Adrenal Dysfunction in Mitochondrial Diseases
Abstract
1. Introduction
2. Adrenals
2.1. Cortisol
2.2. HPA Axis Regulation of Cortisol
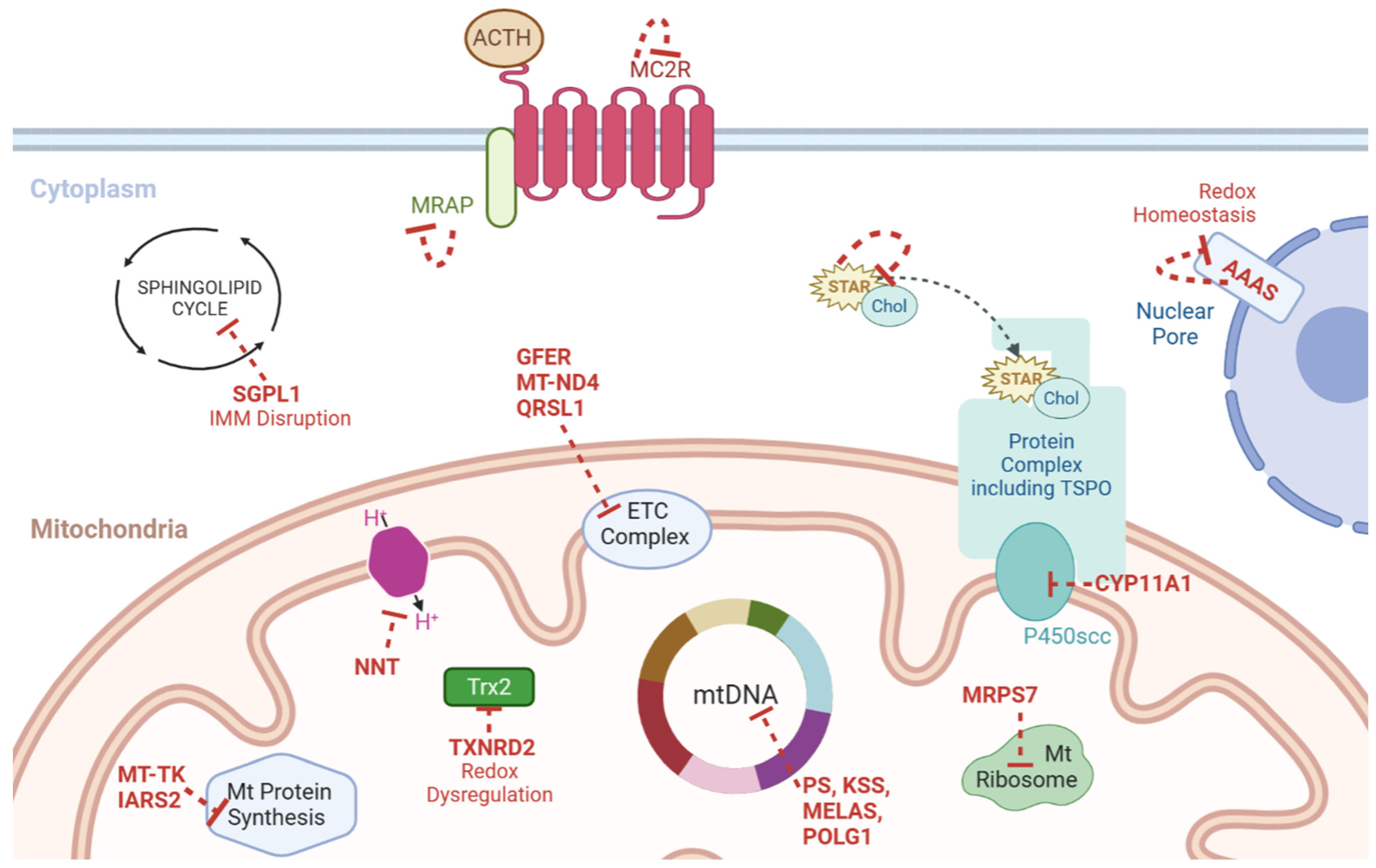
3. Steroidogenesis—Cholesterol Import and Mobilisation
4. Steroidogenesis and Mitochondria
4.1. Mitochondrial Membranes
4.2. Cholesterol Shuttle
4.3. The Start and the End
4.4. Genetic Profiling
4.5. P450 Enzymes
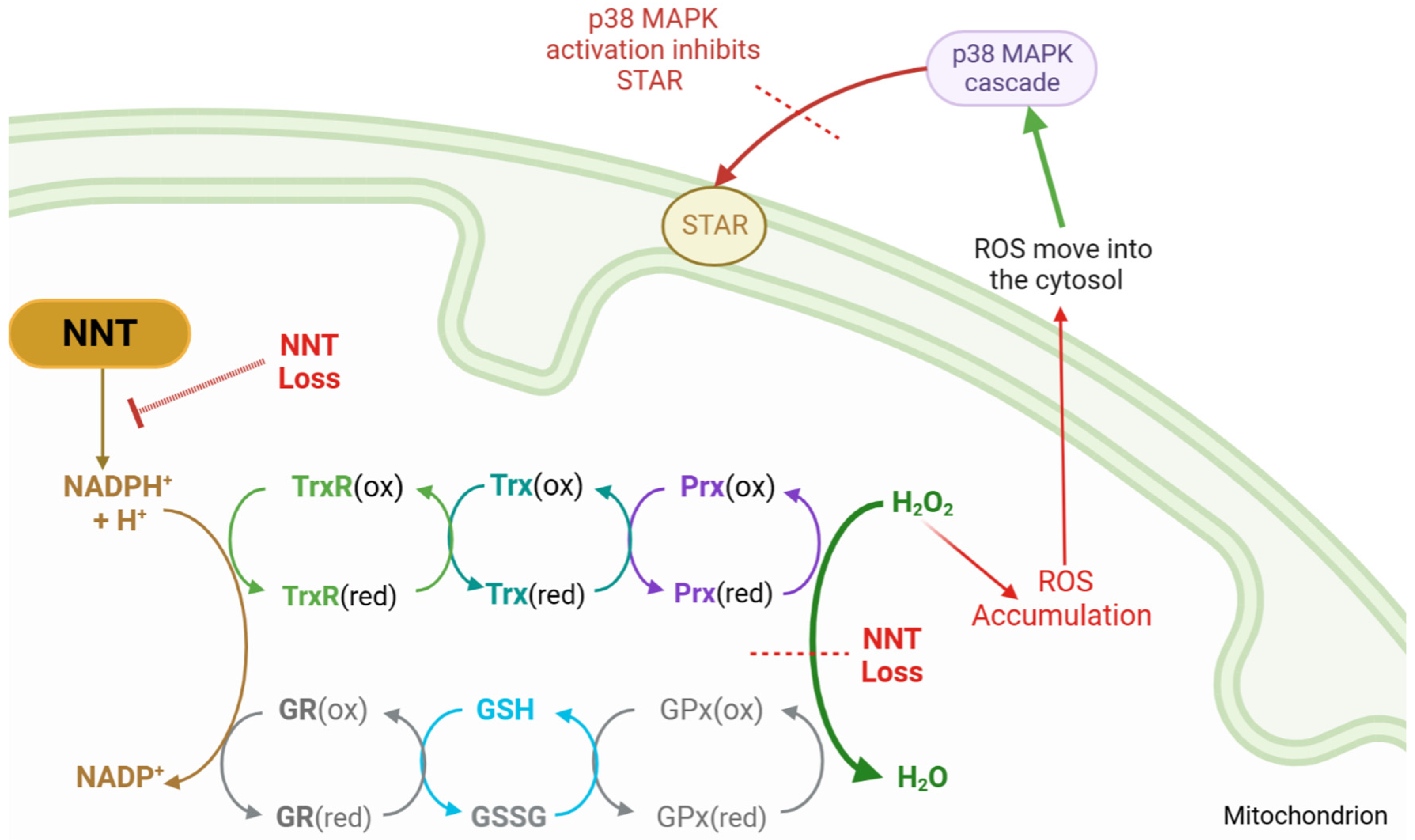
4.6. Mitochondrial Reactive Oxygen Species
5. Mitochondria and Oxidative Stress
5.1. Antioxidant Mechanisms
5.2. Reduction Systems
5.3. IMM Proton Carriers
6. Genetic Profiling of Mitochondrial Genes
6.1. NNT
6.2. TXNRD2
6.3. Syndromic Presentations
7. Literature Review
Syndromes and Endocrine Abnormalities
8. Literature Review Results
8.1. Patient Registries
8.2. mtDNA Deletion Syndromes
8.3. Mutations in Nuclear DNA
9. Discussion
9.1. Deletion Syndromes
9.2. Non-Deletion Mutations
10. Future Research
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Familial Glucocorticoid Deficiency
Appendix A.1. Familial Glucocorticoid Deficiency (FGD)
Appendix A.2. Incidence and Presentation
Appendix A.3. Diagnosis and Management
Appendix B. Kearns–Sayre Syndrome and Pearson Syndrome
References
- Miller, W.L. A brief history of adrenal research: Steroidogenesis—The soul of the adrenal. Mol. Cell. Endocrinol. 2013, 371, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Miller, W.L. Role of mitochondria in steroidogenesis. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 771–790. [Google Scholar] [CrossRef]
- Yoo, H.-W. Diverse etiologies, diagnostic approach, and management of primary adrenal insufficiency in pediatric age. Ann. Pediatr. Endocrinol. Metab. 2021, 26, 149–157. [Google Scholar] [CrossRef]
- Calderwood, L.; Holm, I.A.; Teot, L.A.; Anselm, I. Adrenal Insufficiency in Mitochondrial Disease: A Rare Case of GFER-Related Mitochondrial Encephalomyopathy and Review of the Literature. J. Child Neurol. 2015, 31, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Choi, M.H. Embryonic Development and Adult Regeneration of the Adrenal Gland. Endocrinol. Metab. 2020, 35, 765. [Google Scholar] [CrossRef] [PubMed]
- Pignatti, E.; Flück, C.E. Adrenal cortex development and related disorders leading to adrenal insufficiency. Mol. Cell. Endocrinol. 2021, 527, 111206. [Google Scholar] [CrossRef]
- Abou Nader, N.; Boyer, A. Adrenal Cortex Development and Maintenance: Knowledge Acquired From Mouse Models. Endocrinology 2021, 162, bqab187. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Taves, M.D.; Ashwell, J.D. Glucocorticoids in T cell development, differentiation and function. Nat. Rev. Immunol. 2021, 21, 233–243. [Google Scholar] [CrossRef]
- Turan, S.; Hughes, C.; Atay, Z.; Guran, T.; Haliloglu, B.; Clark, A.J.L.; Bereket, A.; Metherell, L.A. An atypical case of familial glucocorticoid deficiency without pigmentation caused by coexistent homozygous mutations in MC2R (T152K) and MC1R (R160W). J. Clin. Endocrinol. Metab. 2012, 97, E771–E774. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, A.; Maudhoo, A.; Chan, L.F.; Novoselova, T.; Prasad, R.; Metherell, L.A.; Guasti, L. Isolated glucocorticoid deficiency: Genetic causes and animal models. J. Steroid Biochem. Mol. Biol. 2019, 189, 73–80. [Google Scholar] [CrossRef]
- Novoselova, T.V.; King, P.J.; Guasti, L.; Metherell, L.A.; Clark, A.J.L.; Chan, L.F. ACTH signalling and adrenal development: Lessons from mouse models. Endocr. Connect. 2019, 8, R122–R130. [Google Scholar] [CrossRef]
- Fridmanis, D.; Roga, A.; Klovins, J. ACTH Receptor (MC2R) Specificity: What Do We Know About Underlying Molecular Mechanisms? Front. Endocrinol. 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Metherell, L.A.; David, A.; Sharma, R.; Sharma, P.K.; Clark, A.J.L.; Chan, L.F. Neonatal presentation of familial glucocorticoid deficiency resulting from a novel splice mutation in the melanocortin 2 receptor accessory protein. Eur. J. Endocrinol. 2011, 165, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Meimaridou, E.; Hughes, C.R.; Kowalczyk, J.; Guasti, L.; Chapple, J.P.; King, P.J.; Chan, L.F.; Clark, A.J.L.; Metherell, L.A. Familial glucocorticoid deficiency: New genes and mechanisms. Mol. Cell. Endocrinol. 2013, 371, 195–200. [Google Scholar] [CrossRef]
- Flück, C.E. Mechanisms in Endocrinology: Update on pathogenesis of primary adrenal insufficiency: Beyond steroid enzyme deficiency and autoimmune adrenal destruction. Eur. J. Endocrinol. 2017, 177, R99–R111. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, M.; Pyakurel, A.; Glytsou, C.; Scorrano, L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 2020, 21, 204–224. [Google Scholar] [CrossRef]
- Kang, E.; Kim, Y.-M.; Kim, G.-H.; Lee, B.H.; Yoo, H.-W.; Choi, J.-H. Mutation Spectrum of STAR and the Founder Effect of p.Q258* in Korean Patients with Congenital Lipoid Adrenal Hyperplasia. Mol. Med. 2017, 23, 149. [Google Scholar] [CrossRef]
- Kim, C.J. Congenital lipoid adrenal hyperplasia. Ann. Pediatr. Endocrinol. Metab. 2014, 19, 179. [Google Scholar] [CrossRef]
- Bassi, G.; Sidhu, S.K.; Mishra, S. The Expanding Role of Mitochondria, Autophagy and Lipophagy in Steroidogenesis. Cells 2021, 10, 1851. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Fan, J.; Zirkin, B. Translocator Protein (18 kDa): An Update on Its Function in Steroidogenesis. J. Neuroendocrinol. 2018, 30, e12500. [Google Scholar] [CrossRef]
- Stevens, V.L.; Tribble, D.L.; Lambeth, J.D. Regulation of mitochondrial compartment volumes in rat adrenal cortex by ether stress. Arch. Biochem. Biophys. 1985, 242, 324–327. [Google Scholar] [CrossRef]
- Prasad, R.; Kowalczyk, J.C.; Meimaridou, E.; Storr, H.L.; Metherell, L.A. Oxidative stress and adrenocortical insufficiency. J. Endocrinol. 2014, 221, R63–R73. [Google Scholar] [CrossRef]
- Clark, A.J.L.; Grossman, A.; McLoughlin, L. Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet 1993, 341, 461–462. [Google Scholar] [CrossRef]
- Metherell, L.A.; Chapple, J.P.; Cooray, S.; David, A.; Becker, C.; Rüschendorf, F.; Naville, D.; Begeot, M.; Khoo, B.; Nürnberg, P.; et al. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat. Genet. 2005, 37, 166–170. [Google Scholar] [CrossRef]
- Gorrigan, R.J.; Guasti, L.; King, P.; Clark, A.J.; Chan, L.F. Localisation of the melanocortin-2-receptor and its accessory proteins in the developing and adult adrenal gland. J. Mol. Endocrinol. 2011, 46, 227–232. [Google Scholar] [CrossRef]
- Flück, C.E.; Pandey, A.V.; Dick, B.; Camats, N.; Fernández-Cancio, M.; Clemente, M.; Gussinyé, M.; Carrascosa, A.; Mullis, P.E.; Audi, L. Characterization of Novel StAR (Steroidogenic Acute Regulatory Protein) Mutations Causing Non-Classic Lipoid Adrenal Hyperplasia. PLoS ONE 2011, 6, e20178. [Google Scholar] [CrossRef]
- Maharaj, A.; Buonocore, F.; Meimaridou, E.; Ruiz-Babot, G.; Guasti, L.; Peng, H.-M.; Capper, C.P.; Burgos-Tirado, N.; Prasad, R.; Hughes, C.R.; et al. Predicted Benign and Synonymous Variants in CYP11A1 Cause Primary Adrenal Insufficiency Through Missplicing. J. Endocr. Soc. 2019, 3, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Goursaud, C.; Mallet, D.; Janin, A.; Menassa, R.; Tardy-Guidollet, V.; Russo, G.; Lienhardt-Roussie, A.; Lecointre, C.; Plotton, I.; Morel, Y.; et al. Aberrant Splicing Is the Pathogenicity Mechanism of the p.Glu314Lys Variant in CYP11A1 Gene. Front. Endocrinol. 2018, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hirschenson, J.; Melgar-Bermudez, E.; Mailloux, R.J. The Uncoupling Proteins: A Systematic Review on the Mechanism Used in the Prevention of Oxidative Stress. Antioxidants 2022, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, J.B.; Haigis, M.C. The Multifaceted Contributions of Mitochondria to Cellular Metabolism. Nat. Cell Biol. 2018, 20, 745. [Google Scholar] [CrossRef]
- Chanoine, J.-P.; Compagnone, N.A.; Wong, A.C.K.; Mellon, S.H. Modulation of steroidogenesis by selenium in a novel adrenal cell line developed using targeted tumorigenesis. BioFactors 2001, 14, 229–238. [Google Scholar] [CrossRef]
- Cox, A.G.; Winterbourn, C.C.; Hampton, M.B. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem. J. 2010, 425, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Kil, I.S.; Lee, S.K.; Ryu, K.W.; Woo, H.A.; Hu, M.-C.; Bae, S.H.; Rhee, S.G. Feedback Control of Adrenal Steroidogenesis via H2O2-Dependent, Reversible Inactivation of Peroxiredoxin III in Mitochondria. Mol. Cell 2012, 46, 584–594. [Google Scholar] [CrossRef]
- Jazayeri, O.; Liu, X.; van Diemen, C.C.; Bakker-van Waarde, W.M.; Sikkema-Raddatz, B.; Sinke, R.J.; Zhang, J.; van Ravenswaaij-Arts, C.M.A. A novel homozygous insertion and review of published mutations in the NNT gene causing familial glucocorticoid deficiency (FGD). Eur. J. Med. Genet. 2015, 58, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Meimaridou, E.; Kowalczyk, J.; Guasti, L.; Hughes, C.R.; Wagner, F.; Frommolt, P.; Nürnberg, P.; Mann, N.P.; Banerjee, R.; Saka, H.N.; et al. Mutations in NNT encoding nicotinamide nucleotide transhydrogenase cause familial glucocorticoid deficiency. Nat. Genet. 2012, 44, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Meimaridou, E.; Goldsworthy, M.; Chortis, V.; Fragouli, E.; Foster, P.A.; Arlt, W.; Cox, R.; Metherell, L.M. NNT is a key regulator of adrenal redox homeostasis and steroidogenesis in male mice. J. Endocrinol. 2018, 236, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Chan, L.F.; Hughes, C.R.; Kaski, J.P.; Kowalczyk, J.C.; Savage, M.O.; Peters, C.J.; Nathwani, N.; Clark, A.J.L.; Storr, H.L.; et al. Thioredoxin Reductase 2 (TXNRD2) mutation associated with familial glucocorticoid deficiency (FGD). J. Clin. Endocrinol. Metab. 2014, 99, E1556–E1563. [Google Scholar] [CrossRef]
- Chow, J.; Rahman, J.; Achermann, J.C.; Dattani, M.T.; Rahman, S. Mitochondrial disease and endocrine dysfunction. Nat. Rev. Endocrinol. 2017, 13, 92–104. [Google Scholar] [CrossRef]
- Habeb, A.M.; Hughes, C.R.; Al-Arabi, R.; Al-Muhamadi, A.; Clark, A.J.L.; Metherell, L.A. Familial glucocorticoid deficiency: A diagnostic challenge during acute illness. Eur. J. Pediatr. 2013, 172, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, A.; Güran, T.; Buonocore, F.; Achermann, J.C.; Metherell, L.; Prasad, R.; Çetinkaya, S. Insights From Long-term Follow-up of a Girl With Adrenal Insufficiency and Sphingosine-1-Phosphate Lyase Deficiency. J. Endocr. Soc. 2022, 6, bvac020. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, A.; Williams, J.; Bradshaw, T.; Güran, T.; Braslavsky, D.; Casas, J.; Chan, L.F.; Metherell, L.A.; Prasad, R. Sphingosine-1-phosphate lyase (SGPL1) deficiency is associated with mitochondrial dysfunction. J. Steroid Biochem. Mol. Biol. 2020, 202, 105730. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Hadjidemetriou, I.; Maharaj, A.; Meimaridou, E.; Buonocore, F.; Saleem, M.; Hurcombe, J.; Bierzynska, A.; Barbagelata, E.; Bergadá, I.; et al. Sphingosine-1-phosphate lyase mutations cause primary adrenal insufficiency and steroid-resistant nephrotic syndrome. J. Clin. Investig. 2017, 127, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Al-Gadi, I.S.; Haas, R.H.; Falk, M.J.; Goldstein, A.; McCormack, S.E. Endocrine Disorders in Primary Mitochondrial Disease. J. Endocr. Soc. 2018, 2, 361–373. [Google Scholar] [CrossRef]
- Schaefer, A.M.; Walker, M.; Turnbull, D.M.; Taylor, R.W. Endocrine disorders in mitochondrial disease. Mol. Cell. Endocrinol. 2013, 379, 2–11. [Google Scholar] [CrossRef]
- Endres, D.; Süß, P.; Maier, S.J.; Friedel, E.; Nickel, K.; Ziegler, C.; Fiebich, B.L.; Glocker, F.X.; Stock, F.; Egger, K.; et al. New Variant of MELAS Syndrome With Executive Dysfunction, Heteroplasmic Point Mutation in the MT-ND4 Gene (m.12015T>C; p.Leu419Pro) and Comorbid Polyglandular Autoimmune Syndrome Type 2. Front. Immunol. 2019, 10, 412. [Google Scholar] [CrossRef]
- Hopkins, S.E.; Somoza, A.; Gilbert, D.L. Rare autosomal dominant POLG1 mutation in a family with metabolic strokes, posterior column spinal degeneration, and multi-endocrine disease. J. Child Neurol. 2010, 25, 752–756. [Google Scholar] [CrossRef]
- Sanaker, P.S.; Husebye, E.S.; Fondenes, O.; Bindoff, L.A. Clinical evolution of Kearns-Sayre syndrome with polyendocrinopathy and respiratory failure. Acta Neurol. Scand. Suppl. 2007, 187, 64–67. [Google Scholar] [CrossRef]
- Artuch, R.; Pavía, C.; Playán, A.; Vilaseca, M.A.; Colomer, J.; Valls, C.; Rissech, M.; González, M.A.; Pou, A.; Briones, P.; et al. Multiple endocrine involvement in two pediatric patients with Kearns-Sayre syndrome. Horm. Res. 1998, 50, 99–104. [Google Scholar] [CrossRef]
- Son, J.S.; Seo, G.H.; Kim, Y.-M.; Kim, G.-H.; Jin, H.K.; Bae, J.-S.; Im, H.J.; Yoo, H.-W.; Lee, B.H. Clinical and genetic features of four patients with Pearson syndrome: An observational study. Medicine 2022, 101, e28793. [Google Scholar] [CrossRef] [PubMed]
- Farruggia, P.; Di Cataldo, A.; Pinto, R.M.; Palmisani, E.; Macaluso, A.; Valvo, L.L.; Cantarini, M.E.; Tornesello, A.; Corti, P.; Fioredda, F.; et al. Pearson Syndrome: A Retrospective Cohort Study from the Marrow Failure Study Group of A.I.E.O.P. (Associazione Italiana Emato-Oncologia Pediatrica). In JIMD Reports; Morava, E., Baumgartner, M., Patterson, M., Rahman, S., Zschocke, J., Peters, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 26, pp. 37–43. [Google Scholar] [CrossRef]
- O’Grady, M.J.; Monavari, A.A.; Cotter, M.; Murphy, N.P. Sideroblastic anaemia and primary adrenal insufficiency due to a mitochondrial respiratory chain disorder in the absence of mtDNA deletion. BMJ Case Rep. 2015, 2015, bcr2014208514. [Google Scholar] [CrossRef]
- Nicolino, M.; Ferlin, T.; Forest, M.; Godinot, C.; Carrier, H.; David, M.; Chatelain, P.; Mousson, B. Identification of a large-scale mitochondrial deoxyribonucleic acid deletion in endocrinopathies and deafness: Report of two unrelated cases with diabetes mellitus and adrenal insufficiency, respectively. J. Clin. Endocrinol. Metab. 1997, 82, 3063–3067. [Google Scholar] [CrossRef]
- Boles, R.G.; Roe, T.; Senadheera, D.; Mahnovski, V.; Wong, L.J. Mitochondrial DNA deletion with Kearns Sayre syndrome in a child with Addison disease. Eur. J. Pediatr. 1998, 157, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Bruno, C.; Minetti, C.; Tang, Y.; Magalhães, P.J.; Santorelli, F.M.; Shanske, S.; Bado, M.; Cordone, G.; Gatti, R.; DiMauro, S. Primary adrenal insufficiency in a child with a mitochondrial DNA deletion. J. Inherit. Metab. Dis. 1998, 21, 155–161. [Google Scholar] [CrossRef]
- Duran, G.P.; Martinez-Aguayo, A.; Poggi, H.; Lagos, M.; Gutierrez, D.; Harris, P.R. Large Mitochondrial DNA Deletion in an Infant with Addison Disease. JIMD Rep. 2011, 3, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Tzoufi, M.; Makis, A.; Chaliasos, N.; Nakou, I.; Siomou, E.; Tsatsoulis, A.; Zikou, A.; Argyropoulou, M.; Bonnefont, J.P.; Siamopoulou, A. A rare case report of simultaneous presentation of myopathy, Addison’s disease, primary hypoparathyroidism, and Fanconi syndrome in a child diagnosed with Kearns–Sayre syndrome. Eur. J. Pediatr. 2013, 172, 557–561. [Google Scholar] [CrossRef]
- Williams, T.B.; Daniels, M.; Puthenveetil, G.; Chang, R.; Wang, R.Y.; Abdenur, J.E. Pearson syndrome: Unique endocrine manifestations including neonatal diabetes and adrenal insufficiency. Mol. Genet. Metab. 2012, 106, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Ribes, A.; Riudor, E.; Valcárel, R.; Salvá, A.; Castelló, F.; Murillo, S.; Dominguez, C.; Rötig, A.; Jakobs, C. Pearson syndrome: Altered tricarboxylic acid and urea-cycle metabolites, adrenal insufficiency and corneal opacities. J. Inherit. Metab. Dis. 1993, 16, 537–540. [Google Scholar] [CrossRef] [PubMed]
- DeBalsi, K.L.; Longley, M.J.; Hoff, K.E.; Copeland, W.C. Synergistic Effects of the in cis T251I and P587L Mitochondrial DNA Polymerase γ Disease Mutations. J. Biol. Chem. 2017, 292, 4198–4209. [Google Scholar] [CrossRef]
- Da Pozzo, P.; Cardaioli, E.; Rubegni, A.; Gallus, G.N.; Malandrini, A.; Rufa, A.; Battisti, C.; Carluccio, M.A.; Rocchi, R.; Giannini, F.; et al. Novel POLG mutations and variable clinical phenotypes in 13 Italian patients. Neurol. Sci. 2017, 38, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Sugiana, C.; Pagliarini, D.J.; McKenzie, M.; Kirby, D.M.; Salemi, R.; Abu-Amero, K.K.; Dahl, H.-H.M.; Hutchison, W.M.; Vascotto, K.A.; Smith, S.M.; et al. Mutation of C20orf7 Disrupts Complex I Assembly and Causes Lethal Neonatal Mitochondrial Disease. Am. J. Hum. Genet. 2008, 83, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Hannah-Shmouni, F.; Stratakis, C.A. An overview of inborn errors of metabolism manifesting with primary adrenal insufficiency. Rev. Endocr. Metab. Disord. 2018, 19, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Dursun, F.; Genc, H.M.; Mine Yılmaz, A.; Tas, I.; Eser, M.; Pehlivanoglu, C.; Yilmaz, B.K.; Guran, T. Primary adrenal insufficiency in a patient with biallelic QRSL1 mutations. Eur. J. Endocrinol. 2022, 187, K27–K32. [Google Scholar] [CrossRef] [PubMed]
- North, K.; Korson, M.S.; Krawiecki, N.; Shoffner, J.M.; Holm, I.A. Oxidative phosphorylation defect associated with primary adrenal insufficiency. J. Pediatr. 1996, 128, 688–692. [Google Scholar] [CrossRef]
- Menezes, M.J.; Guo, Y.; Zhang, J.; Riley, L.G.; Cooper, S.T.; Thorburn, D.R.; Li, J.; Dong, D.; Li, Z.; Glessner, J.; et al. Mutation in mitochondrial ribosomal protein S7 (MRPS7) causes congenital sensorineural deafness, progressive hepatic and renal failure and lactic acidemia. Hum. Mol. Genet. 2015, 24, 2297–2307. [Google Scholar] [CrossRef]
- Vona, B.; Maroofian, R.; Bellacchio, E.; Najafi, M.; Thompson, K.; Alahmad, A.; He, L.; Ahangari, N.; Rad, A.; Shahrokhzadeh, S.; et al. Expanding the clinical phenotype of IARS2-related mitochondrial disease. BMC Med. Genet. 2018, 19, 196. [Google Scholar] [CrossRef]
- Genetic and Rare Diseases Information Center (GARD). Genetic and Rare Diseases Information Center (GARD) Kearns-Sayre Syndrome|Genetic and Rare Diseases Information Center (GARD)—An NCATS Program [Internet]. 2021. Available online: https://rarediseases.info.nih.gov/diseases/6817/kearns-sayre-syndrome (accessed on 4 May 2022).
- Rötig, A.; Bourgeron, T.; Chretien, D.; Rustin, P.; Munnich, A. Spectrum of mitochondrial DNA rearrangements in the Pearson marrow-pancreas syndrome. Hum. Mol. Genet. 1995, 4, 1327–1330. [Google Scholar] [CrossRef]
- Afroze, B.; Amjad, N.; Ibrahim, S.H.; Humayun, K.N.; Yakob, Y. Adrenal insufficiency in a child with MELAS syndrome. Brain Dev. 2014, 36, 924–927. [Google Scholar] [CrossRef]
- Chan, L.F.; Campbell, D.C.; Novoselova, T.V.; Clark, A.J.L.; Metherell, L.A. Whole-Exome Sequencing in the Differential Diagnosis of Primary Adrenal Insufficiency in Children. Front. Endocrinol. 2015, 6, 113. [Google Scholar] [CrossRef]
- Mohri, I.; Taniike, M.; Fujimura, H.; Matsuoka, T.; Inui, K.; Nagai, T.; Okada, S. A case of Kearns-Sayre syndrome showing a constant proportion of deleted mitochondrial DNA in blood cells during 6 years of follow-up. J. Neurol. Sci. 1998, 158, 106–109. [Google Scholar] [CrossRef] [PubMed]

| Author | Year | Gene Affected/ Mutation | Resultant Pathology | Diagnosis | Gender | Age of PAI | Other Clinical Symptoms | Impaired MC | Impaired GC | PAI? | Other Endocrine Dysfunction? |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ribes | 1993 | 1.5 kb mtDNA deletion | Complex 4 deficiency | Pearson | Male | Metabolic derangement and acidosis, bilateral corneal opacities, anaemia | Y | Y | Unknown | Unknown | |
| Nicolino | 1997 | 7.436 kb mtDNA deletion | Complex 1 deficiency, impaired mtDNA translation | Not stated | Female | 4 | Short stature, cognitive decline, sensorineural hearing loss, ophthalmic involvement, impaired glucose tolerance | Y | Y | Y | Y |
| Artuch | 1998 | 6.7 kb mtDNA deletion | Loss of 10–45% mtDNA | KSS | Male | 16 | Bilateral degenerative retinopathy, developmental delay, complete heart block, T2DM, gynecomastia, obesity, hyperpigmentation | Unknown | Y | Autoimmune | Y |
| Artuch | 1998 | 6.7 kb mtDNA deletion | Loss of 64–66% mtDNA | KSS | Male | 7 | hyperpigmentation, growth retardation, ptosis, hypoparathyroidism | Unknown | Y | Y | Y |
| Boles | 1998 | 4.9 kb mtDNA deletion | 65% mutated adrenal mtDNA, trace scar tissue in adrenals | KSS | Male | 5 | Growth retardation, RTA, developmental delay, ophthalmoplegia, hyperpigmentation, lactic acidosis, | Unknown | Y | Y | N |
| Bruno | 1998 | 6.9 kb mtDNA deletion | Complex 1 & 4 deficiency | Not stated | Female | 4 | Developmental delay, hyperpigmentation, growth retardation, hypotonia, lactic acidosis, hypoparathyroidism | N | Y | Y | Y |
| Sanaker | 2007 | 3–4 kb mtDNA deletion | Impaired Oxidative Phosphorylation | KSS | Female | 32 | Ophthalmoplegia, short stature, RBBB, myopathy, hypothyroidism, Type 2 respiratory failure | Y | Y | Autoimmune | Y |
| Sugiana | 2008 | NDUFAF5 gene—c.719C>T (C20orf) | Impaired complex 1 assembly | Not stated | Male | 7 d | Lactic acidosis, congenital abnormalities, hypotension | Unknown | Y | Y | N |
| Hopkins | 2010 | POLG1 gene—c.1550G>T (p.G517V) | N/A | Not stated | Female | 10 | Type 1 Diabetes, hypothyroidism, seizures, chorea, psychiatric involvement, hypercalcaemia | Unknown | Unknown | Autoimmune | Y |
| Hopkins | 2010 | POLG1 gene—c.1550G>T (p.G517V) | N/A | Not stated | Female | 11 | Type 1 diabetes, hypothyroidism, bilateral basal ganglia pathologies, psychiatric involvement, hypercalcaemia | Unknown | Unknown | Autoimmune | Y |
| Duran | 2011 | 7.3 kb mtDNA deletion | N/A | Not stated | Male | 3 | Short stature, hyperpigmentation, chronic pancreatitis, congenital glaucoma | N | Y | Autoimmune | Y |
| Tzoufi | 2012 | 9 kb mtDNA deletion | N/A | KSS | Male | 5 | Lactic acidosis, myopathy, hyperpigmentation, hypoparathyroidism, Fanconi syndrome | Y | Y | Y | Y |
| Williams | 2012 | 5.1 kb mtDNA deletion | N/A | Pearson | Male | 4 | Anaemia, febrile seizures, hyperpigmentation, hypertonia, lactic acidosis, leucopoenia, thrombocytopaenia | Y | Y | Y | N |
| Afroze | 2014 | m.8344A>G | N/A | MELAS | Male | 5 | Febrile seizures, hyperpigmentation, hypertonia, lactic acidosis | N | Y | Y | N |
| Menes | 2015 | MRPS7 gene—c.550A>G (p.Met184Val) | Impaired mtDNA translation | Not stated | Female | 16 | Sensorineural deafness, lactic acidosis, primary hypogonadism | Unknown | Y | Y | Y |
| North, Calderwood | 1996, 2015 | GFER gene—c.581 G>A (R194 H), c.373 C>T | No complex 1 activity, Impaired 2,3,4 activity | Mitochondrial encephalo-myopathy | Female | 7 m | Lactic acidosis, growth retardation, bilateral cataracts, hyperpigmentation, hepatomegaly | N | Y | Y | N |
| O’Grady | 2015 | Unknown | Complex 4 deficiency | Not Stated | Male | 13 | Sideroblastic anaemia, neurological dysfunction, renal tubulopathy, faltering growth, Fanconi syndrome, T1DM | Y | Y | Y | Y |
| Kohda | 2016 | QRSL1 gene—c.398G>T (p.G133V) | I, II, III, and IV complex deficiencies | lethal infantile mitochondrial disease (LIMD) | Female | <1 m | Hypertrophic cardiomyopathy, hearing loss | Unknown | Y | Unknown | N |
| Farruggia | 2016 | Not described | Unknown | Pearson | Unknown | 2.9 | Hepatomegaly, growth impairment, ventricular wall thickness, neurological involvement, severe infections, hyperlactatemia | Y | Y | Unknown | N |
| Farruggia | 2016 | Not described | Unknown | Pearson | Unknown | Hepatomegaly, splenomegaly, ventricular wall thickness, severe infections, hyperlactatemia | Y | Y | Unknown | N | |
| Vona | 2018 | IARS2 gene—c.2725C>T (p.Pro909Ser) | mt-tRNA malformation | CAGSSS | Male | 20.6 | Congenital cataracts, short stature, GH deficiency, sensorineural hearing loss, peripheral neuropathy, Type II achalasia | N | Y | Y | Y |
| Endres | 2019 | MT-ND4 gene—m.12015T>C (p.Leu419Pro) | Complex 1 subunit malformation | MELAS | Female | 25 | Dysexecutive syndrome, muscular fatigue, Hashimoto’s thyroiditis | Y | y | Autoimmune | Y |
| Son Soo | 2022 | 2.3 kb mtDNA deletion | Unknown | Pearson | Female | 6 | Seizures, ketotic hypoglycaemia, failure to thrive, pancreatic insufficiency, macrocytic anaemia | Unknown | Y | Unknown | Y |
| Dursun | 2022 | c.300T>A; Y100 * and c.610G>A; G204R | QRSL1 | COXPD40 | Male | 6 | Developmental delay sensorineural deafness, cardiomyopathy, renal impairment, lactic acidosis, hypertension | N | Y | Y | N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corkery-Hayward, M.; Metherell, L.A. Adrenal Dysfunction in Mitochondrial Diseases. Int. J. Mol. Sci. 2023, 24, 1126. https://doi.org/10.3390/ijms24021126
Corkery-Hayward M, Metherell LA. Adrenal Dysfunction in Mitochondrial Diseases. International Journal of Molecular Sciences. 2023; 24(2):1126. https://doi.org/10.3390/ijms24021126
Chicago/Turabian StyleCorkery-Hayward, Madeleine, and Louise A. Metherell. 2023. "Adrenal Dysfunction in Mitochondrial Diseases" International Journal of Molecular Sciences 24, no. 2: 1126. https://doi.org/10.3390/ijms24021126
APA StyleCorkery-Hayward, M., & Metherell, L. A. (2023). Adrenal Dysfunction in Mitochondrial Diseases. International Journal of Molecular Sciences, 24(2), 1126. https://doi.org/10.3390/ijms24021126






