Acute and Chronic Systemic Inflammation: Features and Differences in the Pathogenesis, and Integral Criteria for Verification and Differentiation
Abstract
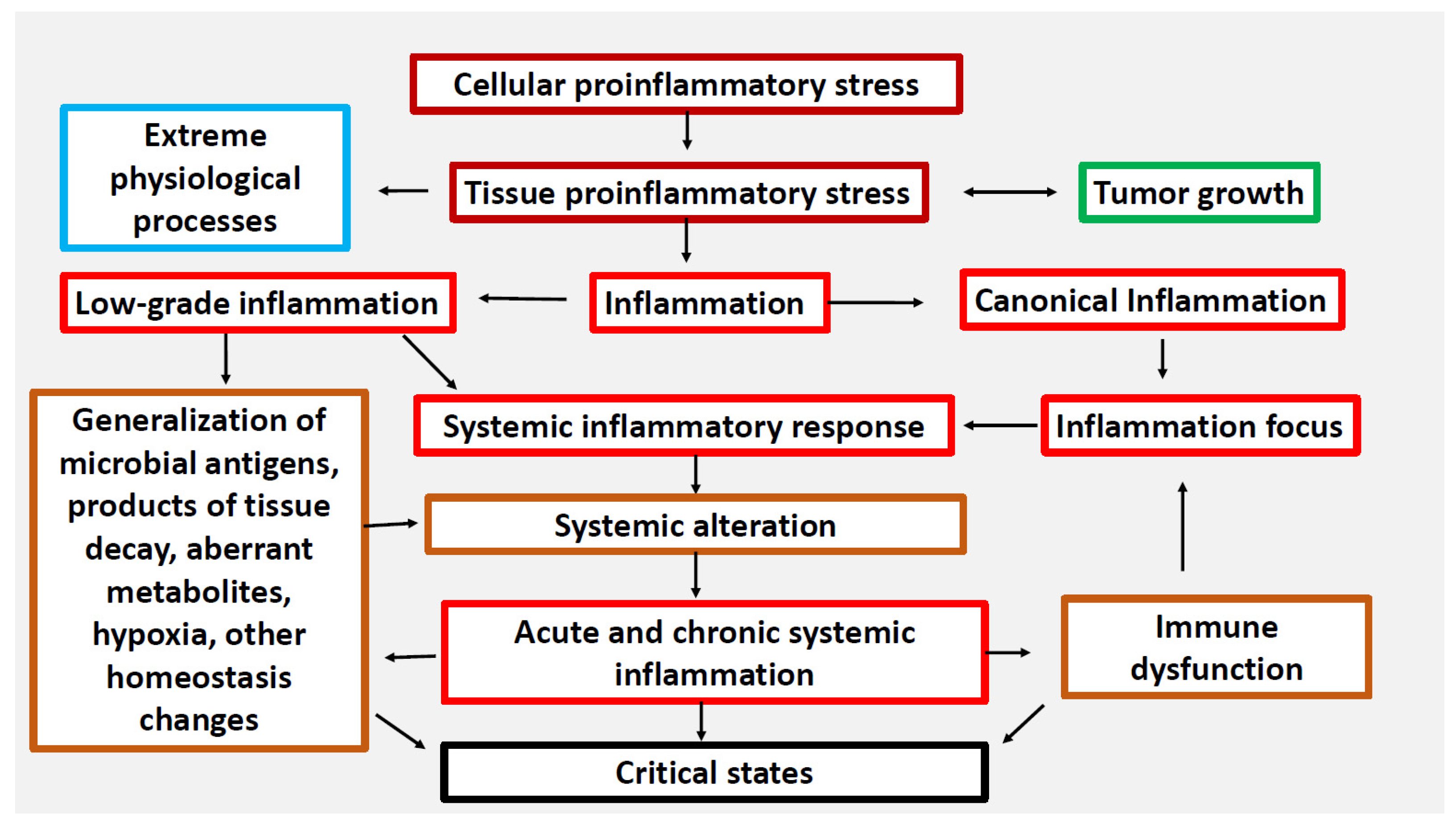
:1. Introduction
- To check the quality of the SI scale by comparing the results of its use with the clinical equivalents of SI, which can be ranked in the following order by the probability of SI development: refractory shock > lethal outcomes in intensive care units (ICU) > MOD without lethal outcomes in ICU patients > MOD in patients of other departments;
- To confirm the general pathological, multi-etiological nature of the acute SI variant by comparing acute critical conditions of infectious (sepsis) and non-infectious genesis (acute polytrauma);
- To verify separate phases of acute SI by comparing the hyperergic (with extremely high levels of SIR) and hypoergic (with relatively moderate manifestations of SIR) variants of SI, taking into account the time of process development and the degree of its criticality;
- To compare the prognostic value of the SI and RL scales and the clinical scores of polytrauma, such as APACHE-II (Acute Physiology and Chronic Health Evaluation II), TRISS (Trauma Score and Injury Severity Score), ISS (Injury Severity Score), GCS (Glasgow Coma Scale), SOFA (Sequential Organ Failure Assessment), TISS (Therapeutic Intervention Scoring System), SAPS II (Simplified Acute Physiology Score II), and criteria of the SIR syndrome (SIRS) for lethal outcomes;
- To evaluate the probability of SI presence in chronic pathologies of different genesis (infectious, autoimmune, endocrine, ischemic gangrene and others) depending on the severity of the disease and peculiarities of its pathogenesis;
- To assess the possibility of differentiation between acute and chronic SI by means of detecting false-positive results of acute SI scale application in patients with chronic diseases;
- To elucidate differences and similarities between certain phenomena of acute and chronic systemic inflammation.
1.1. Methodological Approaches Used in the Work
1.2. Validation Criteria for SI Scales
1.2.1. Agreement of the Results Obtained Using the SI Scale and the ChSI Scale with the Clinical Equivalents of SI
- An analysis of apparent SI manifestations was performed on two cohorts of patients with refractory septic shock (SS) that developed in acute sepsis and in tertiary peritonitis (prolonged and subacute sepsis). These cohorts were characterized by different SIR severity, which makes it possible to assess the probability of verifying various SI phases using the SI scale;
- Sepsis and acute polytrauma (without a confirmed infectious complication) were considered as pathologies with a high risk of SI development; patients were examined on the 1st–2nd and 5th–7th days from the onset of the critical process. A comparison of the groups with infections and aseptic critical conditions points to the general pathological nature of SI;
- Systemic microcirculatory disorders (according to autopsies) are the main cause of mortality in intensive care units. Therefore, a separate aspect of the work was to analyze the relationship between the SI scale results and lethal outcomes. In this case, complications associated with thromboembolisms (according to autopsies), including pulmonary embolisms, secondary myocardial infarctions and strokes, were excluded from the study;
- The comparison group included patients with acute phlegmonas of the lower limbs meeting Sepsis-3 criteria (the presence of the infection focus and MOD) but were treated in a surgical department (without intensive care) and had no lethal outcomes. By the 5th–7th day of surgical and etiological treatment, signs of MOD had resolved in all patients in this cohort. The expected outcome of SI scale testing in this group was a relatively low detection rate of SI, especially of the most life-critical phases of SI;
- The efficacy of the ChSI scale was assessed by examining independent cohorts with 16 chronic pathologies of different genesis, such as infections, autoimmune and endocrine diseases, chronic organ failure, and chronic ischemia of the lower limbs. Patients in these groups had different levels of SIR and condition severity. The focus was on the analysis of the registration rates of certain phenomena of ChSI depending on the severity of the pathological process and the nature of the damaging factors. A particular aim of this study was to evaluate the specificity of the SI scale (designed for the acute variant of SI) by identifying false-positive results of this scale in chronic pathologies.
1.2.2. Clinical Material Collection
1.2.3. Laboratory Analysis of Biomarkers
1.2.4. Selection of Biomarkers
1.2.5. Mathematical Analysis
1.2.6. Therapy
2. Results
2.1. Phenomena of the Acute SI Variant
- A comparison of two cohorts of patients with refractory septic shock (groups of patients with acute sepsis and tertiary peritonitis) showed that SS can proceed in two alternative scenarios (Table 5 and Table 6). The first, i.e., the hyperergic variant, predominates in the groups with acute sepsis and is characterized by RL-4-5. The second, i.e., the hypoergic variant (RL-2-3), predominates in patients with tertiary peritonitis. Lethality in these cohorts was 71% and 94%, respectively (Table 6). These two sepsis variants reflect different SI phases: the phlogogenic stroke phase (cytokine storm) and the depressive (depletion) phase. Meanwhile, the SI scale, despite these differences, confirms SI in both variants of SS in 100% of cases. Thus, the pathogenesis of various SS variants can be a priori considered in terms of SI.
- In contrast to patients with SS, lethality in the groups of intensive care patients with MOD (trauma and sepsis) on days 1–2 and 5–7 was significantly less (21–50%) and SI was not detected in 100% of patients (except for the group “Sepsis 5–7”, in which SI detection reached 100%). In isolated cases (4.4% in acute sepsis and 1.9% in acute trauma), neither SI nor pre-SI was detected on day 1–2 in these patients. A comparative analysis of the groups with sepsis and polytrauma showed a fundamental similarity in the patterns of SI development, but also some differences (Table 5). In particular, the greatest differences between the polytrauma and sepsis groups were found on days 5–7 (Table 5 and Table 6). Thus, in acute trauma, only 55.6% of patients (10 out of 18, Table 6) had SI on days 5–7. However, all nine fatalities in “Multiple injuries 5–7” were associated with SI. The other eight patients (without SI) had a relatively mild manifestation of MOD (SOFA score ≤ 4). All of these patients had not developed SI criteria by day 5–7 and subsequently recovered. Thus, despite some differences, the fundamental patterns of SI development in sepsis and trauma were quite comparable: in both nosologies, the presence of SI and its individual phenomena determined the severity of patients’ condition and mortality in intensive care units.
- Patients with “non-resuscitative” sepsis (diagnosing sepsis was made according to Sepsis-3 criteria) (comparison group) had relatively moderate manifestations of MOD (mean SOFA score—3.6) on days 1–2 from hospitalization, which resolved in all patients by the 5th–7th days (Table 5 and Table 6). This group was characterized by the development of a relatively noncritical SI phase (development phase) in 7.5% of cases; in 18.2% of patients, SIR manifested itself only as classical inflammation; and 65% of patients had a borderline pre-SI state. The intensity of SIR in the patients of this group did not exceed RL-3. However, in two patients (5%) without signs of myocardial infarction, troponin I was detected in plasma: 16.1 ng/mL and 16.2 ng/mL. In these cases, we could assume the presence of organ damage associated with relatively hidden microcirculatory disorders. In general, these data once again confirmed the correlation of SI with the criticality of the patients’ condition.
2.2. Association of Lethality with Specific Phases of SI
2.3. Comparative Prognostic Value of SI Scale and Some Clinical Scales for Fatal Outcomes
2.4. Phenomena of ChSI
2.5. Comparative Analysis of Acute and Chronic SI Variants
2.6. Summary of the Results
3. Discussion
- The clinical criteria for ChSI can be a sign of increasing chronic organ dysfunction. However, long-term multicenter prospective studies of a large number of diseases are needed to assess these dynamics. There is also a need to create a universal scale of chronic organ failure, similar to the SOFA scale;
- More accurate verification of ChSI and identification of specific stages in this process require multiple examinations of each patient over a long period of time. We have performed such studies in a limited number of patients only, so these data are not presented;
- It is necessary to differentiate ChSI not only from classical inflammation SIR, but also from low-grade systemic inflammation characteristic of the metabolic syndrome and type 2 diabetes mellitus. These studies are currently ongoing in our laboratory, but their results have not yet been generalized;
- The broader medical community has no understanding of the fundamental meaning of general pathological processes and their distinction from clinical definitions. Without such understanding, independent multicenter studies of ChSI are impossible.
4. Materials and Methods
4.1. Patients
- Sepsis non-resuscitative on the 1st–2nd days—deep shin phlegmon: III-IV level of soft tissue damage in military men, all patients showing signs of MODS (average score by the SOFA scale—3.6—from 2 to 5 points). The dominant etiological factor was S. aureus. The study was conducted immediately after the surgical treatment of the inflammatory focus. Deaths and shock states in the postoperative period were not observed, treatment was carried out only in the surgical department (not in intensive care unit (ICU)), n = 40, mean age—20.4 ± 2.4 years. According to the Sepsis-3 consensus [11], this group should be attributed to the sepsis group; however, due to the difference in the clinical picture in comparison with the patients of other groups with sepsis, this group was considered separately. Signs of MOD on days 5–7 were absent in this group (MOD was resolved), so the group of patients with deep shin phlegmon on days 5–7 did not meet the inclusion criteria;
- Sepsis (MODS) (n = 46, mean age—47.1 ± 16.6 years, Male/Female = 60.9%/39.1%), 1–2 days after admission to the ICU, SOFA score from 2 to 10 points, mean—5.5. The initial diseases for all groups of patients with sepsis were as follows: severe pneumonia, peritonitis, obstetrical sepsis, and some other; all patients in this and in the other groups went through intensive therapy in the ICU. Lethal outcomes (n = 11) in 23.9% of cases;
- The same + septic shock (SS), the presence of hypotension, not responding to vasopressors, SOFA score from 6 to 14 points, mean—9.75 (n = 14, mean age—49.1 ± 17.8 years, Male/Female = 57.1%/42.9%). Lethal outcomes (n = 10) in 71.4% of cases;
- Sepsis (MODS) (n = 13, mean age—40.2 ± 14.2 years, Male/Female = 61.5%/38.5%) screening on day 5–7 of hospitalization in the ICU, SOFA score from 3 to 10, mean—5.7. Lethal outcomes (n = 4) in 30.7% of cases;
- Tertiary peritonitis (TP) with MODS, and prolonged (14–30 days) and subacute (>30 days from the start of manifestation) septic process (n = 34, mean age—51.5 ± 16.6 years, Male/Female = 58.8%/41.2%), SOFA score was not calculated. Lethal outcomes (n = 10) in 29.4% of cases;
- The same + development of septic shock (TP + SS) (n = 17, mean age—50.2 ± 15.6 years. Male/Female = 64.7%/35.3%), SOFA score was not calculated. Lethal outcomes (n = 16) in 94.1% of cases;
- Polytrauma-acute multiple injuries in two or more different body regions, required intensive therapy in the ICU (n = 51, mean age—37.8 ± 14.9 years, Male/Female = 67.4%/32.6%), on the 1st–2nd days of hospitalization in the ICU, with the development of MODS, SOFA score from 2 to 12, mean—4.96. Lethal outcomes (n = 11) in 21.6% of cases. All patients had values of the ISS scale ≥ 9. According to the ISS criteria, the following injuries prevailed: chest in twenty-three patients; extremities, pelvic organs and abdomen in twenty patients; craniocerebral trauma in five patients; and mixed variants in three patients. All patients with predominant chest injuries had pulmonary insufficiency-ALI/ARDS. ROC analysis was used exclusively to investigate the integral ALI/ARDS group, in which 14 out of 37 patients had signs of pulmonary failure without MOD (Table 8). In all other patients with acute pathologies in this study, the presence of MOD was established according to the criteria of the SOFA scale. ISS scale values were determined on the 1st day of hospitalization, and SOFA and CI scales were scored in dynamics. In patients with chest trauma, the APACHE II and TRISS scales were additionally used for scoring on the first day of follow-up. In other cases, these scales were applied to only some of the patients;
- Multiple injuries with the development of MODS (n = 18, mean age—39.4 ± 15.1 years, Male/Female = 64.7%/35.3%), on days 5–7 after admission to the ICU, SOFA score from 2 to 16, mean—6.75. Traumatic lesions in the following areas: thorax, upper and lower extremities, pelvic region, abdominal injuries, and traumatic brain injury. Lethal outcomes (n = 9) in 50.0% of cases.
- 9.
- Systemic lupus erythematosus—SLE (n = 49, mean age—43.7 ± 13.3 years, Male/Female = 6.1%/93.9%). The patients met the 1982 American College of Rheumatology criteria for SLE [36]. The duration of SLE was (mean ± SD) 11.9 ± 9.4 years. Disease activity was assessed according to the SLE Disease Activity Index (SLEDAI) [37]. SLE patients with SLEDAI ≥ 5 (95.5% of cases) were attributed to the active-disease cohort, and those with SLEDAI < 5 (4.5% of cases) were attributed to the stable-disease cohort;
- 10.
- Rheumatoid arthritis—RA (n = 42, mean age—53.1 ± 14.3 years, Male/Female = 9.5%/90.5%). The patients met the 1987 American College of Rheumatology criteria for RA [38]. Thirty-eight patients (90.5%) were seropositive. RA activity was measured by Disease Activity Score in 28 joints (DAS28) [39]: low activity (DAS28 ≤ 3.2) was detected in 7.2% of cases, moderate activity (3.2 < DAS28 ≤ 5.1) in 35.7%, and high activity (DAS28 > 5.1) in 57.1%. The duration of RA was (mean ± SD) 7.1 ± 7.4 years;
- 11.
- Reactive arthritis associated with Chlamydia trachomatis—ReA (n = 30, mean age—42.4 ± 14.3 years, Male/Female = 39.3%/60.7%). The duration of ReA was (mean ± SD) 7.1 ± 7.4 years;
- 12.
- Ankylosing spondylitis—AS (n = 27, mean age—41.0 ± 13.1 years, Male/Female = 85.2%/14.8%). The patients met the modified New York criteria for AS (1984) [40]. AS activity was measured by Bath Ankylosing spondylitis Disease Activity Index—BASDAI (1994) [41], the median of AS activity was 4.05. High disease activity (BASDAI > 4) was detected in 73% of cases;
- 13.
- Psoriatic arthritis—PsA (n = 12, mean age—52.9 ± 6.1 years, Male/Female = 50%/50%). The patients met the CASPAR (for Classification Criteria for Psoriatic Arthritis) criteria [42];
- 14.
- Chronic rheumatic valvular heart disease—RHD (n = 15, mean age—55.3 ± 13.0 years, Male/Female = 14.3%/85.7%). The diagnosis of RHD was based on echocardiographic findings according to the World Heart Federation criteria [43];
- 15.
- Chronic heart failure—CHF (n = 49, mean age—80.7 ± 4.3 years, Male/Female = 73.5%/26.5%). Patients included in the study were 70 years old or above, diagnosed with CHF, taking medications, having heart failure of New York Heart Association (NYHA) functional class II-IV, and were clinically stable. Most patients had chronic bronchitis, pulmonary emphysema, age-related encephalopathy. The exclusion criteria were autoimmune diseases, tumors, end-stage renal failure, previous stroke with immobility, severe dementia, and acute inflammatory processes at the time of the study;
- 16.
- End-stage renal disease—ESRD (n = 42, mean age—45.4 ± 13.9 years, Male/Female =47.6%/52.4%) caused by chronic glomerulonephritis (n = 22), chronic pyelonephritis (n = 12) and diabetic nephropathy (n = 8). The diagnosis of ESRD was based on the international criteria K/DOQI (2002) [44]. All patients received replacement treatment by programmed hemodialysis-12 h a week. The duration of the dialysis period was 63.0 ± 9.6 months. Blood samples were collected before hemodialysis sessions;
- 17.
- Chronic limb threatening ischemia—CLI—caused by common femoral artery atherosclerotic lesions (n = 38, mean age—65.8 ± 9.1 years, Male/Female = 67.6%/32.4%). All patients had grade III CLI according to Rutherford classification [45]. The study was conducted in preparation for a high thigh amputation. The condition of the patients was assessed as moderate in 68.2% of cases and as severe in 31.8%;
- 18.
- Chronic nonhealing wounds—CNW in military men (n = 42, mean age—19.4 ± 0.5 years) caused by surgical treatment of phlegmon (50.5% of cases), microtraumas and abrasions (44.5% of cases), and erysipelatous inflammation (5% of cases). The duration of the purulent process in all patients was more than 90 days. The depth of soft tissue lesion corresponded to grade II (47.5% of cases), grade III (31.5% of cases), and grade IV (21% of cases) by D. Ahrenholz classification (1991), length: from 10 to 25 cm2;
- 19.
- Autoimmune thyroiditis—AIT (n = 29, mean age—44.2 ± 13.2 years). Thyroid gland hypofunction was detected in twenty-one patients and hyperfunction in eight patients;
- 20.
- Pelvic inflammatory disease—PID (n = 16, mean age—28.9 ± 4.7 years)—chronic infection of the upper genital tract. Patients were treated for recurrent first trimester miscarriage;
- 21.
- Menopausal syndrome: women who were treated for menopausal syndrome (n = 16, mean age—50.7 ± 4.4 years). All patients had stage 2 arterial hypertension (according to the criteria of the American College of Cardiology/American Heart Association [46]);
- 22.
- Chronic renal allograft dysfunction—CRAD (n = 23, mean age—42.0 ± 9.4 years Male/Female = 69.6%/30.4%): patients after renal allotransplantation with morphological (according to Banff classification [47]), clinical, and laboratory signs of CRAD (sustained increase in plasma creatinine over 0.15 mmol/L, persistent proteinuria over 0.5 g/day, arterial hypertension). Signs of allograft dysfunction manifested themselves in all recipients not earlier than 6 months after transplantation. All patients received hemodialysis therapy before renal transplantation;
- 23.
- Normal function of renal allograft: patients with normal renal allograft function, i.e., without clinical and laboratory signs of CRAD (n = 24, mean age—43.5 ± 9.1 years Male/Female = 50%/50%). It was a reference group for the CRAD group;
- 24.
- Primary antiphospholipid syndrome (PAPS) in women with recurrent miscarriage (n = 5). All patients met the PAPS classification criteria [48];
- 25.
- Control group—healthy blood donors aged 18–55 years (n = 50, mean age—34.1 ± 10.4 years, Male/Female = 52%/48%) recruited at Regional Blood Transfusion Station (Ekaterinburg);
- 26.
- Elderly people aged 60–74 years without acute and system destructive diseases, acute attack of chronic diseases, and free from other inflammatory conditions (n = 22, mean age—68.5 ± 5.9 years, Male/Female = 59.1%/40.9%), which underwent a routine medical check-up at Ekaterinburg City Clinical Hospital No. 40.
4.2. Measurement of Biomarkers
4.3. Verification of Systemic Inflammation
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gusev, E.; Zhuravleva, Y. Inflammation: A New Look at an Old Problem. Int. J. Mol. Sci. 2022, 23, 4596. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.Y.; Zotova, N.V. Cellular Stress and General Pathological Processes. Curr. Pharm. Des. 2019, 25, 251–297. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.Y.; Zotova, N.V.; Zhuravleva, Y.A.; Chereshnev, V.A. Physiological and Pathogenic Role of Scavenger Receptors in Humans. Med. Immunol. 2020, 22, 7–48. (In Russian) [Google Scholar] [CrossRef] [Green Version]
- Kimsa, M.C.; Strzalka-Mrozik, B.; Kimsa, M.W.; Gola, J.; Kochanska-Dziurowicz, A.; Zebrowska, A.; Mazurek, U. Differential Expression of Inflammation-related Genes after Intense Exercise. Prague Med. Rep. 2014, 115, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Gusev, E.; Sarapultsev, A.; Hu, D.; Chereshnev, V. Problems of Pathogenesis and Pathogenetic Therapy of COVID-19 from the Perspective of the General Theory of Pathological Systems (General Pathological Processes). Int. J. Mol. Sci. 2021, 22, 7582. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Solomatina, L.; Zhuravleva, Y.; Sarapultsev, A. The Pathogenesis of End-Stage Renal Disease from the Standpoint of the Theory of General Pathological Processes of Inflammation. Int. J. Mol. Sci. 2021, 22, 11453. [Google Scholar] [CrossRef]
- De Backer, D.; Ricottilli, F.; Ospina-Tascón, G.A. Septic shock: A microcirculation disease. Curr. Opin. Anaesthesiol. 2021, 34, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Saygin, D.; Highland, K.B.; Tonelli, A.R. Microvascular involvement in systemic sclerosis and systemic lupus erythematosus. Microcirculation 2019, 26, e12440. [Google Scholar] [CrossRef]
- Caraballo, C.; Jaimes, F. Organ Dysfunction in Sepsis: An Ominous Trajectory From Infection To Death. Yale J. Biol. Med. 2019, 92, 629–640. [Google Scholar]
- Zotova, N.V.; Chereshnev, V.A.; Gusev, E.Y. Systemic Inflammation: Methodological Approaches to Identification of the Common Pathological Process. PLoS ONE 2016, 11, e0155138. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Gusev, E.Y.; Zotova, N.V.; Chereshnev, V.A. Sepsis-3: New edition—Old problems. Analysis from the perspective of general pathology. Russ. J. Infect. Immun. 2021, 11, 649–662. [Google Scholar] [CrossRef]
- Zotova, N.V.; Zhuravleva, Y.A.; Zubova, T.E.; Gusev, E.Y. Integral Estimation of Systemic Inflammatory Response under Sepsis. Gen. Physiol. Biophys. 2020, 39, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.Y.; Yurchenko, L.N.; Chereshnev, V.A.; Zotova, N.V.; Kopalova, Y.A. The Method for Diagnosis and Prognosis of Systemic Inflammation with Phases and Stages Verification. Patent RF 2335771; IPC7 G01N 33/53, 10 October 2008. (In Russian). [Google Scholar]
- Zhang, H.; Liu, Y.; Wang, Y. Observer-Based Finite-Time Adaptive Fuzzy Control for Nontriangular Nonlinear Systems with Full-State Constraints. IEEE Trans Cybern. 2021, 51, 1110–1120. [Google Scholar] [CrossRef]
- Bochkarev, P.Y.; Berdyugina, O.V.; Zhidkova, V.S.; Zubova, T.E.; Gusev, E.Y. The Role of Systemic Inflammation in the Pathogenesis of Hemorrhagic Stroke in the Presence or Absence of Effective Brain Blood Flow. Zh. Nevrol. Psikhiatr. Im. S. Korsakova. 2020, 120, 24–29. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Chereshnev, V.A.; Mazurov, V.D.; Yurchenko, L.N.; Gusev, E.Y.; Kim, A.V. Pattern recognition method in medicine. Russ. J. Num. Anal. Math. Model. 2004, 19, 281–292. (In Russian) [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metz, C.E. Fundamental ROC Analysis. In Handbook of Medical Imaging; Van Metter, R.L., Beutel, J., Kundel, H.L., Eds.; SPIE Press: Bellingham, DC, USA, 2000; Volume 1, pp. 751–770. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Pacholczak-Madej, R.; Kuszmiersz, P.; Iwaniec, T.; Zaręba, L.; Zarychta, J.; Walocha, J.A.; Dropiński, J.; Bazan-Socha, S. Endothelial Dysfunction and Pentraxin-3 in Clinically Stable Adult Asthma Patients. J. Investig. Allergol. Clin. Immunol. 2021, 31, 417–425. [Google Scholar] [CrossRef]
- Nøst, T.H.; Alcala, K.; Urbarova, I.; Byrne, K.S.; Guida, F.; Sandanger, T.M.; Johansson, M. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur. J. Epidemiol. 2021, 36, 841–848. [Google Scholar] [CrossRef]
- Ilangumaran, S.; Moriggl, R.; Kalvakolanu, D.V. Editorial: Cytokines in liver diseases. Cytokine 2019, 124, 154608. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Peng, J.; Enrico, T.; Lu, K.; Cheung, E.C.; Kuo, Z.; He, Q.; Tang, Y.; Liu, A.; Fan, D.; et al. Metabolic syndrome and its component traits present gender-specific association with liver cancer risk: A prospective cohort study. BMC Cancer 2021, 21, 1084. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Tang, F.; Wang, Z.; Qi, G.; Liang, X.; Li, B.; Yuan, S.; Liu, J.; Yu, S.; He, S. Upregulation of Akt/NF-κB-regulated inflammation and Akt/Bad-related apoptosis signaling pathway involved in hepatic carcinoma process: Suppression by carnosic acid nanoparticle. Int. J. Nanomed. 2016, 11, 6401–6420. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Ramadori, P.; Pfister, D.; Seehawer, M.; Zender, L.; Heikenwalder, M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat. Rev. Cancer 2021, 21, 541–557. [Google Scholar] [CrossRef]
- Dubin, A.; Kanoore Edul, V.S.; Caminos Eguillor, J.F.; Ferrara, G. Monitoring Microcirculation: Utility and Barriers—A Point-of-View Review. Vasc. Health Risk. Manag. 2020, 16, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.; Bollmann, M. Soluble syndecans: Biomarkers for diseases and therapeutic options. Br. J. Pharmacol. 2019, 176, 67–81. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, J.Y.; Baek, I.W.; Kim, W.U.; Cho, C.S. Elevated serum levels of syndecan-1 are associated with renal involvement in patients with systemic lupus erythematosus. J. Rheumatol. 2015, 42, 202–209. [Google Scholar] [CrossRef]
- Statz, S.; Sabal, G.; Walborn, A.; Williams, M.; Hoppensteadt, D.; Mosier, M.; Rondina, M.; Fareed, J. Angiopoietin 2 levels in the risk stratification and mortality outcome prediction of sepsis-associated coagulopathy. Clin. Appl. Thromb. Hemost. 2018, 24, 1223–1233. [Google Scholar] [CrossRef] [Green Version]
- Maiese, A.; Bolino, G.; Mastracchio, A.; Frati, P.; Fineschi, V. An immunohistochemical study of the diagnostic value of TREM-1 as marker for fatal sepsis cases. Biotech. Histochem. 2019, 94, 159–166. [Google Scholar] [CrossRef]
- Velissaris, D.; Zareifopoulos, N.; Karamouzos, V.; Karanikolas, E.; Pierrakos, C.; Koniari, I.; Karanikolas, M. Presepsin as a Diagnostic and Prognostic Biomarker in Sepsis. Cureus 2021, 13, e15019. [Google Scholar] [CrossRef]
- Maiese, A.; Scatena, A.; Costantino, A.; Chiti, E.; Occhipinti, C.; La Russa, R.; Di Paolo, M.; Turillazzi, E.; Frati, P.; Fineschi, V. Expression of MicroRNAs in Sepsis-Related Organ Dysfunction: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 9354. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, L.; Zhang, X.; Dutta, P. The role of extracellular vesicles in regulating local and systemic inflammation in cardiovascular disease. Pharmacol. Res. 2021, 170, 105692. [Google Scholar] [CrossRef] [PubMed]
- Caricchio, R.; Gallucci, M.; Dass, C.; Zhang, X.; Gallucci, S.; Fleece, D.; Bromberg, M.; Criner, G.J.; Temple University COVID-19 Research Group. Preliminary Predictive Criteria for COVID-19 Cytokine Storm. Ann. Rheum. Dis. 2021, 80, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.M.; Cohen, A.S.; Fries, J.F.; Masi, A.T.; McShane, D.J.; Rothfield, N.F.; Schaller, J.G.; Talal, N.; Winchester, R.J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982, 25, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Bombardier, C.; Gladman, D.D.; Urowitz, M.B.; Caron, D.; Chang, C.H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992, 35, 630–640. [Google Scholar] [CrossRef]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Prevoo, M.L.; van’t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef] [Green Version]
- van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef]
- Garrett, S.; Jenkinson, T.; Kennedy, L.G.; Whitelock, H.; Gaisford, P.; Calin, A. A new approach to defining disease status in ankylosing spondylitis: The Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 1994, 21, 2286–2291. [Google Scholar]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H.; CASPAR Study Group. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef]
- Remenyi, B.; Wilson, N.; Steer, A.; Ferreira, B.; Kado, J.; Kumar, K.; Lawrenson, J.; Maguire, G.; Marijon, E.; Mirabel, M.; et al. World heart federation criteria for echocardiographic diagnosis of rheumatic heart disease—An evidence-based guideline. Nat. Rev. Cardiol. 2012, 9, 297–309. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. K/DOQI clinical practice guidelines chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39 (Suppl. S1), S1–S266. [Google Scholar]
- Rutherford, R.B.; Baker, J.D.; Ernst, C.; Johnston, K.W.; Porter, J.M.; Ahn, S.; Jones, D.N. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J. Vasc. Surg. 1997, 26, 517–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, R.M.; Whelton, P.K.; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension Guideline. Ann. Intern. Med. 2018, 168, 351–358. [Google Scholar] [CrossRef]
- Solez, K.; Colvin, R.B.; Racusen, L.C.; Haas, M.; Sis, B.; Mengel, M.; Halloran, P.F.; Baldwin, W.; Banfi, G.; Collins, A.B.; et al. Banff 07 classification of renal allograft pathology: Updates and future directions. Am. J. Transplant. 2008, 8, 753–760. [Google Scholar] [CrossRef]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.; DE Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef]






| Marker | Normal | Ranges of Absolute Parameter Values and Corresponding RL | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| IL-6, pg/mL | <5.0 | 5–10 | 10–40 | 40–200 | 200–1000 | >1000 | - |
| IL-8, pg/mL | <10.0 | 10–25 | 25–100 | 100–500 | 500–2500 | >2500 | - |
| IL-10, pg/mL | <5.0 | - | 5–10 | 10–25 | 25–100 | 100–500 | >500 |
| TNFα, pg/mL | <8.0 | 8–16 | 16–40 | 40–160 | 160–800 | >800 | - |
| CRP, mg/dL | <1.0 | 1–3 | 3–15 | >15 | - | - | - |
| Phenomena | Criteria | Points | Note |
|---|---|---|---|
| SIR–Cytokinemia | Values of RL-0-5 | 2–5 | Values of RL-0-1 except for acute SI |
| DIC | D-dimer > 500 ng/mL | 1 | or DIC-syndrome, e.g., DIC scale ≥ 5 score |
| Distress of hypothalamic-pituitary-adrenal axis | Cortisol > 1380 or <100 nmol/L (Norm 170–690 nmol/L) | 1 | In the absence of the criteria; but for glucocorticoid therapy 1, +1 point to score |
| Systemic alteration | Troponin I ≥ 0.2 ng/mL and/or myoglobin ≥ 800 ng/mL 2 | 1 | Troponin does not sum up in case of myocardial infarction |
| MOD | SOFA score and/or criteria of MODS | 1 | Phenomenon and syndrome are non-specific to SI |
| Verification | Scale-RL (Scores) | Scale-SI (Scores) |
|---|---|---|
| SIR without SI and pre-SI | 1–2 | ≤2 |
| Pre-SI | 1–4 | 3–4 |
| SI | 2–5 | 5–9 |
| Phases (SI) development/permission | 2–3 | 5–7 |
| Phlogogenic stroke (PS) | 4–5 * | 5–9 * |
| Depressive phase (DP) (depletion phase) | 2–3 ** | 5–7 ** |
| The structure of the SI process complex | Ratio of individual SI phenomena |
| ChSI Phenomena | Partial ChSI Criteria | Norm | ChSI Scale Points |
|---|---|---|---|
| Systemic inflammatory response | RL scale (Points) (0 to 5) | 0 | 1 RL point = 1 ChSI scale point |
| Microthrombosis | D-dimers > 500 ng/mL | <250 | 1 point |
| Systemic alteration | Myoglobin > 60 ng/mL or | <25 | 1 point |
| Troponin I > 0.2 ng/mL | <0.2 | ||
| Distress of the hypothalamic-pituitary-adrenal system | Cortisol > 690 nmol/L or | 138–690 | 1 point |
| Cortisol < 100 nmol/L |
| Group | RL, % | SIR | Systemic Alteration | DIC | Distress HPA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |||||
| Control 1 (donors), n = 50 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Control 2 (elderly people), n = 22 | 81.8 | 18.2 | 0 | 0 | 0 | 0 | 18.2 1 | 0 | 0 | 0 |
| Sepsis non-resuscitative on days 1–2, n = 40 | 0 | 27.5 | 55.0 | 17.5 | 0 | 0 | 100.0 | 5.0 | 10.0 | 0 |
| Sepsis on days 1–2, n = 46 | 0 | 4.4 | 10.9 | 41.3 | 30.4 | 13.0 | 100.0 | 34.8 | 58.7 | 30.4 |
| Sepsis on days 5–7, n = 13 | 0 | 0 | 7.7 | 46.2 | 46.1 | 0 | 100.0 | 46.2 | 92.3 2,3 | 15.4 |
| Septic shock, n = 14 | 0 | 0 | 7.1 | 14.3 | 42.9 | 35.7 | 100.0 | 42.9 | 85.7 | 64.3 |
| Tertiary peritonitis, n = 34 | 0 | 0 | 14.7 | 64.7 | 17.7 | 2.9 | 100.0 | 32.4 | 85.3 | 5.9 |
| Tertiary peritonitis + SS, n = 17 | 0 | 0 | 35.3 | 58.8 | 5.9 | 0 | 100.0 | 70.8 | 88.2 | 35.3 |
| Multiple injuries on days 1–2, n = 51 | 0 | 2.0 | 25.5 | 37.2 | 31.4 | 3.9 | 100.0 | 52.9 | 72.6 | 31.4 |
| Multiple injuries on days 5–7, n = 18 | 0 | 5.6 | 44.4 | 22.2 | 27.8 | 0 | 100.0 | 50.0 | 55.6 3 | 50.0 |
| Group (n) | Pre-SI, % | Phases of SI (100%—All SI Cases) | SI, % | LO, % | ||
|---|---|---|---|---|---|---|
| Development/Interphase Transition/Permission | Phlogogenic Stroke 1 | Depressive | ||||
| Control 1 (donors), n = 50 | 0 | 0 | 0 | 0 | 0 | 0 |
| Control 2 (elderly people), n = 22 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sepsis non-resuscitative on days 1–2, n = 40 | 65.0 | 7.5 | 0 | 0 | 7.5 | 0 |
| Sepsis on days 1–2, n = 46 | 21.7 | 41.2 | 58.8 | 0 | 73.9 3 | 23.9 |
| Sepsis on days 5–7, n = 13 | 0 | 46.2 | 53.9 | 0 | 100 3,4 | 30.8 |
| Septic shock, n = 14 | 0 | 0 | 78.6 | 21.4 | 100 | 71.4 |
| Tertiary peritonitis, n = 34 | 17.6 | 75.0 | 25.0 2 | 0 | 82.4 | 29.4 |
| Tertiary peritonitis + SS, n = 17 | 0 | 0 | 5.9 2 | 94.1 | 100 | 94.1 |
| Multiple injuries on days 1–2, n = 51 | 21.6 | 53.3 | 46.7 | 0 | 76.5 | 21.6 |
| Multiple injuries on days 5–7, n = 18 | 44.4 | 50.0 | 50.0 2 | 0 | 55.6 4 | 50.0 |
| Scales | Overall Mortality Prognostic Value (AUC) on Days 1–10 after Injury (n-9). Scale Scored on Day 1. | Overall Mortality Prognostic Value (AUC) on 6–10 Days (n-7). Scales Scored on 5–7 Day. |
|---|---|---|
| SI scale | 0.862 ± 0.0514 (p < 0.0001) | 0.874 ± 0.0567 (p < 0.0001) |
| RL scale | 0.867 ± 0.051 (p < 0.0001) | 0.878 ± 0.0574 (p < 0.0001) |
| SOFA | 0.721 ± 0.0792 (p = 0.0052) | 0.68 ± 0.0867 (p = 0.0376) |
| GCS | 0.821 ± 0.0722 (p < 0.0001) | 0.801 ± 0.0771 (p = 0.0001) |
| SIRS | 0.748 ± 0.068 (p = 0.0003) | 0.742 ± 0.072 (p = 0.0008) |
| APACHE II | 0.539 ± 0.153 (p = 0.7995) | We did not. |
| TRISS | 0.77 ± 0.10 (p = 0.0052) | We did not. |
| ISS | 0.78 ± 0.11 (p = 0.008) | We did not. |
| TISS | 0.625 ± 0.2 (p = 0.5329) | We did not. |
| SAPS II | 0.537 ± 0.211 (p = 0.8613) | We did not. |
| Group | RL, % | SIR, % | Systemic Alteration % | Microthrombosis, % | Distress of HPA, % | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |||||
| Control 1 (donors), n = 50 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Control 2 (elderly people), n = 22 | 81.8 | 18.2 | 0 | 0 | 0 | 0 | 18.2 | 0 | 0 | 0 |
| SLE, n = 49 | 8.2 | 4.1 | 16.3 | 32.6 | 34.7 | 4.1 | 91.8 | 6.1 | 40.8 | 28.6 |
| RA, n = 42 | 31.0 | 47.6 | 19.0 | 2.4 | 0 | 0 | 69.0 | 7.1 | 54.8 | 28.6 |
| ReA, n = 30 | 46.7 | 33.3 | 20.0 | 0 | 0 | 0 | 53.3 | 0 | 23.3 | 43.3 |
| AS, n = 27 | 44.5 | 33.3 | 22.2 | 0 | 0 | 0 | 55.5 | 3.7 | 11.1 | 7.4 |
| PsA, n = 12 | 33.3 | 50.0 | 16.7 | 0 | 0 | 0 | 66.7 | 8.3 | 8.3 | 8.3 |
| RHD, n = 15 | 53.5 | 33.3 | 13.2 | 0 | 0 | 0 | 46.7 | 0 | 13.3 | 14.3 |
| CHF, n = 49 | 53.1 | 36.7 | 10.2 | 0 | 0 | 0 | 46.9 | 2.0 | 33.3 | 2.0 |
| ESRD, n = 42 | 4.8 | 16.6 | 54.8 | 21.4 | 2.4 | 0 | 95.2 | 92.9 | 38.1 | 14.3 |
| CLI, n = 38 | 5.3 | 31.6 | 52.6 | 10.5 | 0 | 0 | 94.7 | 50.0 | 47.4 | 34.2 |
| CNW, n = 42 | 19.0 | 78.6 | 2.4 | 0 | 0 | 0 | 81.0 | 2.4 | 9.5 | 45.2 |
| AIT, n = 29 | 79.3 | 20.7 | 0 | 0 | 0 | 0 | 20.7 | 0 | 0 | 3.4 |
| PID, n = 16 | 75.0 | 25.0 | 0 | 0 | 0 | 0 | 25.0 | - * | 0 | - * |
| Menopausal syndrome, n = 16 | 93.7 | 6.3 | 0 | 0 | 0 | 0 | 6.3 | 0 | 0 | 0 |
| CRAD, n = 23 | 8.7 | 69.6 | 17.4 | 4.3 | 0 | 0 | 91.3 | 30.4 | 21.7 | 56.5 |
| Normal function of renal allograft, n = 24 | 58.3 | 25.0 | 16.7 | 0 | 0 | 0 | 41.7 | 0 | 4.2 | 29.2 |
| PAPS, n = 5 | 0 | 0 | 20.0 | 80.0 | 0 | 0 | 100 | - * | 100 | - * |
| Disease Clusters | Nosologies (The Frequency of ChSI Detection, %) |
|---|---|
| Cluster 1 (≤1% of ChSI) | Autoimmune thyroiditis (0 %) 1 |
| Menopausal syndrome (0 %) 1 | |
| Pelvic inflammatory disease (0 %) 1 | |
| Normal function of renal allograft (0 %) 1 | |
| Cluster 2 (1.1–10% of ChSI) | Chronic heart failure (2.0%) |
| Chronic nonhealing wounds (9.5%) | |
| Psoriatic arthritis (8.3%) | |
| Cluster 3 (10.1–50% of ChSI) | Ankylosing spondylitis (11.1%) |
| Chronic rheumatic valvular heart disease (13.3%) | |
| Reactive arthritis (20.0%) | |
| Rheumatoid arthritis (31.0%) | |
| Chronic renal allograft dysfunction (43.5%) | |
| Cluster 4 (>50% of ChSI) | Chronic limb threatening ischemia (57.9%) |
| Systemic lupus erythematosus (75.5%) | |
| End-stage renal disease (88.1%) | |
| Primary antiphospholipid syndrome (100%) |
| Objective | Solution | Result |
|---|---|---|
| 1. Check the quality of the SI scale. | Comparison of the SI scale score with the clinical equivalents of SI. | The SI scale detected SI in 100% of cases of refractory shock, in 96% of fatal outcomes, and in only 7.5% of non-resuscitated sepsis (MOD) cases. |
| 2. Confirm the general pathological nature of SI. | Comparison of two resuscitation cohorts: with sepsis and acute trauma. | Principal similarities in SI development revealed among pathologies with different nature of systemic damage |
| 3. Verify the individual SI phases. | Comparison of hyperergic and hypoergic variants of SI at different terms of critical state development. | Five principal phases of SI identified, of which the phase of secondary phlogogenic stroke (cytokine storm) and the depressive phase were the most critical for patients’ life (lethality > 50%). They were detected only in intensive care patients. |
| 4. Analyze the mortality prognostic value of the SI and clinical scales in polytrauma. | Comparison of the prognostic effectiveness of SI and RL with the clinical scales: APACHE II, TRISS, ISS, GCS, SOFA, TISS, SAPS II, SIRS. | The SI scales were superior or, at least, not inferior to the clinical scales tested in prognostic value for fatal outcomes. |
| 5. Define the role of SI in chronic diseases. | Identification of signs of ChSI in 16 chronic pathologies with alteration factors of different nature. | The diseases were divided into four classes according to the degree of ChSI detection. Assignment to each class was determined by the intensity of systemic damage rather than its nature. |
| 6 Differentiate between acute and chronic SI. | Determination of false-positive SI scale results in chronic diseases | False-positive results recorded in significant numbers (26.5%) only in patients with SLE who had very high levels of cytokinemia. |
| 7. Address the identification of similarities and differences between acute and chronic SI. | Comparison of SI phenomena frequency in integral groups with acute and chronic pathology | In general, the severity of SI phenomena in acute pathologies was higher, but the basic structure of their ratios was comparable. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zotova, N.; Zhuravleva, Y.; Chereshnev, V.; Gusev, E. Acute and Chronic Systemic Inflammation: Features and Differences in the Pathogenesis, and Integral Criteria for Verification and Differentiation. Int. J. Mol. Sci. 2023, 24, 1144. https://doi.org/10.3390/ijms24021144
Zotova N, Zhuravleva Y, Chereshnev V, Gusev E. Acute and Chronic Systemic Inflammation: Features and Differences in the Pathogenesis, and Integral Criteria for Verification and Differentiation. International Journal of Molecular Sciences. 2023; 24(2):1144. https://doi.org/10.3390/ijms24021144
Chicago/Turabian StyleZotova, Natalya, Yulia Zhuravleva, Valeriy Chereshnev, and Evgenii Gusev. 2023. "Acute and Chronic Systemic Inflammation: Features and Differences in the Pathogenesis, and Integral Criteria for Verification and Differentiation" International Journal of Molecular Sciences 24, no. 2: 1144. https://doi.org/10.3390/ijms24021144
APA StyleZotova, N., Zhuravleva, Y., Chereshnev, V., & Gusev, E. (2023). Acute and Chronic Systemic Inflammation: Features and Differences in the Pathogenesis, and Integral Criteria for Verification and Differentiation. International Journal of Molecular Sciences, 24(2), 1144. https://doi.org/10.3390/ijms24021144








