Melatonin Involved in Protective Effects against Cadmium Stress in Wolffia arrhiza
Abstract
1. Introduction
2. Results
2.1. Melatonin Improves the Growth Rate in W. arrhiza Exposed to Cadmium
2.2. Melatonin Decreases the Cadmium Level of W. arrhiza
2.3. Cadmium Enhances the Endogenous Level of Melatonin and Its Precursors in W. arrhiza
2.4. Melatonin Increases the Content of Photosynthetic Pigments, Protein, and Monosaccharides in W. arrhiza Exposed to Cadmium
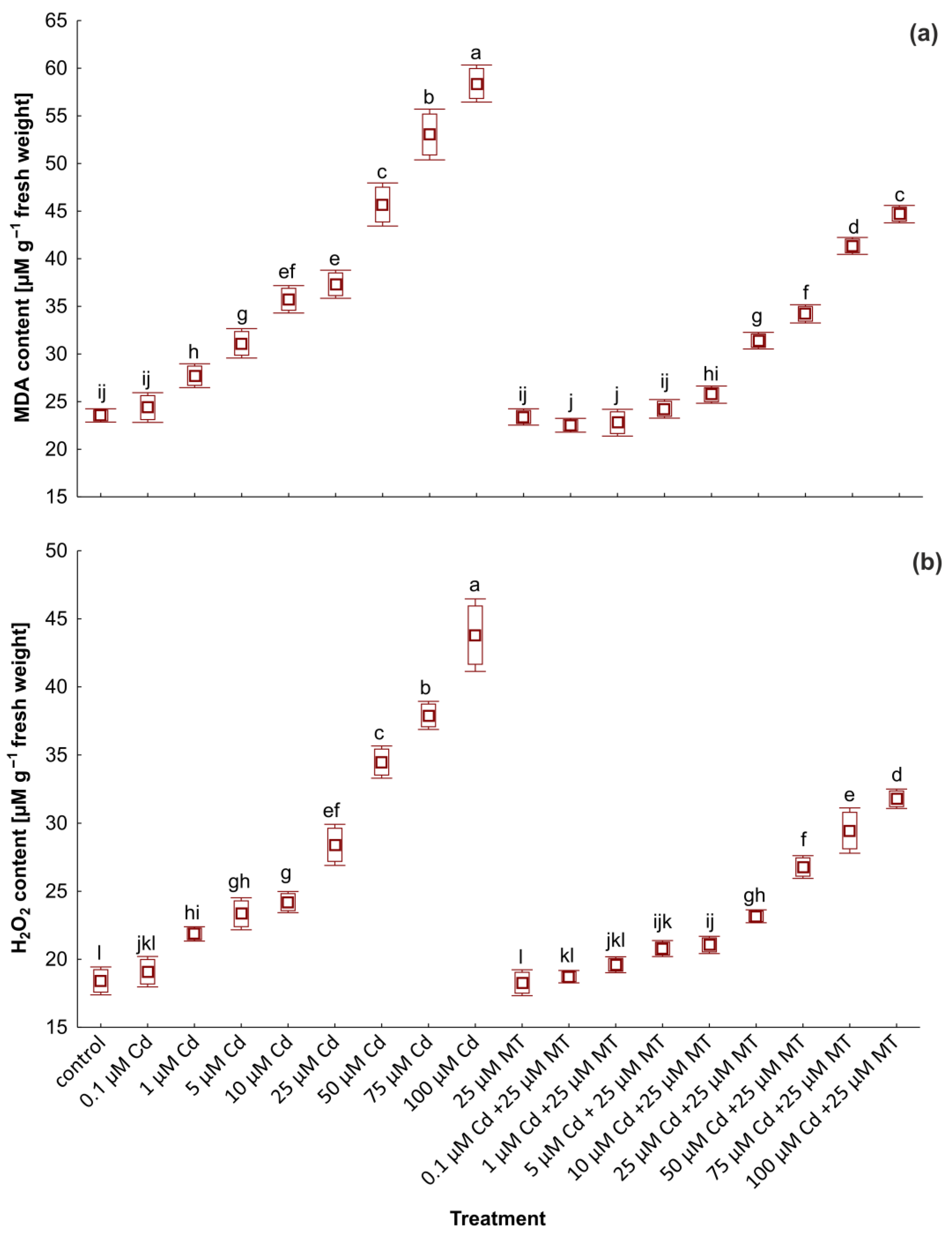
2.5. Melatonin Reduces the Content of Malondialdehyde and H2O2 in W. arrhiza Treated with Cadmium
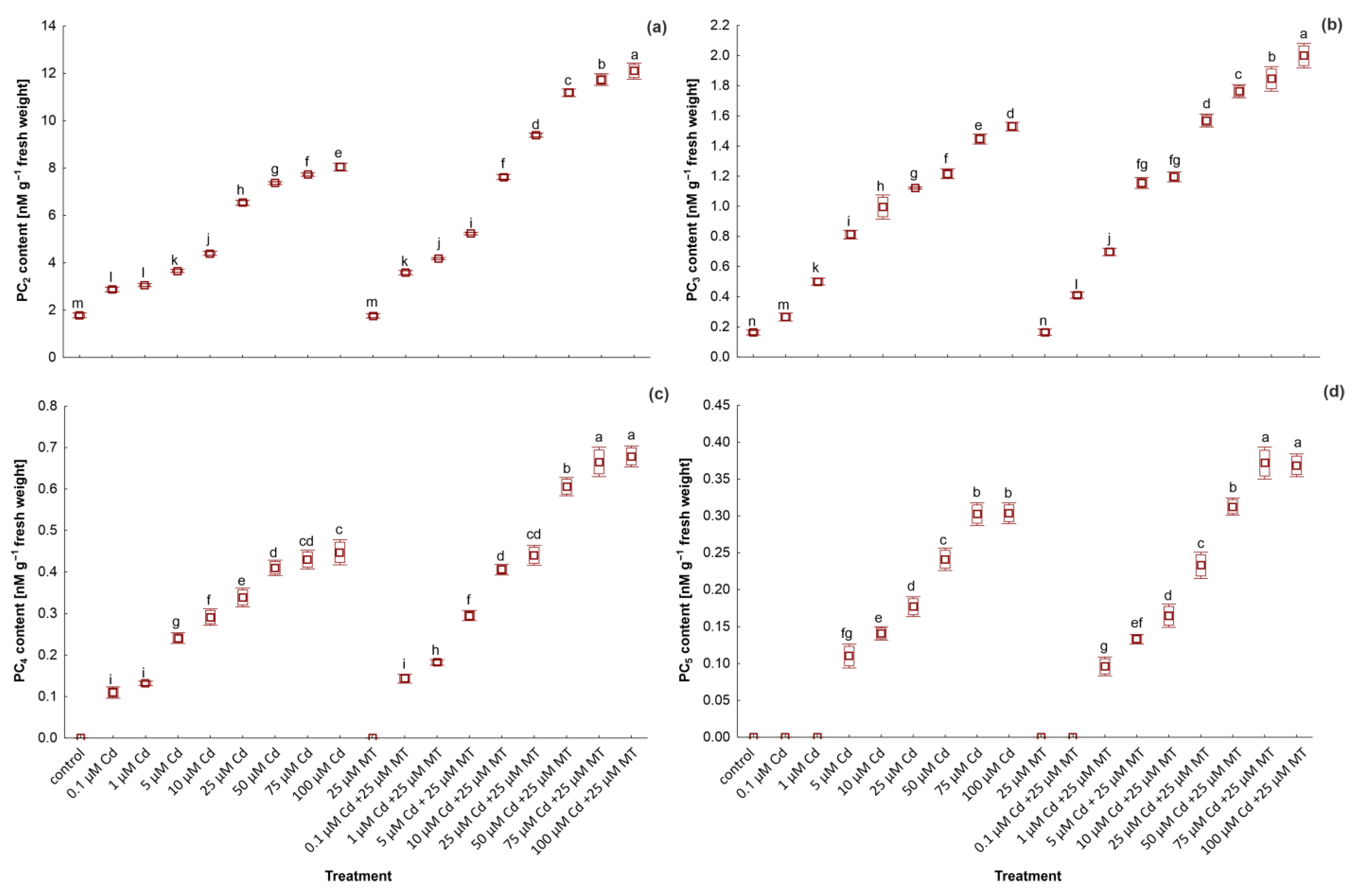
2.6. Melatonin Stimulates the Content of Glutathione and Phytochelatins in W. arrhiza Treated with Cadmium
2.7. Melatonin Intensifies the Antioxidants Activity in W. arrhiza Treated with Cadmium
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Chemicals
4.3. Determination of Cadmium Content
4.4. Quantitative Analysis of Melatonin and Its Intermediates
4.5. Determination of Photosynthetic Pigments
4.6. Determination of Monosaccharides
4.7. Determination of Soluble Proteins
4.8. Determination of Malondialdehyde and H2O2 Content
4.9. Determination of Glutathione and Phytochelatins
4.10. Determination of Antioxidants
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.E.; Reiter, R.J.; Wasdell, M.B.; Bax, M. The role of the thalamus in sleep, pineal melatonin production, and circadian rhythm sleep disorders. J. Pineal Res. 2009, 46, 1–7. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Is phytomelatonin a new plant hormone? Agronomy 2020, 10, 95. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtanikaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Wei, J.; Li, D.X.; Zhang, J.R.; Shan, C.; Rengel, Z.; Song, Z.B.; Chen, Q. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef]
- Mir, A.R.; Faizan, M.; Bajguz, A.; Sami, F.; Siddiqui, H.; Hayat, S. Occurrence and biosynthesis of melatonin and its exogenous effect on plants. Acta Soc. Bot. Pol. 2020, 89, 8922. [Google Scholar] [CrossRef]
- Bose, S.K.; Howlader, P. Melatonin plays multifunctional role in horticultural crops against environmental stresses: A review. Environ. Exp. Bot. 2020, 176, 104063. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernandez-Ruiz, J. Phytomelatonin: An unexpected molecule with amazing performances in plants. J. Exp.Bot. 2022, 73, 5779–5800. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin as a plant biostimulant in crops and during post-harvest: A new approach is needed. J. Sci. Food Agric. 2021, 101, 5297–5304. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Su, W.N.; Kamran, M.; Ahmad, I.; Meng, X.P.; Wu, X.R.; Javed, T.; Han, Q.F. Foliar application of melatonin delay leaf senescence in maize by improving the antioxidant defense system and enhancing photosynthetic capacity under semi-arid regions. Protoplasma 2020, 257, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Wang, G.Y.; Muhammad, I.; Chi, Y.X.; Zeeshan, M.; Nasar, J.; Zhou, X.B. Interactive effects of melatonin and nitrogen improve drought tolerance of maize seedlings by regulating growth and physiochemical attributes. Antioxidants 2022, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Mostafa, S.; Lu, Z.G.; Jin, B. Melatonin-mediated abiotic stress tolerance in plants. Front. Plant Sci. 2022, 13, 847175. [Google Scholar] [CrossRef]
- Song, R.X.; Ritonga, F.N.; Yu, H.Y.; Ding, C.J.; Zhao, X.Y. Plant melatonin: Regulatory and protective role. Horticulturae 2022, 8, 810. [Google Scholar] [CrossRef]
- Huang, X.; Tanveer, M.; Min, Y.; Shabala, S. Melatonin as a regulator of plant ionic homeostasis: Implications for abiotic stress tolerance. J. Exp. Bot. 2022, 73, 5886–5902. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Guan, W.J.; Yu, A.; Li, Z.S.; Wang, Z.J.; Yu, H.Y.; Zou, L.Y. Exogenous melatonin improves cadmium tolerance in Solanum nigrum L. without affecting its remediation potential. Int. J. Phytoremediat. 2022, 24, 1284–1291. [Google Scholar] [CrossRef]
- Wang, K.; He, J.J.; Gao, Y.; Han, K.; Liu, J.Q.; Wang, Y.J. Exogenous melatonin improved the growth and development of naked oat seedlings under cadmium stress. Environ. Sci. Pollut. Res. 2022, 29, 88109–88118. [Google Scholar] [CrossRef]
- Hasan, M.K.; Ahammed, G.J.; Yin, L.L.; Shi, K.; Xia, X.J.; Zhou, Y.H.; Yu, J.Q.; Zhou, J. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 2015, 6, 601. [Google Scholar] [CrossRef]
- Bertin, G.; Averbeck, D. Cadmium: Cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 2006, 88, 1549–1559. [Google Scholar] [CrossRef]
- Baycu, G.; Rognes, S.E.; Ozden, H.; Goren-Saglam, N.; Csatari, I.; Szabo, S. Abiotic stress effects on the antioxidative response profile of Albizia julibrissin Durazz. (Fabaceae). Rev. Bras. Bot. 2017, 40, 21–32. [Google Scholar] [CrossRef]
- Liu, H.J.; Zhang, C.X.; Wang, J.M.; Zhou, C.J.; Feng, H.; Mahajan, M.D.; Han, X.R. Influence and interaction of iron and cadmium on photosynthesis and antioxidative enzymes in two rice cultivars. Chemosphere 2017, 171, 240–247. [Google Scholar] [CrossRef]
- Zong, H.; Liu, S.; Xing, R.; Chen, X.; Li, P. Protective effect of chitosan on photosynthesis and antioxidative defense system in edible rape (Brassica rapa L.) in the presence of cadmium. Ecotoxicol. Environ. Saf. 2017, 138, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, C.; Nan, Z. Effects of cadmium stress on growth and anti-oxidative systems in Achnatherum inebrians symbiotic with Neotyphodium gansuense. J. Hazard. Mater. 2010, 175, 703–709. [Google Scholar] [CrossRef]
- Lazar, D.; Murch, S.J.; Beilby, M.J.; Al Khazaaly, S. Exogenous melatonin affects photosynthesis in characeae Chara australis. Plant Signal. Behav. 2013, 8, e23279. [Google Scholar] [CrossRef]
- Agathokleous, E.; Zhou, B.Y.; Xu, J.N.; Ioannou, A.; Feng, Z.Z.; Saitanis, C.J.; Frei, M.; Calabrese, E.J.; Fotopoulos, V. Exogenous application of melatonin to plants, algae, and harvested products to sustain agricultural productivity and enhance nutritional and nutraceutical value: A meta-analysis. Environ. Res. 2021, 200, 111746. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.Y.; Choi, D.W.; Back, K. Chloroplast-encoded serotonin N-acetyltransferase in the red alga Pyropia yezoensis: Gene transition to the nucleus from chloroplasts. J. Exp. Bot. 2015, 66, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Bog, M.; Appenroth, K.J.; Sree, K.S. Key to the determination of taxa of Lemnaceae: An update. Nord. J. Bot. 2020, 38, e02658. [Google Scholar] [CrossRef]
- Romano, L.E.; Aronne, G. The world smallest plants (Wolffia Sp.) as potential species for bioregenerative life support systems in space. Plants 2021, 10, 1896. [Google Scholar] [CrossRef] [PubMed]
- Soda, S.; Kawahata, Y.; Takai, Y.; Mishima, D.; Fujita, M.; Ike, M. Kinetics of nutrient removal and biomass production by duckweed Wolffia arrhiza in continuous-flow mesocosms. Ecol. Eng. 2013, 57, 210–215. [Google Scholar] [CrossRef]
- Kotowska, U.; Karpinska, J.; Kapelewska, J.; Kowejsza, E.M.; Piotrowska-Niczyporuk, A.; Piekutin, J.; Kotowski, A. Removal of phthalates and other contaminants from municipal wastewater during cultivation of Wolffia arrhiza. Process Saf. Environ. Prot. 2018, 120, 268–277. [Google Scholar] [CrossRef]
- Wang, M.; Duan, S.H.; Zhou, Z.C.; Chen, S.B.; Wang, D. Foliar spraying of melatonin confers cadmium tolerance in Nicotiana tabacum L. Ecotoxicol. Environ. Saf. 2019, 170, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Yang, L.; Ahmad, S.; Mosaad, I.S.M.; Al-Ghamdi, A.A.; Abbasi, A.M.; Zhou, X.B. Melatonin application alleviates atress-induced photosynthetic inhibition and oxidative damage by regulating antioxidant defense system of maize: A meta-analysis. Antioxidants 2022, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, F.; Tang, M.J.; Wang, Y.; Dong, J.H.; Ying, J.L.; Chen, Y.L.; Hu, B.; Li, C.; Liu, L.W. Melatonin confers cadmium tolerance by modulating critical heavy metal chelators and transporters in radish plants. J. Pineal Res. 2020, 69, e12659. [Google Scholar] [CrossRef]
- Gu, Q.; Chen, Z.P.; Yu, X.L.; Cui, W.T.; Pan, J.C.; Zhao, G.; Xu, S.; Wang, R.; Shen, W.B. Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant Sci. 2017, 261, 28–37. [Google Scholar] [CrossRef]
- Sami, A.; Shah, F.A.; Abdullah, M.; Zhou, X.; Yan, Y.; Zhu, Z.; Zhou, K. Melatonin mitigates cadmium and aluminium toxicity through modulation of antioxidant potential in Brassica napus L. Plant Biol. 2020, 22, 679–690. [Google Scholar] [CrossRef]
- Chen, J.Q.; Qin, H.; Zhang, B.F.; Mao, W.H.; Lou, L.P.; Shen, C.F.; Mao, J.D.; Lin, Q. Development of melatonin nano-delivery systems to reduce cadmium accumulation in rice (Oryza sativa L.) seedlings: Insights from photosynthetic efficiency, antioxidative response and gene expression. Environ. Exp. Bot. 2022, 196, 104822. [Google Scholar] [CrossRef]
- Huang, X.; Mazza, G. Simultaneous analysis of serotonin, melatonin, piceid and resveratrol in fruits using liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 3890–3899. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Giridhar, P.; Sankar, K.U.; Ravishankar, G.A. Melatonin and serotonin profiles in beans of Coffea species. J. Pineal Res. 2012, 52, 470–476. [Google Scholar] [CrossRef]
- Ye, T.T.; Yin, X.M.; Yu, L.; Zheng, S.J.; Cai, W.J.; Wu, Y.; Feng, Y.Q. Metabolic analysis of the melatonin biosynthesis pathway using chemical labeling coupled with liquid chromatography-mass spectrometry. J. Pineal Res. 2019, 66, e12531. [Google Scholar] [CrossRef]
- Murch, S.J.; Erland, L.A.E. A systematic review of melatonin in plants: An example of evolution of literature. Front. Plant Sci. 2021, 12, 683047. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Park, S.; Lee, H.Y.; Kim, Y.S.; Back, K. Elevated production of melatonin in transgenic rice seeds expressing rice tryptophan decarboxylase. J. Pineal Res. 2014, 56, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Q.; Hasan, M.K.; Li, C.X.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Reiter, R.J.; Yu, J.Q.; Xu, M.X.; et al. Melatonin mediates selenium-induced tolerance to cadmium stress in tomato plants. J. Pineal Res. 2016, 61, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Xiao, Q.Q.; Chen, Z.P.; Han, Y. Crosstalk between melatonin and reactive oxygen species in plant abiotic stress responses: An update. Int. J. Mol. Sci. 2022, 23, 5666. [Google Scholar] [CrossRef]
- Lee, K.; Choi, G.H.; Back, K. Cadmium-induced melatonin synthesis in rice requires light, hydrogen peroxide, and nitric oxide: Key regulatory roles for tryptophan decarboxylase and caffeic acid O-methyltransferase. J. Pineal Res. 2017, 63, e12441. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Lee, H.Y.; Hwang, O.J.; Lee, H.J.; Lee, K.; Back, K. Coordinated regulation of melatonin synthesis and degradation genes in rice leaves in response to cadmium treatment. J. Pineal Res. 2015, 58, 470–478. [Google Scholar] [CrossRef]
- Chmur, M.; Bajguz, A.; Piotrowska-Niczyporuk, A. Effect of cadmium on the level of isoprenoid-derived phytohormones in duckweed Wolffia arrhiza. J. Plant Growth Regul. 2020, 39, 1518–1530. [Google Scholar] [CrossRef]
- Chmur, M.; Bajguz, A. Brassinolide enhances the level of brassinosteroids, protein, pigments, and monosaccharides in Wolffia arrhiza treated with brassinazole. Plants 2021, 10, 1311. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Zambrzycka-Szelewa, E. Response and the detoxification strategies of green alga Acutodesmus obliquus (Chlorophyceae) under lead stress. Environ. Exp. Bot. 2017, 144, 25–36. [Google Scholar] [CrossRef]
- Talarek-Karwel, M.; Bajguz, A.; Piotrowska-Niczyporuk, A. 24-Epibrassinolide modulates primary metabolites, antioxidants, and phytochelatins in Acutodesmus obliquus exposed to lead stress. J. Appl. Phycol. 2020, 32, 263–276. [Google Scholar] [CrossRef]
- Gillet, S.; Decottignies, P.; Chardonnet, S.; Le Marechal, P. Cadmium response and redoxin targets in Chlamydomonas reinhardtii: A proteomic approach. Photosynth. Res. 2006, 89, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A. Suppression of Chlorella vulgaris growth by cadmium, lead, and copper stress and its restoration by endogenous brassinolide. Arch. Environ. Contam. Toxicol. 2011, 60, 406–416. [Google Scholar] [CrossRef]
- Eckstein, A.; Zieba, P.; Gabrys, H. Sugar and light effects on the condition of the photosynthetic apparatus of Arabidopsis thaliana cultured in vitro. J. Plant Growth Regul. 2012, 31, 90–101. [Google Scholar] [CrossRef]
- Sobkowiak, R.; Deckert, J. The effect of cadmium on cell cycle control in suspension culture cells of soybean. Acta Physiol. Plant. 2004, 26, 335–344. [Google Scholar] [CrossRef]
- Simlat, M.; Ptak, A.; Skrzypek, E.; Warchol, M.; Moranska, E.; Piorkowska, E. Melatonin significantly influences seed germination and seedling growth of Stevia rebaudiana Bertoni. PeerJ 2018, 6, e5009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Sun, Q.Q.; Zhang, H.J.; Cao, Y.Y.; Weeda, S.; Ren, S.X.; Guo, Y.D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015, 66, 647–656. [Google Scholar] [CrossRef]
- Tousi, S.; Zoufan, P.; Ghahfarrokhie, A.R. Alleviation of cadmium-induced phytotoxicity and growth improvement by exogenous melatonin pretreatment in mallow (Malva parviflora) plants. Ecotoxicol. Environ. Saf. 2020, 206, 111403. [Google Scholar] [CrossRef]
- Ahmad, S.; Muhammad, I.; Wang, G.Y.; Zeeshan, M.; Yang, L.; Ali, I.; Zhou, X.B. Ameliorative effect of melatonin improves drought tolerance by regulating growth, photosynthetic traits and leaf ultrastructure of maize seedlings. BMC Plant Biol. 2021, 21, 368. [Google Scholar] [CrossRef]
- Amjadi, Z.; Namdjoyan, S.; Soorki, A.A. Exogenous melatonin and salicylic acid alleviates cadmium toxicity in safflower (Carthamus tinctorius L.) seedlings. Ecotoxicology 2021, 30, 387–401. [Google Scholar] [CrossRef]
- Xie, Q.L.; Zhang, Y.; Cheng, Y.X.; Tian, Y.L.; Luo, J.J.; Hu, Z.L.; Chen, G.P. The role of melatonin in tomato stress response, growth and development. Plant Cell Rep. 2022, 41, 1631–1650. [Google Scholar] [CrossRef] [PubMed]
- Allegrone, G.; Razzano, F.; Pollastro, F.; Grassi, G. Determination of melatonin content of different varieties of hemp (Cannabis sativa L.) by liquid chromatography tandem mass spectrometry. SN Appl. Sci. 2019, 1, 720. [Google Scholar] [CrossRef]
- Yang, X.X.; Chen, J.; Ma, Y.; Huang, M.H.; Qiu, T.; Bian, H.W.; Han, N.; Wang, J.H. Function, mechanism, and application of plant melatonin: An update with a focus on the cereal crop, barley (Hordeum vulgare L.). Antioxidants 2022, 11, 634. [Google Scholar] [CrossRef]
- Ni, J.; Wang, Q.J.; Shah, F.A.; Liu, W.B.; Wang, D.D.; Huang, S.W.; Fu, S.L.; Wu, L.F. Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 2018, 23, 799. [Google Scholar] [CrossRef] [PubMed]
- Nabaei, M.; Amooaghaie, R. Melatonin and nitric oxide enhance cadmium tolerance and phytoremediation efficiency in Catharanthus roseus (L.) G. Don. Environ. Sci. Pollut. Res. 2020, 27, 6981–6994. [Google Scholar] [CrossRef]
- Wu, S.Q.; Wang, Y.; Zhang, J.K.; Gong, X.J.; Zhang, Z.; Sun, J.J.; Chen, X.S.; Wang, Y.L. Exogenous melatonin improves physiological characteristics and promotes growth of strawberry seedlings under cadmium stress. Hortic. Plant J. 2021, 7, 13–22. [Google Scholar] [CrossRef]
- Sharma, R.; Bhardwaj, R.; Handa, N.; Gautam, V.; Kohli, S.K.; Bali, S.; Kaur, P.; Thukral, A.K.; Arora, S.; Ohri, P.; et al. Responses of phytochelatins and metallothioneins in alleviation of heavy metal stress in plants: An overview. In Plant Metal Interaction; Elsevier: Amsterdam, The Netherlands, 2016; pp. 263–283. [Google Scholar] [CrossRef]
- Figueira, E.; Freitas, R.; Guasch, H.; Almeida, S.F.P. Efficiency of cadmium chelation by phytochelatins in Nitzschia palea (Kutzing) W. Smith. Ecotoxicology 2014, 23, 285–292. [Google Scholar] [CrossRef]
- Kawakami, S.K.; Gledhill, M.; Achterberg, E.P. Effects of metal combinations on the production of phytochelatins and glutathione by the marine diatom Phaeodactylum tricornutum. Biometals 2006, 19, 51–60. [Google Scholar] [CrossRef]
- Bellini, E.; Maresca, V.; Betti, C.; Castiglione, M.R.; Fontanini, D.; Capocchi, A.; Sorce, C.; Borso, M.; Bruno, L.; Sorbo, S.; et al. The moss Leptodictyum riparium counteracts severe cadmium stress by activation of glutathione transferase and phytochelatin synthase, but slightly by phytochelatins. Int. J. Mol. Sci. 2020, 21, 1583. [Google Scholar] [CrossRef]
- Zhang, T.G.; Wang, J.; Sun, Y.P.; Zhang, L.; Zheng, S. Versatile roles of melatonin in growth and stress tolerance in plants. J. Plant Growth Regul. 2022, 41, 507–523. [Google Scholar] [CrossRef]
- Hutner, S.H. Comparative physiology of heterotrophic growth in plants. In Growth and Differentiation in Plants; Iowa State College Press: Ames, IA, USA, 1953; pp. 417–446. [Google Scholar]
- Malejko, J.; Deoniziak, K.; Tomczuk, M.; Dlugokencka, J.; Godlewska-Zylkiewicz, B. Puparial cases as toxicological indicators: Bioaccumulation of cadmium and thallium in the forensically important blowfly Lucilia sericata. Front. Chem. 2020, 8, 586067. [Google Scholar] [CrossRef] [PubMed]
- Zapata, M.; Rodriguez, F.; Garrido, J.L. Separation of chlorophylls and carotenoids from marine phytoplankton: A new HPLC method using a reversed phase C-8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 2000, 195, 29–45. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Somogyi, M. Notes on sugar determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar] [CrossRef]
- Blunden, C.A.; Wilson, M.F. A specific method for the determination of soluble sugars in plant extracts using enzymatic analysis and its application to the sugar content of developing pear fruit buds. Anal. Biochem. 1985, 151, 403–408. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. J. Anal. Chem. 2014, 5, 730–736. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Scheidegger, C.; Behra, R.; Sigg, L. Phytochelatin formation kinetics and toxic effects in the freshwater alga Chlamydomonas reinhardtii upon shortand long-term exposure to lead(II). Aquat. Toxicol. 2011, 101, 423–429. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Schaedle, M.; Bassham, J.A. Chloroplast glutathione reductase. Plant Physiol. 1977, 59, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovic, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13; TIBCO Software Inc.: Sydney, Australia, 2017.




| Treatment | Cd Content in W. arrhiza | Cd Content in Medium Day 1 | Cd Content in Medium Day 7 |
|---|---|---|---|
| Control | 0 | 0 | 0 |
| 0.1 µM Cd | 0.026 ± 0.002 k [0.024–0.028] | 0.007 ± 0.001 g [0.006–0.007] | 0.005 ± 0.001 k [0.005–0.006] |
| 1 µM Cd | 0.31 ± 0.016 k [0.292–0.328] | 0.101 ± 0.002 g [0.099–0.103] | 0.088 ± 0.004 k [0.084–0.092] |
| 5 µM Cd | 1.655 ± 0.036 jk [1.614–1.696] | 0.652 ± 0.005 f [0.647–0.657] | 0.542 ± 0.004 j [0.538–0.547] |
| 10 µM Cd | 4.088 ± 0.068 i [4.011–4.165] | 1.435 ± 0.008 e [1.425–1.444] | 1.155 ± 0.049 i [1.099–1.211] |
| 25 µM Cd | 10.788 ± 0.168 g [10.598–10.979] | 3.139 ± 0.005 d [3.133–3.145] | 2.572 ± 0.035 h [2.533–2.612] |
| 50 µM Cd | 24.66 ± 1.424 e [23.049–26.271] | 6.079 ± 0.042 c [6.032–6.126] | 5.178 ± 0.011 f [5.166–5.19] |
| 75 µM Cd | 34.784 ± 0.618 c [34.085–35.483] | 8.130 ± 0.008 b [8.121–8.138] | 7.18 ± 0.03 d [7.146–7.214] |
| 100 µM Cd | 42.076 ± 1.695 a [40.158–43.994] | 11.652 ± 0.308 a [11.303–12.001] | 10.299 ± 0.019 b [10.278–10.32] |
| 0.1 µM Cd + 25 µM MT | 0.021 ± 0.002 k [0.019–0.023] | 0.007 ± 0.001 g [0.006–0.008] | 0.007 ± 0.001 k [0.006–0.007] |
| 1 µM Cd + 25 µM MT | 0.28 ± 0.008 k [0.272–0.289] | 0.103 ± 0.004 g [0.099–0.108] | 0.094 ± 0.002 k [0.092–0.096] |
| 5 µM Cd + 25 µM MT | 1.274 ± 0.101 k [1.159–1.389] | 0.658 ± 0.006 f [0.651–0.666] | 0.596 ± 0.057 j [0.531–0.66] |
| 10µM Cd + 25 µM MT | 3.232 ± 0.118 ij [3.099–3.365] | 1.447 ± 0.008 e [1.438–1.456] | 1.225 ± 0.004 i [1.221–1.229] |
| 25 µM Cd + 25 µM MT | 8.783 ± 0.087 h [8.684–8.882] | 3.099 ± 0.02 d [3.076–3.121] | 2.799 ± 0.081 g [2.707–2.891] |
| 50 µM Cd + 25 µM MT | 22.241 ± 0.393 f [21.796–22.685] | 6.082 ± 0.023 c [6.055–6.108] | 5.586 ± 0.09 e [5.484–5.689] |
| 75 µM Cd + 25 µM MT | 32.366 ± 0.752 d [31.515–33.217] | 8.137 ± 0.012 b [8.124–8.15] | 7.548 ± 0.066 c [7.473–7.622] |
| 100 µM Cd + 25 µM MT | 38.342 ± 0.565 b [37.703–38.981] | 11.556 ± 0.25 a [11.273–11.839] | 10.906 ± 0.085 a [10.81–11.001] |
| Treatment | MT | 5HT | NAS | Trp | 5HTP | TAM | 5MT |
|---|---|---|---|---|---|---|---|
| Control | 12.934 ± 1.01 o [12.053–13.816] | 62.23 ± 0.96 m [61.387–63.067] | 1.195 ± 0.06 n [1.14–1.25] | 31.322 ± 1.83 n [29.717–32.926] | 6.523 ± 0.33 j [6.234–6.811] | 1.426 ± 0.02 m [1.412–1.439] | 4.866 ± 0.18 k [4.704–5.027] |
| 0.1 µM Cd | 15.887 ± 1.01 o [14.999–16.774] | 64.69 ± 0.84 lm [63.954–65.424] | 1.337 ± 0.05 m [1.297–1.377] | 35.087 ± 1.67 m [33.620–36.555] | 7.534 ± 0.07 ij [7.472–7.597] | 1.686 ± 0.03 l [1.663–1.709] | 6.027 ± 0.18 j [5.871–6.182] |
| 1 µM Cd | 24.761 ± 1.92 n [23.075–26.448] | 68.74 ± 0.9 k [67.953–69.531] | 1.57 ± 0.03 kl [1.548–1.593] | 46.269 ± 2.08 k [44.447–48.091] | 9.488 ± 0.11 gh [9.393–9.583] | 2.374 ± 0.05 k [2.329- 2.42] | 7.904 ± 0.56 i [7.413–8.394] |
| 5 µM Cd | 32.286 ± 1.24 m [31.197–33.375] | 87.29 ± 1.29 i [86.164–88.424] | 1.674 ± 0.03 jk [1.645–1.703] | 54.031 ± 2.01 j [52.269–55.794] | 12.440 ± 0.06 f [12.39–12.491] | 3.394 ± 0.05 i [3.352–3.436] | 9.37 ± 0.17 gh [9.222–9.518] |
| 10 µM Cd | 42.739 ± 1.46 l [41.456–44.022] | 97.26 ± 1.61 g [95.85–98.676] | 2.087 ± 0.05 h [2.046–2.129] | 72.012 ± 1.63 h [70.583–73.44] | 14.506 ± 0.42 e [14.141–14.871] | 3.834 ± 0.04 h [3.801–3.867] | 11.036 ± 0.49 f [10.603–11.469] |
| 25 µM Cd | 52.582 ± 1.42 j [51.334–53.829] | 119.17 ± 1.17 e [118.147–120.202] | 2.483 ± 0.02 f [2.464–2.503] | 112.670 ± 1.99 e [110.922–114.417] | 16.816 ± 0.45 d [16.418–17.214] | 4.283 ± 0.04 fg [4.245–4.321] | 13.771 ± 0.37 d [13.447–14.095] |
| 50 µM Cd | 62.585 ± 1.4 i [61.354–63.817] | 128.04 ± 1.44 c [126.776–129.295] | 3.154 ± 0.06 c [3.102–3.205] | 117.259 ± 0.73 d [116.624–117.895] | 18.793 ± 0.52 c [18.338–19.247] | 4.784 ± 0.07 d [4.723–4.846] | 15.998 ± 0.33 c [15.707–16.289] |
| 75 µM Cd | 55.594 ± 1.96 j [53.875–57.312] | 123.75 ± 1.3 d [122.611–124.882] | 3.012 ± 0.01 d [3.007–3.017] | 113.294 ± 1.36 e [112.103–114.486] | 16.286 ± 0.81 d [15.573–16.998] | 4.428 ± 0.03 ef [4.4–4.457] | 15.060 ± 0.31 c [14.786–15.334] |
| 100 µM Cd | 48.83 ± 1.61 k [47.421–50.239] | 119.58 ± 2.13 e [117.718–121.45] | 2.935 ± 0.06 d [2.880–2.991] | 109.058 ± 1.11 f [108.088–110.028] | 13.842 ± 1.02 e [12.950–14.735] | 4.245 ± 0.03 g [4.215–4.275] | 13.681 ± 0.95 d [12.848–14.515] |
| 25 µM MT | 148.624 ± 1.57 h [147.246–150.001] | 65.17 ± 1.11 lm [64.194–66.144] | 1.308 ± 0.03 m [1.286–1.330] | 39.542 ± 1.12 l [38.560–40.523] | 7.535 ± 0.25 ij [7.32–7.75] | 1.5 ± 0.01 m [1.494–1.506] | 5.598 ± 0.06 jk [5.542–5.653] |
| 0.1 µM Cd + 25 µM MT | 153.043 ± 1.64 g [151.602–154.484] | 67.59 ± 1.14 kl [66.595–68.586] | 1.497 ± 0.01 l [1.489–1.506] | 41.298 ± 0.84 l [40.563–42.033] | 8.540 ± 0.3 hi [8.281–8.799] | 1.749 ± 0.04 l [1.715–1.783] | 6.516 ± 0.04 j [6.481–6.552] |
| 1 µM Cd + 25 µM MT | 164.182 ± 1.5 f [162.864–165.501] | 76.97 ± 2.01 j [75.207–78.729] | 1.745 ± 0.02 j [1.724–1.766] | 53.779 ± 1.17 j [52.752–54.805] | 10.268 ± 0.28 g [10.022–10.514] | 3.046 ± 0.11 j [2.952–3.140] | 8.270 ± 0.6 hi [7.747–8.794] |
| 5 µM Cd + 25 µM MT | 172.387 ± 0.8 e [171.686–173.088] | 93.96 ± 1.21 h [92.908–95.021] | 1.902 ± 0.02 i [1.882–1.923] | 65.887 ± 0.45 i [65.494–66.28] | 14.429 ± 0.68 e [13.833–15.024] | 3.889 ± 0.08 h [3.822–3.956] | 10.061 ± 0.12 fg [9.957–10.166] |
| 10 µM Cd + 25 µM MT | 181.929 ± 1.03 d [181.028–182.829] | 104.05 ± 1.8 f [102.474–105.624] | 2.353 ± 0.05 g [2.311–2.395] | 80.687 ± 0.83 g [79.960–81.414] | 17.004 ± 0.52 d [16.546–17.462] | 4.498 ± 0.13 e [4.384–4.611] | 12.404 ± 0.42 e [12.039–12.769] |
| 25 µM Cd + 25 µM MT | 202.728 ± 1.66 c [201.275–204.181] | 130.28 ± 1.81 bc [128.691–131.862] | 2.815 ± 0.04 e [2.784–2.846] | 120.683 ± 1.3 bc [119.546–121.821] | 20.347 ± 0.9 b [19.556–21.138] | 4.840 ± 0.09 d [4.765–4.914] | 16.017 ± 0.6 c [15.491–16.543] |
| 50 µM Cd + 25 µM MT | 232.417 ± 1.32 a [231.262–233.573] | 134.98 ± 1.22 a [133.910–136.049] | 3.494 ± 0.03 a [3.471–3.517] | 126.791 ± 0.88 a [126.020–127.562] | 24.211 ± 0.59 a [23.696–24.726] | 5.473 ± 0.11 a [5.375–5.572] | 19.133 ± 0.43 a [18.755–19.511] |
| 75 µM Cd + 25 µM MT | 233.685 ± 1.47 a [232.399–234.971] | 132.33 ± 1.12 ab [131.346–133.317] | 3.324 ± 0.09 b [3.248–3.4] | 123.005 ± 0.96 b [122.160–123.849] | 21.623 ± 0.94 b [20.801–22.444] | 5.304 ± 0.14 b [5.179–5.429] | 18.829 ± 0.82 a [18.108–19.549] |
| 100 µM Cd + 25 µM MT | 223.099 ± 1.52 b [221.769–224.429] | 122.28 ±1.25 de [121.177–123.375] | 3.118 ± 0.08 c [3.052–3.184] | 117.845 ± 1.09 cd [116.888–118.801] | 18.524 ± 0.86 c [17.771–19.277] | 5.118 ± 0.08 c [5.052–5.185] | 17.714 ± 0.71 b [17.088–18.34] |
| Treatment | Chlorophyll a | Chlorophyll b | α-Carotene | β-Carotene | Neoxanthin |
|---|---|---|---|---|---|
| Control | 162.155 ± 2.69 e [159.798–164.513] | 42.194 ± 2.2 ef [40.262–44.127] | 1.289 ± 0.01 e [1.281–1.297] | 1.78 ± 0.07 d [1.716–1.844] | 0.917 ± 0.04 e [0.884–0.95] |
| 0.1 µM Cd | 159.030 ± 1.13 e [158.040–160.019] | 40.155 ± 0.73 fg [39.514–40.796] | 1.235 ± 0.01 e [1.223–1.247] | 1.701 ± 0.07 de [1.64–1.761] | 0.906 ± 0.02 e [0.89–0.922] |
| 1 µM Cd | 153.193 ± 1.15 f [152.186–154.199] | 39.324 ± 0.56 g [38.837–39.811] | 1.095 ± 0.03 fg [1.072–1.118] | 1.619 ± 0.03 ef [1.596–1.642] | 0.864 ± 0.02 e [0.846–0.882] |
| 5 µM Cd | 146.853 ± 1.09 g [145.896–147.809] | 36.140 ± 0.76 h [35.472–36.809] | 1.04 ± 0.01 g [1.029–1.050] | 1.531 ± 0.05 f [1.486–1.576] | 0.786 ± 0.01 f [0.779–0.792] |
| 10 µM Cd | 140.7 ± 0.97 h [139.852–141.548] | 33.991 ± 0.38 hi [33.659–34.323] | 0.944 ± 0.02 h [0.929–0.959] | 1.411 ± 0.03 g [1.386–1.436] | 0.739 ± 0.02 f [0.724–0.753] |
| 25 µM Cd | 127.306 ± 0.94 i [126.486–128.126] | 30.056 ± 0.53 jk [29.593–30.519] | 0.809 ± 0.01 j [0.796–0.822] | 1.235 ± 0.07 hi [1.174–1.295] | 0.652 ± 0.01 g [0.645–0.66] |
| 50 µM Cd | 92.663 ± 1.36 l [91.467–93.858] | 26.034 ± 0.41 l [25.679–26.389] | 0.724 ± 0.02 k [0.71–0.739] | 1.076 ± 0.07 j [1.01–1.141] | 0.569 ± 0.02 ij [0.55–0.588] |
| 75 µM Cd | 78.585 ± 1.21 m [77.524–79.646] | 21.454 ± 0.90 m [20.667–22.240] | 0.633 ± 0.01 l [0.625–0.641] | 0.949 ± 0.02 k [0.932–0.966] | 0.514 ± 0.01 jk [0.501–0.526] |
| 100 µM Cd | 65.017 ± 1.83 n [63.416–66.619] | 18.041 ± 0.82 n [17.324–18.758] | 0.591 ± 0.02 l [0.576–0.606] | 0.895 ± 0.02 k [0.878–0.913] | 0.473 ± 0.01 k [0.462–0.484] |
| 25 µM MT | 237.154 ± 1.76 a [235.61–238.698] | 67.216 ± 1.26 a [66.11–68.323] | 1.794 ± 0.02 a [1.773–1.814] | 2.911 ± 0.04 a [2.872–2.949] | 1.442 ± 0.03 a [1.411–1.472] |
| 0.1 µM Cd + 25 µM MT | 228.022 ± 1.53 b [226.678–229.365] | 65.336 ± 0.95 ab [64.504–66.167] | 1.722 ± 0.07 b [1.659–1.785] | 2.807 ± 0.03 a [2.784–2.831] | 1.389 ± 0.04 a [1.353–1.424] |
| 1 µM Cd + 25 µM MT | 219.783 ± 0.92 c [218.977–220.588] | 63.692 ± 0.55 b [63.207–64.176] | 1.7 ± 0.01 bc [1.687–1.713] | 2.596 ± 0.06 b [2.545–2.647] | 1.286 ± 0.02 b [1.265–1.308] |
| 5 µM Cd + 25 µM MT | 173.649 ± 1.68 d [172.181–175.118] | 60.752 ± 0.85 c [60.006–61.497] | 1.651 ± 0.05 c [1.603–1.699] | 2.324 ± 0.05 c [2.28–2.369] | 1.222 ± 0.03 c [1.195–1.248] |
| 10 µM Cd + 25 µM MT | 160.743 ± 1.51 e [159.42–162.067] | 48.853 ± 0.90 d [48.068–49.638] | 1.415 ± 0.05 d [1.367–1.463] | 1.705 ± 0.03 de [1.68–1.730] | 1.076 ± 0.04 d [1.044–1.108] |
| 25 µM Cd + 25 µM MT | 147.226 ± 2.71 g [144.847–149.604] | 42.715 ± 0.88 e [41.946–43.483] | 1.121 ± 0.04 f [1.086–1.156] | 1.414 ± 0.04 g [1.379–1.450] | 0.874 ± 0.03 e [0.844–0.903] |
| 50 µM Cd + 25 µM MT | 122.811 ± 1.92 j [121.133–124.490] | 35.349 ± 0.93 h [34.534–36.164] | 0.897 ± 0.02 hi [0.88–0.914] | 1.292 ± 0.04 h [1.26–1.325] | 0.667 ± 0.03 g [0.639–0.695] |
| 75 µM Cd + 25 µM MT | 104.141 ± 0.75 k [103.483–104.799] | 32.233 ± 1.12 ij [31.255–33.210] | 0.838 ± 0.01 ij [0.829–0.847] | 1.180 ± 0.03 ij [1.156–1.205] | 0.629 ± 0.02 gh [0.613–0.645] |
| 100 µM Cd + 25 µM MT | 93.883 ± 2.69 l [91.526–96.240] | 29.073 ± 1.0 k [28.201–29.945] | 0.791 ± 0.02 jk [0.774–0.808] | 1.155 ± 0.02 ij [1.141–1.169] | 0.586 ± 0.02 hi [0.57–0.603] |
| Violaxanthin | Astaxanthin | Zeaxanthin | Cryptoxanthin | Lutein | |
| Control | 0.7 ± 0.01 ef [0.69–0.711] | 0.352 ± 0.02 e [0.331–0.373] | 3.208 ± 0.06 f [3.158–3.258] | 4.237 ± 0.08 de [4.168–4.307] | 0.473 ± 0.01 d [0.465–0.481] |
| 0.1 µM Cd | 0.653 ± 0.03 fg [0.628–0.678] | 0.328 ± 0.01 ef [0.32–0.337] | 3.143 ± 0.01 fg [3.135–3.151] | 4.141 ± 0.05 ef [4.094–4.188] | 0.422 ± 0.01 e [0.411–0.434] |
| 1 µM Cd | 0.625 ± 0.01 g [0.616–0.635] | 0.317 ± 0.01 ef [0.31–0.324] | 3.079 ± 0.01 g [3.068–3.089] | 4.061 ± 0.02 fg [4.04–4.082] | 0.392 ± 0.01 ef [0.381–0.403] |
| 5 µM Cd | 0.554 ± 0.01 hi [0.542–0.565] | 0.292 ± 0.01 fg [0.282–0.302] | 2.904 ± 0.02 h [2.884–2.924] | 3.880 ± 0.06 h [3.826–3.934] | 0.371 ± 0.01 fg [0.364–0.378] |
| 10 µM Cd | 0.526 ± 0.01 hij [0.521–0.531] | 0.265 ± 0.01 gh [0.256–0.274] | 2.770 ± 0.02 i [2.749–2.792] | 3.521 ± 0.02 i [3.502–3.540] | 0.349 ± 0.02 gh [0.334–0.363] |
| 25 µM Cd | 0.486 ± 0.01 jk [0.476–0.496] | 0.238 ± 0.01 hi [0.23–0.246] | 2.512 ± 0.02 k [2.496–2.529] | 3.362 ± 0.02 j [3.344–3.381] | 0.309 ± 0.02 i [0.296–0.323] |
| 50 µM Cd | 0.464 ± 0.01 k [0.454–0.474] | 0.207 ± 0.01 ij [0.197–0.216] | 2.236 ± 0.04 l [2.205–2.267] | 3.001 ± 0.04 l [2.970–3.032] | 0.276 ± 0.01 jk [0.265–0.287] |
| 75 µM Cd | 0.397 ± 0.02 l [0.383–0.412] | 0.182 ± 0.01 jk [0.175–0.188] | 1.953 ± 0.04 m [1.922–1.984] | 2.790 ± 0.03 m [2.761–2.820] | 0.254 ± 0.01 kl [0.249–0.258] |
| 100 µM Cd | 0.356 ± 0.02 l [0.342–0.371] | 0.147 ± 0.01 k [0.142–0.152] | 1.853 ± 0.05 m [1.81–1.896] | 2.634 ± 0.05 n [2.593–2.674] | 0.242 ± 0.01 l [0.235–0.249] |
| 25 µM MT | 1.146 ± 0.04 a [1.109–1.183] | 0.622 ± 0.03 a [0.593–0.650] | 4.693 ± 0.03 a [4.663–4.723] | 6.490 ± 0.03 a [6.465–6.515] | 0.772 ± 0.01 a [0.762–0.782] |
| 0.1 µM Cd + 25 µM MT | 1.077 ± 0.03 b [1.049–1.105] | 0.558 ± 0.02 b [0.540–0.576] | 4.577 ± 0.09 b [4.494–4.660] | 6.389 ± 0.07 a [6.325–6.453] | 0.736 ± 0.03 b [0.711–0.760] |
| 1 µM Cd + 25 µM MT | 0.966 ± 0.02 c [0.945–0.987] | 0.472 ± 0.02 c [0.458–0.486] | 4.442 ± 0.04 c [4.403–4.481] | 5.95 ± 0.07 b [5.886–6.013] | 0.605 ± 0.02 c [0.589–0.620] |
| 5 µM Cd + 25 µM MT | 0.777 ± 0.02 d [0.755–0.798] | 0.395 ± 0.02 d [0.377–0.412] | 4.256 ± 0.04 d [4.221–4.292] | 5.422 ± 0.12 c [5.315–5.529] | 0.464 ± 0.02 d [0.447–0.480] |
| 10 µM Cd + 25 µM MT | 0.702 ± 0.03 e [0.677–0.727] | 0.346 ± 0.02 e [0.33–0.361] | 3.764 ± 0.04 e [3.73–3.799] | 4.325 ± 0.08 d [4.257–4.392] | 0.405 ± 0.02 e [0.391–0.419] |
| 25 µM Cd + 25 µM MT | 0.658 ± 0.02 efg [0.641–0.676] | 0.325 ± 0.01 ef [0.315–0.335] | 3.088 ± 0.03 g [3.06–3.116] | 3.941 ± 0.04 gh [3.908–3.973] | 0.363 ± 0.02 fg [0.349–0.377] |
| 50 µM Cd + 25 µM MT | 0.57 ± 0.02 h [0.551–0.589] | 0.264 ± 0.02 gh [0.249–0.279] | 2.645 ± 0.06 j [2.595–2.695] | 3.387 ± 0.05 j [3.347–3.427] | 0.347 ± 0.01 gh [0.339–0.354] |
| 75 µM Cd + 25 µM MT | 0.561 ± 0.02 hi [0.546–0.576] | 0.248 ± 0.01 h [0.236–0.261] | 2.336 ± 0.1 l [2.253–2.42] | 3.336 ± 0.03 j [3.309–3.363] | 0.327 ± 0.01 hi [0.323–0.332] |
| 100 µM Cd + 25 µM MT | 0.516 ± 0.01 ij [0.504–0.529] | 0.21 ± 0.02 ij [0.195–0.226] | 2.230 ± 0.03 l [2.203–2.256] | 3.177 ± 0.03 k [3.154–3.201] | 0.297 ± 0.02 ij [0.284–0.311] |
| Compound | Scan Mode (ESI) | Precursor m/z | Product m/z | CE | Retention Time |
|---|---|---|---|---|---|
| Melatonin | + | 233.0 | 174.0 | −16 | 7.380 |
| Serotonin | + | 176.85 | 160.15 | −14 | 1.864 |
| N-acetylserotonin | + | 219.0 | 160.25 | −18 | 4.358 |
| Tryptophan | + | 204.9 | 188.15 | −22 | 2.997 |
| 5-hydroxytryptophan | + | 220.9 | 204.0 | −15 | 3.093 |
| Tryptamine | + | 161.0 | 144.2 | −15 | 2.242 |
| 5-metoxytryptamine | + | 190.9 | 174.15 | −13 | 2.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmur, M.; Bajguz, A. Melatonin Involved in Protective Effects against Cadmium Stress in Wolffia arrhiza. Int. J. Mol. Sci. 2023, 24, 1178. https://doi.org/10.3390/ijms24021178
Chmur M, Bajguz A. Melatonin Involved in Protective Effects against Cadmium Stress in Wolffia arrhiza. International Journal of Molecular Sciences. 2023; 24(2):1178. https://doi.org/10.3390/ijms24021178
Chicago/Turabian StyleChmur, Magdalena, and Andrzej Bajguz. 2023. "Melatonin Involved in Protective Effects against Cadmium Stress in Wolffia arrhiza" International Journal of Molecular Sciences 24, no. 2: 1178. https://doi.org/10.3390/ijms24021178
APA StyleChmur, M., & Bajguz, A. (2023). Melatonin Involved in Protective Effects against Cadmium Stress in Wolffia arrhiza. International Journal of Molecular Sciences, 24(2), 1178. https://doi.org/10.3390/ijms24021178







