Effects and Mechanisms Activated by Treatment with Cationic, Anionic and Zwitterionic Liposomes on an In Vitro Model of Porcine Pre-Pubertal Sertoli Cells
Abstract
1. Introduction
2. Results
2.1. Sertoli Cell Isolation and Characterisation
2.2. Liposome Size and Surface Charge
2.3. Encapsulation Efficiency (EE)
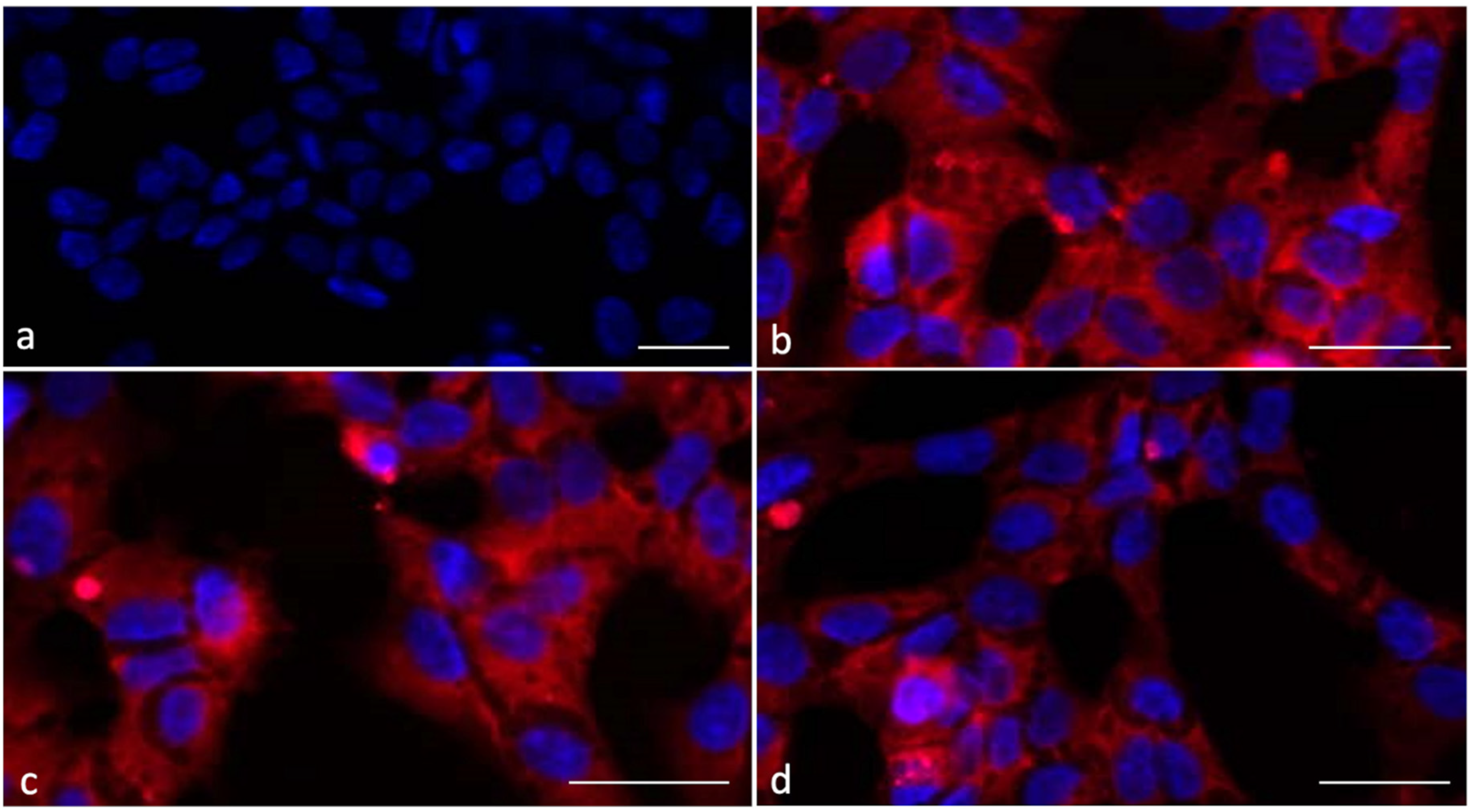
2.4. SC Incubation with Liposomes Loaded with Rhodamine
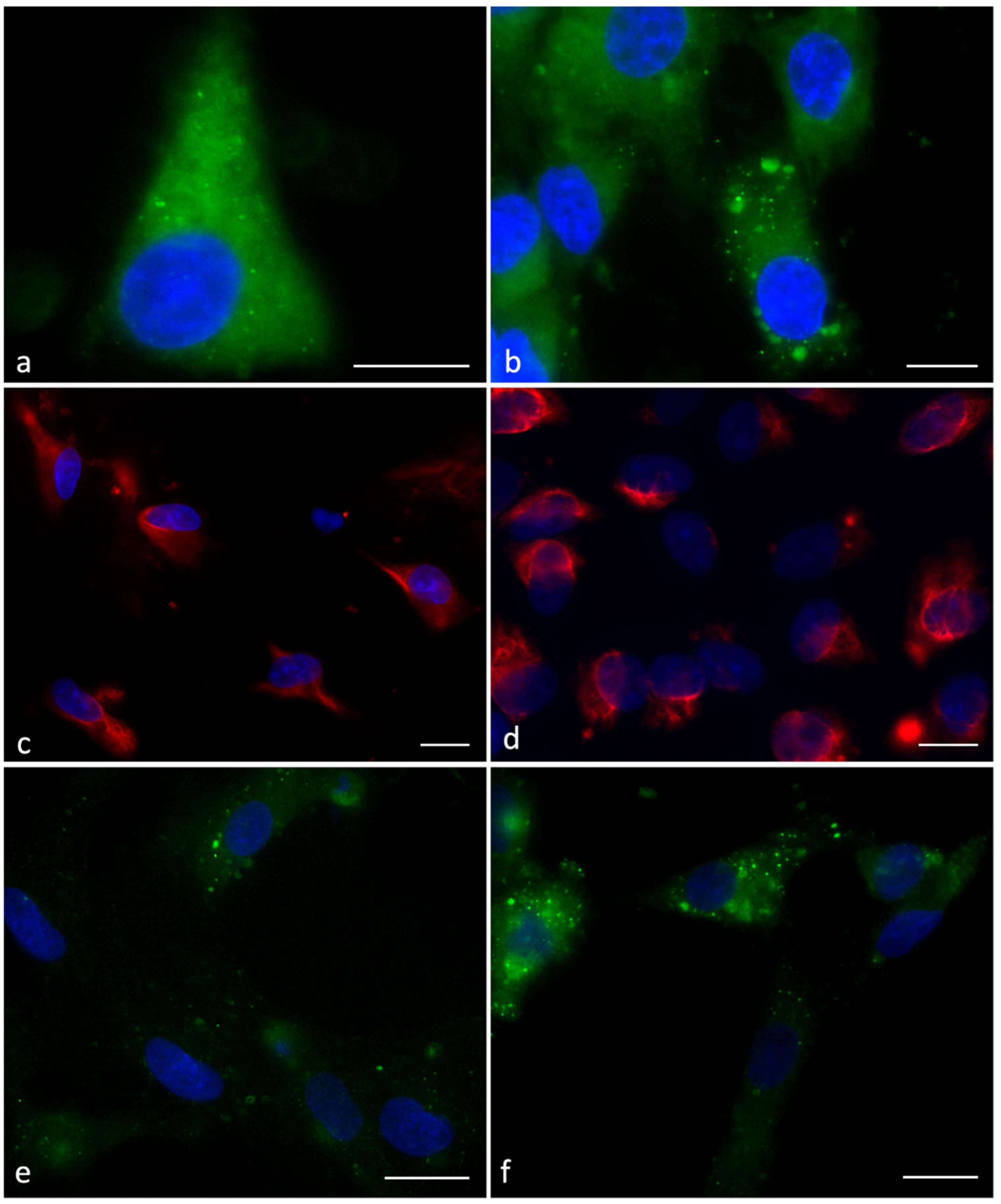
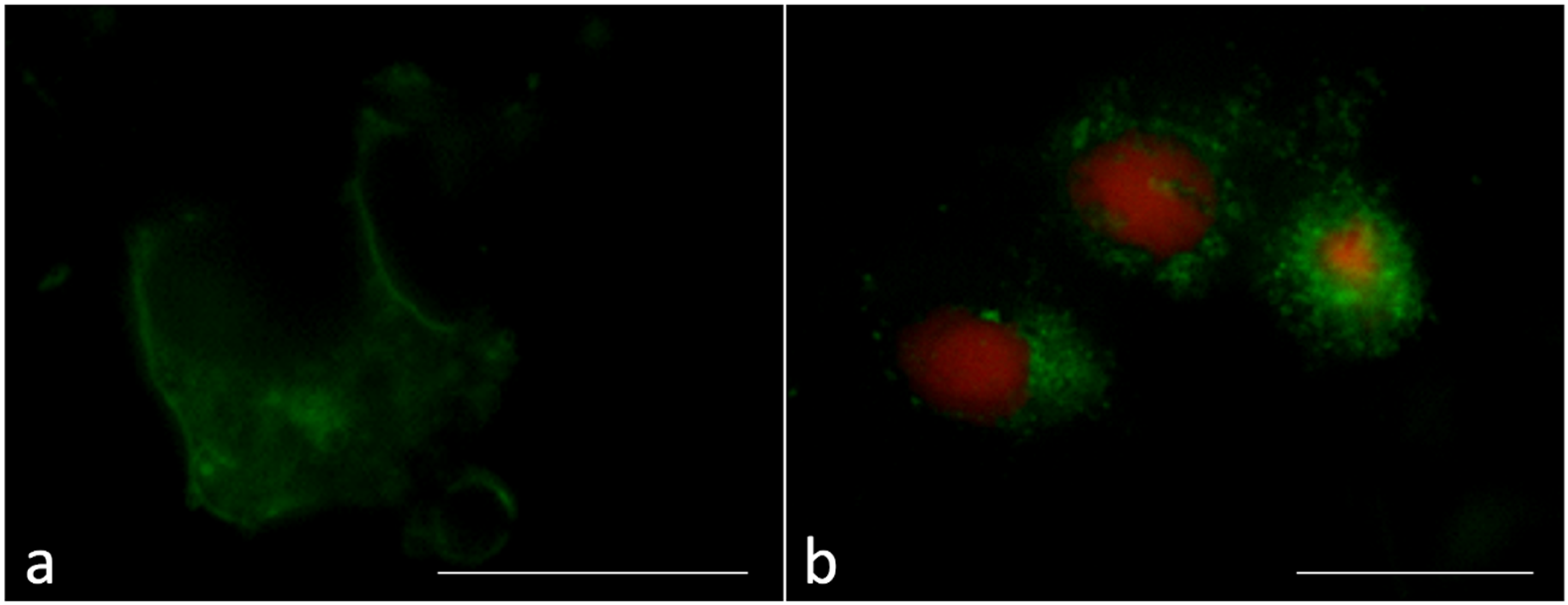
2.5. Immunofluorescence
2.6. Ultrastructural Analysis
3. Discussion
4. Materials and Methods
4.1. Primary Cultures of Neonatal Porcine Sertoli Cells
4.2. Anti-Mullerian Hormone (AMH) and Inhibin B Determination
4.3. Liposome Preparation
4.4. Liposome Preparation: Cationic, Anionic and Zwitterionic
4.5. Size and Surface Charge of Liposomes
4.6. Encapsulation Efficiency (EE) of Rhodamine
4.7. Primary Cultures of Neonatal Porcine Sertoli Cells and Treatments
4.8. Transmission Electron Microscopy (TEM)
4.9. Immunofluorescence Analysis
4.10. Annexin-V and Propidium Iodide Labelling
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wu, S.; Yan, M.; Ge, R.; Cheng, C.Y. Crosstalk between Sertoli and germ cells in male fertility. Trends Mol. Med. 2020, 26, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Thompson, L.A.; Dufour, J.M. Sertoli cells—Immunological sentinels of spermatogenesis. Semin. Cell Dev. Biol. 2014, 30, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Mitchell, R.T.; Kelsey, T.W.; Spears, N.; Telfer, E.E.; Wallace, W.H. Cancer treatment and gonadal function: Experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol. 2015, 3, 556–567. [Google Scholar] [CrossRef]
- Stukenborg, J.B.; Jahnukainen, K.; Hutka, M.; Mitchell, R.T. Cancer treatment in childhood and testicular function: The importance of the somatic environment. Endocr. Connect. 2018, 7, R69–R87. [Google Scholar] [CrossRef]
- Griswold, M.D. Sertoli Cell Biology, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: San Diego, CA, USA, 2015. [Google Scholar]
- Bernardino, R.L.; Alves, M.G.; Oliveira, P.F. Establishment of primary culture of Sertoli cells. Methods Mol. Biol. 2018, 1748, 1–8. [Google Scholar] [CrossRef]
- Mancuso, F.; Arato, I.; Lilli, C.; Bellucci, C.; Bodo, M.; Calvitti, M.; Aglietti, M.C.; dell’Omo, M.; Nastruzzi, C.; Calafiore, R.; et al. Acute effects of lead on porcine neonatal Sertoli cells in vitro. Toxicol. Vitr. 2018, 48, 45–52. [Google Scholar] [CrossRef]
- Marinucci, L.; Balloni, S.; Bellucci, C.; Lilli, C.; Stabile, A.M.; Calvitti, M.; Aglietti, M.C.; Gambelunghe, A.; Muzi, G.; Rende, M.; et al. Effects of nicotine on porcine pre-pubertal Sertoli cells: An in vitro study. Toxicol. Vitr. 2020, 67, 104882. [Google Scholar] [CrossRef]
- Luca, G.; Lilli, C.; Bellucci, C.; Mancuso, F.; Calvitti, M.; Arato, I.; Falabella, G.; Giovagnoli, S.; Aglietti, M.C.; Lumare, A.; et al. Toxicity of cadmium on Sertoli cell functional competence: An in vitro study. J. Biol. Regul. Homeost. Agents 2013, 27, 805–816. [Google Scholar]
- Arato, I.; Ceccarelli, V.; Mancuso, F.; Bellucci, C.; Lilli, C.; Ferolla, P.; Perruccio, K.; D’Arpino, A.; Aglietti, M.C.; Calafiore, R.; et al. Effect of EPA on neonatal pig Sertoli cells “in vitro”: A possible treatment to help maintain fertility in pre-pubertal boys undergoing treatment with gonadotoxic therapies. Front. Endocrinol. 2021, 12, 694796. [Google Scholar] [CrossRef]
- Bartolini, D.; Arato, I.; Mancuso, F.; Giustarini, D.; Bellucci, C.; Vacca, C.; Aglietti, M.C.; Stabile, A.M.; Rossi, R.; Cruciani, G.; et al. Melatonin modulates Nrf2 activity to protect porcine pre-pubertal Sertoli cells from the abnormal H2O2 generation and reductive stress effects of cadmium. J. Pineal. Res. 2022, 73, e12806. [Google Scholar] [CrossRef]
- Arato, I.; Milardi, D.; Giovagnoli, S.; Grande, G.; Bellucci, C.; Lilli, C.; Bartoli, S.; Corneli, S.; Mazzone, P.; Calvitti, M.; et al. In “vitro” Lps-stimulated Sertoli cells pre-loaded with microparticles: Intracellular activation pathways. Front. Endocrinol. 2021, 11, 611932. [Google Scholar] [CrossRef] [PubMed]
- Luca, G.; Mancuso, F.; Calvitti, M.; Arato, I.; Falabella, G.; Bufalari, A.; De Monte, V.; Tresoldi, E.; Nastruzzi, C.; Basta, G.; et al. Long-term stability, functional competence and safety of microencapsulated specific pathogen-free neonatal porcine Sertoli cells: A potential product for cell transplant therapy. Xenotransplantation 2015, 22, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.M.; Jeong, H.J. Liposomes: Biomedical applications. Chonnam Med. J. 2021, 57, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Arisaka, M.; Nakamura, T.; Yamada, A.; Negishi, Y.; Aramaki, Y. Involvement of protein kinase Cdelta in induction of apoptosis by cationic liposomes in macrophage-like RAW264.7 cells. FEBS Lett. 2010, 584, 1016–1020. [Google Scholar] [CrossRef]
- Balazs, D.A.; Godbey, W. Liposomes for use in gene delivery. J. Drug Deliv. 2011, 2011, 326497. [Google Scholar] [CrossRef]
- Zhigaltsev, I.V.; Maurer, N.; Wong, K.F.; Cullis, P.R. Triggered release of doxorubicin following mixing of cationic and anionic liposomes. Biochim. Biophys. Acta 2002, 1565, 129–135. [Google Scholar] [CrossRef]
- Bonechi, C.; Donati, A.; Tamasi, G.; Leone, G.; Consumi, M.; Rossi, C.; Lamponi, S.; Magnani, A. Protective effect of quercetin and rutin encapsulated liposomes on induced oxidative stress. Biophys. Chem. 2018, 233, 55–63. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019, 20180032. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Luan, S.; Zhou, J.; Xiao, X.; Yang, Y.; Mao, C.; Fang, P.; Chen, L.; Zeng, X.; et al. The development and progress of nanomedicine for oesophageal cancer diagnosis and treatment. Semin. Cancer Biol. 2022, 86, 873–885. [Google Scholar] [CrossRef]
- Fonseca Bezerra, C.; de Alencar Júnior, J.G.; de Lima Honorato, R.; Dos Santos, A.T.L.; Pereira da Silva, J.C.; Silva, T.G.D.; Leal, A.L.A.B.; de Freitas, T.S.; Vieira, T.A.T.; Esmeraldo Rocha, J.; et al. Antifungal properties of nerolidol-containing liposomes in association with fluconazole. Membranes 2020, 10, 194. [Google Scholar] [CrossRef]
- Pireddu, R.M.; Pibiri, M.; Valenti, D.; Sinico, C.; Fadda, A.M.; Simbula, G.; Lai, F. A novel lactoferrin-modified stealth liposome for hepatoma-delivery of triiodothyronine. Int. J. Pharm. 2018, 537, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Pratsinis, A.; Zuercher, S.; Forster, V.; Fischer, E.J.; Luciani, P.; Leroux, J.C. Liposome-supported enzymatic peritoneal dialysis. Biomaterials 2017, 145, 128–137. [Google Scholar] [CrossRef]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of lipid nanoparticles for RNA delivery. Acc. Chem. Res. 2022, 55, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Taouzinet, L.; Fatmi, S.; Khellouf, A.; Lahiani-Skiba, M.; Skiba, M.; Iguer-Ouada, M. Drug release, stability and efficiency of vitamin E loaded in liposomes for bovine sperm protection in cryopreservation medium. CryoLetters 2022, 43, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, Y.; Ji, X.; He, W.; Fan, J.; Huang, Y.; Wang, Y. NGF rescues spermatogenesis in azoospermic mice. Reprod. Sci. 2021, 28, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- França, L.R.; Hess, R.A.; Dufour, J.M.; Hofmann, M.C.; Griswold, M.D. The Sertoli cell: One hundred fifty years of beauty and plasticity. Andrology 2016, 4, 189–212. [Google Scholar] [CrossRef]
- Yokonishi, T.; McKey, J.; Ide, S.; Capel, B. Sertoli cell ablation and replacement of the spermatogonial niche in mouse. Nat. Commun. 2020, 11, 40. [Google Scholar] [CrossRef]
- Koruji, M.; Movahedin, M.; Mowla, S.J.; Gourabi, H.; Arfaee, A.J. Efficiency of adult mouse spermatogonial stem cell colony formation under several culture conditions. Vitr. Cell Dev. Biol. Anim. 2009, 45, 281–289. [Google Scholar] [CrossRef]
- Mirzapour, T.; Movahedin, M.; Tengku Ibrahim, T.A.; Koruji, M.; Haron, A.W.; Nowroozi, M.R.; Rafieian, S.H. Effects of basic fibroblast growth factor and leukaemia inhibitory factor on proliferation and short-term culture of human spermatogonial stem cells. Andrologia 2012, 44 (Suppl. S1), 41–55. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical characterisation of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Pascarelli, N.A.; Collodel, G.; Moretti, E.; Cheleschi, S.; Fioravanti, A. Changes in ultrastructure and cytoskeletal aspects of human normal and osteoarthritic chondrocytes exposed to interleukin-1β and cyclical hydrostatic pressure. Int. J. Mol. Sci. 2015, 16, 26019–26034. [Google Scholar] [CrossRef] [PubMed]
- Micheli, L.; Collodel, G.; Moretti, E.; Noto, D.; Menchiari, A.; Cerretani, D.; Crispino, S.; Signorini, C. Redox imbalance induced by docetaxel in the neuroblastoma SH-SY5Y cells: A study of docetaxel-induced neuronal damage. Redox Rep. 2021, 26, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Aramaki, Y.; Takano, S.; Tsuchiya, S. Cationic liposomes induce macrophage apoptosis through mitochondrial pathway. Arch. Biochem. Biophys. 2001, 392, 245–250. [Google Scholar] [CrossRef]
- Takano, S.; Aramaki, Y.; Tsuchiya, S. Physicochemical properties of liposomes affecting apoptosis induced by cationic liposomes in macrophages. Pharm. Res. 2003, 20, 962–968. [Google Scholar] [CrossRef]
- Iwaoka, S.; Nakamura, T.; Takano, S.; Tsuchiya, S.; Aramaki, Y.J. Cationic liposomes induce apoptosis through p38 MAP kinase-caspase-8-Bid pathway in macrophage-like RAW264.7 cells. J. Leukoc. Biol. 2006, 79, 184–191. [Google Scholar] [CrossRef]
- Takano, K.; Sato, K.; Negishi, Y.; Aramaki, Y. Involvement of actin cytoskeleton in macrophage apoptosis induced by cationic liposomes. Arch. Biochem. Biophys. 2012, 518, 89–94. [Google Scholar] [CrossRef]
- Meroni, S.B.; Galardo, M.N.; Rindone, G.; Gorga, A.; Riera, M.F.; Cigorraga, S.B. Molecular Mechanisms and Signaling Pathways Involved in Sertoli Cell Proliferation. Front. Endocrinol. 2019, 10, 224. [Google Scholar] [CrossRef]
- Moretti, E.; Mazzi, L.; Bonechi, C.; Salvatici, M.C.; Iacoponi, F.; Rossi, C.; Collodel, G. Effect of Quercetin-loaded liposomes on induced oxidative stress in human spermatozoa. Reprod. Toxicol. 2016, 60, 140–147. [Google Scholar] [CrossRef]
- Luca, G.; Fallarino, F.; Calvitti, M.; Mancuso, F.; Nastruzzi, C.; Arato, I.; Falabella, G.; Grohmann, U.; Becchetti, E.; Puccetti, P.; et al. Xenograft of microencapsulated Sertoli cells reverses T1DM in NOD mice by inducing neogenesis of beta-cells. Transplantation 2010, 90, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, F.; Arato, I.; Di Michele, A.; Antognelli, C.; Angelini, L.; Bellucci, C.; Lilli, C.; Boncompagni, S.; Fusella, A.; Bartolini, D.; et al. Effects of titanium dioxide nanoparticles on porcine prepubertal Sertoli cells: An “in vitro” study. Front. Endocrinol. 2022, 12, 751915. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Application, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Langley, K.H. Developments in electrophoretic laser light scattering and some of biochemical applications. In Laser Light Scattering in Biochemistry, 1st ed.; Harding, S.E., Sattelle, D.B., Bloomfield, V.A., Eds.; The Royal Society of Chemistry: London, UK, 1992; pp. 151–160. [Google Scholar]
- Cerretani, D.; Collodel, G.; Brizzi, A.; Fiaschi, A.I.; Menchiari, A.; Moretti, E.; Moltoni, L.; Micheli, L. Cytotoxic effects of cannabinoids on human HT-29 colorectal adenocarcinoma cells: Different mechanisms of THC, CBD and CB. Int. J. Mol. Sci. 2020, 21, 5533. [Google Scholar] [CrossRef] [PubMed]

| Liposome Composition | Mean Diameter (nm) ± SD | P.D.I. | ζ-Potential (mV) ± SD |
|---|---|---|---|
| DOPC/DOPE (1:0.5) | 125.9 ± 15.4 | 0.12 | −18.27 ± 3.72 |
| DOPC/DOPE + rhodamine | 159.8 ± 10.4 | 0.18 | −17.93 ± 2.41 |
| DOPC/DOTAP (1:0.5) | 139.0 ±16.2 | 0.08 | 60.12 ± 3.67 |
| DOPC/DOTAP + rhodamine | 165.2 ± 9.4 | 0.16 | 52.34 ± 4.65 |
| DOPE/DOPA (1:0.5) | 149.1 ± 11.3 | 0.12 | −37.28 ± 2.67 |
| DOPE/DOPA + rhodamine | 164.8 ± 13.7 | 0.15 | −35.94 ± 2.31 |
| Liposome Composition | Encapsulation Efficiency (EE%) |
|---|---|
| DOPC/DOPE + rhodamine | 25.3 ± 6.1 |
| DOPC/DOTAP + rhodamine | 29.9 ± 9.5 |
| DOPE/DOPA + rhodamine | 23.9 ± 8.7 |
| Samples | Vol. | Total Cells (n° × 10⁶) | Live (n° × 10⁶) | Dead (n° × 105) | Vitality % |
|---|---|---|---|---|---|
| SCs | 2 mL | 3.17 ± 0.64 | 2.97 ± 0.55 | 1.93 ± 0.85 | 94.00 ± 0.02 |
| SCs + Zwitterionic liposomes | 2 mL | 3.17 ± 0.47 | 3.00 ± 0.36 | 1.83 ± 0.83 | 94.00 ± 2.01 |
| SCs + Anionic liposomes | 2 mL | 2.26 ± 0.60 | 2.10 ± 0.53 | 1.76 ± 0.710 | 93.00 ± 3.20 |
| SCs + Cationic liposomes | 2 mL | 2.70 ± 0.10 | 2.46 ± 0.06 | 1.83 ± 0.40 | 93.00 ± 1.00 |
| SCs | SCs + Zwitterionic Liposomes | SCs + Anionic Liposomes | SCs + Cationic Liposomes | |
|---|---|---|---|---|
| AMH | 0.096 ± 0.056 | 0.77 ± 0.042 | 0.62 ± 0.08 | 0.66 ± 0.07 |
| Inhibin B | 21.41 ± 9.60 | 21.20 ± 7.54 | 23.8 ± 1.55 | 24.02 ± 3.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collodel, G.; Moretti, E.; Noto, D.; Corsaro, R.; Signorini, C.; Bonechi, C.; Cangeloni, L.; Luca, G.; Arato, I.; Mancuso, F. Effects and Mechanisms Activated by Treatment with Cationic, Anionic and Zwitterionic Liposomes on an In Vitro Model of Porcine Pre-Pubertal Sertoli Cells. Int. J. Mol. Sci. 2023, 24, 1201. https://doi.org/10.3390/ijms24021201
Collodel G, Moretti E, Noto D, Corsaro R, Signorini C, Bonechi C, Cangeloni L, Luca G, Arato I, Mancuso F. Effects and Mechanisms Activated by Treatment with Cationic, Anionic and Zwitterionic Liposomes on an In Vitro Model of Porcine Pre-Pubertal Sertoli Cells. International Journal of Molecular Sciences. 2023; 24(2):1201. https://doi.org/10.3390/ijms24021201
Chicago/Turabian StyleCollodel, Giulia, Elena Moretti, Daria Noto, Roberta Corsaro, Cinzia Signorini, Claudia Bonechi, Lorenzo Cangeloni, Giovanni Luca, Iva Arato, and Francesca Mancuso. 2023. "Effects and Mechanisms Activated by Treatment with Cationic, Anionic and Zwitterionic Liposomes on an In Vitro Model of Porcine Pre-Pubertal Sertoli Cells" International Journal of Molecular Sciences 24, no. 2: 1201. https://doi.org/10.3390/ijms24021201
APA StyleCollodel, G., Moretti, E., Noto, D., Corsaro, R., Signorini, C., Bonechi, C., Cangeloni, L., Luca, G., Arato, I., & Mancuso, F. (2023). Effects and Mechanisms Activated by Treatment with Cationic, Anionic and Zwitterionic Liposomes on an In Vitro Model of Porcine Pre-Pubertal Sertoli Cells. International Journal of Molecular Sciences, 24(2), 1201. https://doi.org/10.3390/ijms24021201









