotton Fabric. Colloids Surf. A-Physicochem. Eng. Asp. 2021, 630, 127601. [Google Scholar] [CrossRef]
- Li, Z.-F.; Zhang, C.-J.; Cui, L.; Zhu, P.; Yan, C.; Liu, Y. Fire Retardant and Thermal Degradation Properties of Cotton Fabrics Based on Aptes and Sodium Phytate through Layer-by-Layer Assembly. J. Anal. Appl. Pyrolysis 2016, 123, 216–223. [Google Scholar] [CrossRef]
- Schartel, B. Phosphorus-Based Flame Retardancy Mechanisms-Old Hat or a Starting Point for Future Development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Wang, B.; Xu, Y.J.; Jiang, Z.; Dong, C.; Liu, Y.; Zhu, P. Ecofriendly Flame-Retardant Cotton Fabrics: Preparation, Flame Retardancy, Thermal Degradation Properties, and Mechanism. ACS Sustain. Chem. Eng. 2019, 7, 19246–19256. [Google Scholar] [CrossRef]
- Alongi, J.; Carletto, R.A.; Di Blasio, A.; Carosio, F.; Bosco, F.; Malucelli, G. DNA: A Novel, Green, Natural Flame Retardant and Suppressant for Cotton. J. Mater. Chem. A 2013, 1, 4779–4785. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, W.; Liu, L.; Cheng, W.; Wang, W.; Liew, K.M.; Wang, B.; Hu, Y. Eco-Friendly Flame Retardant and Electromagnetic Interference Shielding Cotton Fabrics with Multi-Layered Coatings. Chem. Eng. J. 2019, 372, 1077–1090. [Google Scholar] [CrossRef]
- Angel, R.; Tamim, N.M.; Applegate, T.J.; Dhandu, A.S.; Ellestad, L.E. Phytic Acid Chemistry: Influence on Phytin-Phosphorus Availability and Phytase Efficacy. J. Appl. Poult. Res. 2002, 11, 471–480. [Google Scholar] [CrossRef]
- Ma, Y.; Luo, X.; Liu, L.; Zhang, C.; Shang, X.; Yao, J. Eco-Friendly, Efficient and Durable Fireproof Cotton Fabric Prepared by a Feasible Phytic Acid Grafting Route. Cellulose 2021, 28, 3887–3899. [Google Scholar] [CrossRef]
- Liu, X.-H.; Zhang, Q.-Y.; Cheng, B.-W.; Ren, Y.-L.; Zhang, Y.-G.; Ding, C. Durable Flame Retardant Cellulosic Fibers Modified with Novel, Facile and Efficient Phytic Acid-Based Finishing Agent. Cellulose 2018, 25, 799–811. [Google Scholar] [CrossRef]
- Li, Z.; Expósito, D.F.; González, A.J.; Wang, D.Y. Natural Halloysite Nanotube Based Functionalized Nanohybrid Assembled via Phosphorus-Containing Slow Release Method: A Highly Efficient Way to Impart Flame Retardancy to Polylactide. Eur. Polym. J. 2017, 93, 458–470. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, M.; Huang, H.; Dai, Z.; Cheng, B.; Hao, S. A Phytic Acid-Based Chelating Coordination Embedding Structure of Phosphorus-Boron-Nitride Synergistic Flame Retardant to Enhance Durability and Flame Retardancy of Cotton. Cellulose 2020, 27, 4817–4829. [Google Scholar] [CrossRef]
- Xu, W.; Li, Y. Cotton Fabric Strength Loss from Treatment with Polycarboxylic Acids for Durable Press Performance. Text. Res. J. 2000, 70, 957–961. [Google Scholar] [CrossRef]
- Cheng, X.W.; Guan, J.P.; Kiekens, P.; Yang, X.H.; Tang, R.C. Preparation and Evaluation of an Eco-Friendly, Reactive, and Phytic Acid-Based Flame Retardant for Wool. React. Funct. Polym. 2019, 134, 58–66. [Google Scholar] [CrossRef]
- Yang, C.Q.; Wang, X. Formation of Cyclic Anhydride Intermediates and Esterification of Cotton Cellulose by Multifunctional Carboxylic Acids: An Infrared Spectroscopy Study. Text. Res. J. 1996, 66, 595–603. [Google Scholar] [CrossRef]
- Zhang, K.; Zong, L.; Tan, Y.; Ji, Q.; Yun, W.; Shi, R.; Xia, Y. Improve the Flame Retardancy of Cellulose Fibers by Grafting Zinc Ion. Carbohydr. Polym. 2016, 136, 121–127. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, B.; Duan, L.; Gui, Z.; Wang, B.; Hu, Y.; Yuen, R.K.K. Graphitic Carbon Nitride/Phosphorus-Rich Aluminum Phosphinates Hybrids as Smoke Suppressants and Flame Retardants for Polystyrene. J. Hazard. Mater. 2017, 332, 87–96. [Google Scholar] [CrossRef]
- Cheng, X.-W.; Tang, R.-C.; Guan, J.-P.; Zhou, S.-Q. An Eco-Friendly and Effective Flame Retardant Coating for Cotton Fabric Based on Phytic Acid Doped Silica Sol Approach. Prog. Org. Coat. 2020, 141, 105539. [Google Scholar] [CrossRef]
- Zhou, K.; Gui, Z.; Hu, Y. The Influence of Graphene Based Smoke Suppression Agents on Reduced Fire Hazards of Polystyrene Composites. Compos. Part A-Appl. Sci. Manuf. 2016, 80, 217–227. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, P.; Wang, B.; She, J.-H.; Liu, Y.; Zhu, P. Inorganic-Organic Hybrid Coatings from Tea Polyphenols and Laponite to Improve the Fire Safety of Flexible Polyurethane Foams. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 655, 105539. [Google Scholar] [CrossRef]
- Zhou, L.L.; Li, W.X.; Zhao, H.B.; Wang, J.S.; Zhao, B. Niti-Layered Double Hydroxide Nanosheets toward High-Efficiency Flame Retardancy and Smoke Suppression for Silicone Foam. Polym. Degrad. Stab. 2022, 204, 105539. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.; Jiao, C. A Recycled Environmental Friendly Flame Retardant by Modifying Para-Aramid Fiber with Phosphorus Acid for Thermoplastic Polyurethane Elastomer. J. Hazard. Mater. 2017, 331, 257–264. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, L.; Zhang, Y.; Song, L.; Hu, Y.; Jiang, S.; Zhao, H. Effect of Genipin Crosslinked Layer-by-Layer Self-Assembled Coating on the Thermal Stability, Flammability and Wash Durability of Cotton Fabric. Carbohydr. Polym. 2019, 206, 396–402. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.; Chen, Z.G. Surface Structure of Pyrolytic Char of Cellulose Based on Xps Analysis. J. Combust. Sci. Technol. 2015, 21, 378–381. [Google Scholar]
- Kundu, C.K.; Song, L.; Hu, Y. Sol-Gel Coatings from Dopo-Alkoxysilanes: Efficacy in Fire Protection of Polyamide 66 Textiles. Eur. Polym. J. 2020, 125, 109483. [Google Scholar] [CrossRef]
- Li, P.; Wang, B.; Liu, Y.-Y.; Xu, Y.-J.; Jiang, Z.-M.; Dong, C.-H.; Zhang, L.; Liu, Y.; Zhu, P. Fully Bio-Based Coating from Chitosan and Phytate for Fire-Safety and Antibacterial Cotton Fabrics. Carbohydr. Polym. 2020, 237, 109483. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, Y.; Li, D.; He, S.; Zhang, F.; Zhang, G. A Plant-Based Reactive Ammonium Phytate for Use as a Flame-Retardant for Cotton Fabric. Carbohydr. Polym. 2017, 175, 636–644. [Google Scholar] [CrossRef]
- Wang, S.; Xu, D.; Zhu, P.; Jiang, Z. Facile Fabrication of Antibacterial and Fire-Safety Multifunctional Cotton Fabric with a Triazine-Phosphonate N-Halamine. Ind. Crops Prod. 2022, 186, 115261. [Google Scholar] [CrossRef]
- Lou, J.; Wang, D.; Yuan, J.; Xu, J.; Fan, X. Improving the Anti-Wrinkle and Hydrophilicity Performance of Cotton Fabric via Crosslinking Cellulose with Carboxylated Polyaldehyde Trehalose. Cellulose 2021, 28, 5135–5149. [Google Scholar] [CrossRef]
- Lou, J.; Wang, D.; Fan, X. Study on the Cross-Linking Process of Carboxylated Polyaldehyde Sucrose as an Anti-Wrinkle Finishing Agent for Cotton Fabric. Sci. Rep. 2022, 12, 5379. [Google Scholar] [CrossRef]
- Huang, K.-S.; Hwang, M.-C.; Chen, J.-S.; Lin, S.-J.; Wang, S.-P. Application of Mixed Gel Solution in the Anti-Wrinkle Finishing of Cotton Fabrics. J. Text. Inst. 2007, 98, 169–176. [Google Scholar] [CrossRef]
- Ghanadpour, M.; Carosio, F.; Larsson, P.T.; Wågberg, L. Phosphorylated Cellulose Nanofibrils: A Renewable Nanomaterial for the Preparation of Intrinsically Flame-Retardant Materials. Biomacromolecules 2015, 16, 3399–3410. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Qiao, J.; Hong, F. Application of Phosphoric Acid and Phytic Acid-Doped Bacterial Cellulose as Novel Proton-Conducting Membranes to PEMFC. Int. J. Hydrogen Energy 2012, 37, 9182–9192. [Google Scholar] [CrossRef]
- Guo, X.; Miao, Y.; Ye, P.; Wen, Y.; Yang, H. Multi-Walled Carbon Nanotubes in Aqueous Phytic Acid for Enhancing Biosensor. Mater. Res. Express 2014, 1, 025403. [Google Scholar] [CrossRef]
- Bebot-Brigaud, A.; Dange, C.; Fauconnier, N.; Gérard, C. P-31 Nmr, Potentiometric and Spectrophotometric Studies of Phytic Acid Ionization and Complexation Properties toward Co2+, Ni2+, Cu2+, Zn2+ and Cd2+. J. Inorg. Biochem. 1999, 75, 71–78. [Google Scholar] [CrossRef]


| Samples | Concentration of Flame Retardants (g/L) | Weight Gain (%) | Afterflame Time (s) | Afterglow Time (s) | Damaged Length (mm) | LOI (%) |
|---|---|---|---|---|---|---|
| Control | / | 0.0 | 17 | 34 | 300 | 18.0 |
| PAP-100 | 100 | 15.9 ± 1.1 | 0 | 0 | 77 ± 5 | 29.7 |
| PAPBTCA-100 | 100 | 17.3 ± 1.5 | 0 | 0 | 79 ± 6 | 29.5 |
| PAP-200 | 200 | 21.2 ± 0.4 | 0 | 0 | 71 ± 4 | 32.6 |
| PAPBTCA-200 | 200 | 20.3 ± 1.4 | 0 | 0 | 62 ± 5 | 31.5 |
| PAP-200-5Ls | 200 | 3.2 ± 0.3 | 6 | 0 | 300 ± 0 | 20.5 |
| PAPBTCA-200-5Ls | 200 | 16.8 ± 0.7 | 0 | 0 | 98 ± 6 | 29.3 |
| Atmosphere | Samples | T5% (°C) | T10% (°C) | Tmax1 (°C) | Rmax1 (%/°C) | Tmax2 (°C) | Rmax2 (%/°C) | Residue at 700 °C (%) |
|---|---|---|---|---|---|---|---|---|
| N2 | Control cotton | 322 | 367 | 359 | 1.36 | - | - | 3.65 |
| PAP-200 | 213 | 233 | 249 | 0.48 | 345 | 0.10 | 37.68 | |
| PAPBTCA-200 | 288 | 315 | 317 | 0.74 | 409 | 0.16 | 21.82 | |
| Air | Control cotton | 311 | 331 | 343 | 1.32 | 462 | 0.17 | 0.48 |
| PAP-200 | 210 | 232 | 248 | 0.48 | 521 | 0.17 | 7.24 | |
| PAPBTCA-200 | 283 | 310 | 312 | 0.71 | 500 | 0.24 | 2.86 |
| Samples | TTI (s) | PHRR (kW/m2) | Av-HRR (kW/m2) | THR (MJ/m2) | TSP (m2) | FIGRA kW/(m2·s) | Residue (wt%) |
|---|---|---|---|---|---|---|---|
| Control cotton | 18 ± 3 | 110 | 15.9 | 4.5 | 2.8 | 2.76 | 5.5 |
| PAP-200 | - * | 14 | 7.3 | 2.3 | 0.4 | 0.12 | 13.1 |
| PAPBTCA-200 | - * | 11 | 6.8 | 2.1 | 0.5 | 0.07 | 20.7 |
| Samples | Crease Sharp Return Angle (°) | Total (°) | Crease Slow Return Angle (°) | Total (°) | ||
|---|---|---|---|---|---|---|
| Warp | Weft | Warp | Weft | |||
| Control cotton | 79.9 | 80.9 | 160.8 | 83.5 | 85.2 | 168.7 |
| PAP-200 | 81.2 | 86.1 | 167.3 | 86.6 | 91.1 | 177.7 |
| PAPBTCA-200 | 91.6 | 93.1 | 184.7 | 114.7 | 116.2 | 230.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.-M.; Zhang, L.-Y.; Li, P.; Liu, Y. High-Efficient Flame-Retardant Finishing of Cotton Fabrics Based on Phytic Acid. Int. J. Mol. Sci. 2023, 24, 1093. https://doi.org/10.3390/ijms24021093
Song W-M, Zhang L-Y, Li P, Liu Y. High-Efficient Flame-Retardant Finishing of Cotton Fabrics Based on Phytic Acid. International Journal of Molecular Sciences. 2023; 24(2):1093. https://doi.org/10.3390/ijms24021093
Chicago/Turabian StyleSong, Wan-Meng, Li-Yao Zhang, Ping Li, and Yun Liu. 2023. "High-Efficient Flame-Retardant Finishing of Cotton Fabrics Based on Phytic Acid" International Journal of Molecular Sciences 24, no. 2: 1093. https://doi.org/10.3390/ijms24021093




