Enhancement of High-Density Lipoprotein (HDL) Quantity and Quality by Regular and Habitual Exercise in Middle-Aged Women with Improvements in Lipid and Apolipoprotein Profiles: Larger Particle Size and Higher Antioxidant Ability of HDL
Abstract
1. Introduction
2. Results
2.1. Anthropometric Profiles
| Group 1 n = 20 | Group 2 n = 16 | Group 3 n = 21 | p | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | |
|---|---|---|---|---|---|---|---|
| Moderate-intensity exercise/week (times) | 0.0 (0.0; 0.0) | 1.5 (1.5; 3.5) | 3.5 (1.5, 5.5) | <0.001 | 0.000 | 0.000 | 0.671 |
| High-intensity exercise/week (times) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 1.5 (1.5, 3.5) | <0.001 | 1.000 | <0.001 | <0.001 |
| Total number of exercises/week (times) | 0.0 (0.0; 0.0) | 1.5 (1.5; 3.5) | 5.0 (3.0, 7.0) | <0.001 | <0.001 | <0.001 | 0.009 |
| Total time of exercise/week (minutes) | 0 (0; 0) | 60 (60; 150) | 90 (75; 150) | <0.001 | <0.001 | <0.001 | 0.357 |
| MET equivalents of common physical activities (score) | 2.5 (2.5; 2.5) | 4.8 (3.3; 5.5) | 9.5 (8.0; 10.0) | 0.000 | 0.000 | 0.000 | 0.000 |
| Alcohol intake/month (g) | 63 (5; 210) | 21 (0; 94.5) | 21 (0, 137) | 0.458 | 0.801 | 0.901 | 1.000 |
| Age (year), (min., max.) | 53.5 (50.0; 56.0) (41, 58) | 45.0 (40.8; 54.8) (35, 63) | 52.0 (43.0, 55.5) (36, 62) | 0.132 | 0.171 | 0.301 | 0.924 |
| Height (cm) | 160.0 (157; 163.8) | 161.0 (158.3; 165.0) | 159.0 (156.0, 164.5) | 0.804 | 1.000 | 1.000 | 1.000 |
| Weight (kg) | 55.7 (50.9; 59.9) | 56.9 (53.3; 66.8) | 54.5 (51.2; 61.1) | 0.116 | 0.242 | 1.000 | 0.169 |
| BMI (kg/m2), (min., max.) | 21.4 (20.1; 22.8) (19.5, 26.1) | 23.0 (20.4; 24.2) (18.4, 30.9) | 21.4 (20.2, 23.0) (17.0, 26.8) | 0.446 | 0.797 | 1.000 | 0.773 |
| Heart rate (BPM) | 70.0 (66.8; 75.8) | 73.0 (66.0; 84.8) | 75.0 (72.0, 81.0) | 0.142 | 0.549 | 0.167 | 1.000 |
| SBP (mmHg) | 120.5 (110.0; 129.8) | 123.0 (108.5; 127.5) | 120.0 (117.0, 124.0) | 0.930 | 1.000 | 1.000 | 1.000 |
| DBP (mmHg) | 75.5 (63.3; 87.8) | 68.0 (62.8; 77.0) | 69.0 (65.5; 78.0) | 0.207 | 0.262 | 0.673 | 1.000 |
| Muscle mass (kg) | 37.5 (35.8; 38.8) | 39.7 (35.4; 42.8) | 38.1 (34.5; 40.1) | 0.172 | 0.248 | 1.000 | 0.357 |
| Fat mass_subcutaneous (kg) | 13.6 (12.2; 15.9) | 14.4 (12.7; 18.1) | 13.7 (11.0; 16.4) | 0.240 | 0.860 | 1.000 | 0.282 |
| Fat mass_visceral (kg) | 1.5 (1.2; 2.0) | 1.8 (1.2; 2.4) | 1.5 (1.1; 2.0) | 0.406 | 1.000 | 1.000 | 0.542 |
| HS (kg), (min., max.) | 26.5 (23.0; 28.0) (16.0, 30.0) | 25.0 (23.0; 27.8) (22.0, 37.0) | 28.0 (25.5; 30.0) (13.0, 32.0) | 0.158 | 1.000 | 0.275 | 0.334 |
| Body water (kg) | 29.2 (27.4; 30.3) | 30.9 (27.6; 33.5) | 29.9 (31.5) | 0.087 | 0.103 | 1.000 | 0.263 |
2.2. Blood Parameters
2.3. LDL Compositions and Particle Analysis
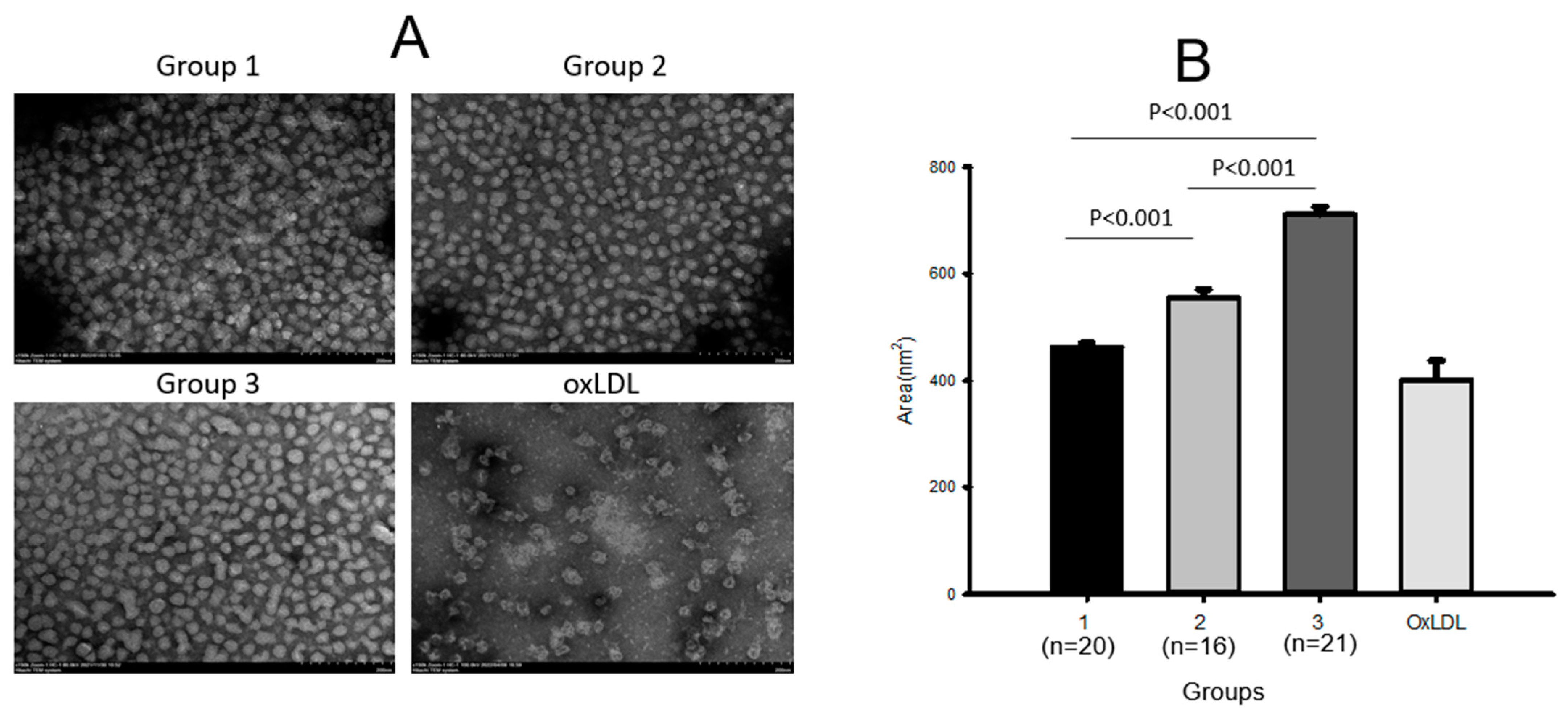
2.4. Electron Microscopic Analysis of LDL
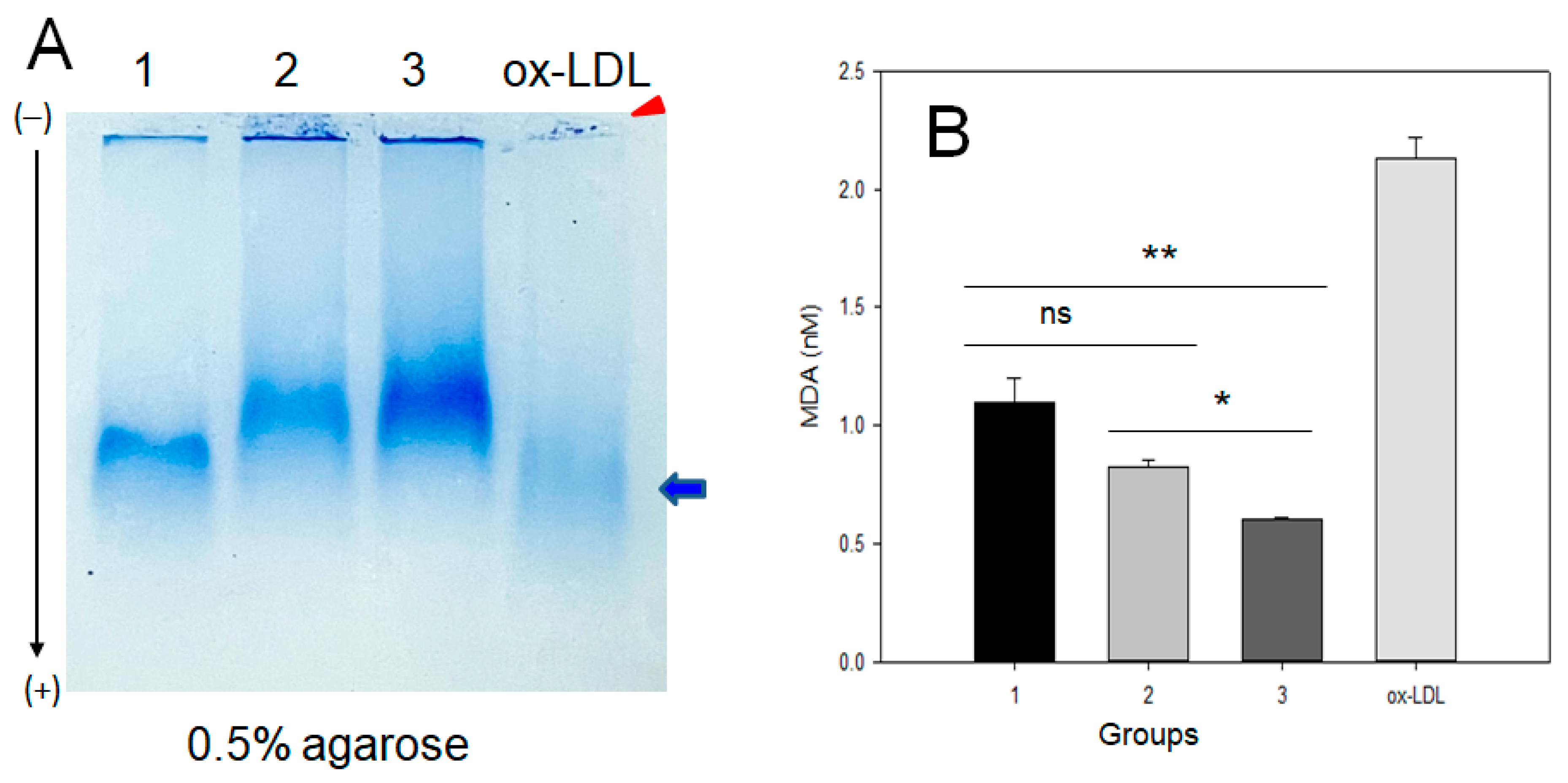
2.5. Electromobility of LDL
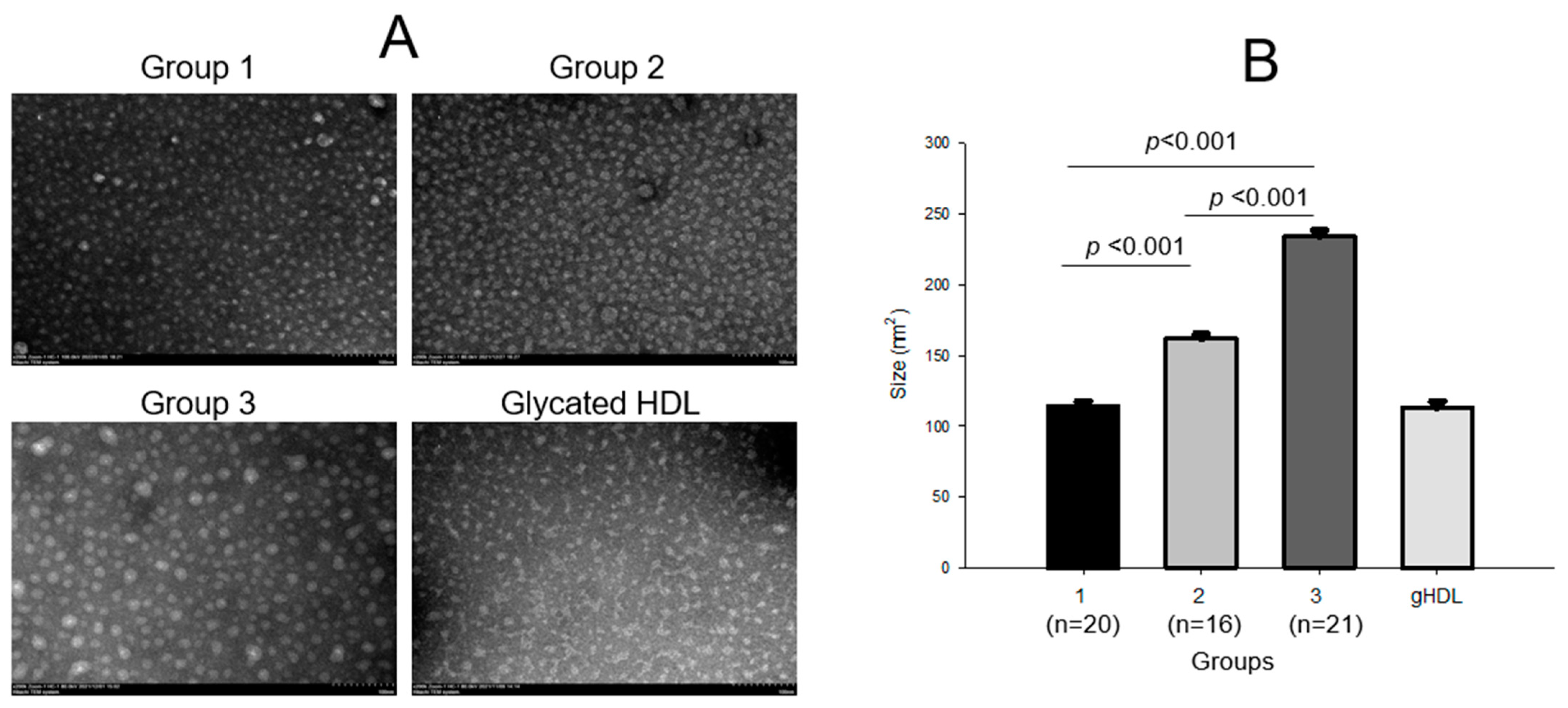
2.6. HDL2 Compositions and Particle Morphology
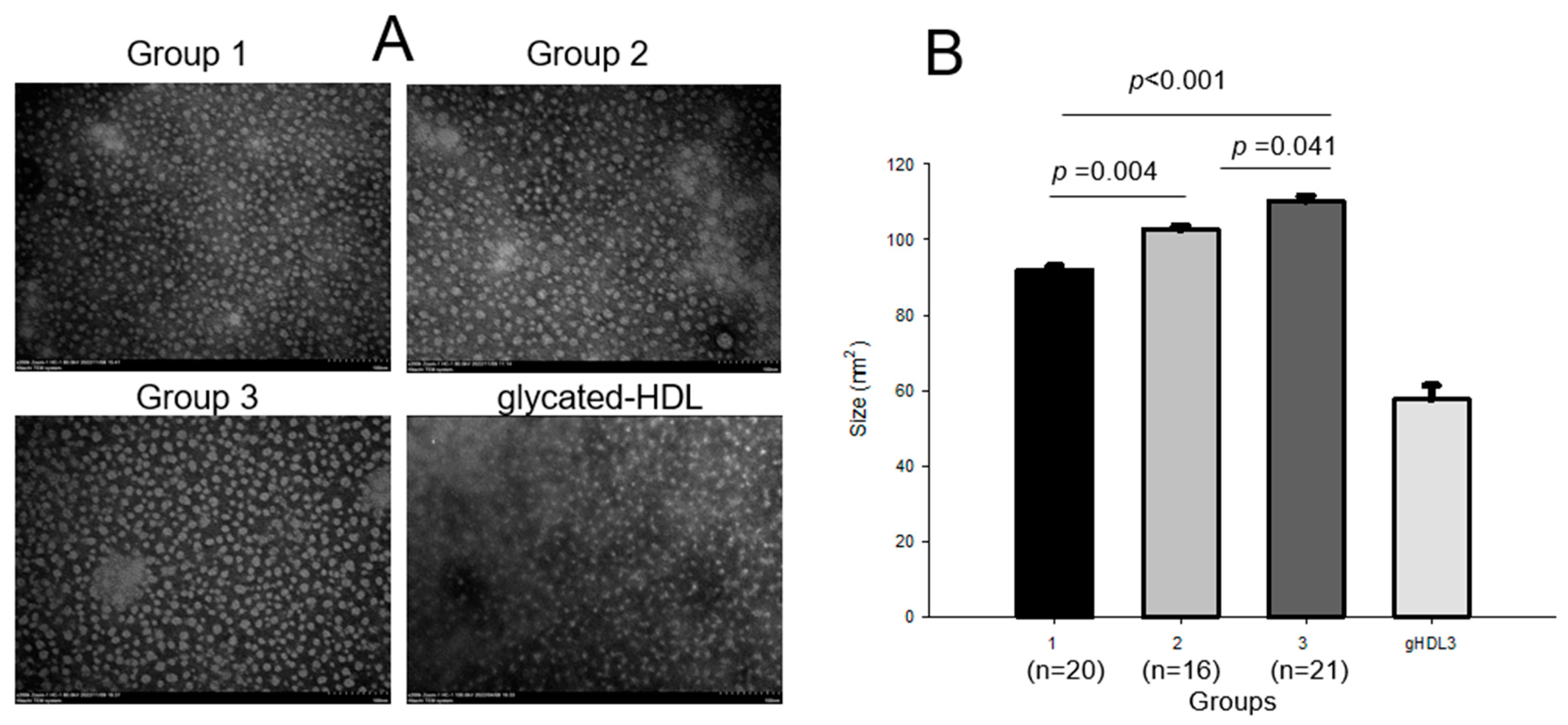
2.7. HDL3 Compositions and Particle Analysis
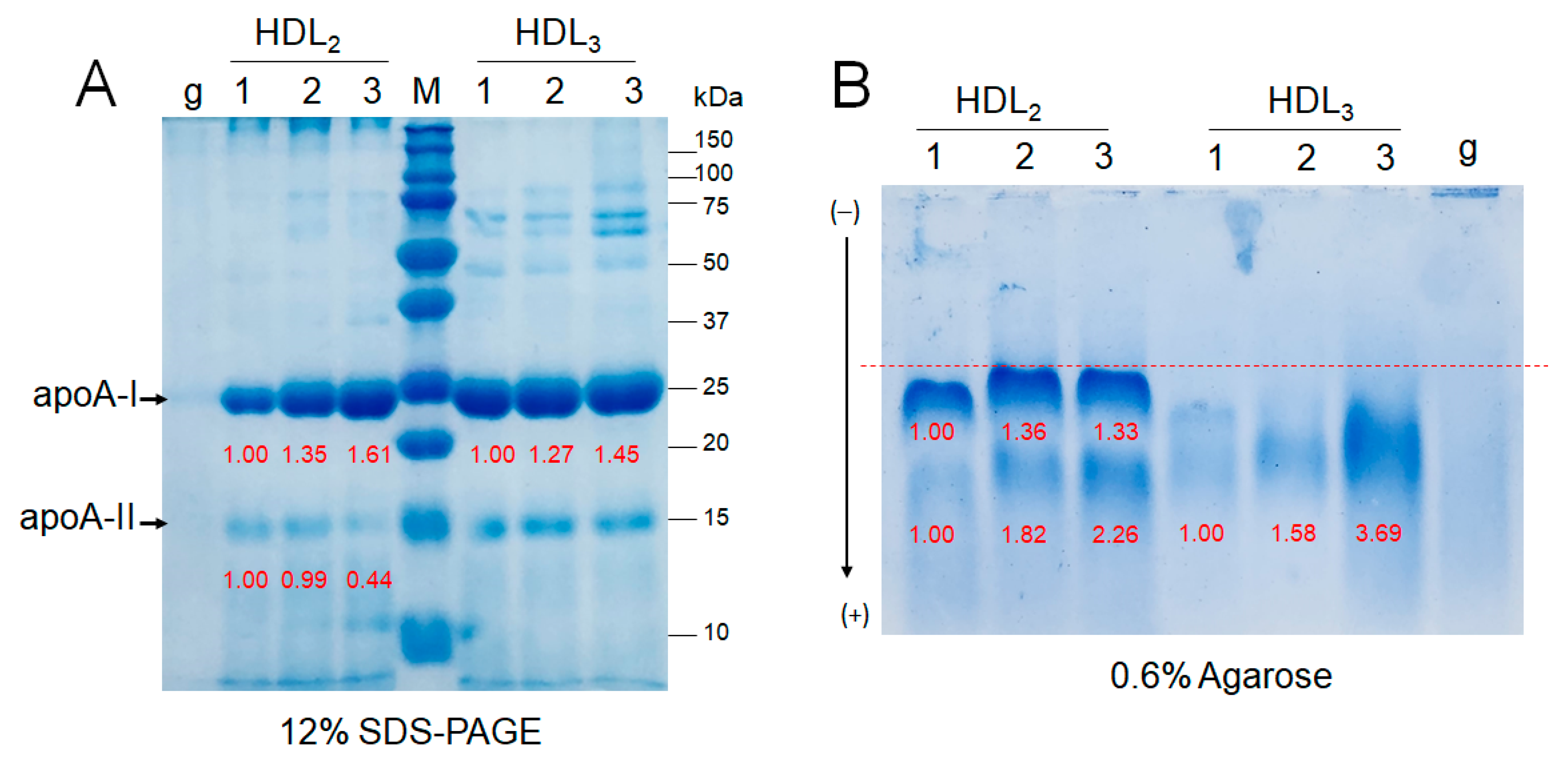
2.8. Electrophoretic Patterns of HDL2 and HDL3
2.9. Correlation Analysis
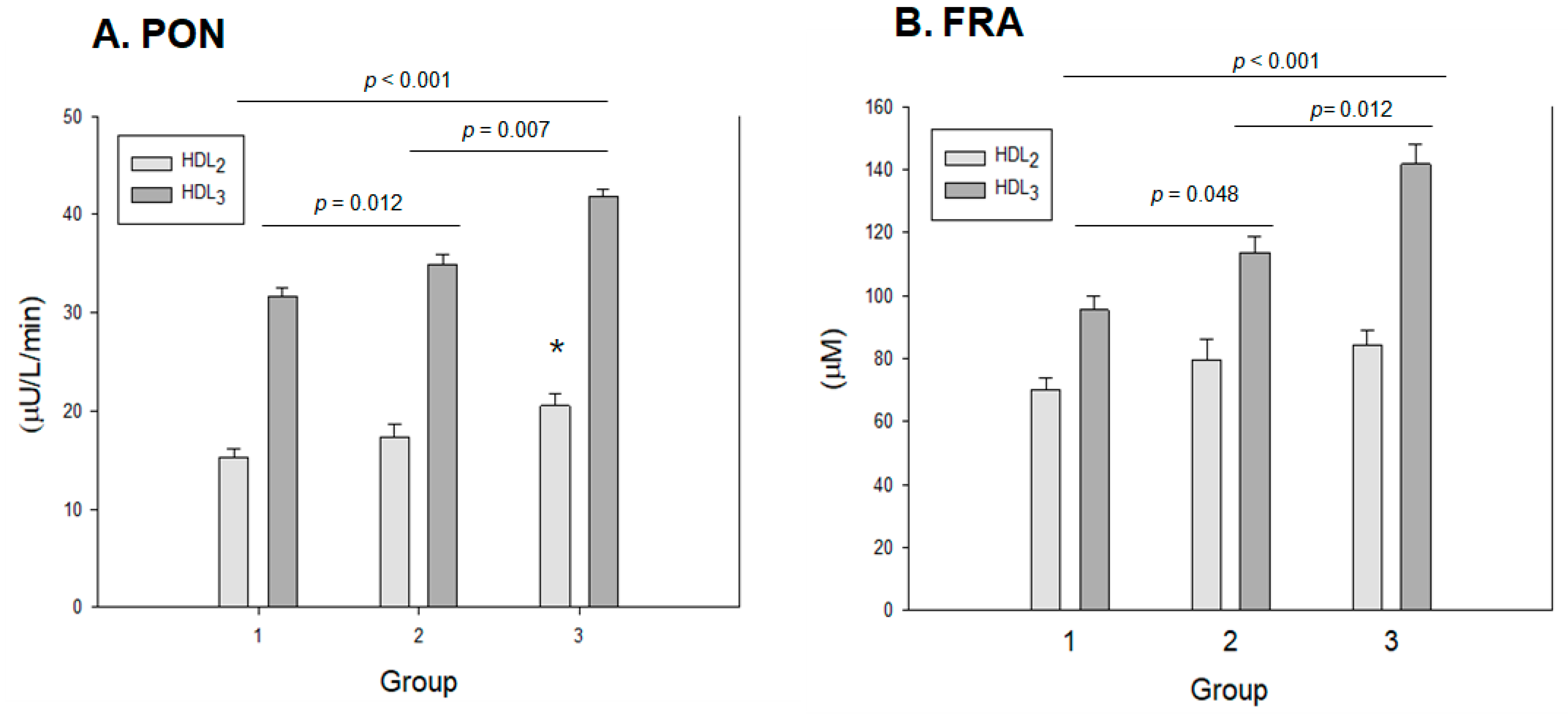
2.10. Antioxidant Activity in HDL2 and HDL3
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Anthropometric Analysis
4.3. Blood Analysis
4.4. Isolation of Lipoproteins
4.5. LDL Oxidation and Quantification
4.6. Paraoxonase Assay
4.7. Ferric-Ion-Reducing Ability Assay
4.8. Electromobility of Lipoproteins
4.9. Glycation of HDL
4.10. Electron Microscopy
4.11. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mainous, A.G., III; Tanner, R.J.; Rahmanian, K.P.; Jo, A.; Carek, P.J. Effect of sedentary lifestyle on cardiovascular disease risk among healthy adults with body mass indexes 18.5 to 29.9 kg/m2. Am. J. Cardiol. 2019, 123, 764–768. [Google Scholar] [CrossRef]
- Paffenbarger, R.S., Jr.; Hyde, R.T.; Wing, A.L.; Lee, I.-M.; Jung, D.L.; Kampert, J.B. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N. Engl. J. Med. 1993, 328, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Ozato, N.; Yamaguchi, T.; Sudo, M.; Yamashiro, Y.; Mori, K.; Ishida, M.; Katsuragi, Y.; Sasai, H.; Yasukawa, T. Association of sedentary behaviour and physical activity with cardiometabolic health in Japanese adults. Sci. Rep. 2022, 12, 2262. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Hein, H.O.; Suadicani, P.; Gyntelberg, F. Relation of high TG–low HDL cholesterol and LDL cholesterol to the incidence of ischemic heart disease: An 8-year follow-up in the Copenhagen male study. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Paffenbarger, R.S., Jr.; Hyde, R.; Wing, A.L.; Hsieh, C.-C. Physical activity, all-cause mortality, and longevity of college alumni. N. Engl. J. Med. 1986, 314, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Blair, S.N.; Kohl, H.W.; Paffenbarger, R.S.; Clark, D.G.; Cooper, K.H.; Gibbons, L.W. Physical fitness and all-cause mortality: A prospective study of healthy men and women. JAMA 1989, 262, 2395–2401. [Google Scholar] [CrossRef]
- Ruiz-Ramie, J.J.; Barber, J.L.; Sarzynski, M.A. Effects of exercise on HDL functionality. Curr. Opin. Lipidol. 2019, 30, 16. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017, 16, 132. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Kim, J.; Choi, I.; Kim, J.-R.; Cho, K.-H. Breast Milk from frequent trans fatty acid consumers shows high triglyceride and glucose levels, but low cholesterol and apolipoprotein AI levels, with resulting impaired in vitro zebrafish embryo growth and survival. Breastfeed. Med. 2016, 11, 239–246. [Google Scholar] [CrossRef]
- Cho, K.-H.; Nam, H.-S.; Kang, D.-J.; Park, M.-H.; Kim, J.-H. Long-Term Alcohol Consumption Caused a Significant Decrease in Serum High-Density Lipoprotein (HDL)-Cholesterol and Apolipoprotein AI with the Atherogenic Changes of HDL in Middle-Aged Korean Women. Int. J. Mol. Sci. 2022, 23, 8623. [Google Scholar] [CrossRef]
- Park, K.H.; Shin, D.G.; Cho, K.H. Dysfunctional lipoproteins from young smokers exacerbate cellular senescence and atherogenesis with smaller particle size and severe oxidation and glycation. Toxicol. Sci. 2014, 140, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D.; Haas, M.J.; Wehmeier, K.R.; Wong, N.C. Obesity-related changes in high-density lipoprotein metabolism. Obesity 2008, 16, 1152–1160. [Google Scholar] [CrossRef]
- Cho, K.-H. The Current Status of Research on High-Density Lipoproteins (HDL): A Paradigm Shift from HDL Quantity to HDL Quality and HDL Functionality. Int. J. Mol. Sci. 2022, 23, 3967. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Shin, D.G.; Kim, J.R.; Cho, K.H. Senescence-related truncation and multimerization of apolipoprotein A-I in high-density lipoprotein with an elevated level of advanced glycated end products and cholesteryl ester transfer activity. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 600–610. [Google Scholar] [CrossRef]
- Park, K.H.; Cho, K.H. High-density lipoprotein (HDL) from elderly and reconstituted HDL containing glycated apolipoproteins A-I share proatherosclerotic and prosenescent properties with increased cholesterol influx. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-H.; Park, J.-E.; Choi, I.-H.; Cho, K.-H. Enhanced functional and structural properties of high-density lipoproteins from runners and wrestlers compared to throwers and lifters. BMB Rep. 2009, 42, 605–610. [Google Scholar] [CrossRef]
- Franceschini, G.; Calabresi, L.; Maderna, P.; Galli, C.; Gianfranceschi, G.; Sirtori, C.R. ω-3 fatty acids selectively raise high-density lipoprotein 2 levels in healthy volunteers. Metabolism 1991, 40, 1283–1286. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, S.-M.; Kim, S.-J.; Lee, E.-Y.; Kim, J.-R.; Cho, K.-H. Consumption of policosanol enhances HDL functionality via CETP inhibition and reduces blood pressure and visceral fat in young and middle-aged subjects. Int. J. Mol. Med. 2017, 39, 889–899. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, S.-J.; Yadav, D.; Kim, J.-Y.; Kim, J.-R. Consumption of cuban policosanol improves blood pressure and lipid profile via enhancement of HDL functionality in healthy women subjects: Randomized, double-blinded, and placebo-controlled study. Oxidative Med. Cell. Longev. 2018, 2018, 4809525. [Google Scholar] [CrossRef]
- Kim, S.-J.; Yadav, D.; Park, H.-J.; Kim, J.-R.; Cho, K.-H. Long-term consumption of cuban policosanol lowers central and brachial blood pressure and improves lipid profile with enhancement of lipoprotein properties in healthy korean participants. Front. Physiol. 2018, 9, 412. [Google Scholar] [CrossRef]
- Park, H.-J.; Yadav, D.; Jeong, D.-J.; Kim, S.-J.; Bae, M.-A.; Kim, J.-R.; Cho, K.-H. Short-term consumption of Cuban policosanol lowers aortic and peripheral blood pressure and ameliorates serum lipid parameters in healthy Korean participants: Randomized, double-blinded, and placebo-controlled study. Int. J. Environ. Res. Public Health 2019, 16, 809. [Google Scholar] [CrossRef]
- Zhao, S.; Zhong, J.; Sun, C.; Zhang, J. Effects of aerobic exercise on TC, HDL-C, LDL-C and TG in patients with hyperlipidemia: A protocol of systematic review and meta-analysis. Medicine 2021, 100, e25103. [Google Scholar] [CrossRef]
- Haskell, W.L.; Lee, I.-M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007, 116, 1081. [Google Scholar] [CrossRef] [PubMed]
- Durstine, J.L.; Grandjean, P.W.; Davis, P.G.; Ferguson, M.A.; Alderson, N.L.; DuBose, K.D. Blood lipid and lipoprotein adaptations to exercise. Sport. Med. 2001, 31, 1033–1062. [Google Scholar] [CrossRef] [PubMed]
- Ben Ounis, O.; Elloumi, M.; Makni, E.; Zouhal, H.; Amri, M.; Tabka, Z.; Lac, G. Exercise improves the ApoB/ApoA-I ratio, a marker of the metabolic syndrome in obese children. Acta Paediatr. 2010, 99, 1679–1685. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M. Comparison of effects of diet versus exercise weight loss regimens on LDL and HDL particle size in obese adults. Lipids Health Dis. 2011, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Sarzynski, M.A.; Burton, J.; Rankinen, T.; Blair, S.N.; Church, T.S.; Després, J.-P.; Hagberg, J.M.; Landers-Ramos, R.; Leon, A.S.; Mikus, C.R. The effects of exercise on the lipoprotein subclass profile: A meta-analysis of 10 interventions. Atherosclerosis 2015, 243, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Liu, Y.; Lebaka, V.R.; Mohammed, A.; Ye, W.; Chen, B.; Korivi, M. Effect of Exercise Training on Serum Transaminases in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front. Physiol. 2022, 1260. [Google Scholar] [CrossRef]
- Senior, J. Alanine aminotransferase: A clinical and regulatory tool for detecting liver injury–past, present, and future. Clin. Pharmacol. Ther. 2012, 92, 332–339. [Google Scholar] [CrossRef]
- Chalasani, N. Clinical Meaning of Elevated Aminotransferase Activity; Hepatotoxicity Special Interest Group Meeting: Silver Springs, MD, USA, 2008. [Google Scholar]
- Kido, T.; Kondo, K.; Kurata, H.; Fujiwara, Y.; Urata, T.; Itakura, H.; Yokoyama, S. ApoA-I/A-II-HDL positively associates with apoB-lipoproteins as a potential atherogenic indicator. Lipids Health Dis. 2017, 16, 225. [Google Scholar] [CrossRef]
- Kuchta, A.; Strzelecki, A.; Ćwiklińska, A.; Gruchała, M.; Zdrojewski, Z.; Kortas-Stempak, B.; Wieczorek, E.; Gliwińska, A.; Dąbkowski, K.; Jankowski, M. HDL subpopulations containing apoA-I without apoA-II (LpA-I) in patients with angiographically proven coronary artery disease. J. Cardiol. 2017, 69, 523–528. [Google Scholar] [CrossRef]
- Kido, T.; Kurata, H.; Kondo, K.; Itakura, H.; Okazaki, M.; Urata, T.; Yokoyama, S. Bioinformatic analysis of plasma apolipoproteins AI and A-II revealed unique features of AI/A-II HDL particles in human plasma. Sci. Rep. 2016, 6, 31532. [Google Scholar] [CrossRef] [PubMed]
- LeMura, L.M.; von Duvillard, S.P.; Andreacci, J.; Klebez, J.M.; Chelland, S.A.; Russo, J. Lipid and lipoprotein profiles, cardiovascular fitness, body composition, and diet during and after resistance, aerobic and combination training in young women. Eur. J. Appl. Physiol. 2000, 82, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Stanton, K.M.; Kienzle, V.; Dinnes, D.L.M.; Kotchetkov, I.; Jessup, W.; Kritharides, L.; Celermajer, D.S.; Rye, K.A. Moderate- and High-Intensity Exercise Improves Lipoprotein Profile and Cholesterol Efflux Capacity in Healthy Young Men. J. Am. Heart Assoc. 2022, 11, e023386. [Google Scholar] [CrossRef]
- Fonseca, M.I.H.; da Silva, I.T.; Ferreira, S.R.G. Impact of menopause and diabetes on atherogenic lipid profile: Is it worth to analyse lipoprotein subfractions to assess cardiovascular risk in women? Diabetol. Metab. Syndr. 2017, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Park, H.J.; Kim, S.J.; Kim, J.R. Decrease in HDL-C is Associated with Age and Household Income in Adults from the Korean National Health and Nutrition Examination Survey 2017: Correlation Analysis of Low HDL-C and Poverty. Int. J. Environ. Res. Public Health 2019, 16, 3329. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef]
- Markwell, M.A.K.; Haas, S.M.; Bieber, L.; Tolbert, N. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Garin, M.-C.B.; Moren, X.; James, R.W. Paraoxonase-1 and serum concentrations of HDL-cholesterol and apoA-I. J. Lipid Res. 2006, 47, 515–520. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-R.; Lee, I.-C.; Kwon, H.-J. Native high-density lipoproteins (HDL) with higher paraoxonase exerts a potent antiviral effect against SARS-CoV-2 (COVID-19), while glycated HDL lost the antiviral activity. Antioxidants 2021, 10, 209. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 15–27. [Google Scholar]
- Noble, R.P. Electrophoretic separation of plasma lipoproteins in agarose gel. J. Lipid Res. 1968, 9, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Kang, D.-J.; Nam, H.-S.; Kim, J.-H.; Kim, S.-Y.; Lee, J.-O.; Kim, B.-J. Ozonated sunflower oil exerted protective effect for embryo and cell survival via potent reduction power and antioxidant activity in HDL with strong antimicrobial activity. Antioxidants 2021, 10, 1651. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-H.; Kim, J.-Y.; Choi, I.; Kim, J.-R.; Won, K.C.; Cho, K.-H. Fructated apolipoprotein AI exacerbates cellular senescence in human umbilical vein endothelial cells accompanied by impaired insulin secretion activity and embryo toxicity. Biochem. Cell Biol. 2016, 94, 337–345. [Google Scholar] [CrossRef] [PubMed]
- McPherson, J.D.; Shilton, B.H.; Walton, D.J. Role of fructose in glycation and cross-linking of proteins. Biochemistry 1988, 27, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
| Group 1 n = 20 | Group 2 n = 16 | Group 3 n = 21 | p | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | |
|---|---|---|---|---|---|---|---|
| MET equivalents of common physical activities (score) | 2.5 (2.5; 2.5) | 4.8 (3.3; 5.5) | 9.5 (8.0; 10.0) | 0.000 | 0.000 | 0.000 | 0.000 |
| TC (mg/dL) | 222.0 (195.2; 244.1) | 210.8 (170.3; 251.0) | 206.0 (184.3; 220.8) | 0.179 | 1.000 | 0.199 | 0.924 |
| HDL-C (mg/dL) | 46.7 (42.3; 56.5) | 52.5 (45.4; 59.7) | 54.0 (47.8; 61.9) | 0.030 | 0.133 | 0.040 | 1.000 |
| HDL-C/TC (ratio) | 0.22 (0.19; 0.24) | 0.26 (0.20; 0.34) | 0.28 (0.24; 0.30) | 0.002 | 0.099 | <0.001 | 0.921 |
| LDL-C (mg/dL) | 151.5 (131.8; 170.3) | 141.0 (100.5; 178.8) | 137.0 (115.5; 141.5) | 0.172 | 0.764 | 0.104 | 0.850 |
| TG (mg/dL) | 107.5 (85.4; 136.6) | 78.0 (57.5; 114.9) | 57.2 (44.5; 65.5) | <0.001 | 0.165 | <0.001 | 0.080 |
| TG/HDL-C (ratio) | 2.4 (1.5; 3.3) | 1.5 (1.0; 2.5) | 1.0 (0.9; 1.4) | <0.001 | 0.183 | <0.001 | 0.112 |
| LDL-C/HDL-C (ratio) | 3.1 (2.8; 3.7) | 2.3 (1.8; 3.7) | 2.4 (2.2; 3.0) | 0.004 | 0.277 | 0.001 | 0.652 |
| ApoA-I (mg/dL) | 163.0 (153.0; 180.5) | 167.5 (152.5; 210.8) | 183.0 (161.5; 200.0) | 0.072 | 0.223 | 0.101 | 1.000 |
| ApoB (mg/dL) | 103.5 (91.5; 117.8) | 104.5 (73.8; 118.3) | 91.0 (80.5; 100.0) | 0.132 | 0.919 | 0.136 | 1.000 |
| ApoB/ApoA-I (ratio) | 0.64 (0.50; 0.82) | 0.55 (0.41; 0.70) | 0.54 (0.44; 0.61) | 0.014 | 0.261 | 0.009 | 0.797 |
| RC (mg/dL) | 21.1 (16.9;27.2) | 15.6 (11.3;22.6) | 11.4 (8.9;13.0) | <0.001 | 0.166 | <0.001 | 0.097 |
| Glucose (mg/dL) | 96.0 (90.0; 101.8) | 89.0 (82.5; 93.0) | 84.0 (80.5; 92.0) | 0.003 | 0.028 | 0.003 | 1.000 |
| hs-CRP (mg/L) | 0.24 (0.15; 0.55) | 0.28 (0.23; 0.48) | 0.32 (0.21; 0.51) | 0.896 | 1.000 | 1.000 | 1.000 |
| AST (Unit/L) | 20.0 (17.0; 22.8) | 16.5 (11.5; 18.0) | 14.0 (12.5; 19.0) | 0.003 | 0.028 | 0.003 | 1.000 |
| ALT (Unit/L) | 14.0 (11.0; 16.0) | 11.0 (7.3; 16.8) | 11.0 (8.0; 14.0) | 0.088 | 0.314 | 0.110 | 1.000 |
| γ-GTP (Unit/L) | 14.5 (10.3; 25.0) | 12.5 (9.3; 18.0) | 10.0 (8.0; 15.0) | 0.064 | 0.943 | 0.058 | 0.708 |
| Group 1 Sedentary | Group 2 Low- Exercise | Group 3 High- Exercise | p | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | ||
|---|---|---|---|---|---|---|---|---|
| MET score/week | 2.48 ± 0.03 | 4.55 ± 0.27 | 9.13 ± 0.35 | <0.001 | <0.001 | <0.001 | <0.001 | |
| LDL | TC (mg/mL) | 0.99 ± 0.17 | 1.09 ± 0.09 | 1.12 ± 0.10 | 0.801 | 1.000 | 1.000 | 1.000 |
| TG (mg/mL) | 0.22 ± 0.03 | 0.21 ± 0.02 | 0.17 ± 0.01 | 0.023 | 0.095 | 0.041 | 0.192 | |
| MDA (nM) | 1.78 ± 0.06 | 1.43 ± 0.11 | 1.07 ± 0.14 | <0.001 | 0.095 | <0.001 | 0.073 | |
| FI (glycated) | 1406 ± 37 | 1403 ± 78 | 1317 ± 70 | 0.572 | 1.000 | 1.000 | 1.000 | |
| Diameter (nm) | 23.9 ± 0.4 | 24.6 ± 0.3 | 27.9 ± 0.5 | <0.001 | <0.001 | <0.001 | <0.001 | |
| HDL2 | TC (mg/mL) | 0.55 ± 0.07 | 0.71 ± 0.13 | 0.85 ± 0.07 | 0.031 | 0.166 | 0.042 | 1.000 |
| TG (mg/mL) | 0.14 ± 0.01 | 0.13 ± 0.02 | 0.10 ± 0.02 | 0.541 | 0.860 | 0.125 | 1.000 | |
| MDA (nM) | 0.40 ± 0.10 | 0.31 ± 0.07 | 0.36 ± 0.09 | 0.775 | 1.000 | 1.000 | 1.000 | |
| FI (glycated) | 934 ± 92.8 | 853 ± 35 | 843 ± 60 | 0.592 | 1.000 | 1.000 | 1.000 | |
| Diameter (nm) | 12.6 ± 0.3 | 15.0 ± 0.3 | 17.6 ± 0.3 | <0.001 | <0.001 | <0.001 | <0.001 | |
| HDL3 | TC (mg/mL) | 0.37 ± 0.03 | 0.36 ± 0.02 | 0.36 ± 0.02 | 0.881 | 0.943 | 0.988 | 0.987 |
| TG (mg/mL) | 0.09 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.022 | 0.051 | 0.017 | 0.873 | |
| MDA (nM) | 0.29 ± 0.05 | 0.25 ± 0.06 | 0.24 ± 0.04 | 0.138 | 0.074 | 0.145 | 0.150 | |
| FI (glycated) | 642 ± 46 | 663 ± 87 | 713 ± 29 | 0.473 | 1.000 | 0.699 | 1.000 | |
| Diameter (nm) | 10.9 ± 0.2 | 12.3 ± 0.2 | 14.6 ± 0.3 | <0.001 | <0.001 | <0.001 | <0.001 | |
| r | p | ||
|---|---|---|---|
| Anthropometric profiles | BMI (kg/m2) | −0.072 | 0.592 |
| Fat mass_subcutaneous (kg) | −0.166 | 0.217 | |
| Fat mass_visceral (kg) | −0.167 | 0.216 | |
| Heart rate (BPM) | 0.344 | 0.009 | |
| SBP (mmHg) | −0.075 | 0.577 | |
| DBP (mmHg) | −0.178 | 0.186 | |
| HS (kg) | 0.126 | 0.351 | |
| Blood lipid and inflammatory parameters | HDL-C (mg/dL) | 0.365 | 0.005 |
| ApoA-I (mg/dL) | 0.354 | 0.007 | |
| ApoB (mg/dL) | −0.264 | 0.047 | |
| ApoB/ApoA-I (ratio) | −0.373 | 0.004 | |
| LDL-C (mg/dL) | −0.246 | 0.065 | |
| LDL-C/HDL-C (ratio) | −0.437 | 0.001 | |
| Glucose | −0.522 | 0.000 | |
| TC (mg/dL) | −0.234 | 0.080 | |
| HDL-C/TC (ratio) | 0.484 | 0.000 | |
| TG (mg/dL) | −0.577 | 0.000 | |
| TG/HDL-C (ratio) | −0.612 | 0.000 | |
| Remnant cholesterol | −0.572 | 0.000 | |
| AST (Unit/L) | −0.555 | 0.000 | |
| ALT (Unit/L) | −0.351 | 0.007 | |
| γ-GTP (Unit/L) | −0.365 | 0.005 | |
| hs-CRP (mg/L) | 0.116 | 0.389 | |
| Lipoprotein profiles and characteristics | LDL-TC (mg/mL/protein) | 0.151 | 0.491 |
| LDL-TG (mg/mL/protein) | 0.263 | 0.225 | |
| LDL_MDA (nM) | −0.238 | 0.000 | |
| LDL_size (nm) | 0.272 | 0.000 | |
| HDL2-TC (mg/mL/protein) | 0.621 | 0.002 | |
| HDL2-TG (mg/mL/protein) | 0.240 | 0.270 | |
| HDL2_MDA (nM) | −0.005 | 0.981 | |
| HDL2_size (nm) | 0.650 | 0.000 | |
| HDL2_FRA | 0.308 | 0.153 | |
| HDL2_PON | 0.446 | 0.033 | |
| HDL3-TC (mg/mL/protein) | −0.084 | 0.702 | |
| HDL3-TG (mg/mL/protein) | −0.172 | 0.433 | |
| HDL3-MDA (nM) | 0.370 | 0.082 | |
| HDL3-size (nm) | 0.485 | 0.000 | |
| HDL3-FRA | 0.578 | 0.004 | |
| HDL3-PON | 0.811 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.-H.; Nam, H.-S.; Kang, D.-J.; Zee, S.; Park, M.-H. Enhancement of High-Density Lipoprotein (HDL) Quantity and Quality by Regular and Habitual Exercise in Middle-Aged Women with Improvements in Lipid and Apolipoprotein Profiles: Larger Particle Size and Higher Antioxidant Ability of HDL. Int. J. Mol. Sci. 2023, 24, 1151. https://doi.org/10.3390/ijms24021151
Cho K-H, Nam H-S, Kang D-J, Zee S, Park M-H. Enhancement of High-Density Lipoprotein (HDL) Quantity and Quality by Regular and Habitual Exercise in Middle-Aged Women with Improvements in Lipid and Apolipoprotein Profiles: Larger Particle Size and Higher Antioxidant Ability of HDL. International Journal of Molecular Sciences. 2023; 24(2):1151. https://doi.org/10.3390/ijms24021151
Chicago/Turabian StyleCho, Kyung-Hyun, Hyo-Seon Nam, Dae-Jin Kang, Seonggeun Zee, and Min-Hee Park. 2023. "Enhancement of High-Density Lipoprotein (HDL) Quantity and Quality by Regular and Habitual Exercise in Middle-Aged Women with Improvements in Lipid and Apolipoprotein Profiles: Larger Particle Size and Higher Antioxidant Ability of HDL" International Journal of Molecular Sciences 24, no. 2: 1151. https://doi.org/10.3390/ijms24021151
APA StyleCho, K.-H., Nam, H.-S., Kang, D.-J., Zee, S., & Park, M.-H. (2023). Enhancement of High-Density Lipoprotein (HDL) Quantity and Quality by Regular and Habitual Exercise in Middle-Aged Women with Improvements in Lipid and Apolipoprotein Profiles: Larger Particle Size and Higher Antioxidant Ability of HDL. International Journal of Molecular Sciences, 24(2), 1151. https://doi.org/10.3390/ijms24021151






