Data-Driven Approaches Used for Compound Library Design for the Treatment of Parkinson’s Disease
Abstract
1. Introduction
2. Results
2.1. Compounds and Pharmacological Targets against PD
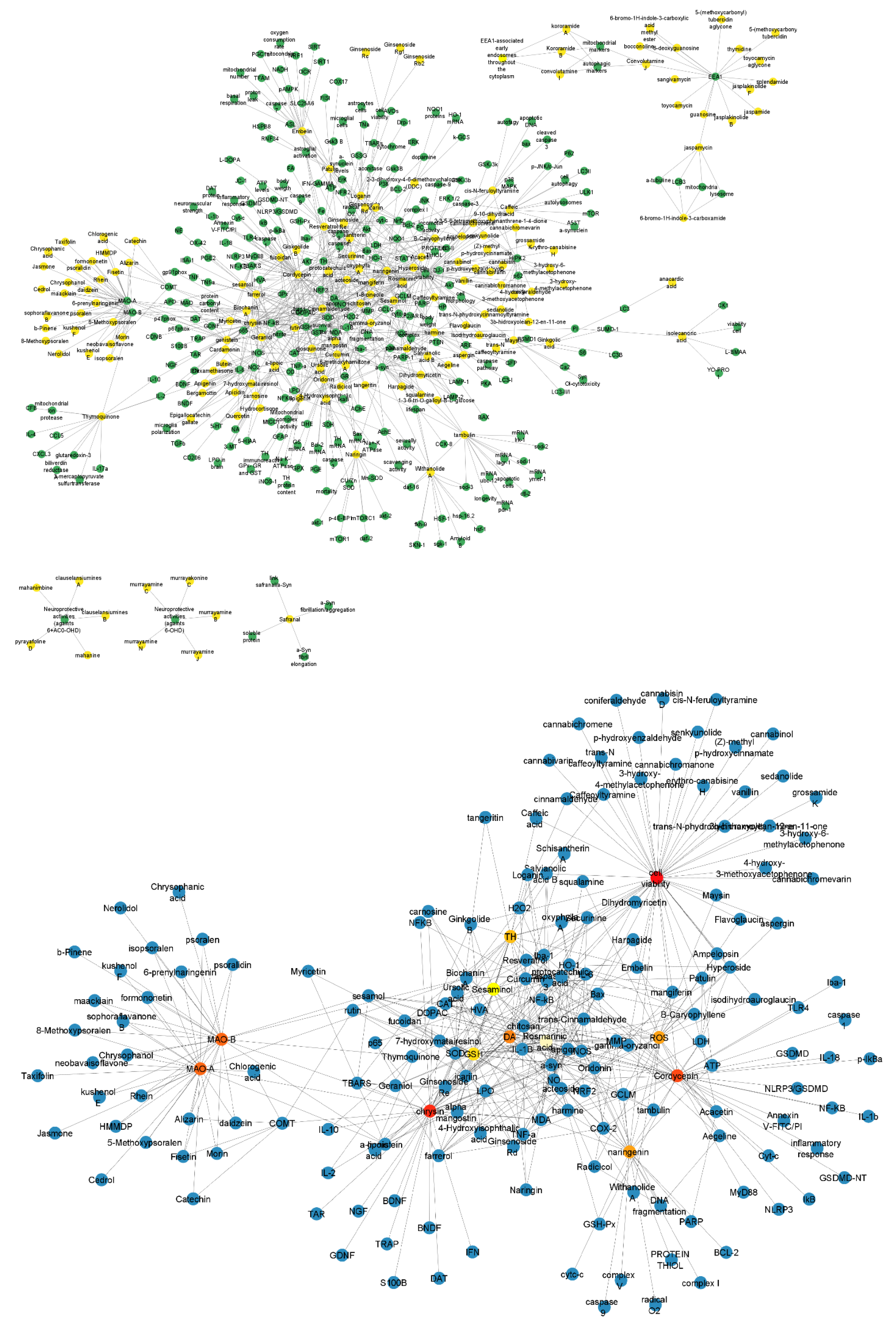
2.2. A Network between Drugs and Targets for Parkinson’s
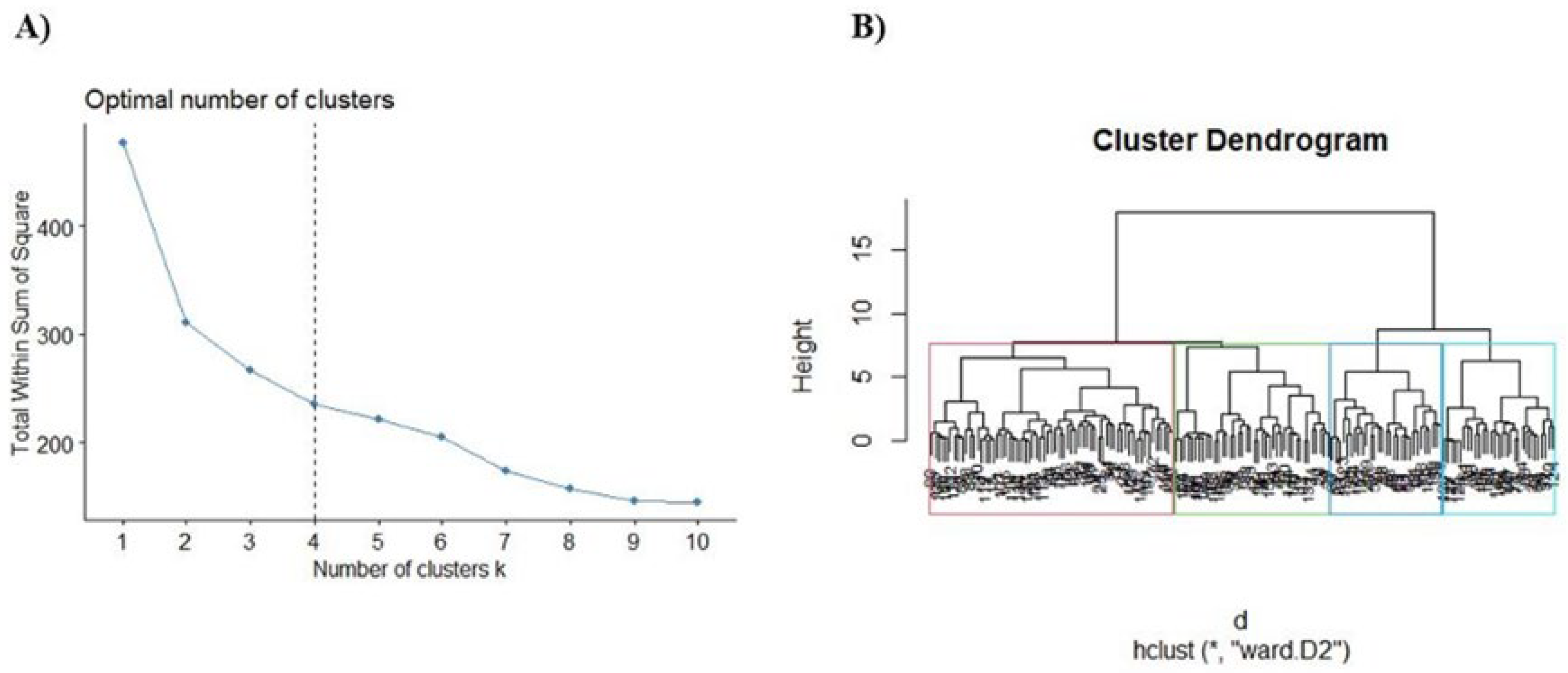
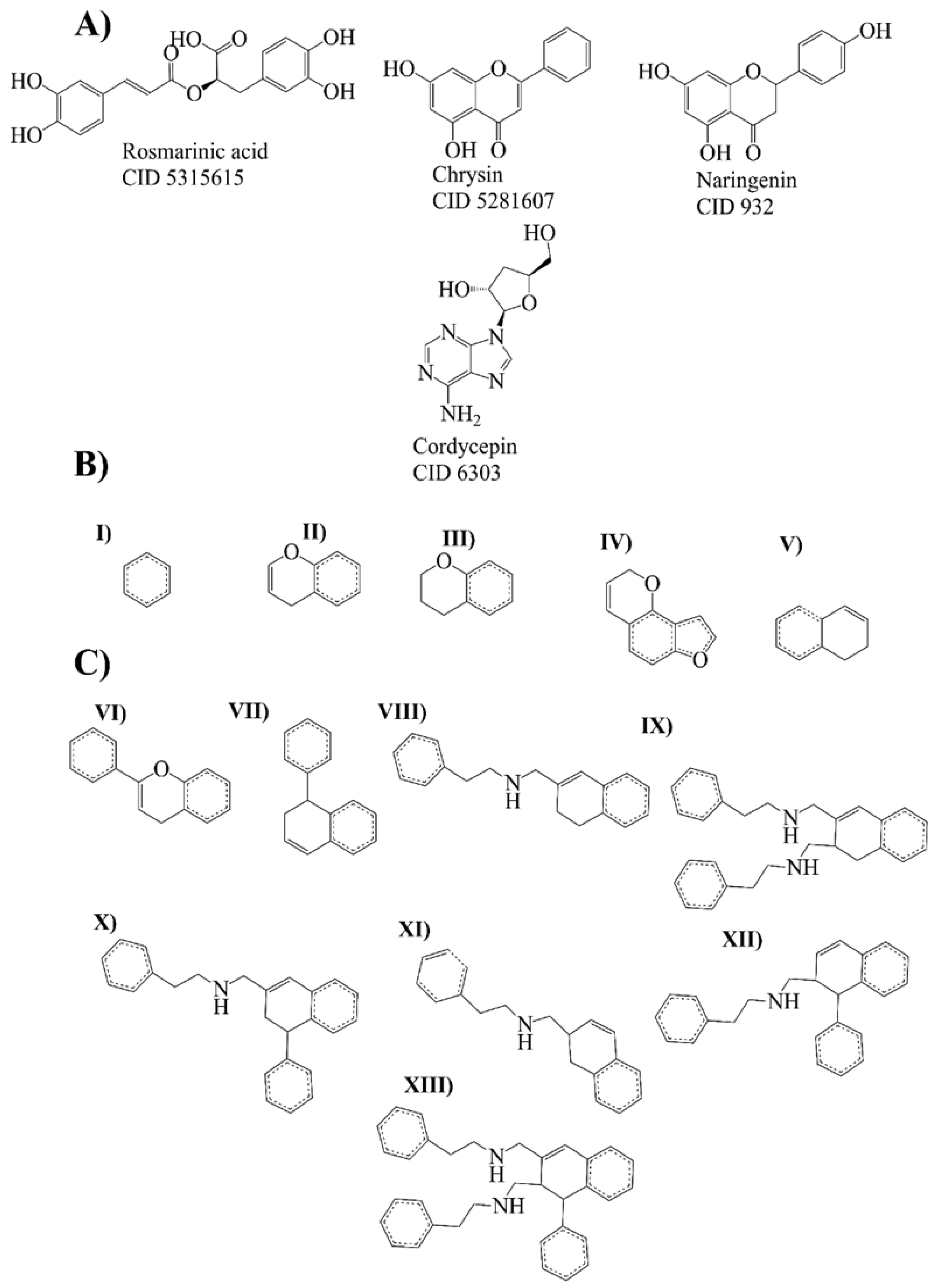
2.3. Bemis-Murcko Scaffold Analysis
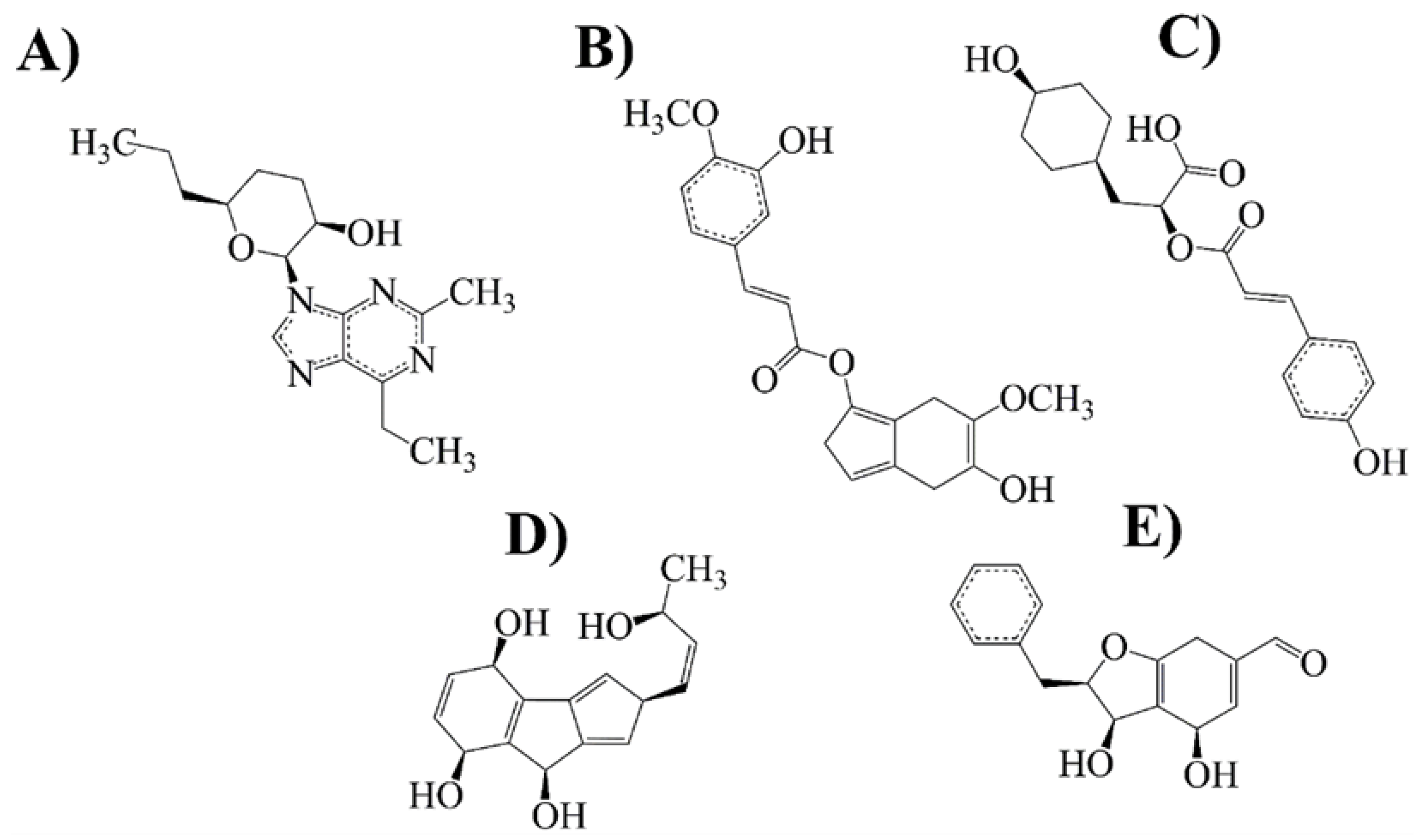
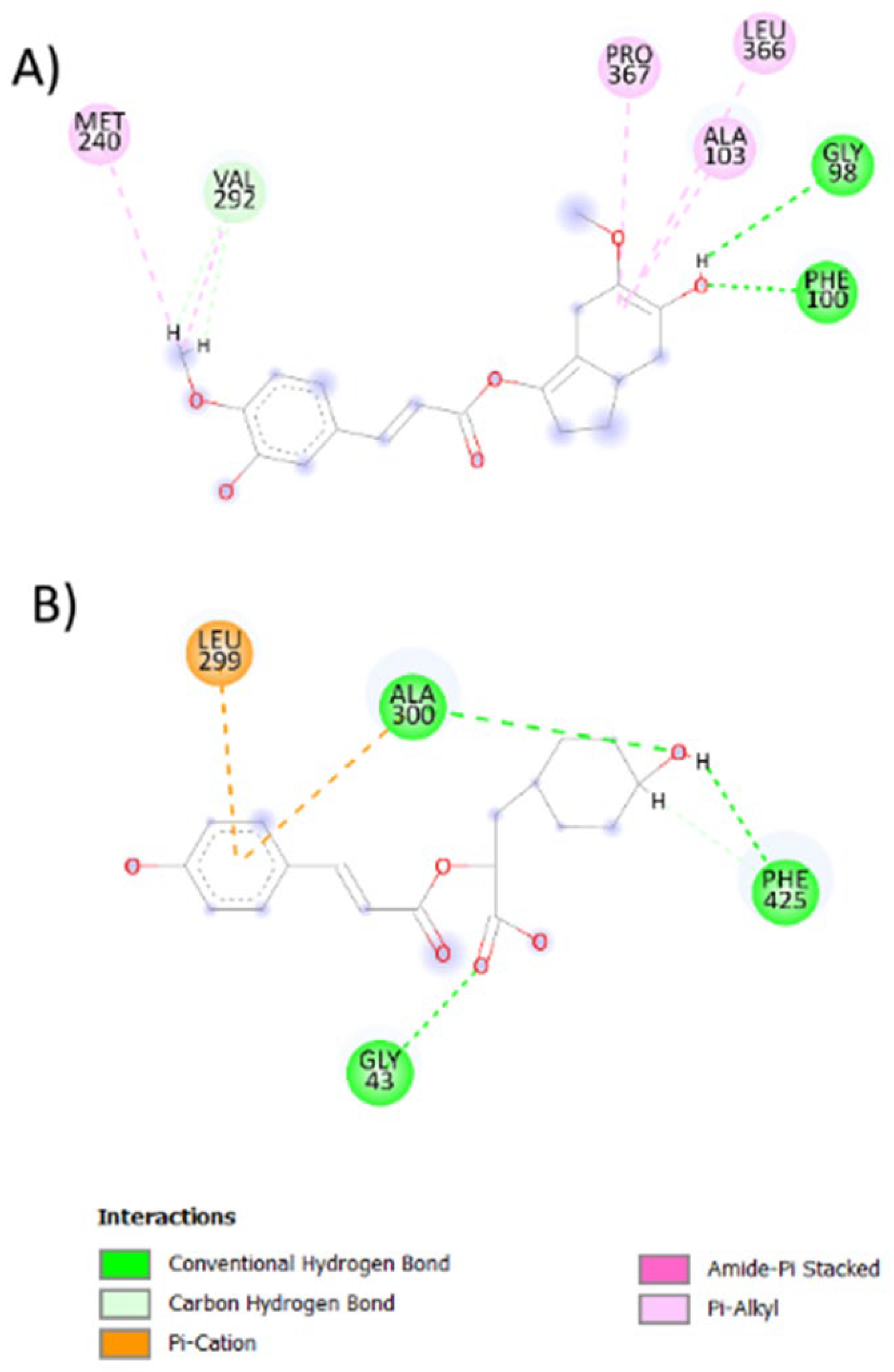
2.4. Evolutionary Library and Docking with Targets of PD
2.5. Drug-Likeness and Lead-Likeness Analysis
2.6. Antioxidant Activity against P450 and NO
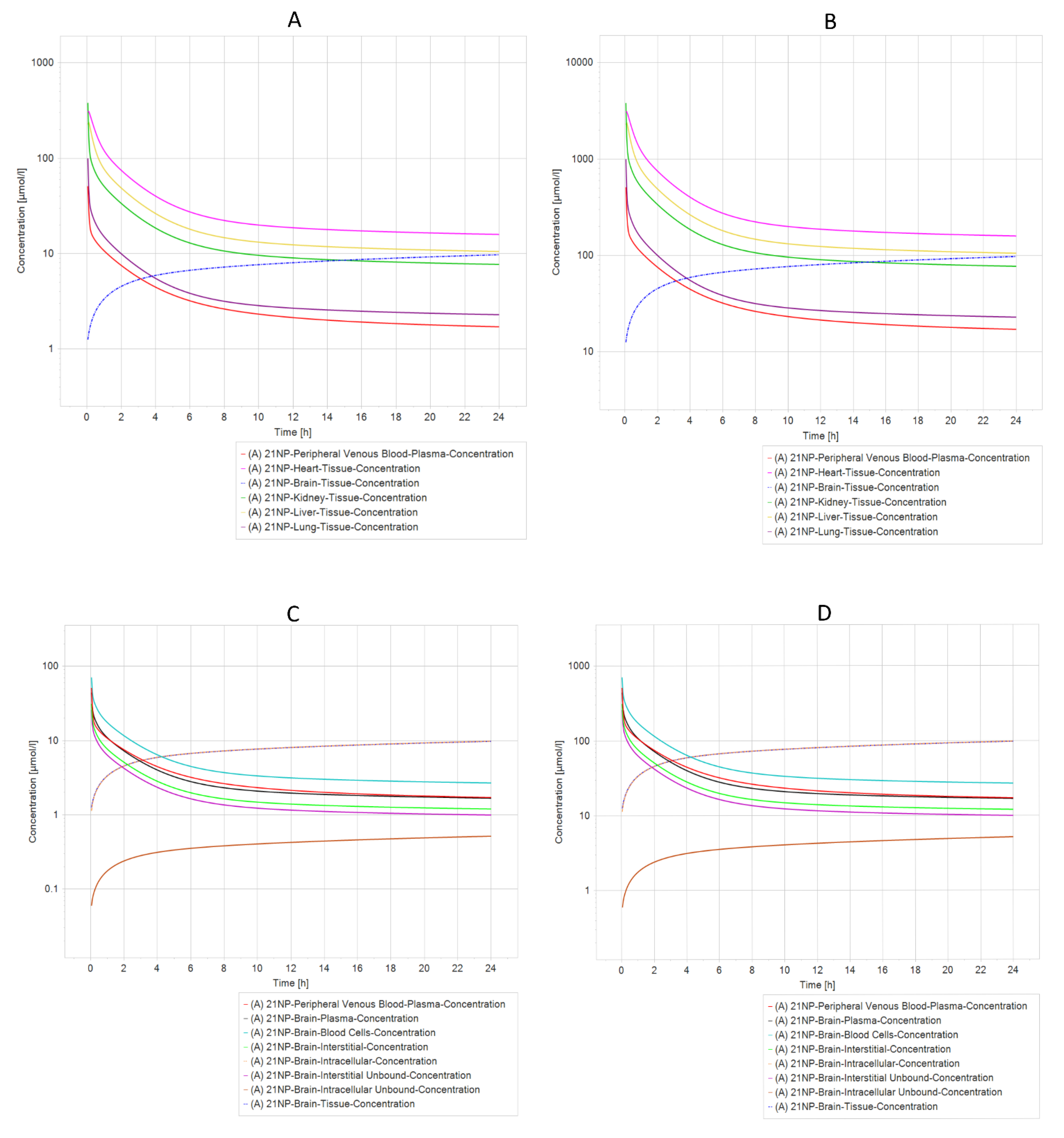
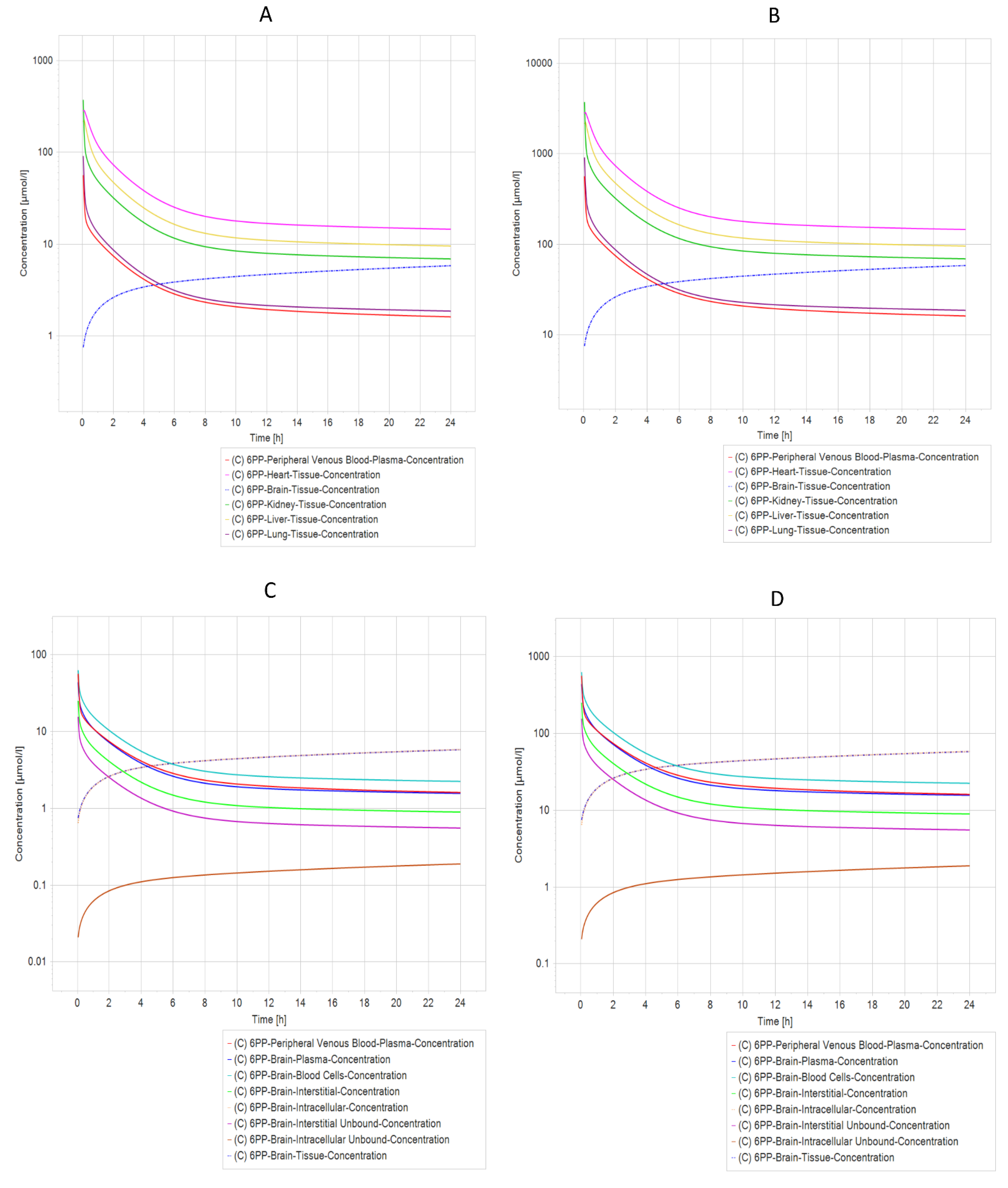
2.7. Pharmacokinetic Prediction (PBPK Modeling)
3. Discussion
4. Materials and Methods
4.1. Data Curation
4.2. Structural Network Analysis
4.3. Fingerprints Analysis
4.4. Bemis Murcko Scaffold Analysis
4.5. Evolutionary Library Construction
4.6. Docking Modeling
4.7. Drug-Likeness and Lead-Likeness Analysis
4.8. In Silico Prediction of Toxicity
4.9. In Silico Antioxidant Activity
4.9.1. Ligand Preparation
4.9.2. Protein Structure Preparation
4.10. PBPK Model Building
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kouli, A.; Torsney, K.M.; Kuan, W.-L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publication: Brisbane, Australia, 2018; Chapter 1. [Google Scholar] [CrossRef]
- Maiti, P.; Manna, J.; Dunbar, G.L. Current Understanding of the Molecular Mechanisms in Parkinson’s Disease: Targets for Potential Treatments. Transl. Neurodegener. 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Sharan, S.; Kumar, P.; Ambasta, R.K. Discovery of novel compounds targeting DJ-1 as neuroprotectants for Parkinson’s disease by virtual screening and in silico method. Curr. Comput. Aided Drug Des. 2021, 17, 351–359. [Google Scholar] [CrossRef]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef]
- Chakraborty, A.; Brauer, S.; Diwan, A. A review of possible therapies for Parkinson’s disease. J. Clin. Neurosci. 2020, 76, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Desai, B.S.; Monahan, A.J.; Carvey, P.M.; Hendey, B. Blood–Brain Barrier Pathology in Alzheimer’s and Parkinson’s Disease: Implications for Drug Therapy. Cell Transplant. 2007, 16, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, A.S.; Dietrich, D.; Fricker, G.; Lamprecht, A. Blood-brain barrier models: Rationale for selection. Adv. Drug Deliv. Rev. 2021, 176, 113859. [Google Scholar] [CrossRef]
- Radan, M.; Djikic, T.; Obradovic, D.; Nikolic, K. Application of in vitro PAMPA technique and in silico computational methods for blood-brain barrier permeability prediction of novel CNS drug candidates. Eur. J. Pharm. Sci. 2022, 168, 106056. [Google Scholar] [CrossRef]
- Villoutreix, B.O.; Krishnamoorthy, R.; Tamouza, R.; Leboyer, M.; Beaune, P. Chemoinformatic Analysis of Psychotropic and Antihistaminic Drugs in the Light of Experimental Anti-SARS-CoV-2 Activities. Adv. Appl. Bioinform. Chem. AABC 2021, 14, 71–85. [Google Scholar] [CrossRef]
- Barrera-Vázquez, O.S.; Gómez-Verjan, J.C.; Magos-Guerrero, G.A. Chemoinformatic Screening for the Selection of Potential Senolytic Compounds from Natural Products. Biomolecules 2021, 11, 467. [Google Scholar] [CrossRef]
- Rivero-Segura, N.A.; Gomez-Verjan, J.C. In Silico Screening of Natural Products Isolated from Mexican Herbal Medicines against COVID-19. Biomolecules 2021, 11, 216. [Google Scholar] [CrossRef]
- Hawash, M.; Jaradat, N.; Abualhasan, M.; Amer, J.; Levent, S.; Issa, S.; Ibrahim, S.; Ayaseh, A.; Shtayeh, T.; Mousa, A. Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives. Open Chem. 2021, 19, 855–863. [Google Scholar] [CrossRef]
- Britz, H.; Hanke, N.; Volz, A.; Spigset, O.; Schwab, M.; Eissing, T.; Wendl, T.; Frechen, S.; Lehr, T. Physiologically-Based Pharmacokinetic Models for CYP 1A2 Drug–Drug Interaction Prediction: A Modeling Network of Fluvoxamine, Theophylline, Caffeine, Rifampicin, and Midazolam. CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Tajabadi, F.M.; Pouwer, R.H.; Liu, M.; Dashti, Y.; Campitelli, M.R.; Murtaza, M.; Mellick, G.D.; Wood, S.A.; Jenkins, I.D.; Quinn, R.J. Design and Synthesis of Natural Product Inspired Libraries Based on the Three-Dimensional (3D) Cedrane Scaffold: Toward the Exploration of 3D Biological Space. J. Med. Chem. 2018, 61, 6609–6628. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Monteagudo, M.; Borges, F.; Cordeiro, M.D.S.; Helguera, A.M.; Tejera, E.; Paz-Y-Mino, C.; Sanchez-Rodriguez, A.; Perera-Sardina, Y.; Perez-Castillo, Y. Chemoinformatics Profiling of the Chromone Nucleus as a MAO-B/A2AAR Dual Binding Scaffold. Curr. Neuropharmacol. 2017, 15, 1117–1135. [Google Scholar] [CrossRef]
- Makhouri, F.R.; Ghasemi, J.B. In Silico Studies in Drug Research Against Neurodegenerative Diseases. Curr. Neuropharmacol. 2018, 16, 664–725. [Google Scholar] [CrossRef]
- Azam, F.; Madi, A.M.; Ali, H.I. Molecular Docking and Prediction of Pharmacokinetic Properties of Dual Mechanism Drugs that Block MAO-B and Adenosine A2A Receptors for the Treatment of Parkinson’s Disease. J. Young Pharm. 2012, 4, 184–192. [Google Scholar] [CrossRef]
- Mallajosyula, J.K.; Kaur, D.; Chinta, S.J.; Rajagopalan, S.; Rane, A.; Nicholls, D.G.; Di Monte, D.; MacArthur, H.; Andersen, J.K. MAO-B Elevation in Mouse Brain Astrocytes Results in Parkinson’s Pathology. PLoS ONE 2008, 3, e1616. [Google Scholar] [CrossRef]
- Pflüger, P.; Pereira, P.; Loza, M.I.; Brea, J.; Viña, D.; Kumar, A.; Fontenla, J.A. Gamma-decanolactone: Preliminary evaluation as potential antiparkinsonian drug. Eur. J. Pharmacol. 2021, 906, 174276. [Google Scholar] [CrossRef]
- Crisan, L.; Istrate, D.; Bora, A.; Pacureanu, L. Virtual screening and drug repurposing experiments to identify potential novel selective MAO-B inhibitors for Parkinson’s disease treatment. Mol. Divers. 2021, 25, 1775–1794. [Google Scholar] [CrossRef]
- Polishchuk, P. CReM: Chemically reasonable mutations framework for structure generation. J. Cheminform 2020, 12, 28. [Google Scholar] [CrossRef]
- Hathout, R.M.; Abdelhamid, S.G.; Metwally, A.A. Chloroquine and hydroxychloroquine for combating COVID-19: Investigating efficacy and hypothesizing new formulations using Bio/chemoinformatics tools. Inform. Med. Unlocked 2020, 21, 100446. [Google Scholar] [CrossRef]
- Maiti, P.; Sharma, P.; Nand, M.; Bhatt, I.D.; Ramakrishnan, M.A.; Mathpal, S.; Joshi, T.; Pant, R.; Mahmud, S.; Simal-Gandara, J.; et al. Integrated Machine Learning and Chemoinformatics-Based Screening of Mycotic Compounds against Kinesin Spindle ProteinEg5 for Lung Cancer Therapy. Molecules 2022, 27, 1639. [Google Scholar] [CrossRef]
- Manfredsson, F.P.; Tansey, M.G.; Golde, T.E. Challenges in Passive Immunization Strategies to Treat Parkinson Disease. JAMA Neurol. 2018, 75, 1180–1181. [Google Scholar] [CrossRef]
- Tan, L.; Song, X.; Ren, Y.; Wang, M.; Guo, C.; Guo, D.; Gu, Y.; Li, Y.; Cao, Z.; Deng, Y. Anti-inflammatory effects of cordycepin: A review. Phytotherapy Res. 2021, 35, 1284–1297. [Google Scholar] [CrossRef]
- Li, B.; Hou, Y.; Zhu, M.; Bao, H.; Nie, J.; Zhang, G.Y.; Shan, L.; Yao, Y.; Du, K.; Yang, H.; et al. 3'-Deoxyadenosine (Cordycepin) Produces a Rapid and Robust Antidepressant Effect via Enhancing Prefrontal AMPA Receptor Signaling Pathway. Int. J. Neuropsychopharmacol. 2016, 19, pyv112. [Google Scholar] [CrossRef]
- Tuli, H.S.; Sandhu, S.S.; Sharma, A.K. Pharmacological and therapeutic potential of Cordyceps with special reference to Cordycepin. 3 Biotech 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Panya, A.; Songprakhon, P.; Panwong, S.; Jantakee, K.; Kaewkod, T.; Tragoolpua, Y.; Sawasdee, N.; Lee, V.; Nimmanpipug, P.; Yenchitsomanus, P.-T. Cordycepin Inhibits Virus Replication in Dengue Virus-Infected Vero Cells. Molecules 2021, 26, 3118. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Huang, W.-M.; Tang, P.-C.; Sun, Y.; Zhang, X.; Qiu, L.; Yu, B.-C.; Hong, Y.-X.; He, Y.; Ge, X.-Q. Anti-inflammatory and neuroprotective effects of natural cordycepin in rotenone-induced PD models through inhibiting Drp1-mediated mitochondrial fission. Neurotoxicology 2021, 84, 1–13. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, W.-M.; Tang, P.-C.; Zhang, X.; Zhang, X.-Y.; Yu, B.-C.; Fan, Y.-Y.; Ge, X.-Q.; Zhang, X.-L. Neuroprotective effects of natural Cordycepin on LPS-induced Parkinson’s disease through suppressing TLR4/NF-κB/NLRP3-mediated pyroptosis. J. Funct. Foods 2020, 75, 104274. [Google Scholar] [CrossRef]
- Cheng, C.; Zhu, X. Cordycepin mitigates MPTP-induced Parkinson’s disease through inhibiting TLR/NF-κB signaling pathway. Life Sci. 2019, 223, 120–127. [Google Scholar] [CrossRef]
- Lv, R.; Du, L.; Liu, X.; Zhou, F.; Zhang, Z.; Zhang, L. Rosmarinic acid attenuates inflammatory responses through inhibiting HMGB1/TLR4/NF-κB signaling pathway in a mouse model of Parkinson’s disease. Life Sci. 2019, 223, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Du, L.; Zhou, F.; Yuan, X.; Liu, X.; Zhang, L. Rosmarinic Acid Alleviates Inflammation, Apoptosis, and Oxidative Stress through Regulating miR-155-5p in a Mice Model of Parkinson’s Disease. ACS Chem. Neurosci. 2020, 11, 3259–3266. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Chen, H.; Wang, J.; Liu, G.; Cui, S.W.; Kang, J.; Jiang, Y.; Wang, H. Naringenin prolongs lifespan and delays aging mediated by IIS and MAPK in Caenorhabditis elegans. Food Funct. 2021, 12, 12127–12141. [Google Scholar] [CrossRef]
- Ghofrani, S.; Joghataei, M.-T.; Mohseni, S.; Baluchnejadmojarad, T.; Bagheri, M.; Khamse, S.; Roghani, M. Naringenin improves learning and memory in an Alzheimer’s disease rat model: Insights into the underlying mechanisms. Eur. J. Pharmacol. 2015, 764, 195–201. [Google Scholar] [CrossRef]
- Mani, S.; Sekar, S.; Barathidasan, R.; Manivasagam, T.; Thenmozhi, A.J.; Sevanan, M.; Chidambaram, S.B.; Essa, M.M.; Guillemin, G.J.; Sakharkar, M.K. Naringenin Decreases α-Synuclein Expression and Neuroinflammation in MPTP-Induced Parkinson’s Disease Model in Mice. Neurotox. Res. 2018, 33, 656–670. [Google Scholar] [CrossRef]
- Zbarsky, V.; Datla, K.P.; Parkar, S.; Rai, D.K.; Aruoma, O.I.; Dexter, D.T. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free. Radic. Res. 2005, 39, 1119–1125. [Google Scholar] [CrossRef]
- Pushpavalli, G.; Kalaiarasi, P.; Veeramani, C.; Pugalendi, K.V. Effect of chrysin on hepatoprotective and antioxidant status in d-galactosamine-induced hepatitis in rats. Eur. J. Pharmacol. 2010, 631, 36–41. [Google Scholar] [CrossRef]
- Brown, E.; Hurd, N.S.; McCall, S.; Ceremuga, T.E. Evaluation of the anxiolytic effects of chrysin, a Passiflora incarnata extract, in the laboratory rat. AANA J. 2007, 75, 333–337. [Google Scholar]
- Moghadam, E.R.; Ang, H.L.; Asnaf, S.E.; Zabolian, A.; Saleki, H.; Yavari, M.; Esmaeili, H.; Zarrabi, A.; Ashrafizadeh, M.; Kumar, A.P. Broad-Spectrum Preclinical Antitumor Activity of Chrysin: Current Trends and Future Perspectives. Biomolecules 2020, 10, 1374. [Google Scholar] [CrossRef]
- Naz, S.; Imran, M.; Rauf, A.; Orhan, I.E.; Shariati, M.A.; Shahbaz, M.; Qaisrani, T.B.; Shah, Z.A.; Plygun, S.; Heydari, M.; et al. Chrysin: Pharmacological and therapeutic properties. Life Sci. 2019, 235, 116797. [Google Scholar] [CrossRef] [PubMed]
- Critchfield, J.W.; Butera, S.T.; Folks, T.M. Inhibition of HIV Activation in Latently Infected Cells by Flavonoid Compounds. AIDS Res. Hum. Retroviruses 1996, 12, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Goes, A.T.; Jesse, C.R.; Antunes, M.S.; Ladd, F.V.L.; Ladd, A.A.L.; Luchese, C.; Paroul, N.; Boeira, S.P. Protective role of chrysin on 6-hydroxydopamine-induced neurodegeneration a mouse model of Parkinson’s disease: Involvement of neuroinflammation and neurotrophins. Chem. Interactions 2018, 279, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Sander, T.; Freyss, J.; Von Korff, M.; Rufener, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Naveja, J.J.; Vogt, M. Automatic Identification of Analogue Series from Large Compound Data Sets: Methods and Applications. Molecules 2021, 26, 5291. [Google Scholar] [CrossRef]
- Bemis, G.W.; Murcko, M.A. The Properties of Known Drugs. 1. Molecular Frameworks. J. Med. Chem. 1996, 39, 2887–2893. [Google Scholar] [CrossRef]
- Connolly, B.S.; Lang, A. Pharmacological Treatment of Parkinson Disease: A review. JAMA 2014, 311, 1670–1683. [Google Scholar] [CrossRef]
- Edmondson, D.E.; Binda, C. Monoamine Oxidases. Membr. Protein Complexes Struct. Funct. 2018, 87, 117–139. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Xia, Y.-L.; Ai, S.-M.; Liang, J.; Sang, P.; Ji, X.-L.; Liu, S.-Q. Insights into Protein–Ligand Interactions: Mechanisms, Models, and Methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef]
- Ormachea, C.; Ferretti, C.A. In Silico Evaluation of Antioxidant Properties of Cinnamaldehyde Phenylhydrazone. Chem. Proc. 2021, 8, 10. [Google Scholar] [CrossRef]
- Boulaamane, Y.; Ibrahim, M.A.A.; Britel, M.R.; Maurady, A. In silico studies of natural product-like caffeine derivatives as potential MAO-B inhibitors/AA2AR antagonists for the treatment of Parkinson’s disease. J. Integr. Bioinform. 2022, 20210027. [Google Scholar] [CrossRef]
- Basu, S.; Lien, Y.T.; Vozmediano, V.; Schlender, J.-F.; Eissing, T.; Schmidt, S.; Niederalt, C. Physiologically Based Pharmacokinetic Modeling of Monoclonal Antibodies in Pediatric Populations Using PK-Sim. Front. Pharmacol. 2020, 11, 868. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4). [Google Scholar] [CrossRef]
- Voicu, A.; Duteanu, N.; Voicu, M.; Vlad, D.; Dumitrascu, V. The rcdk and cluster R packages applied to drug candidate selection. J. Cheminform 2020, 12, 3. [Google Scholar] [CrossRef]
- Bickerton, G.R.; Paolini, G.V.; Besnard, J.; Muresan, S.; Hopkins, A.L. Quantifying the chemical beauty of drugs. Nat. Chem. 2012, 4, 90–98. [Google Scholar] [CrossRef]
- Hetényi, C.; Spoel, D.V.D. Toward prediction of functional protein pockets using blind docking and pocket search algorithms. Protein Sci. 2011, 20, 880–893. [Google Scholar] [CrossRef]
- Chaurasiya, N.D.; Zhao, J.; Pandey, P.; Doerksen, R.J.; Muhammad, I.; Tekwani, B.L. Selective Inhibition of Human Monoamine Oxidase B by Acacetin 7-Methyl Ether Isolated from Turnera diffusa (Damiana). Molecules 2019, 24, 810. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Guex, N. Swiss-PdbViewer: A fast and easy-to-use PDB viewer for Macintosh and PC. Protein Data Bank Q. Newsl. 1996, 77, 7. [Google Scholar]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Dallmann, A.; Ince, I.; Coboeken, K.; Eissing, T.; Hempel, G. A Physiologically Based Pharmacokinetic Model for Pregnant Women to Predict the Pharmacokinetics of Drugs Metabolized Via Several Enzymatic Pathways. Clin. Pharmacokinet. 2018, 57, 749–768. [Google Scholar] [CrossRef]
| MAO-B | |||
|---|---|---|---|
| Compounds | Full Fitness (kcal/mol) | Estimated ΔG (kcal/mol) | Binding Energy (kcal/mol) |
| Selegiline (MAOB-I) | −2242.3184 | −6.79202 | −12.4572 |
| Rasagiline (MAOB-I) | −2254.627 | −6.8181977 | −12.2604 |
| Cordycepin-derived compound 21 NP (1) | −2293.7556 | −9.754256 | −13.6346 |
| Rosmarinic acid-derived compound 14 NP (2) | −2206.5972 | −9.025398 | 5.34911 |
| Rosmarinic acid-derived compound 6 APP (3) | −2264.7612 | −8.981724 | −21.0128 |
| Naringenin-derived compound 20 NP (4) | −2259.6467 | −8.539444 | −17.4789 |
| Chrysin-derived compound 22 NP (5) | −2265.2837 | −8.242364 | −23.0139 |
| Chrysin-derived compound 20 APP (6) | −2233.857 | −8.174571 | 1.13213 |
| Rosmarinic acid-derived compound 18 NP (7) | −2204.3083 | −8.12989 | 12.598 |
| Chrysin-derived compound 19 NP (8) | −2241.1152 | −7.9971757 | 3.67269 |
| Naringenin-derived compound 18 NP (9) | −2282.8008 | −7.717949 | −22.522 |
| Chrysin-derived compound 11 APP (10) | −2235.878 | −7.5757804 | 15.3924 |
| Chrysin fragment 16 NP (11) | −2230.024 | −7.515393 | 4.66808 |
| Chrysin fragment 20 NP (12) | −2268.7068 | −7.207463 | 5.43122 |
| Naringenin fragment 19 APP (13) | −2258.6753 | −7.1575794 | −18.8473 |
| Rosmarinic acid fragment 15 NP (14) | −2217.6724 | −7.0223274 | 4.64089 |
| Rosmarinic acid fragment 21 NP (15) | −2235.1602 | −7.012647 | −17.5915 |
| Rosmarinic acid fragment 16 APP (16) | −2259.545 | −6.911508 | −12.4415 |
| Rosmarinic acid-derived compound 19 APP (17) | −2237.1956 | −6.8166437 | 12.0367 |
| Naringenin fragment 23 NP (18) | −2238.6543 | −6.244289 | 1.68624 |
| Cytochrome P450 | |||
|---|---|---|---|
| Compounds | Full Fitness (kcal/mol) | Estimated ΔG (kcal/mol) | Binding Energy (kcal/mol) |
| Cordycepin-derived compound (21 NP) | −4482.541 | −7.754484 | −1.887 |
| Rosmarinic acid-derived compound (14 NP) | −4437.125 | −8.617973 | 17.7735 |
| Rosmarinic acid-derived compound (6 APP) | −4468.041 | −8.255595 | −4.12677 |
| Naringenin-derived compound (20 NP) | −4481.2944 | −7.580227 | −5.77106 |
| Chrysin-derived compound (22 NP) | −4449.683 | −7.7609963 | −3.99852 |
| NADPH Oxidase (NO) | |||
| Compounds | Full Fitness (kcal/mol) | Estimated ΔG (kcal/mol) | Binding Energy (kcal/mol) |
| Cordycepin-derived compound (21 NP) | −4495.0576 | −8.262831 | −4.28877 |
| Rosmarinic acid-derived compound (14 NP) | −4442.3994 | −8.091313 | 21.4074 |
| Rosmarinic acid-derived compound (6 APP) | −4481.2437 | −8.725966 | −12.686 |
| Naringenin-derived compound (20 NP) | −4494.463 | −7.7760835 | −16.9568 |
| Chrysin-derived compound (22 NP) | −4459.724 | −7.618342 | −5.32596 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrera-Vazquez, O.; Santiago-de-la-Cruz, J.A.; Rivero-Segura, N.A.; Estrella-Parra, E.A.; Morales-Paoli, G.S.; Flores-Soto, E.; Gomez-Verjan, J.C. Data-Driven Approaches Used for Compound Library Design for the Treatment of Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 1134. https://doi.org/10.3390/ijms24021134
Barrera-Vazquez O, Santiago-de-la-Cruz JA, Rivero-Segura NA, Estrella-Parra EA, Morales-Paoli GS, Flores-Soto E, Gomez-Verjan JC. Data-Driven Approaches Used for Compound Library Design for the Treatment of Parkinson’s Disease. International Journal of Molecular Sciences. 2023; 24(2):1134. https://doi.org/10.3390/ijms24021134
Chicago/Turabian StyleBarrera-Vazquez, Oscar, Jose Alberto Santiago-de-la-Cruz, Nadia Alejandra Rivero-Segura, Edgar Antonio Estrella-Parra, Genaro Salvador Morales-Paoli, Edgar Flores-Soto, and Juan Carlos Gomez-Verjan. 2023. "Data-Driven Approaches Used for Compound Library Design for the Treatment of Parkinson’s Disease" International Journal of Molecular Sciences 24, no. 2: 1134. https://doi.org/10.3390/ijms24021134
APA StyleBarrera-Vazquez, O., Santiago-de-la-Cruz, J. A., Rivero-Segura, N. A., Estrella-Parra, E. A., Morales-Paoli, G. S., Flores-Soto, E., & Gomez-Verjan, J. C. (2023). Data-Driven Approaches Used for Compound Library Design for the Treatment of Parkinson’s Disease. International Journal of Molecular Sciences, 24(2), 1134. https://doi.org/10.3390/ijms24021134










