Microbiome Dysbiosis: A Pathological Mechanism at the Intersection of Obesity and Glaucoma
Abstract
1. Introduction
2. Glaucoma: Intraocular Pressure/Ocular Hypertension and Relationship with Obesity
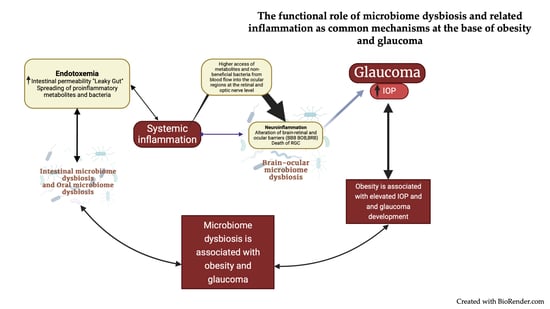
3. Microbiome Dysbiosis at the Intersection of Obesity and Glaucoma
3.1. Role of Dysbiosis in Obesity Development
3.1.1. Gut Dysbiosis
3.1.2. Oral Dysbiosis
3.2. Role of Microbiome Dysbiosis in Glaucoma Development
3.2.1. Gut Dysbiosis
3.2.2. Oral Dysbiosis
3.2.3. Ocular Dysbiosis
3.3. Inflammation Mediated by Microbiome Dysbiosis in Obesity
3.4. Inflammation Mediated by Microbiome Dysbiosis in Glaucoma Pathogenesis
4. Potential Therapies for Obesity and Glaucoma
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boutari, C.; Mantzoros, C.S. A 2022 Update on the Epidemiology of Obesity and a Call to Action: As Its Twin COVID-19 Pandemic Appears to Be Receding, the Obesity and Dysmetabolism Pandemic Continues to Rage On. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef] [PubMed]
- Dinsa, G.; Goryakin, Y.; Fumagalli, E.; Suhrcke, M. Obesity and Socioeconomic Status in Developing Countries: A Systematic Review. Obes. Rev. 2012, 13, 1067–1079. [Google Scholar] [CrossRef]
- Head, G.A. Cardiovascular and Metabolic Consequences of Obesity. Front. Physiol. 2015, 6, 32. [Google Scholar] [CrossRef]
- Fava, M.-C.; Agius, R.; Fava, S. Obesity and Cardio-Metabolic Health. Br. J. Hosp. Med. 2019, 80, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Fruh, S.M. Obesity: Risk Factors, Complications, and Strategies for Sustainable Long-term Weight Management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H.; on behalf of the World Obesity Federation. Obesity: A Chronic Relapsing Progressive Disease Process. A Position Statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and Cancer Risk: Emerging Biological Mechanisms and Perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Yang, F.; Yang, C.; Liu, Y.; Peng, S.; Liu, B.; Gao, X.; Tan, X. Associations between Body Mass Index and Visual Impairment of School Students in Central China. Int. J. Environ. Res. Public Health 2016, 13, 1024. [Google Scholar] [CrossRef]
- Jung, Y.; Han, K.; Park, H.Y.L.; Lee, S.H.; Park, C.K. Metabolic Health, Obesity, and the Risk of Developing Open-Angle Glaucoma: Metabolically Healthy Obese Patients versus Metabolically Unhealthy but Normal Weight Patients. Diabetes Metab. J. 2020, 44, 414–425. [Google Scholar] [CrossRef]
- Marshall, H.; Berry, E.C.; Torres, S.D.; Mullany, S.; Schmidt, J.; Thomson, D.; Nguyen, T.T.; Knight, L.S.; Hollitt, G.; Qassim, A.; et al. Association between Body Mass Index and Primary Open Angle Glaucoma in Three Cohorts. Am. J. Ophthalmol. 2022, 245, 126–133. [Google Scholar] [CrossRef]
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A. Glaucoma. Lancet 2011, 377, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Storgaard, L.; Tran, T.L.; Freiberg, J.C.; Hauser, A.S.; Kolko, M. Glaucoma Clinical Research: Trends in Treatment Strategies and Drug Development. Front. Med. 2021, 8, 733080. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A. Gut Microbiota: A New Path to Treat Obesity. Int. J. Obes. Supp. 2019, 9, 10–19. [Google Scholar] [CrossRef]
- Guo, L.; Yang, K.; Zhou, P.; Yong, W. Gut Microbiota in Obesity and Nonalcoholic Fatty Liver Disease. Surg. Pract. Sci. 2021, 5, 100030. [Google Scholar] [CrossRef]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-Alcoholic Fatty Liver Disease (NAFLD): A Review of Pathophysiology, Clinical Management and Effects of Weight Loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef]
- Pezzino, S.; Sofia, M.; Faletra, G.; Mazzone, C.; Litrico, G.; La Greca, G.; Latteri, S. Gut-Liver Axis and Non-Alcoholic Fatty Liver Disease: A Vicious Circle of Dysfunctions Orchestrated by the Gut Microbiome. Biology 2022, 11, 1622. [Google Scholar] [CrossRef]
- Pellegrini, C.; Antonioli, L.; Calderone, V.; Colucci, R.; Fornai, M.; Blandizzi, C. Microbiota-Gut-Brain Axis in Health and Disease: Is NLRP3 Inflammasome at the Crossroads of Microbiota-Gut-Brain Communications? Prog. Neurobiol. 2020, 191, 101806. [Google Scholar] [CrossRef]
- Rowan, S.; Taylor, A. The Role of Microbiota in Retinal Disease. Adv. Exp. Med. Biol. 2018, 1074, 429–435. [Google Scholar] [CrossRef]
- Horai, R.; Caspi, R.R. Microbiome and Autoimmune Uveitis. Front. Immunol. 2019, 10, 232. [Google Scholar] [CrossRef]
- Scuderi, G.; Troiani, E.; Minnella, A.M. Gut Microbiome in Retina Health: The Crucial Role of the Gut-Retina Axis. Front. Microbiol. 2022, 12, 726792. [Google Scholar] [CrossRef]
- Napolitano, P.; Filippelli, M.; Davinelli, S.; Bartollino, S.; dell’Omo, R.; Costagliola, C. Influence of Gut Microbiota on Eye Diseases: An Overview. Ann. Med. 2021, 53, 750–761. [Google Scholar] [CrossRef]
- Shivaji, S. A Systematic Review of Gut Microbiome and Ocular Inflammatory Diseases: Are They Associated? Indian J. Ophthalmol. 2021, 69, 535–542. [Google Scholar] [CrossRef]
- Xue, W.; Li, J.J.; Zou, Y.; Zou, B.; Wei, L. Microbiota and Ocular Diseases. Front. Cell. Infect. Microbiol. 2021, 11, 759333. [Google Scholar] [CrossRef] [PubMed]
- Artemniak-Wojtowicz, D.; Kucharska, A.M.; Pyrżak, B. Obesity and Chronic Inflammation Crosslinking. Cent. Eur. J. Immunol. 2020, 45, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.-E. Impact of the Gut Microbiota on Inflammation, Obesity, and Metabolic Disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 2546. [Google Scholar] [CrossRef]
- Caspi, R.R. A Look at Autoimmunity and Inflammation in the Eye. J. Clin. Investig. 2010, 120, 3073–3083. [Google Scholar] [CrossRef] [PubMed]
- Okunuki, Y.; Mukai, R.; Nakao, T.; Tabor, S.J.; Butovsky, O.; Dana, R.; Ksander, B.R.; Connor, K.M. Retinal Microglia Initiate Neuroinflammation in Ocular Autoimmunity. Proc. Natl. Acad. Sci. USA 2019, 116, 9989–9998. [Google Scholar] [CrossRef]
- Mac Nair, C.E.; Nickells, R.W. Neuroinflammation in Glaucoma and Optic Nerve Damage. Prog. Mol. Biol. Transl. Sci. 2015, 134, 343–363. [Google Scholar] [CrossRef]
- Williams, P.A.; Marsh-Armstrong, N.; Howell, G.R. Neuroinflammation in Glaucoma: A New Opportunity. Exp. Eye Res. 2017, 157, 20–27. [Google Scholar] [CrossRef]
- Link, C.D. Is There a Brain Microbiome? Neurosci. Insights 2021, 16, 26331055211018708. [Google Scholar] [CrossRef]
- Deng, Y.; Ge, X.; Li, Y.; Zou, B.; Wen, X.; Chen, W.; Lu, L.; Zhang, M.; Zhang, X.; Li, C.; et al. Identification of an Intraocular Microbiota. Cell. Discov. 2021, 7, 13. [Google Scholar] [CrossRef]
- Crawley, L.; Zamir, S.M.; Cordeiro, M.F.; Guo, L. Clinical Options for the Reduction of Elevated Intraocular Pressure. Ophthalmol. Eye Dis. 2012, 4, 43–64. [Google Scholar] [CrossRef]
- Kang, J.M.; Tanna, A.P. Glaucoma. Med. Clin. N. Am. 2021, 105, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Flammer, J.; Orgül, S.; Costa, V.P.; Orzalesi, N.; Krieglstein, G.K.; Serra, L.M.; Renard, J.-P.; Stefánsson, E. The Impact of Ocular Blood Flow in Glaucoma. Prog. Retin. Eye Res. 2002, 21, 359–393. [Google Scholar] [CrossRef] [PubMed]
- Evangelho, K.; Mogilevskaya, M.; Losada-Barragan, M.; Vargas-Sanchez, J.K. Pathophysiology of Primary Open-Angle Glaucoma from a Neuroinflammatory and Neurotoxicity Perspective: A Review of the Literature. Int. Ophthalmol. 2019, 39, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, L.R. Vascular and Autonomic Dysregulation in Primary Open-Angle Glaucoma. Curr. Opin. Ophthalmol. 2016, 27, 94–101. [Google Scholar] [CrossRef]
- Yilmaz, K.C.; Sur Gungor, S.; Ciftci, O.; Akman, A.; Muderrisoglu, H. Relationship between Primary Open Angle Glaucoma and Blood Pressure. Acta Cardiol. 2020, 75, 54–58. [Google Scholar] [CrossRef]
- Chiotoroiu, S.M.; Stefaniu, O.; Noaghi, M.; Teodorescu, A.; Taina, L. The Role of Systemic Blood Pressure in Glaucoma Progression. Rom. J. Ophthalmol. 2015, 59, 141–147. [Google Scholar]
- Lima-Fontes, M.; Barata, P.; Falcão, M.; Carneiro, Â. Ocular Findings in Metabolic Syndrome: A Review. Porto Biomed. J. 2020, 5, 104. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wong, T.Y.; Mitchell, P.; Friedman, D.S.; He, M.; Aung, T. Distribution of Ocular Perfusion Pressure and Its Relationship with Open-Angle Glaucoma: The Singapore Malay Eye Study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3399–3404. [Google Scholar] [CrossRef]
- Newman-Casey, P.A.; Talwar, N.; Nan, B.; Musch, D.C.; Stein, J.D. The Relationship between Components of Metabolic Syndrome and Open-Angle Glaucoma. Ophthalmology 2011, 118, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Hodge, D.O.; White, L.J.; McDonald, J.; Roddy, G.W. Association of Metabolic Syndrome with Glaucoma and Ocular Hypertension in a Midwest United States Population. J. Glaucoma 2022, 31, e18–e31. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jeoung, J.W.; Park, K.H.; Oh, W.H.; Choi, H.J.; Kim, D.M. Metabolic Syndrome as a Risk Factor in Normal-Tension Glaucoma. Acta Ophthalmol. 2014, 92, e637–e643. [Google Scholar] [CrossRef]
- Mori, K.; Ando, F.; Nomura, H.; Sato, Y.; Shimokata, H. Relationship between Intraocular Pressure and Obesity in Japan. Int. J. Epidemiol. 2000, 29, 661–666. [Google Scholar] [CrossRef]
- Klein, B.E.; Klein, R.; Linton, K.L. Intraocular Pressure in an American Community. The Beaver Dam Eye Study. Investig. Ophthalmol. Vis. Sci 1992, 33, 2224–2228. [Google Scholar]
- Karadag, R.; Arslanyilmaz, Z.; Aydin, B.; Hepsen, I.F. Effects of Body Mass Index on Intraocular Pressure and Ocular Pulse Amplitude. Int. J. Ophthalmol 2012, 5, 605–608. [Google Scholar]
- Panon, N.; Luangsawang, K.; Rugaber, C.; Tongchit, T.; Thongsepee, N.; Cheaha, D.; Kongjaidee, P.; Changtong, A.; Daradas, A.; Chotimol, P. Correlation between Body Mass Index and Ocular Parameters. OPTH 2019, 13, 763–769. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, X.; Luo, W.; Jiang, B.; Lin, Q.; Tang, M.; Li, X.; Xie, L. The Causal Association between Obesity and Primary Open-Angle Glaucoma: A Two-Sample Mendelian Randomization Study. Front. Genet. 2022, 13, 835524. [Google Scholar] [CrossRef]
- Reddy, A.; Halenda, K.; Cromer, P.; Chen, L.; Butler, J.; Raed, A.; Bhagatwala, J.; Sponseller, T.; Bollinger, K.; Zhu, H.; et al. The Association of Intraocular Pressure with Obesity and Cardiometabolic Risk in a Young Farmworker Population. J. Glaucoma 2021, 30, 24–31. [Google Scholar] [CrossRef]
- Ahn, M.W.; Lee, J.W.; Shin, J.H.; Lee, J.S. Relationship between Intraocular Pressure and Parameters of Obesity in Ocular Hypertension. Int. J. Ophthalmol. 2020, 13, 794–800. [Google Scholar] [CrossRef]
- Bulpitt, C.J.; Hodes, C.; Everitt, M.G. Intraocular Pressure and Systemic Blood Pressure in the Elderly. Br. J. Ophthalmol. 1975, 59, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lee, S.H.; Oum, B.S.; Chung, J.S.; Cho, B.M.; Hong, J.W. Relationship between Intraocular Pressure and Systemic Health Parameters in a Korean Population. Clin. Exp. Ophthalmol. 2002, 30, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Ishikawa, M.; Kokaze, A.; Sekine, Y.; Matsunaga, N.; Uchida, Y.; Takashima, Y. Association of Life-Style with Intraocular Pressure in Middle-Aged and Older Japanese Residents. Jpn. J. Ophthalmol. 2003, 47, 191–198. [Google Scholar] [CrossRef]
- Asaoka, R.; Obana, A.; Murata, H.; Fujino, Y.; Omoto, T.; Aoki, S.; Muto, S.; Takayanagi, Y.; Inoue, T.; Tanito, M. The Association between Age and Systemic Variables and the Longitudinal Trend of Intraocular Pressure in a Large-Scale Health Examination Cohort. Investig. Ophthalmol. Vis. Sci. 2022, 63, 22. [Google Scholar] [CrossRef]
- Lee, I.-T.; Wang, J.-S.; Fu, C.-P.; Chang, C.-J.; Lee, W.-J.; Lin, S.-Y.; Sheu, W.H.-H. The Synergistic Effect of Inflammation and Metabolic Syndrome on Intraocular Pressure. Medicine 2017, 96, e7851. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Koh, H.; Son, J. The Association between Intraocular Pressure and Different Combination of Metabolic Syndrome Components. BMC Ophthalmol. 2016, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Yokomichi, H.; Kashiwagi, K.; Kitamura, K.; Yoda, Y.; Tsuji, M.; Mochizuki, M.; Sato, M.; Shinohara, R.; Mizorogi, S.; Suzuki, K.; et al. Evaluation of the Associations between Changes in Intraocular Pressure and Metabolic Syndrome Parameters: A Retrospective Cohort Study in Japan. BMJ Open 2016, 6, e010360. [Google Scholar] [CrossRef]
- Zhao, D.; Kim, M.H.; Pastor-Barriuso, R.; Chang, Y.; Ryu, S.; Zhang, Y.; Rampal, S.; Shin, H.; Kim, J.M.; Friedman, D.S.; et al. A Longitudinal Study of Association between Adiposity Markers and Intraocular Pressure: The Kangbuk Samsung Health Study. PLoS ONE 2016, 11, e0146057. [Google Scholar] [CrossRef]
- Kim, Y.K.; Choi, H.J.; Jeoung, J.W.; Park, K.H.; Kim, D.M. Five-Year Incidence of Primary Open-Angle Glaucoma and Rate of Progression in Health Center-Based Korean Population: The Gangnam Eye Study. PLoS ONE 2014, 9, e114058. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-D.; Kim, D.H.; Han, K.; Ha, S.G.; Kim, Y.H.; Kim, J.W.; Park, J.Y.; Yoon, S.J.; Jung, D.W.; Park, S.W.; et al. Relationship between Intraocular Pressure and Parameters of Obesity in Korean Adults: The 2008-2010 Korea National Health and Nutrition Examination Survey. Curr. Eye Res. 2015, 40, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-D.; Lai, L.-J.; Lee, K.-L.; Chen, T.-J.; Liu, C.-Y.; Yang, Y.-H. Is Obesity a Risk or Protective Factor for Open-Angle Glaucoma in Adults? A Two-Database, Asian, Matched-Cohort Study. J. Clin. Med. 2021, 10, 4021. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Villanueva, C.; Milla, E.; Bolarin, J.M.; García-Medina, J.J.; Cruz-Espinosa, J.; Benítez-Del-Castillo, J.; Salgado-Borges, J.; Hernández-Martínez, F.J.; Bendala-Tufanisco, E.; Andrés-Blasco, I.; et al. Impact of Systemic Comorbidities on Ocular Hypertension and Open-Angle Glaucoma, in a Population from Spain and Portugal. J. Clin. Med. 2022, 11, 5649. [Google Scholar] [CrossRef] [PubMed]
- Pileggi, C.; Papadopoli, R.; De Sarro, C.; Nobile, C.G.A.; Pavia, M. Obesity, Blood Pressure, and Intraocular Pressure: A Cross-Sectional Study in Italian Children. Obes. Facts 2021, 14, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Guiraudou, M.; Varlet-Marie, E.; Raynaud de Mauverger, E.; Brun, J.-F. Obesity-Related Increase in Whole Blood Viscosity Includes Different Profiles According to Fat Localization. Clin. Hemorheol. Microcirc. 2013, 55, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M. What Controls Aqueous Humour Outflow Resistance? Exp. Eye Res. 2006, 82, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.; Baretella, O.; Meyer, M.R. Obesity and Risk of Vascular Disease: Importance of Endothelium-Dependent Vasoconstriction. Br. J. Pharmacol. 2012, 165, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Csige, I.; Ujvárosy, D.; Szabó, Z.; Lőrincz, I.; Paragh, G.; Harangi, M.; Somodi, S. The Impact of Obesity on the Cardiovascular System. J. Diabetes Res. 2018, 2018, e3407306. [Google Scholar] [CrossRef] [PubMed]
- Orgül, S. Blood Flow in Glaucoma. Br. J. Ophthalmol. 2007, 91, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.; Harris, A.; WuDunn, D.; Kheradiya, N.; Siesky, B. Dysfunctional Regulation of Ocular Blood Flow: A Risk Factor for Glaucoma? Clin. Ophthalmol. 2008, 2, 849–861. [Google Scholar]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Kastl, A.J.; Terry, N.A.; Wu, G.D.; Albenberg, L.G. The Structure and Function of the Human Small Intestinal Microbiota: Current Understanding and Future Directions. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 33–45. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Murphy, E.F.; Cotter, P.D.; Healy, S.; Marques, T.M.; O’Sullivan, O.; Fouhy, F.; Clarke, S.F.; O’Toole, P.W.; Quigley, E.M.; Stanton, C.; et al. Composition and Energy Harvesting Capacity of the Gut Microbiota: Relationship to Diet, Obesity and Time in Mouse Models. Gut 2010, 59, 1635–1642. [Google Scholar] [CrossRef]
- Kashani, A.; Brejnrod, A.D.; Jin, C.; Kern, T.; Madsen, A.N.; Holm, L.A.; Gerber, G.K.; Holm, J.-C.; Hansen, T.; Holst, B.; et al. Impaired Glucose Metabolism and Altered Gut Microbiome despite Calorie Restriction of Ob/Ob Mice. Anim. Microbiome 2019, 1, 11. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Everard, A.; Cani, P.D. Diabetes, Obesity and Gut Microbiota. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 73–83. [Google Scholar] [CrossRef]
- Khan, M.J.; Gerasimidis, K.; Edwards, C.A.; Shaikh, M.G. Role of Gut Microbiota in the Aetiology of Obesity: Proposed Mechanisms and Review of the Literature. J. Obes. 2016, 2016, e7353642. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Madan, J.C.; Hoen, A.G.; Lundgren, S.N.; Farzan, S.F.; Cottingham, K.L.; Morrison, H.G.; Sogin, M.L.; Li, H.; Moore, J.H.; Karagas, M.R. Association of Cesarean Delivery and Formula Supplementation with the Intestinal Microbiome of 6-Week-Old Infants. JAMA Pediatr. 2016, 170, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Ajami, N.J.; O’Brien, J.L.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.; Metcalf, G.A.; et al. Temporal Development of the Gut Microbiome in Early Childhood from the TEDDY Study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef]
- Coker, M.O.; Laue, H.E.; Hoen, A.G.; Hilliard, M.; Dade, E.; Li, Z.; Palys, T.; Morrison, H.G.; Baker, E.; Karagas, M.R.; et al. Infant Feeding Alters the Longitudinal Impact of Birth Mode on the Development of the Gut Microbiota in the First Year of Life. Front. Microbiol. 2021, 12, 642197. [Google Scholar] [CrossRef] [PubMed]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; et al. Comparison of the Gut Microbiota Composition between Obese and Non-Obese Individuals in a Japanese Population, as Analyzed by Terminal Restriction Fragment Length Polymorphism and next-Generation Sequencing. BMC Gastroenterol. 2015, 15, 100. [Google Scholar] [CrossRef]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The Human Gut Microbiota: Metabolism and Perspective in Obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef]
- Indiani, C.M.D.S.P.; Rizzardi, K.F.; Castelo, P.M.; Ferraz, L.F.C.; Darrieux, M.; Parisotto, T.M. Childhood Obesity and Firmicutes/Bacteroidetes Ratio in the Gut Microbiota: A Systematic Review. Child Obes. 2018, 14, 501–509. [Google Scholar] [CrossRef]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the Gut Microbiota of Adults with Obesity: A Systematic Review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut Microbiota Markers Associated with Obesity and Overweight in Italian Adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Lobley, G.E.; Holtrop, G.; Ince, J.; Johnstone, A.M.; Louis, P.; Flint, H.J. Human Colonic Microbiota Associated with Diet, Obesity and Weight Loss. Int. J. Obes. 2008, 32, 1720–1724. [Google Scholar] [CrossRef]
- Andoh, A.; Nishida, A.; Takahashi, K.; Inatomi, O.; Imaeda, H.; Bamba, S.; Kito, K.; Sugimoto, M.; Kobayashi, T. Comparison of the Gut Microbial Community between Obese and Lean Peoples Using 16S Gene Sequencing in a Japanese Population. J. Clin. Biochem. Nutr. 2016, 59, 65–70. [Google Scholar] [CrossRef]
- Payne, A.N.; Chassard, C.; Zimmermann, M.; Müller, P.; Stinca, S.; Lacroix, C. The Metabolic Activity of Gut Microbiota in Obese Children Is Increased Compared with Normal-Weight Children and Exhibits More Exhaustive Substrate Utilization. Nutr. Diabetes 2011, 1, e12. [Google Scholar] [CrossRef]
- Pinart, M.; Dötsch, A.; Schlicht, K.; Laudes, M.; Bouwman, J.; Forslund, S.K.; Pischon, T.; Nimptsch, K. Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis. Nutrients 2021, 14, 12. [Google Scholar] [CrossRef]
- Ng, K.M.; Ferreyra, J.A.; Higginbottom, S.K.; Lynch, J.B.; Kashyap, P.C.; Gopinath, S.; Naidu, N.; Choudhury, B.; Weimer, B.C.; Monack, D.M.; et al. Microbiota-Liberated Host Sugars Facilitate Post-Antibiotic Expansion of Enteric Pathogens. Nature 2013, 502, 96–99. [Google Scholar] [CrossRef]
- Engevik, M.A.; Engevik, A.C.; Engevik, K.A.; Auchtung, J.M.; Chang-Graham, A.L.; Ruan, W.; Luna, R.A.; Hyser, J.M.; Spinler, J.K.; Versalovic, J. Mucin-Degrading Microbes Release Monosaccharides That Chemoattract Clostridioides Difficile and Facilitate Colonization of the Human Intestinal Mucus Layer. ACS Infect. Dis. 2021, 7, 1126–1142. [Google Scholar] [CrossRef]
- Suki, M.; Leibovici Weissman, Y.; Boltin, D.; Itskoviz, D.; Tsadok Perets, T.; Comaneshter, D.; Cohen, A.; Niv, Y.; Dotan, I.; Leibovitzh, H.; et al. Helicobacter Pylori Infection Is Positively Associated with an Increased BMI, Irrespective of Socioeconomic Status and Other Confounders: A Cohort Study. Eur. J. Gastroenterol. Hepatol. 2018, 30, 143–148. [Google Scholar] [CrossRef]
- Baradaran, A.; Dehghanbanadaki, H.; Naderpour, S.; Pirkashani, L.M.; Rajabi, A.; Rashti, R.; Riahifar, S.; Moradi, Y. The Association between Helicobacter Pylori and Obesity: A Systematic Review and Meta-Analysis of Case–Control Studies. Clin. Diabetes Endocrinol. 2021, 7, 15. [Google Scholar] [CrossRef]
- Xu, M.-Y.; Liu, L.; Yuan, B.-S.; Yin, J.; Lu, Q.-B. Association of Obesity with Helicobacter Pylori Infection: A Retrospective Study. World J. Gastroenterol. 2017, 23, 2750–2756. [Google Scholar] [CrossRef]
- Xu, X.; Li, W.; Qin, L.; Yang, W.; Yu, G.; Wei, Q. Relationship between Helicobacter Pylori Infection and Obesity in Chinese Adults: A Systematic Review with Meta-Analysis. PLoS ONE 2019, 14, e0221076. [Google Scholar] [CrossRef]
- Kyriazanos, I.D.; Sfiniadakis, I.; Gizaris, V.; Hountis, P.; Hatziveis, K.; Dafnopoulou, A.; Datsakis, K. The Incidence of Helicobacter Pylori Infection Is Not Increased Among Obese Young Individuals in Greece. J. Clin. Gastroenterol. 2002, 34, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-S.; Lee, W.-J.; Wang, H.-H.; Huang, S.-P.; Lin, J.-T. A Case-Control Study of Association of Helicobacter Pylori Infection with Morbid Obesity in Taiwan. Arch. Intern. Med. 2005, 165, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Arslan, E.; Atılgan, H.; Yavaşoğlu, İ. The Prevalence of Helicobacter Pylori in Obese Subjects. Eur. J. Intern. Med. 2009, 20, 695–697. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, T.; Chen, X.; Yu, X.; Tu, L.; Zhang, C. Association between Helicobacter Pylori Infection and Overweight or Obesity in a Chinese Population. J. Infect. Dev. Ctries 2015, 9, 945–953. [Google Scholar] [CrossRef] [PubMed]
- den Hollander, W.J.; Broer, L.; Schurmann, C.; Meyre, D.; den Hoed, C.M.; Mayerle, J.; Hofman, A.; Homuth, G.; Uitterlinden, A.G.; Lerch, M.M.; et al. Helicobacter Pylori Colonization and Obesity – a Mendelian Randomization Study. Sci. Rep. 2017, 7, 14467. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Z.; Zhang, M.; Meng, L.; Ming, Z.; Liu, J. Bioinspired Oral Delivery of Gut Microbiota by Self-Coating with Biofilms. Sci. Adv. 2020, 6, eabb1952. [Google Scholar] [CrossRef]
- Deo, P.N.; Deshmukh, R. Oral Microbiome: Unveiling the Fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef]
- Vonaesch, P.; Morien, E.; Andrianonimiadana, L.; Sanke, H.; Mbecko, J.-R.; Huus, K.E.; Naharimanananirina, T.; Gondje, B.P.; Nigatoloum, S.N.; Vondo, S.S.; et al. Stunted Childhood Growth Is Associated with Decompartmentalization of the Gastrointestinal Tract and Overgrowth of Oropharyngeal Taxa. Proc. Natl. Acad. Sci. USA 2018, 115, E8489–E8498. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.J.C.; Blankenberg, D.; Parodi, A.C.L.; Paul, I.M.; Birch, L.L.; Savage, J.S.; Marini, M.E.; Stokes, J.L.; Nekrutenko, A.; Reimherr, M.; et al. Child Weight Gain Trajectories Linked to Oral Microbiota Composition. Sci. Rep. 2018, 8, 14030. [Google Scholar] [CrossRef] [PubMed]
- Janem, W.F.; Scannapieco, F.A.; Sabharwal, A.; Tsompana, M.; Berman, H.A.; Haase, E.M.; Miecznikowski, J.C.; Mastrandrea, L.D. Salivary Inflammatory Markers and Microbiome in Normoglycemic Lean and Obese Children Compared to Obese Children with Type 2 Diabetes. PLoS ONE 2017, 12, e0172647. [Google Scholar] [CrossRef]
- Besnard, P.; Christensen, J.E.; Brignot, H.; Bernard, A.; Passilly-Degrace, P.; Nicklaus, S.; Pais de Barros, J.-P.; Collet, X.; Lelouvier, B.; Servant, F.; et al. Obese Subjects with Specific Gustatory Papillae Microbiota and Salivary Cues Display an Impairment to Sense Lipids. Sci. Rep. 2018, 8, 6742. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, Q.; Zheng, W.; Steinwandel, M.; Blot, W.J.; Shu, X.-O.; Long, J. Oral Microbiome and Obesity in a Large Study of Low-Income and African-American Populations. J. Oral Microbiol. 2019, 11, 1650597. [Google Scholar] [CrossRef] [PubMed]
- Mameli, C.; Cattaneo, C.; Panelli, S.; Comandatore, F.; Sangiorgio, A.; Bedogni, G.; Bandi, C.; Zuccotti, G.; Pagliarini, E. Taste Perception and Oral Microbiota Are Associated with Obesity in Children and Adolescents. PLoS ONE 2019, 14, e0221656. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Rohde, J.F.; Raymond, K.; Heitmann, B.L. Association between Periodontal Disease and Overweight and Obesity: A Systematic Review. J. Periodontol. 2015, 86, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Alonso, B.; León, R.; Roldán, S.; Sanz, M. Antimicrobial Therapy in Periodontitis: The Use of Systemic Antimicrobials against the Subgingival Biofilm. J. Clin. Periodontol. 2008, 35, 45–66. [Google Scholar] [CrossRef]
- Mbakwa, C.A.; Hermes, G.D.A.; Penders, J.; Savelkoul, P.H.M.; Thijs, C.; Dagnelie, P.C.; Mommers, M.; Zoetendal, E.G.; Smidt, H.; Arts, I.C.W. Gut Microbiota and Body Weight in School-Aged Children: The KOALA Birth Cohort Study. Obesity 2018, 26, 1767–1776. [Google Scholar] [CrossRef]
- Larsen, J.M. The Immune Response to Prevotella Bacteria in Chronic Inflammatory Disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef]
- Raju, S.C.; Lagström, S.; Ellonen, P.; de Vos, W.M.; Eriksson, J.G.; Weiderpass, E.; Rounge, T.B. Gender-Specific Associations between Saliva Microbiota and Body Size. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, C.; Riso, P.; Laureati, M.; Gargari, G.; Pagliarini, E. Exploring Associations between Interindividual Differences in Taste Perception, Oral Microbiota Composition, and Reported Food Intake. Nutrients 2019, 11, 1167. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Kramer, M.; Shochat, T.; Goldberg, E.; Garty, M.; Krause, I. Relationship between Body Mass Index and Intraocular Pressure in Men and Women: A Population-Based Study. J. Glaucoma 2016, 25, e509. [Google Scholar] [CrossRef]
- Tham, Y.-C.; Cheng, C.-Y. Associations between Chronic Systemic Diseases and Primary Open Angle Glaucoma: An Epidemiological Perspective. Clin. Exp. Ophthalmol. 2017, 45, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Andriessen, E.M.; Wilson, A.M.; Mawambo, G.; Dejda, A.; Miloudi, K.; Sennlaub, F.; Sapieha, P. Gut Microbiota Influences Pathological Angiogenesis in Obesity-Driven Choroidal Neovascularization. EMBO Mol. Med. 2016, 8, 1366–1379. [Google Scholar] [CrossRef]
- Janowitz, C.; Nakamura, Y.K.; Metea, C.; Gligor, A.; Yu, W.; Karstens, L.; Rosenbaum, J.T.; Asquith, M.; Lin, P. Disruption of Intestinal Homeostasis and Intestinal Microbiota during Experimental Autoimmune Uveitis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 420–429. [Google Scholar] [CrossRef]
- Yoon, C.H.; Ryu, J.S.; Moon, J.; Kim, M.K. Association between Aging-Dependent Gut Microbiome Dysbiosis and Dry Eye Severity in C57BL/6 Male Mouse Model: A Pilot Study. BMC Microbiol. 2021, 21, 106. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Wang, Z.; Xie, G.; Liu, M.; Yuan, B.; Chai, H.; Wang, W.; Cheng, P. Implications of Gut Microbiota in Neurodegenerative Diseases. Front. Immunol. 2022, 13, 785644. [Google Scholar] [CrossRef]
- Bhattarai, Y. Microbiota-Gut-Brain Axis: Interaction of Gut Microbes and Their Metabolites with Host Epithelial Barriers. Neurogastroenterol. Motil. 2018, 30, e13366. [Google Scholar] [CrossRef]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut Microbes and Metabolites as Modulators of Blood-Brain Barrier Integrity and Brain Health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef]
- Mossad, O.; Erny, D. The Microbiota-Microglia Axis in Central Nervous System Disorders. Brain Pathol. 2020, 30, 1159–1177. [Google Scholar] [CrossRef]
- Cook, J.; Prinz, M. Regulation of Microglial Physiology by the Microbiota. Gut Microbes 2022, 14, 2125739. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, G.; Burgaletto, C.; Serapide, M.F.; Caltabiano, R.; Munafò, A.; Bellanca, C.M.; Di Mauro, R.; Bernardini, R.; Cantarella, G. TRAIL-R Deficient Mice Are Protected from Neurotoxic Effects of Amyloid-β. Int. J. Mol. Sci. 2022, 23, 11625. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, G.; Burgaletto, C.; Bellanca, C.M.; Munafò, A.; Bernardini, R.; Cantarella, G. Role of Microglia and Astrocytes in Alzheimer’s Disease: From Neuroinflammation to Ca2+ Homeostasis Dysregulation. Cells 2022, 11, 2728. [Google Scholar] [CrossRef] [PubMed]
- Ghiso, J.A.; Doudevski, I.; Ritch, R.; Rostagno, A.A. Alzheimer’s Disease and Glaucoma: Mechanistic Similarities and Differences. J. Glaucoma 2013, 22, S36–S38. [Google Scholar] [CrossRef]
- Marchesi, N.; Fahmideh, F.; Boschi, F.; Pascale, A.; Barbieri, A. Ocular Neurodegenerative Diseases: Interconnection between Retina and Cortical Areas. Cells 2021, 10, 2394. [Google Scholar] [CrossRef]
- Chen, H.; Cho, K.-S.; Vu, T.H.K.; Shen, C.-H.; Kaur, M.; Chen, G.; Mathew, R.; McHam, M.L.; Fazelat, A.; Lashkari, K.; et al. Commensal Microflora-Induced T Cell Responses Mediate Progressive Neurodegeneration in Glaucoma. Nat. Commun. 2018, 9, 3209. [Google Scholar] [CrossRef]
- Gong, H.; Zhang, S.; Li, Q.; Zuo, C.; Gao, X.; Zheng, B.; Lin, M. Gut Microbiota Compositional Profile and Serum Metabolic Phenotype in Patients with Primary Open-Angle Glaucoma. Exp. Eye Res. 2020, 191, 107921. [Google Scholar] [CrossRef]
- Collins, D.W.; Gudiseva, H.V.; Trachtman, B.; Bowman, A.S.; Sagaser, A.; Sankar, P.; Miller-Ellis, E.; Lehman, A.; Addis, V.; O’Brien, J.M. Association of Primary Open-Angle Glaucoma with Mitochondrial Variants and Haplogroups Common in African Americans. Mol. Vis. 2016, 22, 454–471. [Google Scholar]
- Ma, J.; Coarfa, C.; Qin, X.; Bonnen, P.E.; Milosavljevic, A.; Versalovic, J.; Aagaard, K. MtDNA Haplogroup and Single Nucleotide Polymorphisms Structure Human Microbiome Communities. BMC Genom. 2014, 15, 257. [Google Scholar] [CrossRef]
- Izzotti, A.; Saccà, S.C.; Longobardi, M.; Cartiglia, C. Mitochondrial Damage in the Trabecular Meshwork of Patients with Glaucoma. Arch. Ophthalmol. 2010, 128, 724–730. [Google Scholar] [CrossRef]
- Abu-Amero, K.K.; Morales, J.; Bosley, T.M. Mitochondrial Abnormalities in Patients with Primary Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2533–2541. [Google Scholar] [CrossRef]
- Lauwen, S.; de Jong, E.K.; Lefeber, D.J.; den Hollander, A. Omics Biomarkers in Ophthalmology. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO88–BIO98. [Google Scholar] [CrossRef]
- Buisset, A.; Gohier, P.; Leruez, S.; Muller, J.; Amati-Bonneau, P.; Lenaers, G.; Bonneau, D.; Simard, G.; Procaccio, V.; Annweiler, C.; et al. Metabolomic Profiling of Aqueous Humor in Glaucoma Points to Taurine and Spermine Deficiency: Findings from the Eye-D Study. J. Proteome Res. 2019, 18, 1307–1315. [Google Scholar] [CrossRef]
- Myer, C.; Abdelrahman, L.; Banerjee, S.; Khattri, R.B.; Merritt, M.E.; Junk, A.K.; Lee, R.K.; Bhattacharya, S.K. Aqueous Humor Metabolite Profile of Pseudoexfoliation Glaucoma Is Distinctive. Mol. Omics 2020, 16, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Barbosa Breda, J.; Croitor Sava, A.; Himmelreich, U.; Somers, A.; Matthys, C.; Rocha Sousa, A.; Vandewalle, E.; Stalmans, I. Metabolomic Profiling of Aqueous Humor from Glaucoma Patients—The Metabolomics in Surgical Ophthalmological Patients (MISO) Study. Exp. Eye Res. 2020, 201, 108268. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Pan, Y.; Chen, Y.; Kong, X.; Chen, J.; Zhang, H.; Tang, G.; Wu, J.; Sun, X. Metabolomic Profiling of Aqueous Humor and Plasma in Primary Open Angle Glaucoma Patients Points towards Novel Diagnostic and Therapeutic Strategy. Front. Pharmacol. 2021, 12, 621146. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hou, X.-W.; Liang, G.; Pan, C.-W. Metabolomics in Glaucoma: A Systematic Review. Investig. Ophthalmol. Vis. Sci. 2021, 62, 9. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Lu, Y. Gut Microbiota and Derived Metabolomic Profiling in Glaucoma with Progressive Neurodegeneration. Front. Cell. Infect. Microbiol. 2022, 12, 968992. [Google Scholar] [CrossRef]
- Kountouras, J.; Mylopoulos, N.; Boura, P.; Bessas, C.; Chatzopoulos, D.; Venizelos, J.; Zavos, C. Relationship between Helicobacter Pylori Infection and Glaucoma11The Authors Have No Commercial Interests in the Products or Devices Mention Herein. Ophthalmology 2001, 108, 599–604. [Google Scholar] [CrossRef]
- Zullo, A.; Ridola, L.; Hassan, C.; Bruzzese, V.; Papini, F.; Vaira, D. Glaucoma and Helicobacter Pylori: Eyes Wide Shut? ? Dig. Liver Dis. 2012, 44, 627–628. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, H.; Liu, X.; Ding, C. The Relationship between Helicobacter Pylori Infection and Open-Angle Glaucoma: A Meta-Analysis. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5238–5245. [Google Scholar] [CrossRef] [PubMed]
- Doulberis, M.; Papaefthymiou, A.; Polyzos, S.A.; Bargiotas, P.; Liatsos, C.; Srivastava, D.S.; Zavos, C.; Katsinelos, P.; Kountouras, J. Association between Active Helicobacter Pylori Infection and Glaucoma: A Systematic Review and Meta-Analysis. Microorganisms 2020, 8, 894. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, E.; Meguro, A.; Ota, M.; Kashiwagi, K.; Mabuchi, F.; Iijima, H.; Kawase, K.; Yamamoto, T.; Nakamura, M.; Negi, A.; et al. Association of Toll-like Receptor 4 Gene Polymorphisms with Normal Tension Glaucoma. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4453–4457. [Google Scholar] [CrossRef]
- Kountouras, J.; Mylopoulos, N.; Konstas, A.G.P.; Zavos, C.; Chatzopoulos, D.; Boukla, A. Increased Levels of Helicobacter Pylori IgG Antibodies in Aqueous Humor of Patients with Primary Open-Angle and Exfoliation Glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2003, 241, 884–890. [Google Scholar] [CrossRef]
- Zavos, C.; Kountouras, J.; Sakkias, G.; Venizelos, I.; Deretzi, G.; Arapoglou, S. Histological Presence of Helicobacter Pylori Bacteria in the Trabeculum and Iris of Patients with Primary Open-Angle Glaucoma. ORE 2012, 47, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Ala, S.; Maleki, I.; Sanjari Araghi, A.; Sahebnasagh, A.; Shahraki, A. Helicobacter Pylori Eradication in the Management of Glaucoma. Caspian J. Intern. Med. 2020, 11, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Kountouras, J.; Mylopoulos, N.; Chatzopoulos, D.; Zavos, C.; Boura, P.; Konstas, A.G.P.; Venizelos, J. Eradication of Helicobacter Pylori May Be Beneficial in the Management of Chronic Open-Angle Glaucoma. Arch. Intern. Med. 2002, 162, 1237–1244. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lin, C.-L.; Chen, W.-C.; Kao, C.-H. Does Helicobacter Pylori Eradication Reduce the Risk of Open Angle Glaucoma in Patients with Peptic Ulcer Disease? Medicine 2015, 94, e1578. [Google Scholar] [CrossRef]
- Henein, C.; Khaw, P.T. The Interplay between Inflammation, Immunity and Commensal Microflora in Glaucomatous Neurodegeneration. Ann. Eye Sci. 2019, 4, 10. [Google Scholar] [CrossRef]
- Astafurov, K.; Elhawy, E.; Ren, L.; Dong, C.Q.; Igboin, C.; Hyman, L.; Griffen, A.; Mittag, T.; Danias, J. Oral Microbiome Link to Neurodegeneration in Glaucoma. PLoS ONE 2014, 9, e104416. [Google Scholar] [CrossRef] [PubMed]
- Polla, D.; Astafurov, K.; Hawy, E.; Hyman, L.; Hou, W.; Danias, J. A Pilot Study to Evaluate the Oral Microbiome and Dental Health in Primary Open-Angle Glaucoma. J. Glaucoma 2017, 26, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, L.R.; Hyman, L.; Wiggs, J.L.; Rosner, B.A.; Joshipura, K.; McEvoy, M.; McPherson, Z.E.; Danias, J.; Kang, J.H. Prospective Study of Oral Health and Risk of Primary Open-Angle Glaucoma in Men: Data from the Health Professionals Follow-up Study. Ophthalmology 2016, 123, 2318–2327. [Google Scholar] [CrossRef]
- Yoon, B.W.; Lim, S.-H.; Shin, J.H.; Lee, J.-W.; Lee, Y.; Seo, J.H. Analysis of Oral Microbiome in Glaucoma Patients Using Machine Learning Prediction Models. J. Oral Microbiol. 2021, 13, 1962125. [Google Scholar] [CrossRef] [PubMed]
- Egger, S.F.; Buxbaum, A.; Georgopoulos, M.; Scholda, C.; Vecsei, V.P.; Huber-spitzy, V.; Georgopoulos, A. Bacterial Growth in Human Vitreous Humor. Exp. Eye Res. 1997, 65, 791–795. [Google Scholar] [CrossRef]
- Goto, H.; Usui, Y.; Umazume, A.; Uchida, K.; Eishi, Y. Propionibacterium Acnes as a Possible Pathogen of Granuloma in Patients with Ocular Sarcoidosis. Br. J. Ophthalmol. 2017, 101, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Eishi, Y.; Uchida, K.; Yoneda, K.; Hatanaka, H.; Yasuhara, T.; Nagata, M.; Sotozono, C.; Kinoshita, S. Immunohistochemical Detection of Propionibacterium Acnes in the Retinal Granulomas in Patients with Ocular Sarcoidosis. Sci. Rep. 2017, 7, 15226. [Google Scholar] [CrossRef]
- Logan, R. Adherence of Helicobacter Pylori. Aliment. Pharmacol. Ther. 1996, 10, 3–15. [Google Scholar] [CrossRef]
- Chen, M.; Luo, C.; Zhao, J.; Devarajan, G.; Xu, H. Immune Regulation in the Aging Retina. Prog. Retin. Eye Res. 2019, 69, 159–172. [Google Scholar] [CrossRef]
- Jiang, S.; Kametani, M.; Chen, D.F. Adaptive Immunity: New Aspects of Pathogenesis Underlying Neurodegeneration in Glaucoma and Optic Neuropathy. Front. Immunol. 2020, 11, 65. [Google Scholar] [CrossRef]
- Shestopalov, V.I.; Spurlock, M.; Gramlich, O.W.; Kuehn, M.H. Immune Responses in the Glaucomatous Retina: Regulation and Dynamics. Cells 2021, 10, 1973. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory Mechanisms in Obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Akash, M.S.H. Mechanisms of Inflammatory Responses and Development of Insulin Resistance: How Are They Interlinked? J. Biomed. Sci. 2016, 23, 87. [Google Scholar] [CrossRef]
- Solt, L.A.; May, M.J. The IκB Kinase Complex: Master Regulator of NF-ΚB Signaling. Immunol. Res. 2008, 42, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 Contributes to Macrophage Infiltration into Adipose Tissue, Insulin Resistance, and Hepatic Steatosis in Obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Meng, Y.; He, S.; Tan, X.; Zhang, Y.; Zhang, X.; Wang, L.; Zheng, W. Macrophages, Chronic Inflammation, and Insulin Resistance. Cells 2022, 11, 3001. [Google Scholar] [CrossRef]
- Kern, L.; Mittenbühler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFα and IL-6 Signaling: The Missing Link between Obesity and Inflammation—Driven Liver and Colorectal Cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef]
- Harte, A.L.; Varma, M.C.; Tripathi, G.; McGee, K.C.; Al-Daghri, N.M.; Al-Attas, O.S.; Sabico, S.; O’Hare, J.P.; Ceriello, A.; Saravanan, P.; et al. High Fat Intake Leads to Acute Postprandial Exposure to Circulating Endotoxin in Type 2 Diabetic Subjects. Diabetes Care 2012, 35, 375–382. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell. Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Kamada, N.; Chen, G.Y.; Inohara, N.; Núñez, G. Control of Pathogens and Pathobionts by the Gut Microbiota. Nat. Immunol. 2013, 14, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Tlaskalová-Hogenová, H.; Štěpánková, R.; Kozáková, H.; Hudcovic, T.; Vannucci, L.; Tučková, L.; Rossmann, P.; Hrnčíř, T.; Kverka, M.; Zákostelská, Z.; et al. The Role of Gut Microbiota (Commensal Bacteria) and the Mucosal Barrier in the Pathogenesis of Inflammatory and Autoimmune Diseases and Cancer: Contribution of Germ-Free and Gnotobiotic Animal Models of Human Diseases. Cell. Mol. Immunol. 2011, 8, 110–120. [Google Scholar] [CrossRef]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and Systemic Insulin Resistance Resulting from Hepatic Activation of IKK-β and NF-ΚB. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2021, 11, 3–18. [Google Scholar] [CrossRef]
- Vreugdenhil, A.C.E.; Rousseau, C.H.; Hartung, T.; Greve, J.W.M.; van ‘t Veer, C.; Buurman, W.A. Lipopolysaccharide (LPS)-Binding Protein Mediates LPS Detoxification by Chylomicrons 1. J. Immunol. 2003, 170, 1399–1405. [Google Scholar] [CrossRef]
- Thiriet, M. Hyperlipidemias and Obesity. Vasculopathies 2019, 8, 331–548. [Google Scholar] [CrossRef]
- Lebrun, L.J.; Pallot, G.; Nguyen, M.; Tavernier, A.; Dusuel, A.; Pilot, T.; Deckert, V.; Dugail, I.; Le Guern, N.; Pais De Barros, J.-P.; et al. Increased Weight Gain and Insulin Resistance in HF-Fed PLTP Deficient Mice Is Related to Altered Inflammatory Response and Plasma Transport of Gut-Derived LPS. Int. J. Mol. Sci. 2022, 23, 13226. [Google Scholar] [CrossRef]
- Neal, M.D.; Leaphart, C.; Levy, R.; Prince, J.; Billiar, T.R.; Watkins, S.; Li, J.; Cetin, S.; Ford, H.; Schreiber, A.; et al. Enterocyte TLR4 Mediates Phagocytosis and Translocation of Bacteria Across the Intestinal Barrier. J. Immunol. 2006, 176, 3070–3079. [Google Scholar] [CrossRef]
- Vijay-Kumar, M.; Aitken, J.D.; Carvalho, F.A.; Cullender, T.C.; Mwangi, S.; Srinivasan, S.; Sitaraman, S.V.; Knight, R.; Ley, R.E.; Gewirtz, A.T. Metabolic Syndrome and Altered Gut Microbiota in Mice Lacking Toll-like Receptor 5. Science 2010, 328, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Tanti, J.-F.; Ceppo, F.; Jager, J.; Berthou, F. Implication of Inflammatory Signaling Pathways in Obesity-Induced Insulin Resistance. Front. Endocrinol. 2013, 3, 181. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.F.; Fiamoncini, J.; Prist, I.H.; Ariga, S.K.; de Souza, H.P.; de Lima, T.M. Novel Role of TLR4 in NAFLD Development: Modulation of Metabolic Enzymes Expression. Biochim. Biophys. Acta 2015, 1851, 1353–1359. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The Roles of TLRs, RLRs and NLRs in Pathogen Recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef] [PubMed]
- Vaure, C.; Liu, Y. A Comparative Review of Toll-like Receptor 4 Expression and Functionality in Different Animal Species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef]
- Song, M.J.; Kim, K.H.; Yoon, J.M.; Kim, J.B. Activation of Toll-like Receptor 4 Is Associated with Insulin Resistance in Adipocytes. Biochem. Biophys. Res. Commun. 2006, 346, 739–745. [Google Scholar] [CrossRef]
- Liang, C.-F.; Liu, J.T.; Wang, Y.; Xu, A.; Vanhoutte, P.M. Toll-like Receptor 4 Mutation Protects Obese Mice Against Endothelial Dysfunction by Decreasing NADPH Oxidase Isoforms 1 and 4. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 777–784. [Google Scholar] [CrossRef]
- Pierre, N.; Deldicque, L.; Barbé, C.; Naslain, D.; Cani, P.D.; Francaux, M. Toll-like Receptor 4 Knockout Mice Are Protected against Endoplasmic Reticulum Stress Induced by a High-Fat Diet. PLoS ONE 2013, 8, e65061. [Google Scholar] [CrossRef]
- Kim, J.-A.; Jang, H.-J.; Hwang, D.H. Toll-like Receptor 4-Induced Endoplasmic Reticulum Stress Contributes to Impairment of Vasodilator Action of Insulin. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E767–E776. [Google Scholar] [CrossRef]
- Milanski, M.; Degasperi, G.; Coope, A.; Morari, J.; Denis, R.; Cintra, D.E.; Tsukumo, D.M.L.; Anhe, G.; Amaral, M.E.; Takahashi, H.K.; et al. Saturated Fatty Acids Produce an Inflammatory Response Predominantly through the Activation of TLR4 Signaling in Hypothalamus: Implications for the Pathogenesis of Obesity. J. Neurosci. 2009, 29, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.; Pham, M.; Luttrell, I.; Bannerman, D.D.; Tupper, J.; Thaler, J.; Hawn, T.R.; Raines, E.W.; Schwartz, M.W. Toll-like Receptor-4 Mediates Vascular Inflammation and Insulin Resistance in Diet-Induced Obesity. Cir. Res. 2007, 100, 1589–1596. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 Links Innate Immunity and Fatty Acid-Induced Insulin Resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Renovato-Martins, M.; Moreira-Nunes, C.; Atella, G.C.; Barja-Fidalgo, C.; de Moraes, J.A. Obese Adipose Tissue Secretion Induces Inflammation in Preadipocytes: Role of Toll-like Receptor-4. Nutrients 2020, 12, 2828. [Google Scholar] [CrossRef] [PubMed]
- Boden, G. Obesity and Free Fatty Acids. Endocrinol. Metab. Clin. N. Am. 2008, 37, 635–646. [Google Scholar] [CrossRef]
- Huang, S.; Rutkowsky, J.M.; Snodgrass, R.G.; Ono-Moore, K.D.; Schneider, D.A.; Newman, J.W.; Adams, S.H.; Hwang, D.H. Saturated Fatty Acids Activate TLR-Mediated Proinflammatory Signaling Pathways. J. Lipid. Res. 2012, 53, 2002–2013. [Google Scholar] [CrossRef]
- Henderson, G.C. Plasma Free Fatty Acid Concentration as a Modifiable Risk Factor for Metabolic Disease. Nutrients 2021, 13, 2590. [Google Scholar] [CrossRef]
- Reyna, S.M.; Ghosh, S.; Tantiwong, P.; Meka, C.S.R.; Eagan, P.; Jenkinson, C.P.; Cersosimo, E.; Defronzo, R.A.; Coletta, D.K.; Sriwijitkamol, A.; et al. Elevated Toll-like Receptor 4 Expression and Signaling in Muscle from Insulin-Resistant Subjects. Diabetes 2008, 57, 2595–2602. [Google Scholar] [CrossRef]
- Vitseva, O.I.; Tanriverdi, K.; Tchkonia, T.T.; Kirkland, J.L.; McDonnell, M.E.; Apovian, C.M.; Freedman, J.; Gokce, N. Inducible Toll-like Receptor and NF-KappaB Regulatory Pathway Expression in Human Adipose Tissue. Obesity 2008, 16, 932–937. [Google Scholar] [CrossRef]
- Creely, S.J.; McTernan, P.G.; Kusminski, C.M.; Fisher, f.M.; Da Silva, N.F.; Khanolkar, M.; Evans, M.; Harte, A.L.; Kumar, S. Lipopolysaccharide Activates an Innate Immune System Response in Human Adipose Tissue in Obesity and Type 2 Diabetes. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E740–E747. [Google Scholar] [CrossRef]
- Poulain-Godefroy, O.; Le Bacquer, O.; Plancq, P.; Lecœur, C.; Pattou, F.; Frühbeck, G.; Froguel, P. Inflammatory Role of Toll-like Receptors in Human and Murine Adipose Tissue. Mediat. Inflamm. 2010, 2010, 823486. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Kochumon, S.; Thomas, R.; Atizado, V.; Sindhu, S. Increased Adipose Tissue Expression of TLR8 in Obese Individuals with or without Type-2 Diabetes: Significance in Metabolic Inflammation. J. Inflamm. 2016, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Mraz, M.; Lacinova, Z.; Drapalova, J.; Haluzikova, D.; Horinek, A.; Matoulek, M.; Trachta, P.; Kavalkova, P.; Svacina, S.; Haluzik, M. The Effect of Very-Low-Calorie Diet on MRNA Expression of Inflammation-Related Genes in Subcutaneous Adipose Tissue and Peripheral Monocytes of Obese Patients with Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2011, 96, E606–E613. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.J.; Salvador, J.; Frühbeck, G. Increased Tenascin C and Toll-like Receptor 4 Levels in Visceral Adipose Tissue as a Link between Inflammation and Extracellular Matrix Remodeling in Obesity. J. Clin. Endocrinol. Metab. 2012, 97, E1880–E1889. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.Á.; Ojeda-Ojeda, M.; Rodríguez-Martín, E.; Insenser, M.; Moncayo, S.; Álvarez-Blasco, F.; Luque-Ramírez, M.; Escobar-Morreale, H.F. TLR2 and TLR4 Surface and Gene Expression in White Blood Cells after Fasting and Oral Glucose, Lipid and Protein Challenges: Influence of Obesity and Sex Hormones. Biomolecules 2020, 10, 111. [Google Scholar] [CrossRef]
- Weyrich, P.; Staiger, H.; Stančáková, A.; Machicao, F.; Machann, J.; Schick, F.; Stefan, N.; Kuusisto, J.; Laakso, M.; Schäfer, S.; et al. The D299G/T399I Toll-like Receptor 4 Variant Associates with Body and Liver Fat: Results from the TULIP and METSIM Studies. PLoS ONE 2010, 5, e13980. [Google Scholar] [CrossRef]
- Schneider, S.; Hoppmann, P.; Koch, W.; Kemmner, S.; Schmaderer, C.; Renders, L.; Kastrati, A.; Laugwitz, K.-L.; Heemann, U.; Baumann, M. Obesity-Associated Hypertension Is Ameliorated in Patients with TLR4 Single Nucleotide Polymorphism (SNP) Rs4986790. J. Inflamm. 2015, 12, 57. [Google Scholar] [CrossRef]
- Sharif, E.; Al-Wakeel, M.; Mohamed, A.; Kerkadi, A.; Rizk, N. TLR4 Receptor D299G/T399I Haplotype Polymorphism Is Associated with Insulin Resistance in Obese Female Subjects. Genes 2020, 11, 814. [Google Scholar] [CrossRef]
- Dogan, B.; Kazim Erol, M.; Dogan, U.; Habibi, M.; Bulbuller, N.; Turgut Coban, D.; Bulut, M. The Retinal Nerve Fiber Layer, Choroidal Thickness, and Central Macular Thickness in Morbid Obesity: An Evaluation Using Spectral-Domain Optical Coherence Tomography. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 886–891. [Google Scholar]
- Baran, R.; Baran, S.; Toraman, N.; Filiz, S.; Demirbilek, H. Evaluation of Intraocular Pressure and Retinal Nerve Fiber Layer, Retinal Ganglion Cell, Central Macular Thickness, and Choroidal Thickness Using Optical Coherence Tomography in Obese Children and Healthy Controls. Niger. J. Clin. Pract. 2019, 22, 539. [Google Scholar] [CrossRef]
- Gonul, S.; Yilmaz, H.; Gedik, S.; Ozturk, B.T.; Oflaz, A.B.; Sahin, M. Evaluation of the Choroidal Thickness and Retinal Nerve Fiber Layer and Visual Fields in Morbid Obesity: Does Bariatric Surgery Affect Retinal Structure and Function? Indian J. Ophthalmol. 2021, 69, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Gramlich, O.W.; Beck, S.; von Thun und Hohenstein-Blaul, N.; Boehm, N.; Ziegler, A.; Vetter, J.M.; Pfeiffer, N.; Grus, F.H. Enhanced Insight into the Autoimmune Component of Glaucoma: IgG Autoantibody Accumulation and Pro-Inflammatory Conditions in Human Glaucomatous Retina. PLoS ONE 2013, 8, e57557. [Google Scholar] [CrossRef]
- Margeta, M.A.; Lad, E.M.; Proia, A.D. CD163+ Macrophages Infiltrate Axon Bundles of Postmortem Optic Nerves with Glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 2449–2456. [Google Scholar] [CrossRef]
- Yuan, L.; Neufeld, A.H. Tumor Necrosis Factor-Alpha: A Potentially Neurodestructive Cytokine Produced by Glia in the Human Glaucomatous Optic Nerve Head. Glia 2000, 32, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Neufeld, A.H. Activated Microglia in the Human Glaucomatous Optic Nerve Head. J. Neurosci. Res. 2001, 64, 523–532. [Google Scholar] [CrossRef]
- Yang, J.; Patil, R.V.; Yu, H.; Gordon, M.; Wax, M.B. T Cell Subsets and SIL-2R/IL-2 Levels in Patients with Glaucoma. Am. J. Ophthalmol. 2001, 131, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Rolle, T.; Ponzetto, A.; Malinverni, L. The Role of Neuroinflammation in Glaucoma: An Update on Molecular Mechanisms and New Therapeutic Options. Front. Neurol. 2021, 11, 612422. [Google Scholar] [CrossRef]
- Baudouin, C.; Kolko, M.; Melik-Parsadaniantz, S.; Messmer, E.M. Inflammation in Glaucoma: From the Back to the Front of the Eye, and Beyond. Prog. Retin. Eye Res. 2021, 83, 100916. [Google Scholar] [CrossRef]
- Joachim, S.C.; Bruns, K.; Lackner, K.J.; Pfeiffer, N.; Grus, F.H. Antibodies to α B-Crystallin, Vimentin, and Heat Shock Protein 70 in Aqueous Humor of Patients with Normal Tension Glaucoma and IgG Antibody Patterns Against Retinal Antigen in Aqueous Humor. Cur. Eye Res. 2007, 32, 501–509. [Google Scholar] [CrossRef]
- Kondkar, A.A.; Sultan, T.; Almobarak, F.A.; Kalantan, H.; Al-Obeidan, S.A.; Abu-Amero, K.K. Association of Increased Levels of Plasma Tumor Necrosis Factor Alpha with Primary Open-Angle Glaucoma. Clin. Ophthalmol. 2018, 12, 701–706. [Google Scholar] [CrossRef]
- Sharma, S.; Bollinger, K.E.; Kodeboyina, S.K.; Zhi, W.; Patton, J.; Bai, S.; Edwards, B.; Ulrich, L.; Bogorad, D.; Sharma, A. Proteomic Alterations in Aqueous Humor from Patients with Primary Open Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Hubens, W.H.G.; Beckers, H.J.M.; Gorgels, T.G.M.F.; Webers, C.A.B. Increased Ratios of Complement Factors C3a to C3 in Aqueous Humor and Serum Mark Glaucoma Progression. Exp. Eye Res. 2021, 204, 108460. [Google Scholar] [CrossRef] [PubMed]
- Rowan, S.; Jiang, S.; Korem, T.; Szymanski, J.; Chang, M.-L.; Szelog, J.; Cassalman, C.; Dasuri, K.; McGuire, C.; Nagai, R.; et al. Involvement of a Gut–Retina Axis in Protection against Dietary Glycemia-Induced Age-Related Macular Degeneration. Proc. Natl. Acad. Sci. USA 2017, 114, E4472–E4481. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Yi, S.; Wei, L. Ocular Microbiota and Intraocular Inflammation. Frony. Immunol. 2020, 11, 609765. [Google Scholar] [CrossRef]
- Mölzer, C.; Heissigerova, J.; Wilson, H.M.; Kuffova, L.; Forrester, J.V. Immune Privilege: The Microbiome and Uveitis. Front. Immunol. 2021, 11, 608377. [Google Scholar] [CrossRef]
- Citi, S. Intestinal Barriers Protect against Disease. Science 2018, 359, 1097–1098. [Google Scholar] [CrossRef]
- Manfredo Vieira, S.; Hiltensperger, M.; Kumar, V.; Zegarra-Ruiz, D.; Dehner, C.; Khan, N.; Costa, F.R.C.; Tiniakou, E.; Greiling, T.; Ruff, W.; et al. Translocation of a Gut Pathobiont Drives Autoimmunity in Mice and Humans. Science 2018, 359, 1156–1161. [Google Scholar] [CrossRef]
- Chen, M.; Xu, H. Parainflammation, Chronic Inflammation, and Age-Related Macular Degeneration. J. Leukoc. Biol. 2015, 98, 713–725. [Google Scholar] [CrossRef]
- Donabedian, P.; Dawson, E.; Li, Q.; Chen, J. Gut Microbes and Eye Disease. Ophthalmic Res. 2022, 65, 245–253. [Google Scholar] [CrossRef]
- Kutsyr, O.; Maestre-Carballa, L.; Lluesma-Gomez, M.; Martinez-Garcia, M.; Cuenca, N.; Lax, P. Retinitis Pigmentosa Is Associated with Shifts in the Gut Microbiome. Sci. Rep. 2021, 11, 6692. [Google Scholar] [CrossRef]
- Tang, J.; Tang, Y.; Yi, I.; Chen, D.F. The Role of Commensal Microflora-Induced T Cell Responses in Glaucoma Neurodegeneration. Prog. Brain. Res. 2020, 256, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Howell, G.R.; Macalinao, D.G.; Sousa, G.L.; Walden, M.; Soto, I.; Kneeland, S.C.; Barbay, J.M.; King, B.L.; Marchant, J.K.; Hibbs, M.; et al. Molecular Clustering Identifies Complement and Endothelin Induction as Early Events in a Mouse Model of Glaucoma. J. Clin. Investig. 2011, 121, 1429–1444. [Google Scholar] [CrossRef]
- Alarcon-Martinez, L.; Shiga, Y.; Villafranca-Baughman, D.; Belforte, N.; Quintero, H.; Dotigny, F.; Cueva Vargas, J.L.; Di Polo, A. Pericyte Dysfunction and Loss of Interpericyte Tunneling Nanotubes Promote Neurovascular Deficits in Glaucoma. Proc. Natl. Acad. Sci. USA 2022, 119, e2110329119. [Google Scholar] [CrossRef]
- Williams, P.A.; Braine, C.E.; Foxworth, N.E.; Cochran, K.E.; John, S.W.M. GlyCAM1 Negatively Regulates Monocyte Entry into the Optic Nerve Head and Contributes to Radiation-Based Protection in Glaucoma. J. Neuroinflamm. 2017, 14, 93. [Google Scholar] [CrossRef]
- Williams, P.A.; Braine, C.E.; Kizhatil, K.; Foxworth, N.E.; Tolman, N.G.; Harder, J.M.; Scott, R.A.; Sousa, G.L.; Panitch, A.; Howell, G.R.; et al. Inhibition of Monocyte-like Cell Extravasation Protects from Neurodegeneration in DBA/2J Glaucoma. Mol. Neurodegener. 2019, 14, 6. [Google Scholar] [CrossRef]
- Kong, X.; Liu, X.; Huang, X.; Mao, Z.; Zhong, Y.; Chi, W. Damage to the Blood-Aqueous Barrier in Eyes with Primary Angle Closure Glaucoma. Mol. Vis. 2010, 16, 2026–2032. [Google Scholar] [PubMed]
- Katamay, R.; Nussenblatt, R.B. Blood–Retinal Barrier, Immune Privilege, and Autoimmunity. Retina 2013, 1, 579–589. [Google Scholar] [CrossRef]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front. Cell. Neurosci. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, X.-W.; Zhang, D.-D.; Fan, Z.-G. Blood-Retinal Barrier as a Converging Pivot in Understanding the Initiation and Development of Retinal Diseases. Chin. Med. J. 2020, 133, 2586–2594. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Yang, X.; Luo, C.; Kain, A.D.; Powell, D.W.; Kuehn, M.H.; Kaplan, H.J. Oxidative Stress and the Regulation of Complement Activation in Human Glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5071–5082. [Google Scholar] [CrossRef]
- Trinchieri, G.; Sher, A. Cooperation of Toll-like Receptor Signals in Innate Immune Defence. Nat. Rev. Immunol. 2007, 7, 179–190. [Google Scholar] [CrossRef]
- Shazly, T.A.; Aljajeh, M.; Latina, M.A. Autoimmune Basis of Glaucoma. Semin. Ophthalmol. 2011, 26, 278–281. [Google Scholar] [CrossRef]
- Tezel, G. The Immune Response in Glaucoma: A Perspective on the Roles of Oxidative Stress. Exp. Eye Res. 2011, 93, 178–186. [Google Scholar] [CrossRef]
- Rieck, J. The Pathogenesis of Glaucoma in the Interplay with the Immune System. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2393–2409. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-Q.; Wang, Y.-S. The Role of Toll-like Receptors in Retinal Ischemic Diseases. Int. J. Ophthalmol. 2016, 9, 1343–1351. [Google Scholar] [CrossRef]
- Morzaev, D.; Nicholson, J.D.; Caspi, T.; Weiss, S.; Hochhauser, E.; Goldenberg-Cohen, N. Toll-like Receptor-4 Knockout Mice Are More Resistant to Optic Nerve Crush Damage than Wild-Type Mice. Clin. Exp. Ophthalmol. 2015, 43, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Poyomtip, T. Roles of Toll-like Receptor 4 for Cellular Pathogenesis in Primary Open-Angle Glaucoma: A Potential Therapeutic Strategy. J. Microbiol. Immunol. Infect. 2019, 52, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, B.; Hu, Y.; Lu, L.; Lu, X.; Wang, J.; Xu, F.; Yu, S.; Huang, J.; Liang, X. Wogonin Prevents TLR4-NF-ΚB-Medicated Neuro-Inflammation and Improves Retinal Ganglion Cells Survival in Retina after Optic Nerve Crush. Oncotarget 2016, 7, 72503–72517. [Google Scholar] [CrossRef] [PubMed]
- Vernazza, S.; Oddone, F.; Tirendi, S.; Bassi, A.M. Risk Factors for Retinal Ganglion Cell Distress in Glaucoma and Neuroprotective Potential Intervention. Int. J. Mol. Sci. 2021, 22, 7994. [Google Scholar] [CrossRef]
- Nakano, Y.; Shimazawa, M.; Ojino, K.; Izawa, H.; Takeuchi, H.; Inoue, Y.; Tsuruma, K.; Hara, H. Toll-like Receptor 4 Inhibitor Protects against Retinal Ganglion Cell Damage Induced by Optic Nerve Crush in Mice. J. Pharmacol. Sci. 2017, 133, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Zinkernagel, M.S.; Zysset-Burri, D.C.; Keller, I.; Berger, L.E.; Leichtle, A.B.; Largiadèr, C.R.; Fiedler, G.M.; Wolf, S. Association of the Intestinal Microbiome with the Development of Neovascular Age-Related Macular Degeneration. Sci. Rep. 2017, 7, 40826. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; McCluskey, P.J.; Wakefield, D. Toll-like Receptors in Ocular Immunity and the Immunopathogenesis of Inflammatory Eye Disease. Br. J. Ophthalmol. 2006, 90, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Dvoriantchikova, G.; Santos, A.R.C.; Saeed, A.M.; Dvoriantchikova, X.; Ivanov, D. Putative Role of Protein Kinase C in Neurotoxic Inflammation Mediated by Extracellular Heat Shock Protein 70 after Ischemia-Reperfusion. J. Neuroinflamm. 2014, 11, 81. [Google Scholar] [CrossRef]
- Dvoriantchikova, G.; Hernandez, E.; Grant, J.; Santos, A.R.C.; Yang, H.; Ivanov, D. The High-Mobility Group Box-1 Nuclear Factor Mediates Retinal Injury after Ischemia Reperfusion. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7187–7194. [Google Scholar] [CrossRef] [PubMed]
- Anshu, A.; Price, M.O.; Richardson, M.R.; Segu, Z.M.; Lai, X.; Yoder, M.C.; Price, F.W. Alterations in the Aqueous Humor Proteome in Patients with a Glaucoma Shunt Device. Mol. Vis. 2011, 17, 1891–1900. [Google Scholar]
- Lee, J.-J.; Wang, P.-W.; Yang, I.-H.; Wu, C.-L.; Chuang, J.-H. Amyloid-Beta Mediates the Receptor of Advanced Glycation End Product-Induced pro-Inflammatory Response via Toll-like Receptor 4 Signaling Pathway in Retinal Ganglion Cell Line RGC-5. Int. J. Biochem. Cell Biol. 2015, 64, 1–10. [Google Scholar] [CrossRef]
- Yan, Y.; Yao, D.; Li, X. Immunological Mechanism and Clinical Application of PAMP Adjuvants. Recent Pat. Anti-Cancer Drug Discov. 2021, 16, 30–43. [Google Scholar] [CrossRef]
- Colaco, C.A.; Bailey, C.R.; Walker, K.B.; Keeble, J. Heat Shock Proteins: Stimulators of Innate and Acquired Immunity. BioMed Res. Int. 2013, 2013, 461230. [Google Scholar] [CrossRef]
- Tezel, G. Molecular Regulation of Neuroinflammation in Glaucoma: Current Knowledge and the Ongoing Search for New Treatment Targets. Prog. Retin. Eye Res. 2022, 87, 100998. [Google Scholar] [CrossRef]
- Luo, C.; Yang, X.; Kain, A.D.; Powell, D.W.; Kuehn, M.H.; Tezel, G. Glaucomatous Tissue Stress and the Regulation of Immune Response through Glial Toll-like Receptor Signaling. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5697–5707. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Acioglu, C.; Heary, R.F.; Elkabes, S. Role of Astroglial Toll-like Receptors (TLRs) in Central Nervous System Infections, Injury and Neurodegenerative Diseases. Brain Behav. Immun. 2021, 91, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Fang, D.; Zhou, H.; Su, S.B. The Expression of Toll-like Receptors in Murine Müller Cells, the Glial Cells in Retina. Neurol. Sci. 2013, 34, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Mzyk, P.; Hernandez, H.; Le, T.; Ramirez, J.R.; McDowell, C.M. Toll-like Receptor 4 Signaling in the Trabecular Meshwork. Front. Cell. Dev. Biol. 2022, 10, 936115. [Google Scholar] [CrossRef] [PubMed]
- Choquet, H.; Paylakhi, S.; Kneeland, S.C.; Thai, K.K.; Hoffmann, T.J.; Yin, J.; Kvale, M.N.; Banda, Y.; Tolman, N.G.; Williams, P.A.; et al. A Multiethnic Genome-Wide Association Study of Primary Open-Angle Glaucoma Identifies Novel Risk Loci. Nat. Commun. 2018, 9, 2278. [Google Scholar] [CrossRef]
- Liu, H.; Qi, S.; He, W.; Chang, C.; Chen, Y.; Yu, J. Association of Single-Nucleotide Polymorphisms in TLR4 Gene and Gene-Environment Interaction with Primary Open Angle Glaucoma in a Chinese Northern Population. J. Gene Med. 2020, 22, e3139. [Google Scholar] [CrossRef]
- Navarro-Partida, J.; Martinez-Rizo, A.B.; Ramirez-Barrera, P.; Velazquez-Fernandez, J.B.; Mondragon-Jaimes, V.A.; Santos-Garcia, A.; Benites-Godinez, V. Association of Toll-like Receptor 4 Single-Nucleotide Polymorphisms Asp299Gly and Thr399Ile with the Risk of Primary Open Angle Glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Partida, J.; Alvarado Castillo, B.; Martinez-Rizo, A.B.; Rosales-Diaz, R.; Velazquez-Fernandez, J.B.; Santos, A. Association of Single-Nucleotide Polymorphisms in Non-Coding Regions of the TLR4 Gene with Primary Open Angle Glaucoma in a Mexican Population. Ophthalmic Genet. 2017, 38, 325–329. [Google Scholar] [CrossRef]
- Abu-Amero, K.K.; Kondkar, A.A.; Mousa, A.; Azad, T.A.; Sultan, T.; Osman, E.A.; Al-Obeidan, S.A. Analysis of Toll-like Receptor Rs4986790 Polymorphism in Saudi Patients with Primary Open Angle Glaucoma. Ophthalmic Genet. 2017, 38, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Kondkar, A.A.; Al-Obeidan, S.A.; Azad, T.A.; Sultan, T.; Osman, E.A.; Abu-Amero, K.K. Lack of Association between Polymorphism Rs4986791 in TLR4 and Primary Open-Angle Glaucoma in a Saudi Cohort. Genet. Test. Mol. Biomark. 2016, 20, 556–559. [Google Scholar] [CrossRef]
- Chen, L.J.; Tam, P.O.S.; Leung, D.Y.L.; Fan, A.H.; Zhang, M.; Tham, C.C.Y.; Chiang, S.W.Y.; Fan, B.J.; Wang, N.; Pang, C.P. SNP Rs1533428 at 2p16.3 as a Marker for Late-Onset Primary Open-Angle Glaucoma. Mol. Vis. 2012, 18, 1629–1639. [Google Scholar] [PubMed]
- Takano, Y.; Shi, D.; Shimizu, A.; Funayama, T.; Mashima, Y.; Yasuda, N.; Fukuchi, T.; Abe, H.; Ideta, H.; Zheng, X.; et al. Association of Toll-like Receptor 4 Gene Polymorphisms in Japanese Subjects with Primary Open-Angle, Normal-Tension, and Exfoliation Glaucoma. Am. J. Ophthalmol. 2012, 154, 825–832.e1. [Google Scholar] [CrossRef] [PubMed]
- Suh, W.; Kim, S.; Ki, C.-S.; Kee, C. Toll-like Receptor 4 Gene Polymorphisms Do Not Associate with Normal Tension Glaucoma in a Korean Population. Mol. Vis. 2011, 17, 2343–2348. [Google Scholar] [PubMed]
- Androutsakos, T.; Bakasis, A.-D.; Pouliakis, A.; Gazouli, M.; Vallilas, C.; Hatzis, G. Single Nucleotide Polymorphisms of Toll-like Receptor 4 in Hepatocellular Carcinoma-A Single-Center Study. Int. J. Mol. Sci. 2022, 23, 9430. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Loke, J.; Zheng, F.; Hong, F.; Yea, S.; Fukata, M.; Tarocchi, M.; Abar, O.T.; Huang, H.; Sninsky, J.J.; et al. Functional Linkage of Cirrhosis-Predictive Single Nucleotide Polymorphisms of Toll-like Receptor 4 to Hepatic Stellate Cell Responses. Hepatology 2009, 49, 960–968. [Google Scholar] [CrossRef]
- Ohto, U.; Yamakawa, N.; Akashi-Takamura, S.; Miyake, K.; Shimizu, T. Structural Analyses of Human Toll-like Receptor 4 Polymorphisms D299G and T399I *. J. Biol. Chem. 2012, 287, 40611–40617. [Google Scholar] [CrossRef]
- Hodgkinson, C.P.; Patel, K.; Ye, S. Functional Toll-like Receptor 4 Mutations Modulate the Response to Fibrinogen. Thromb. Haemost. 2008, 100, 301–307. [Google Scholar] [CrossRef]
- Kundi, Z.M.; Lee, J.C.; Pihlajamäki, J.; Chan, C.B.; Leung, K.S.; So, S.S.Y.; Nordlund, E.; Kolehmainen, M.; El-Nezami, H. Dietary Fiber from Oat and Rye Brans Ameliorate Western Diet–Induced Body Weight Gain and Hepatic Inflammation by the Modulation of Short-Chain Fatty Acids, Bile Acids, and Tryptophan Metabolism. Mol. Nutr. Food Res. 2020, 65, e1900580. [Google Scholar] [CrossRef] [PubMed]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M.T. Adipose Tissue: An Endocrine Organ Playing a Role in Metabolic Regulation. Horm. Mol. Biol. Clin. Investig. 2016, 26, 25–42. [Google Scholar] [CrossRef]
- Kim, B.; Choi, H.-N.; Yim, J.-E. Effect of Diet on the Gut Microbiota Associated with Obesity. J. Obes. Metab. Syndr. 2019, 28, 216–224. [Google Scholar] [CrossRef]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxidative Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and Associated Dietary Extremes Impact on Gut Microbial Diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; de Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S.; et al. Exercise Training Modulates the Gut Microbiota Profile and Impairs Inflammatory Signaling Pathways in Obese Children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef] [PubMed]
- Naito, E.; Yoshida, Y.; Makino, K.; Kounoshi, Y.; Kunihiro, S.; Takahashi, R.; Matsuzaki, T.; Miyazaki, K.; Ishikawa, F. Beneficial Effect of Oral Administration of Lactobacillus Casei Strain Shirota on Insulin Resistance in Diet-Induced Obesity Mice. J. Appl. Microbiol. 2011, 110, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Ferrarese, R.; Ceresola, E.R.; Preti, A.; Canducci, F. Probiotics, Prebiotics and Synbiotics for Weight Loss and Metabolic Syndrome in the Microbiome Era. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7588–7605. [Google Scholar] [CrossRef]
- Cerdó, T.; García-Santos, J.A.; Bermúdez, M.G.; Campoy, C. The Role of Probiotics and Prebiotics in the Prevention and Treatment of Obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Nutraceuticals as Modulators of Gut Microbiota: Role in Therapy. Br. J. Pharmacol. 2020, 177, 1351–1362. [Google Scholar] [CrossRef]
- Ben Salah, R.; Trabelsi, I.; Hamden, K.; Chouayekh, H.; Bejar, S. Lactobacillus Plantarum TN8 Exhibits Protective Effects on Lipid, Hepatic and Renal Profiles in Obese Rat. Anaerobe 2013, 23, 55–61. [Google Scholar] [CrossRef]
- Miyoshi, M.; Ogawa, A.; Higurashi, S.; Kadooka, Y. Anti-Obesity Effect of Lactobacillus Gasseri SBT2055 Accompanied by Inhibition of pro-Inflammatory Gene Expression in the Visceral Adipose Tissue in Diet-Induced Obese Mice. Eur. J. Nutr. 2014, 53, 599–606. [Google Scholar] [CrossRef]
- Park, D.-Y.; Ahn, Y.-T.; Park, S.-H.; Huh, C.-S.; Yoo, S.-R.; Yu, R.; Sung, M.-K.; McGregor, R.A.; Choi, M.-S. Supplementation of Lactobacillus Curvatus HY7601 and Lactobacillus Plantarum KY1032 in Diet-Induced Obese Mice Is Associated with Gut Microbial Changes and Reduction in Obesity. PLoS ONE 2013, 8, e59470. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Matamoros, S.; Geurts, L.; Delzenne, N.M.; Cani, P.D. Saccharomyces Boulardii Administration Changes Gut Microbiota and Reduces Hepatic Steatosis, Low-Grade Inflammation, and Fat Mass in Obese and Type 2 Diabetic Db/Db Mice. mBio 2014, 5, e01011-14. [Google Scholar] [CrossRef] [PubMed]
- Bernini, L.J.; Simão, A.N.C.; Alfieri, D.F.; Lozovoy, M.A.B.; Mari, N.L.; de Souza, C.H.B.; Dichi, I.; Costa, G.N. Beneficial Effects of Bifidobacterium Lactis on Lipid Profile and Cytokines in Patients with Metabolic Syndrome: A Randomized Trial. Effects of Probiotics on Metabolic Syndrome. Nutrition 2016, 32, 716–719. [Google Scholar] [CrossRef]
- Tenorio-Jiménez, C.; Martínez-Ramírez, M.J.; Del Castillo-Codes, I.; Arraiza-Irigoyen, C.; Tercero-Lozano, M.; Camacho, J.; Chueca, N.; García, F.; Olza, J.; Plaza-Díaz, J.; et al. Lactobacillus Reuteri V3401 Reduces Inflammatory Biomarkers and Modifies the Gastrointestinal Microbiome in Adults with Metabolic Syndrome: The PROSIR Study. Nutrients 2019, 11, 1761. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Ou, Z.; Huang, D.; Li, C.; Lu, Z.; Liu, W.; Wu, F.; Nong, C.; Gao, J.; Peng, Y. Diverse Effects of Different Akkermansia Muciniphila Genotypes on Brown Adipose Tissue Inflammation and Whitening in a High-Fat-Diet Murine Model. Microb. Pathog. 2020, 147, 104353. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia Muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A Purified Membrane Protein from Akkermansia Muciniphila or the Pasteurized Bacterium Improves Metabolism in Obese and Diabetic Mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Roy, T.L.; de Hase, E.M.; Hul, M.V.; Paquot, A.; Pelicaen, R.; Régnier, M.; Depommier, C.; Druart, C.; Everard, A.; Maiter, D.; et al. Dysosmobacter Welbionis Is a Newly Isolated Human Commensal Bacterium Preventing Diet-Induced Obesity and Metabolic Disorders in Mice. Gut 2022, 71, 534–543. [Google Scholar] [CrossRef]
- Delday, M.; Mulder, I.; Logan, E.T.; Grant, G. Bacteroides Thetaiotaomicron Ameliorates Colon Inflammation in Preclinical Models of Crohn’s Disease. Inflamm. Bowel Dis. 2019, 25, 85–96. [Google Scholar] [CrossRef]
- Martín, R.; Miquel, S.; Chain, F.; Natividad, J.M.; Jury, J.; Lu, J.; Sokol, H.; Theodorou, V.; Bercik, P.; Verdu, E.F.; et al. Faecalibacterium Prausnitzii Prevents Physiological Damages in a Chronic Low-Grade Inflammation Murine Model. BMC Microbiol. 2015, 15, 67. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides Distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary Fiber Intervention on Gut Microbiota Composition in Healthy Adults: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2018, 107, 965–983. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, S.; Sofia, M.; Agosta, M.; Litrico, G.; Sarvà, I.; La Greca, G.; Latteri, S. The Impact of Bariatric Surgery on Colorectal Cancer Risk. Surg. Obes. Relat. Dis. 2022; In Press. [Google Scholar] [CrossRef] [PubMed]
- Lautenbach, A.; Stoll, F.; Mann, O.; Busch, P.; Huber, T.B.; Kielstein, H.; Bähr, I.; Aberle, J. Long-Term Improvement of Chronic Low-Grade Inflammation After Bariatric Surgery. Obes. Surg. 2021, 31, 2913–2920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human Gut Microbiota in Obesity and after Gastric Bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- ULKER, İ.; YILDIRAN, H. The Effects of Bariatric Surgery on Gut Microbiota in Patients with Obesity: A Review of the Literature. Biosci. Microbiota Food Health 2019, 38, 3–9. [Google Scholar] [CrossRef]
- Vrieze, A.; Nood, E.V.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga–Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota from Lean Donors Increases Insulin Sensitivity in Individuals with Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Gomes, A.C.; Bueno, A.A.; de Souza, R.G.M.; Mota, J.F. Gut Microbiota, Probiotics and Diabetes. Nutr. J. 2014, 13, 60. [Google Scholar] [CrossRef]
- Divya Ganeshan, S.; Hosseinidoust, Z. Phage Therapy with a Focus on the Human Microbiota. Antibiotics 2019, 8, 131. [Google Scholar] [CrossRef]
- Guerin, E.; Hill, C. Shining Light on Human Gut Bacteriophages. Front. Cell. Infect. Microbiol. 2020, 10, 481. [Google Scholar] [CrossRef]
- Rasmussen, T.S.; Koefoed, A.K.; Jakobsen, R.R.; Deng, L.; Castro-Mejía, J.L.; Brunse, A.; Neve, H.; Vogensen, F.K.; Nielsen, D.S. Bacteriophage-Mediated Manipulation of the Gut Microbiome—Promises and Presents Limitations. FEMS Microbiol. Rev. 2020, 44, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Federici, S.; Nobs, S.P.; Elinav, E. Phages and Their Potential to Modulate the Microbiome and Immunity. Cell. Mol. Immunol. 2021, 18, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Federici, S.; Kredo-Russo, S.; Valdés-Mas, R.; Kviatcovsky, D.; Weinstock, E.; Matiuhin, Y.; Silberberg, Y.; Atarashi, K.; Furuichi, M.; Oka, A.; et al. Targeted Suppression of Human IBD-Associated Gut Microbiota Commensals by Phage Consortia for Treatment of Intestinal Inflammation. Cell 2022, 185, 2879–2898.e24. [Google Scholar] [CrossRef] [PubMed]
- De Vincentis, A.; Pedone, C.; Vespasiani-Gentilucci, U.; Picardi, A.; Derosa, G.; Maffioli, P.; Sahebkar, A. Effect of Sibutramine on Plasma C-Reactive Protein, Leptin and Adipon Ectin Concentrations: A Systematic Review and Meta-Analysis of Randomized Contr Olled Trials. Curr. Pharm. Des. 2017, 23, 870–878. [Google Scholar] [CrossRef]
- Charpentier, J.; Briand, F.; Lelouvier, B.; Servant, F.; Azalbert, V.; Puel, A.; Christensen, J.E.; Waget, A.; Branchereau, M.; Garret, C.; et al. Liraglutide Targets the Gut Microbiota and the Intestinal Immune System to Regulate Insulin Secretion. Acta Diabetol. 2021, 58, 881–897. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Duffy, L.; O’Reilly, S.C. Toll-like Receptors in the Pathogenesis of Autoimmune Diseases: Recent and Emerging Translational Developments. Immunotargets Ther. 2016, 5, 69–80. [Google Scholar] [CrossRef]
- ul Ain, Q.; Batool, M.; Choi, S. TLR4-Targeting Therapeutics: Structural Basis and Computer-Aided Drug Discovery Approaches. Molecules 2020, 25, 627. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Lee, K.-C.; Wu, P.-S.; Huo, T.-I.; Huang, Y.-H.; Hou, M.-C.; Lin, H.-C. Eritoran Attenuates Hepatic Inflammation and Fibrosis in Mice with Chronic Liver Injury. Cells 2021, 10, 1562. [Google Scholar] [CrossRef]
- Moser, V.A.; Uchoa, M.F.; Pike, C.J. TLR4 Inhibitor TAK-242 Attenuates the Adverse Neural Effects of Diet-Induced Obesity. J. Neuroinflamm. 2018, 15, 306. [Google Scholar] [CrossRef]
- Matsunaga, N.; Tsuchimori, N.; Matsumoto, T.; Ii, M. TAK-242 (Resatorvid), a Small-Molecule Inhibitor of Toll-like Receptor (TLR) 4 Signaling, Binds Selectively to TLR4 and Interferes with Interactions between TLR4 and Its Adaptor Molecules. Mol. Pharmacol. 2011, 79, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.M.; Huen, M.S.Y.; Wang, H.Y.; Zheng, H.; Sigel, E.; Baur, R.; Ren, H.; Li, Z.W.; Wong, J.T.-F.; Xue, H. Anxiolytic Effect of Wogonin, a Benzodiazepine Receptor Ligand Isolated from Scutellaria Baicalensis Georgi. Biochem. Pharmacol. 2002, 64, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.; Nath, L.R. Inevitable Role of TGF-Β1 in Progression of Nonalcoholic Fatty Liver Disease. J. Recept. Signal. Transduct. 2020, 40, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Roh, Y.S.; Song, J.; Zhang, B.; Liu, C.; Loomba, R.; Seki, E. TGF-β Signaling in Hepatocytes Participates in Steatohepatitis Through Regulation of Cell Death and Lipid Metabolism. Hepatology 2014, 59, 483–495. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, K.J.; Jung, K.S.; Chon, Y.E.; Huh, J.H.; Park, K.H.; Chung, J.B.; Kim, C.O.; Han, K.-H.; Park, J.Y. The Relationship between Visceral Obesity and Hepatic Steatosis Measured by Controlled Attenuation Parameter. PLoS ONE 2017, 12, e0187066. [Google Scholar] [CrossRef]
- Hinz, B. The Extracellular Matrix and Transforming Growth Factor-Β1: Tale of a Strained Relationship. Matrix Biol. 2015, 47, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Daher, A.M.; Agarwal, R. Aqueous Humor TGF-Β2 Levels in Patients with Open-Angle Glaucoma: A Meta-Analysis. Mol. Vis. 2015, 21, 612–620. [Google Scholar]
- Fleenor, D.L.; Shepard, A.R.; Hellberg, P.E.; Jacobson, N.; Pang, I.-H.; Clark, A.F. TGFβ2-Induced Changes in Human Trabecular Meshwork: Implications for Intraocular Pressure. Investig. Ophthalmol. Vis. Sci. 2006, 47, 226–234. [Google Scholar] [CrossRef]
- Montecchi-Palmer, M.; Bermudez, J.Y.; Webber, H.C.; Patel, G.C.; Clark, A.F.; Mao, W. TGFβ2 Induces the Formation of Cross-Linked Actin Networks (CLANs) in Human Trabecular Meshwork Cells Through the Smad and Non-Smad Dependent Pathways. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1288–1295. [Google Scholar] [CrossRef]
- Pervan, C.L. Smad-Independent TGF-Β2 Signaling Pathways in Human Trabecular Meshwork Cells. Exp. Eye Res. 2017, 158, 137–145. [Google Scholar] [CrossRef]
- McDowell, C.M.; Tebow, H.E.; Wordinger, R.J.; Clark, A.F. Smad3 Is Necessary for Transforming Growth Factor-Beta2 Induced Ocular Hypertension in Mice. Exp. Eye Res. 2013, 116, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.P.; Curry, S.; McDowell, C.M. Effects of Toll-like Receptor 4 Inhibition on Transforming Growth Factor-Β2 Signaling in the Human Trabecular Meshwork. J. Ocul. Pharmacol. Ther. 2020, 36, 170–178. [Google Scholar] [CrossRef] [PubMed]
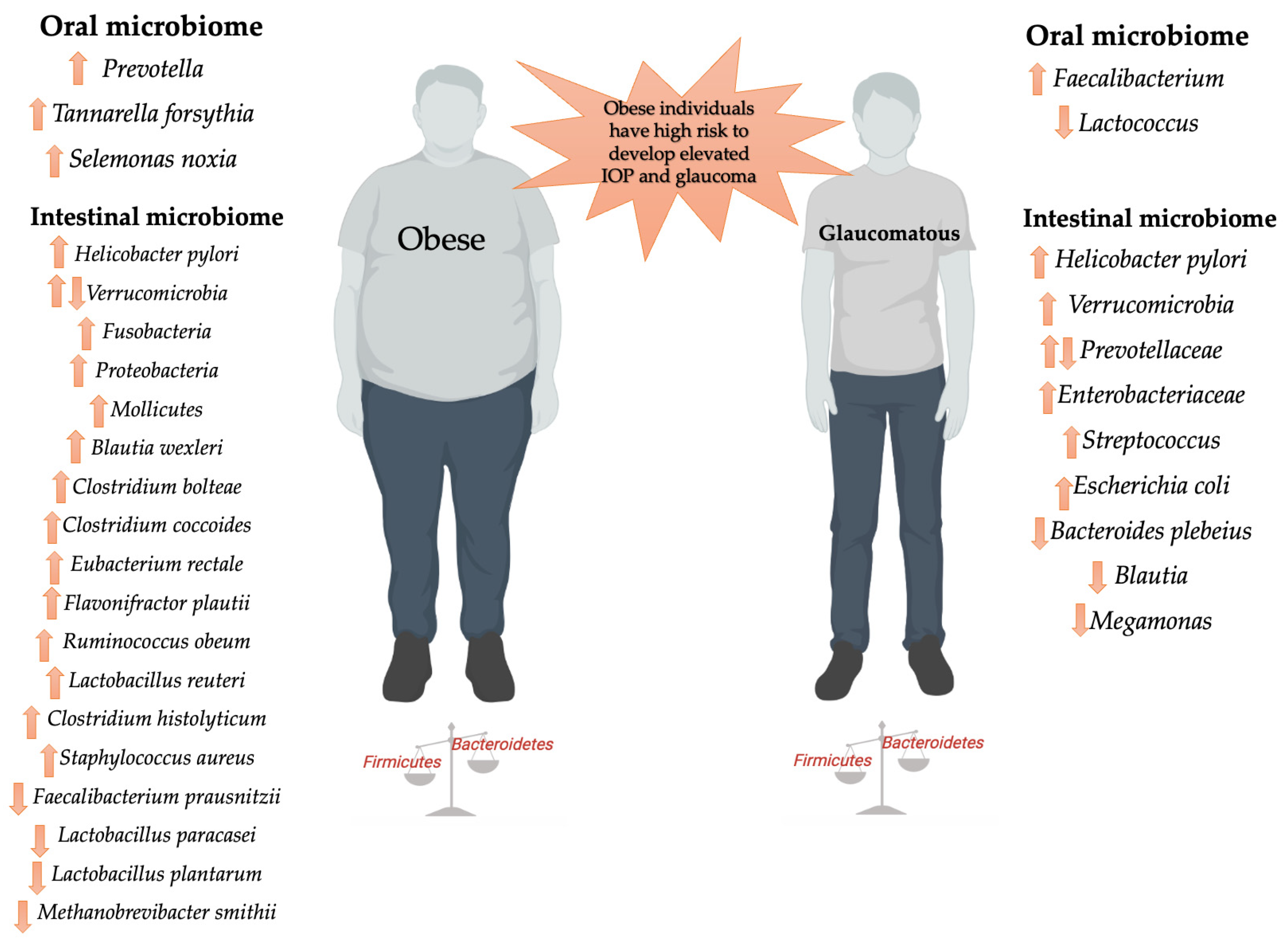
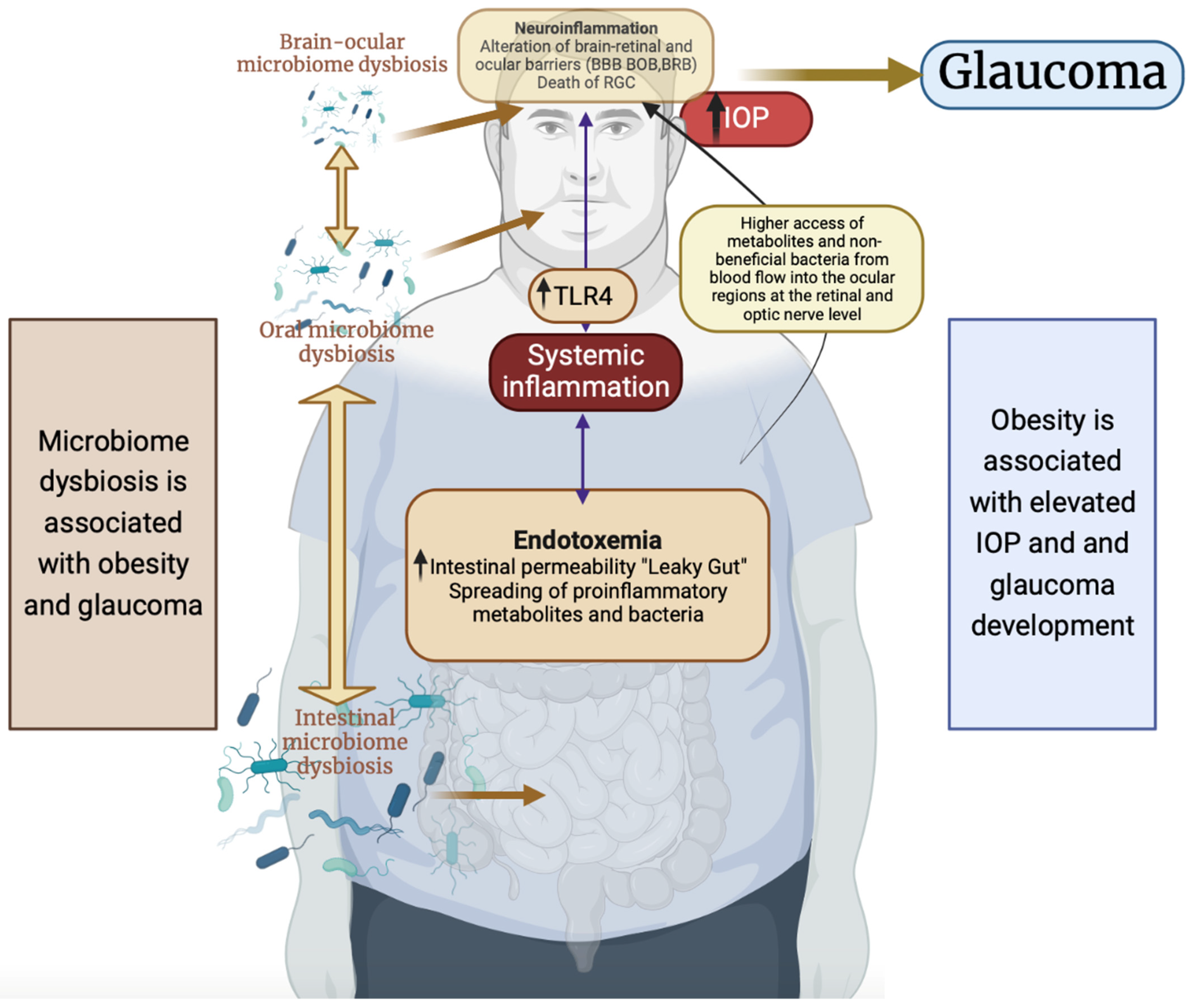
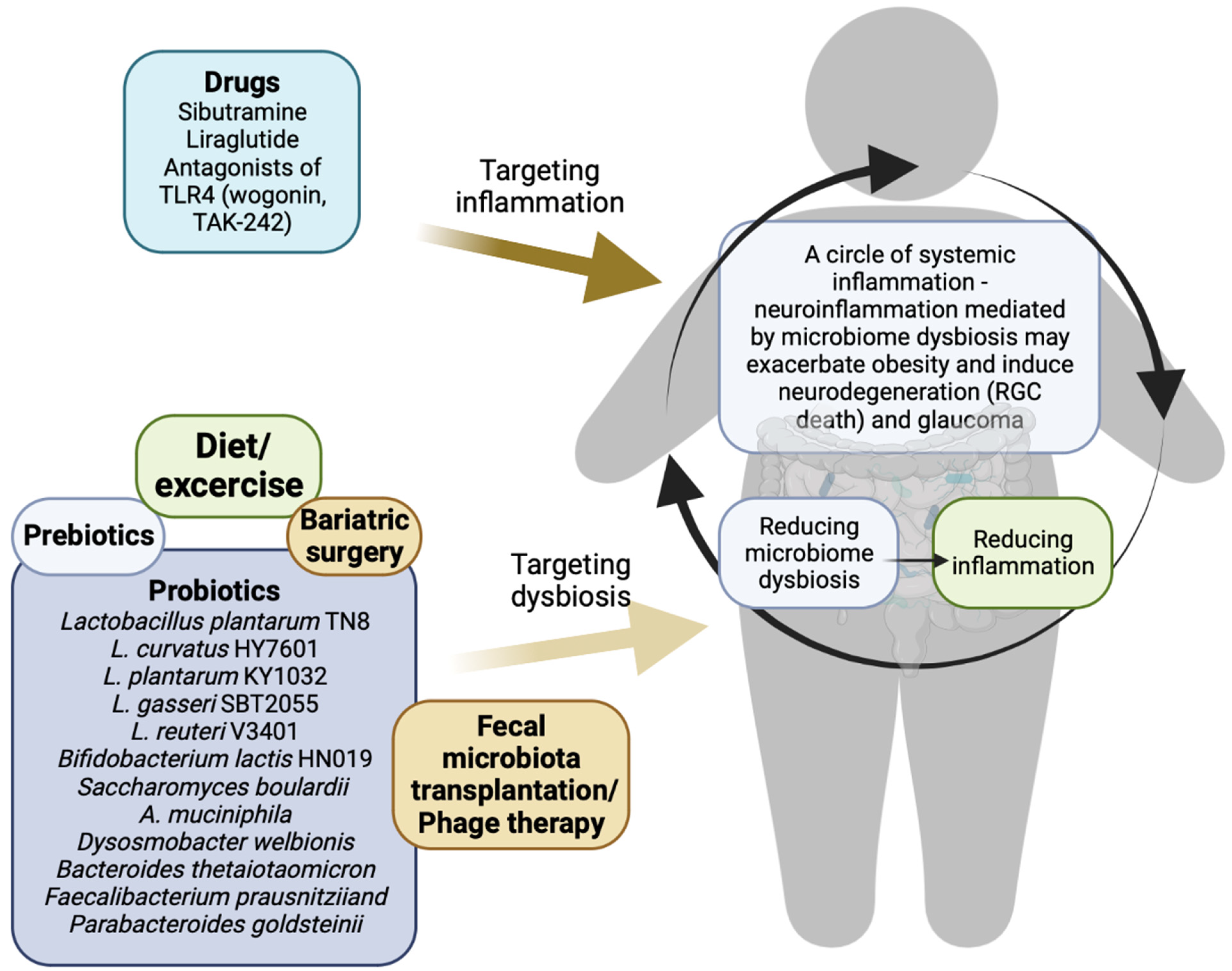
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzino, S.; Sofia, M.; Greco, L.P.; Litrico, G.; Filippello, G.; Sarvà, I.; La Greca, G.; Latteri, S. Microbiome Dysbiosis: A Pathological Mechanism at the Intersection of Obesity and Glaucoma. Int. J. Mol. Sci. 2023, 24, 1166. https://doi.org/10.3390/ijms24021166
Pezzino S, Sofia M, Greco LP, Litrico G, Filippello G, Sarvà I, La Greca G, Latteri S. Microbiome Dysbiosis: A Pathological Mechanism at the Intersection of Obesity and Glaucoma. International Journal of Molecular Sciences. 2023; 24(2):1166. https://doi.org/10.3390/ijms24021166
Chicago/Turabian StylePezzino, Salvatore, Maria Sofia, Luigi Piero Greco, Giorgia Litrico, Giulia Filippello, Iacopo Sarvà, Gaetano La Greca, and Saverio Latteri. 2023. "Microbiome Dysbiosis: A Pathological Mechanism at the Intersection of Obesity and Glaucoma" International Journal of Molecular Sciences 24, no. 2: 1166. https://doi.org/10.3390/ijms24021166
APA StylePezzino, S., Sofia, M., Greco, L. P., Litrico, G., Filippello, G., Sarvà, I., La Greca, G., & Latteri, S. (2023). Microbiome Dysbiosis: A Pathological Mechanism at the Intersection of Obesity and Glaucoma. International Journal of Molecular Sciences, 24(2), 1166. https://doi.org/10.3390/ijms24021166