Characteristic Evaluation of Recombinant MiSp/Poly(lactic-co-glycolic) Acid (PLGA) Nanofiber Scaffolds as Potential Scaffolds for Bone Tissue Engineering
Abstract
:1. Introduction
2. Results and Discussion
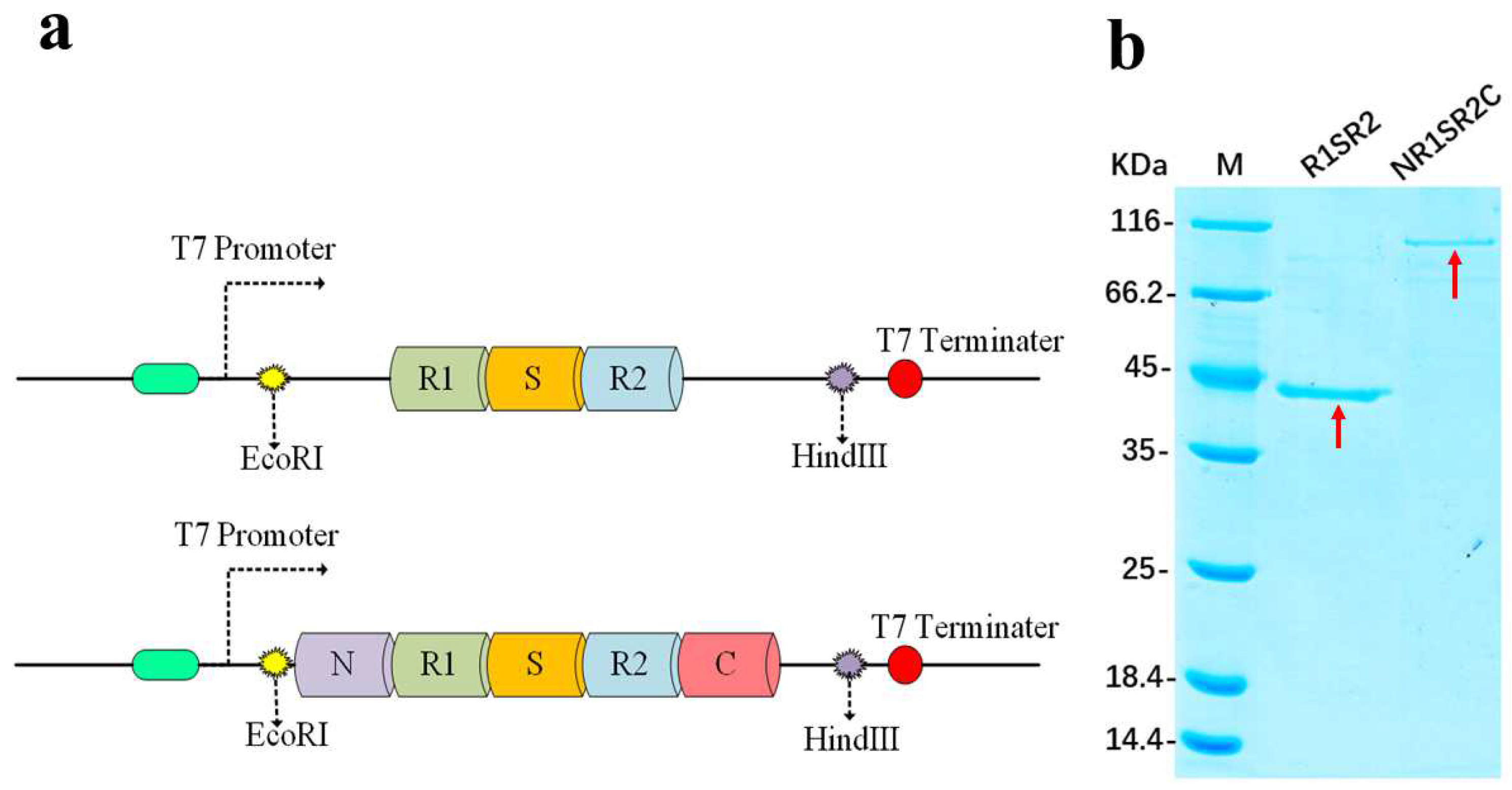
2.1. Structures of Proteins
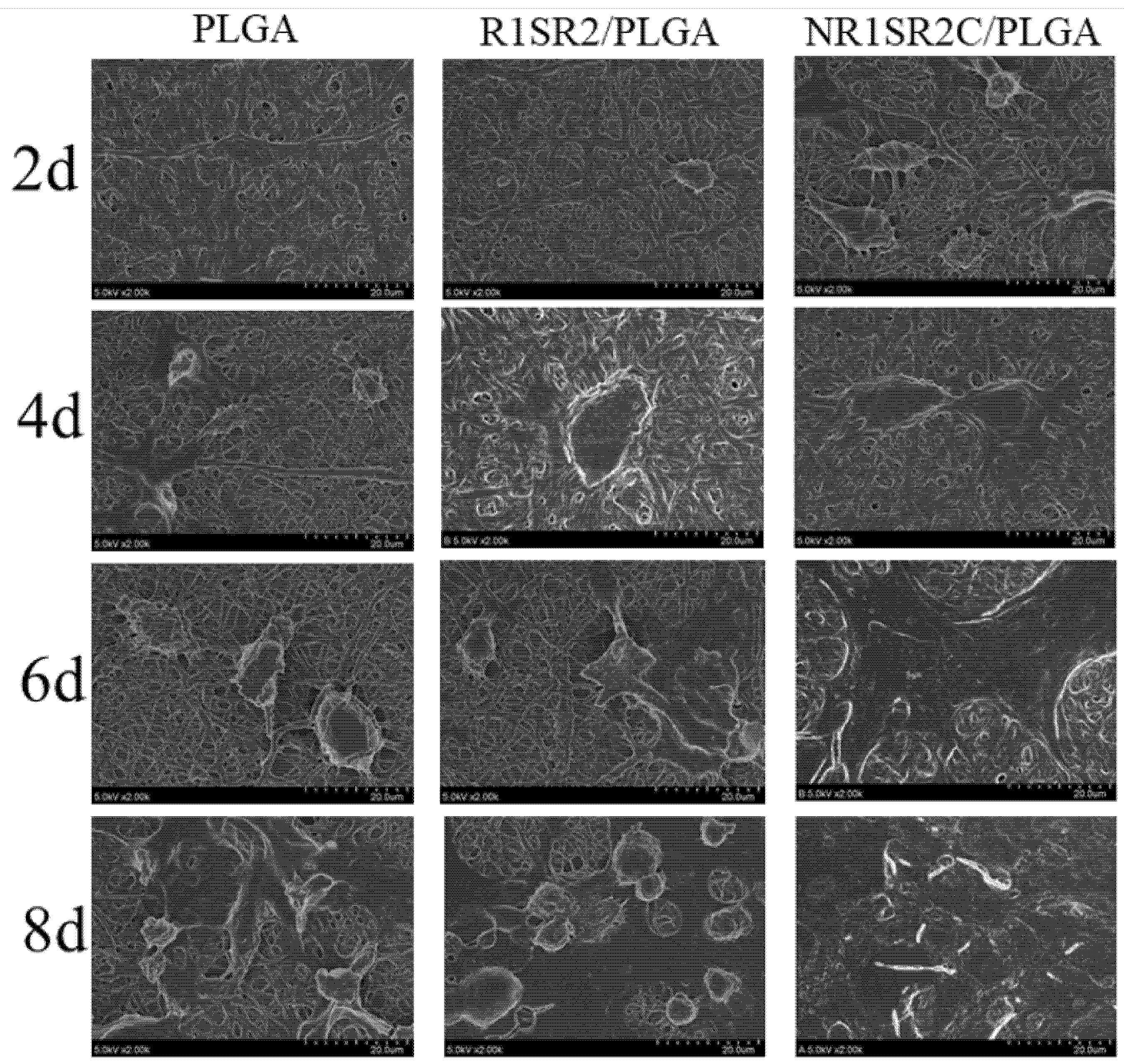
2.2. Morphology of Nanofibrous Scaffolds
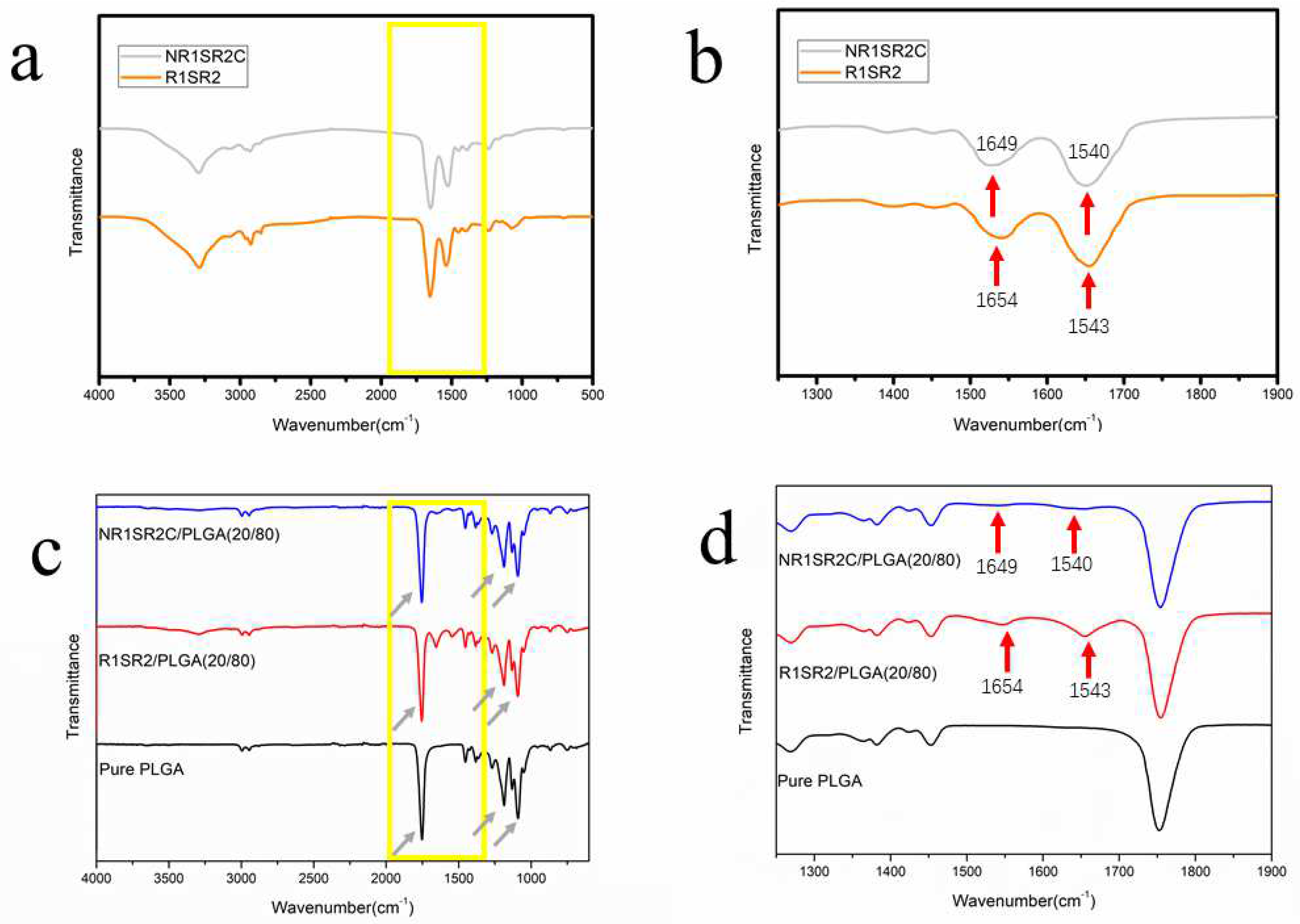
2.3. Structures
2.4. Mechanical Properties
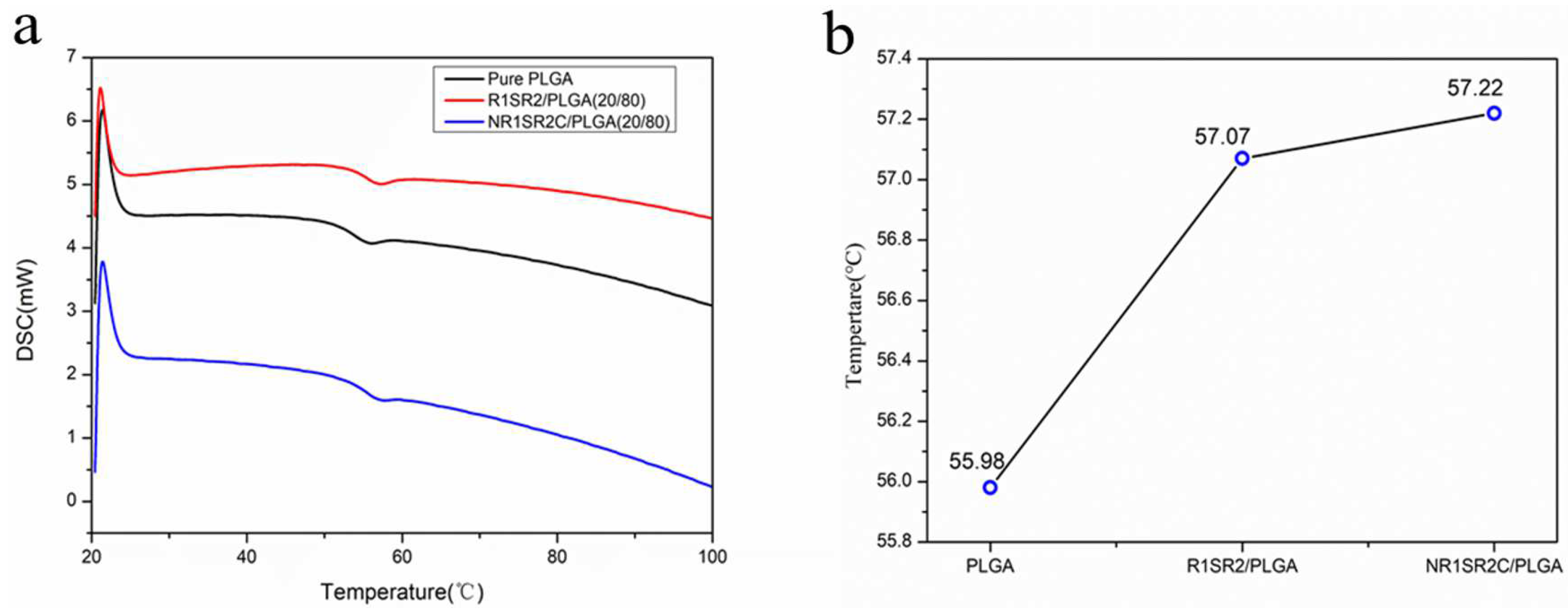
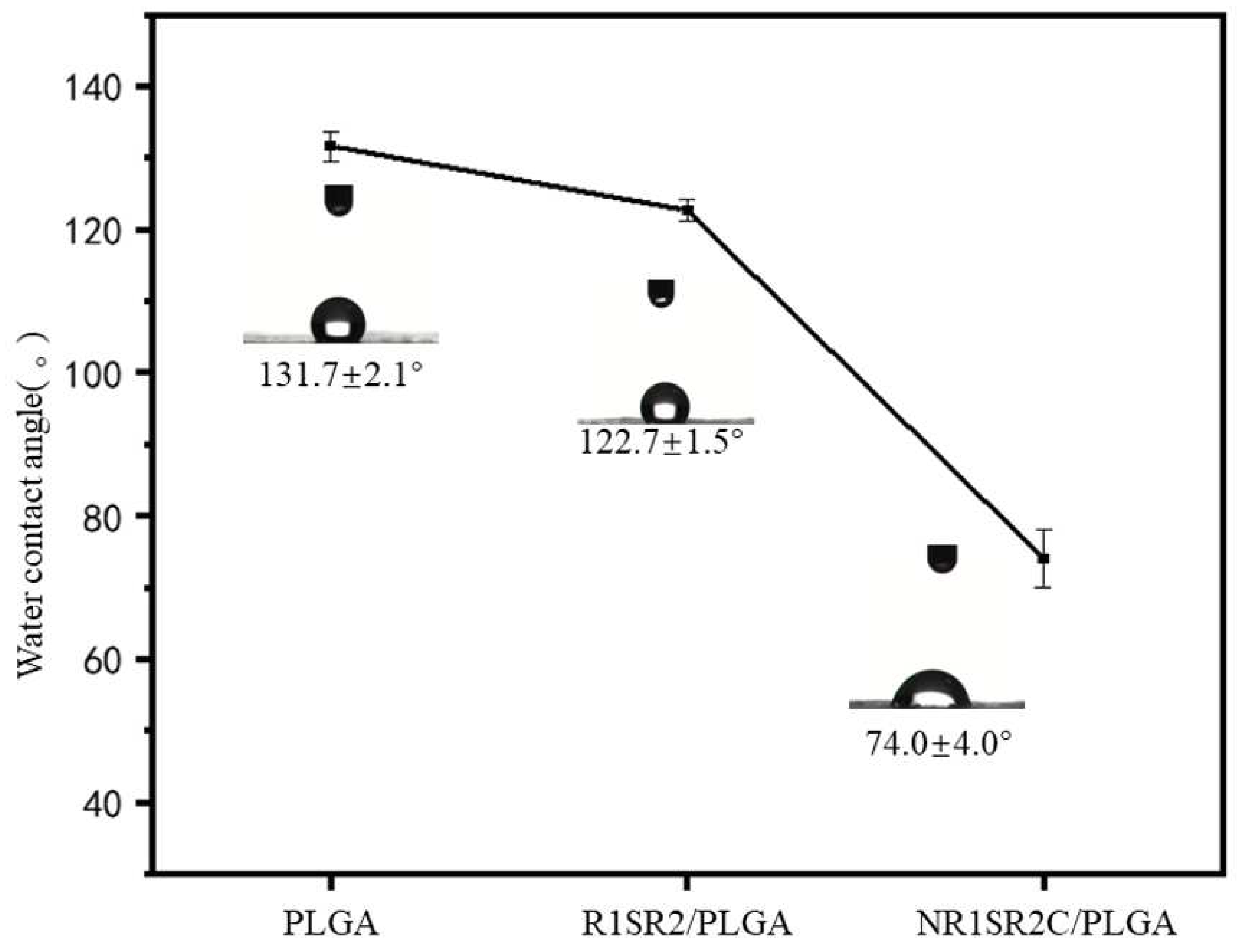
2.5. Hydrophilicity of Nanofibrous Scaffolds
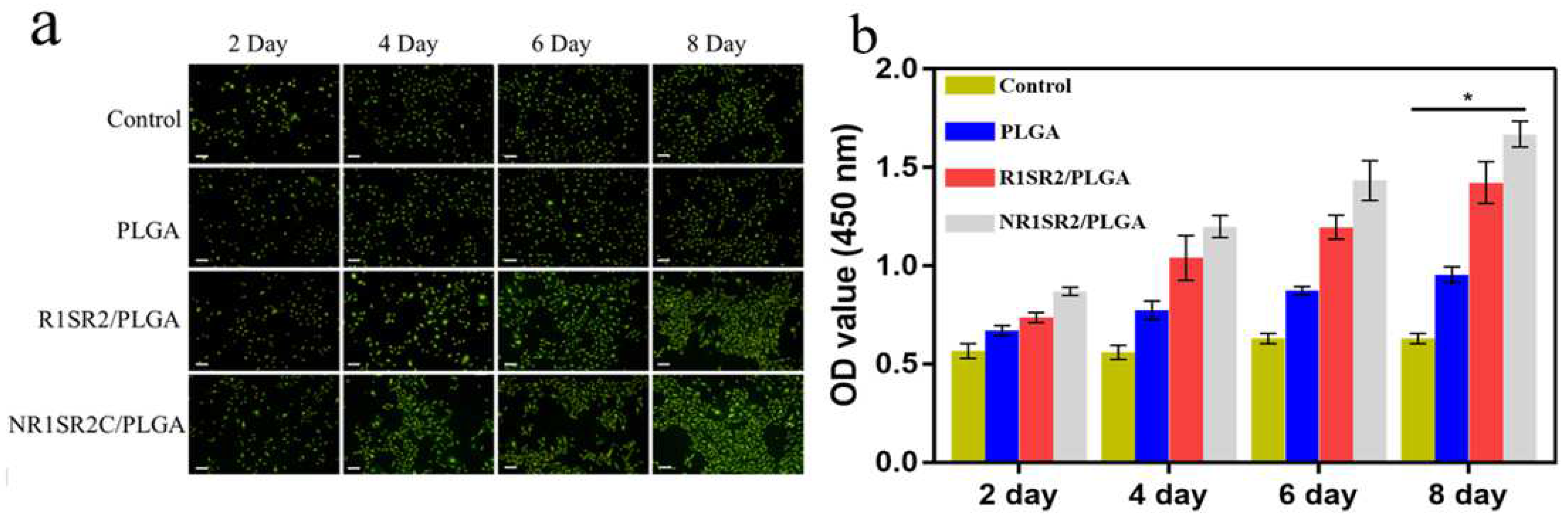
2.6. Biocompatibility of Nanofibrous Scaffolds
3. Materials and Methods
3.1. Materials
3.2. Plasmid Construction
3.3. Protein Production
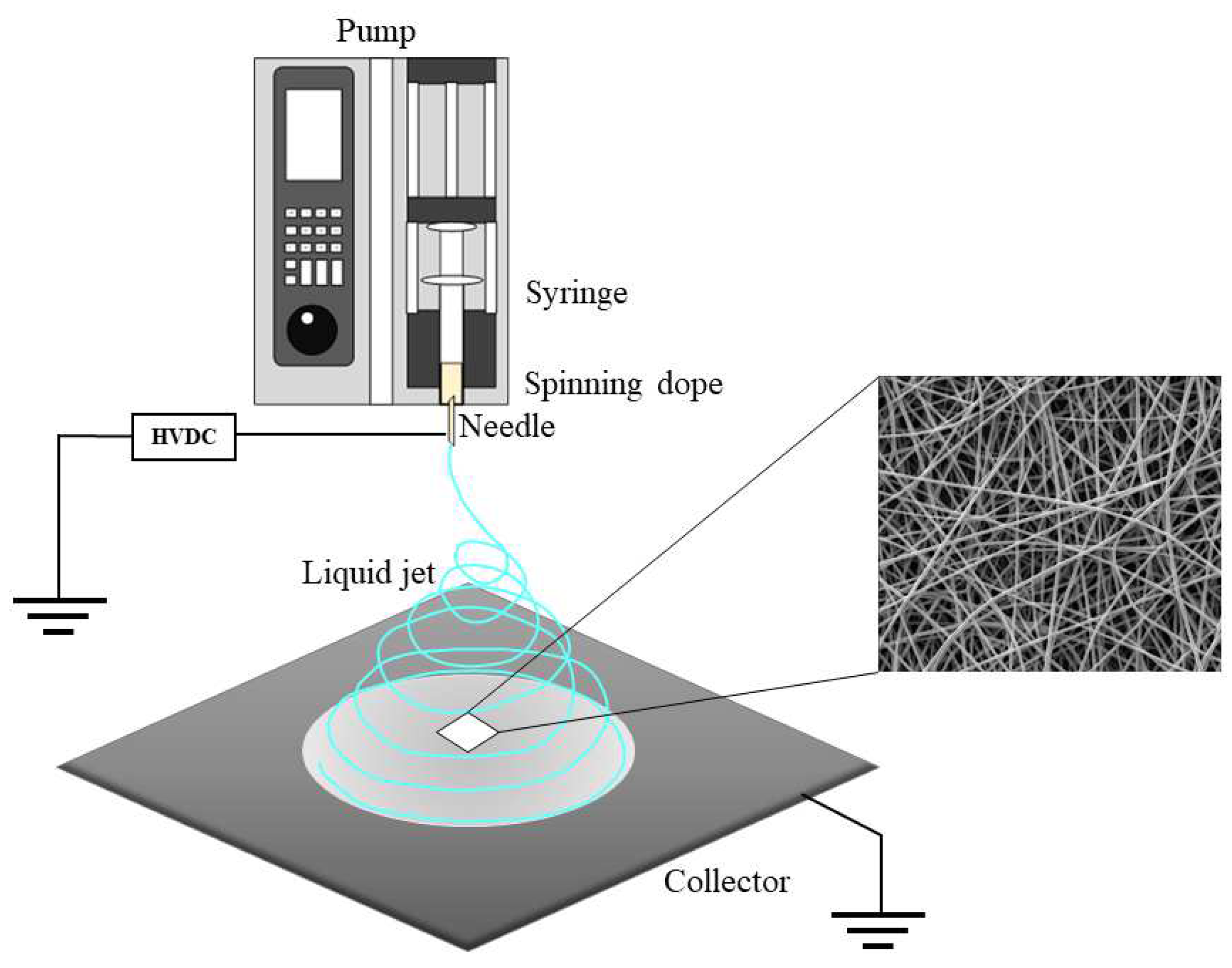
3.4. Preparation of Synthetic Spider-Silk Fibers
3.5. Scanning Electron Microscope (SEM) of Nanofibrous Scaffolds
3.6. Fourier Transform Infrared (FTIR) Spectroscopy
3.7. Differential Scanning Calorimetry (DSC)
3.8. Water Contact Angle Measurements
3.9. Mechanical Testing
3.10. Cell Culture on Nanofibrous Scaffolds
3.11. Cell Proliferation and Attachment Assays
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MiSp | Minor ampullate spidroins |
| PLGA | Poly(lactic-co-glycolic) Acid |
| SEM | Scanning electron microscopy |
| FTIR | Fourier transform infrared spectroscopy |
| DSC | Differential scanning calorimetry |
| HBMSCs | Human bone mesenchymal stem cells |
References
- Habibovic, P. Strategic Directions in Osteoinduction and Biomimetics. Tissue Eng. Part A 2017, 23, 1295–1296. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A. 13—3D bioprinting bone. In 3D Bioprinting for Reconstructive Surgery; Thomas, D., Jessop, Z., Whitaker, I., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 245–275. [Google Scholar]
- Andersson, M.; Chen, G.; Otikovs, M.; Landreh, M.; Nordling, K.; Kronqvist, N.; Westermark, P.; Jörnvall, H.; Knight, S.; Ridderstråle, Y.; et al. Carbonic anhydrase generates CO2 and H+ that drive spider silk formation via opposite effects on the terminal domains. PLoS Biol. 2014, 12, e1001921. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Anjum, S.; Rahman, F.; Pandey, P.; Arya, D.K.; Alam, M.; Rajinikanth, P.S.; Ao, Q. Electrospun Biomimetic Nanofibrous Scaffolds: A Promising Prospect for Bone Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 9206. [Google Scholar] [CrossRef] [PubMed]
- Udomluck, N.; Koh, W.-G.; Lim, D.-J.; Park, H. Recent Developments in Nanofiber Fabrication and Modification for Bone Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 99. [Google Scholar] [CrossRef] [Green Version]
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.; Nukavarapu, S. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [Green Version]
- Ferrone, M.L.; Raut, C. Modern surgical therapy: Limb salvage and the role of amputation for extremity soft-tissue sarcomas. Surg. Oncol. Clin. N. Am. 2012, 21, 201–213. [Google Scholar] [CrossRef]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. In The Biomaterials: Silver Jubilee Compendium; Williams, D.F., Ed.; Elsevier Science: Oxford, UK, 2000; pp. 175–189. [Google Scholar]
- Anwar, A.; Petrino, D.J., Jr.; Alstine, N.V.; Yu, X. Biodegradable Electrospun Nanofibrous Scaffolds for Bone Tissue Engineering. Methods Mol. Biol. 2022, 2394, 693–711. [Google Scholar] [PubMed]
- Wang, K.; Albert, K.; Mosser, G.; Haye, B.; Percot, A.; Paris, C.; Peccate, C.; Trichet, L.; Coradin, T. Self-assembly/condensation interplay in nano-to-microfibrillar silicified fibrin hydrogels. Int. J. Biol. Macromol. 2020, 164, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zeng, Y. Measurement and Comparison of Melt-Blowing Airflow Fields: Nozzle Modifications to Reduce Turbulence and Fibre Whipping. Polymers 2021, 13, 719. [Google Scholar] [CrossRef]
- Lin, W.; Chen, M.; Qu, T.; Li, J.; Man, Y. Three-dimensional electrospun nanofibrous scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ma, P. Poly(alpha-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J. Biomed. Mater. Res. 1999, 44, 446–455. [Google Scholar] [CrossRef]
- Koss, K.M.; Unsworth, L. Neural tissue engineering: Bioresponsive nanoscaffolds using engineered self-assembling peptides. Acta Biomater. 2016, 44, 2–15. [Google Scholar] [CrossRef]
- Lin, S.-J.; Xue, Y.-P.; Chang, G.; Han, Q.-L.; Chen, L.-F.; Jia, Y.-B.; Zheng, Y.-G. Layer-by-layer 3-dimensional nanofiber tissue scaffold with controlled gap by electrospinning. Mater. Res. Express 2018, 5, 025401. [Google Scholar] [CrossRef]
- Liu, W.; Ni, C.; Chase, D.B.; Rabolt, J.F. Preparation of Multilayer Biodegradable Nanofibers by Triaxial Electrospinning. ACS Macro Lett. 2013, 2, 466–468. [Google Scholar] [CrossRef]
- Siddiqui, N.; Kishori, B.; Rao, S.; Anjum, M.; Hemanth, V.; Das, S.; Jabbari, E. Electropsun Polycaprolactone FibRes. in Bone Tissue Engineering: A Review. Mol. Biotechnol. 2021, 63, 363–388. [Google Scholar] [CrossRef]
- Sun, B.; Long, Y.Z.; Zhang, H.D.; Li, M.M.; Duvail, J.L.; Jiang, X.Y.; Yin, H.L. Advances in three-dimensional nanofibrous macrostructuRes. via electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Gong, T.; Liu, T.; Zhang, L.; Ye, W.; Guo, X.; Wang, L.; Quan, L.; Pan, C. Design Redox-Sensitive Drug-Loaded Nanofibers for Bone Reconstruction. ACS Biomater. Sci. Eng. 2018, 4, 240–247. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Yu, D.-G.; Wang, G.; Williams, G.R.; Zhang, Z. Tunable drug release from nanofibers coated with blank cellulose acetate layers fabricated using tri-axial electrospinning. Carbohydr. Polym. 2019, 203, 228–237. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, L.; Zhao, K.; Wu, Z.; Wang, Y.; Tan, Q. Fabrication of PLGA/HA (core)-collagen/amoxicillin (shell) nanofiber membranes through coaxial electrospinning for guided tissue regeneration. Compos. Sci. Technol. 2016, 125, 100–107. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H.W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017, 63, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sadat Tabatabaei Mirakabad, F.; Nejati-Koshki, K.; Akbarzadeh, A.; Yamchi, M.R.; Milani, M.; Zarghami, N.; Zeighamian, V.; Rahimzadeh, A.; Alimohammadi, S.; Hanifehpour, Y.; et al. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac. J. Cancer Prev. 2014, 15, 517–535. [Google Scholar] [CrossRef] [Green Version]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Mano, J.F.; Sousa, R.A.; Boesel, L.F.; Neves, N.M.; Reis, R.L. Bioinert, biodegradable and injectable polymeric matrix composites for hard tissue replacement: State of the art and recent developments. Compos. Sci. Technol. 2004, 64, 789–817. [Google Scholar] [CrossRef] [Green Version]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Ding, J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus 2012, 2, 366–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikh, F.A.; Ju, H.W.; Moon, B.M.; Lee, O.J.; Kim, J.H.; Park, H.J.; Kim, D.W.; Kim, D.K.; Jang, J.E.; Khang, G.; et al. Hybrid scaffolds based on PLGA and silk for bone tissue engineering. J. Tissue Eng. Regen. Med. 2016, 10, 209–221. [Google Scholar] [CrossRef]
- Doğan, A.; Demirci, S.; Bayir, Y.; Halici, Z.; Karakus, E.; Aydin, A.; Cadirci, E.; Albayrak, A.; Demirci, E.; Karaman, A.; et al. Boron containing poly-(lactide-co-glycolide) (PLGA) scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 246–253. [Google Scholar] [CrossRef]
- Khojasteh, A.; Fahimipour, F.; Eslaminejad, M.B.; Jafarian, M.; Jahangir, S.; Bastami, F.; Tahriri, M.; Karkhaneh, A.; Tayebi, L. Development of PLGA-coated β-TCP scaffolds containing VEGF for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 780–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Drug and Gene Delivery. Pharmaceutics 2019, 11, 494. [Google Scholar] [CrossRef] [Green Version]
- Salehi, S.; Koeck, K.; Scheibel, T. Spider Silk for Tissue Engineering Applications. Molecules 2020, 25, 737. [Google Scholar] [CrossRef] [Green Version]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Humenik, M.; Pawar, K.; Scheibel, T. Nanostructured, Self-Assembled Spider Silk Materials for Biomedical Applications. In Biological and Bio-inspired Nanomaterials; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1174, pp. 187–221. [Google Scholar]
- Spiess, K.; Lammel, A.; Scheibel, T. Recombinant spider silk proteins for applications in biomaterials. Macromol. Biosci. 2010, 10, 998–1007. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Nateghi, S.S.; Gazani, M.M.; Dehghani, Z.; Mohammadzadeh, F. A review on advances in the applications of spider silk in biomedical issues. Int. J. Biol. Macromol. 2021, 192, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef]
- Vienneau-Hathaway, J.M.; Brassfield, E.R.; Lane, A.K.; Collin, M.A.; Correa-Garhwal, S.M.; Clarke, T.H.; Schwager, E.E.; Garb, J.E.; Hayashi, C.Y.; Ayoub, N.A. Duplication and concerted evolution of MiSp-encoding genes underlie the material properties of minor ampullate silks of cobweb weaving spiders. BMC Evol. Biol. 2017, 17, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef] [PubMed]
- Müller-Herrmann, S.; Scheibel, T. Enzymatic Degradation of Films, Particles, and Nonwoven Meshes Made of a Recombinant Spider Silk Protein. ACS Biomater. Sci. Eng. 2015, 1, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shen, Q.; Lin, Y.; Xu, S.; Meng, Q. Evaluation of the potential of chimeric spidroins/poly(L-lactic-co-ε-caprolactone) (PLCL) nanofibrous scaffolds for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110752. [Google Scholar] [CrossRef] [PubMed]
- Otikovs, M.; Chen, G.; Nordling, K.; Landreh, M.; Meng, Q.; Jörnvall, H.; Kronqvist, N.; Rising, A.; Johansson, J.; Jaudzems, K. Diversified Structural Basis of a Conserved Molecular Mechanism for pH-Dependent Dimerization in Spider Silk N-Terminal Domains. Chembiochem 2015, 16, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, X.; Zhang, Y.; Lin, S.; Yang, Z.; Johansson, J.; Rising, A.; Meng, Q. Full-length minor ampullate spidroin gene sequence. PLoS ONE 2012, 7, e52293. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Huang, W.; Yang, D. Structure and function of C-terminal domain of aciniform spidroin. Biomacromolecules 2014, 15, 468–477. [Google Scholar] [CrossRef]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining information about protein secondary structuRes. in aqueous solution using Fourier transform IR spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef]
- He, S.; Shi, J.; Walid, E.; Zhang, H.; Ma, Y.; Xue, S.J. Reverse micellar extraction of lectin from black turtle bean (Phaseolus vulgaris): Optimisation of extraction conditions by response surface methodology. Food Chem. 2015, 166, 93–100. [Google Scholar] [CrossRef]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; An, E.y.; Lee, J.B.; Park, J.C.; Shin, J.W.; Kim, J.K. Enhanced cell affinity of poly(d,l-lactic-co-glycolic acid) (50/50) by plasma treatment with β-(1→3) (1→6)-glucan. Surf. Coat. Technol. 2007, 201, 5128–5131. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, H.; Shen, Y.; Tang, H.; Yi, B.; Qin, C.; Zhang, Y. Shape Memory and Osteogenesis Capabilities of the Electrospun Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Modified Poly(l-Lactide) Fibrous Mats. Tissue Eng. Part A 2021, 27, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, H.; Huang, C.; Su, Y.; Mo, X.; Ikada, Y. Fabrication of silk fibroin blended P(LLA-CL) nanofibrous scaffolds for tissue engineering. J. Biomed. Mater. Res. A 2010, 93, 984–993. [Google Scholar] [CrossRef] [PubMed]
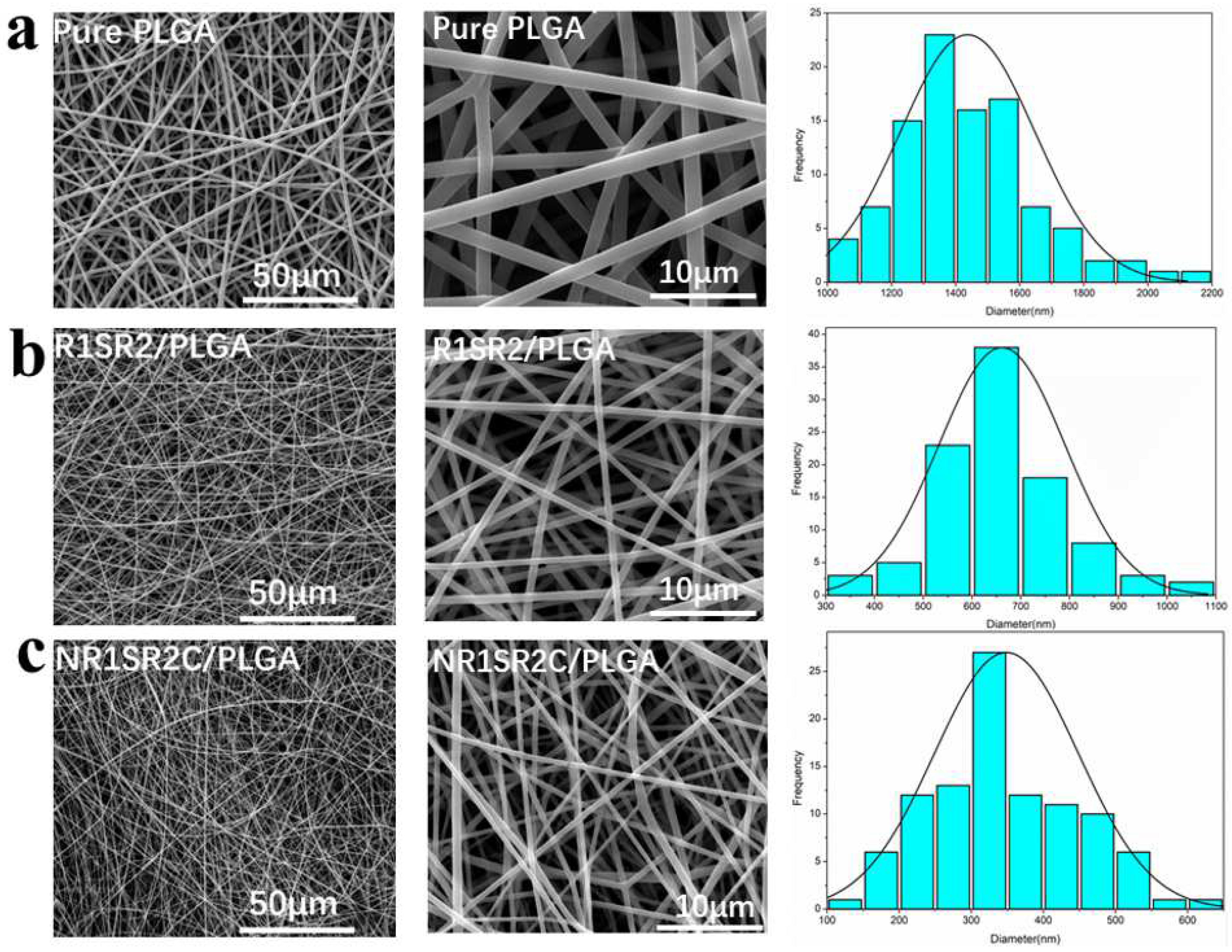

| Scaffold Type | Diameter (nm) | Porosity (%) |
|---|---|---|
| PLGA | 1437 ± 211 nm | 56.7 ± 7.9% |
| R1SR2/PLGA (20/80) | 661 ± 130 nm | 75.5 ± 3.7% |
| NR1SR2C/PLGA (20/80) | 349 ± 100 nm | 85.9 ± 6.1% |
| Scaffold Type | Stress (MPa) | Strain (%) | Tensile Modulus (MPa) |
|---|---|---|---|
| PLGA | 5.4 ± 0.5 | 142.3 ± 10.5 | 123.1 ± 17.6 |
| R1SR2/PLGA (20/80) | 5.8 ± 0.8 | 170.9 ± 8.4 | 127.6 ± 21.7 |
| NR1SR2C/PLGA (20/80) | 5.8 ± 1.3 | 181.6 ± 18.7 | 150.1 ± 28.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Jia, X.; Meng, Q. Characteristic Evaluation of Recombinant MiSp/Poly(lactic-co-glycolic) Acid (PLGA) Nanofiber Scaffolds as Potential Scaffolds for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 1219. https://doi.org/10.3390/ijms24021219
Sun Y, Jia X, Meng Q. Characteristic Evaluation of Recombinant MiSp/Poly(lactic-co-glycolic) Acid (PLGA) Nanofiber Scaffolds as Potential Scaffolds for Bone Tissue Engineering. International Journal of Molecular Sciences. 2023; 24(2):1219. https://doi.org/10.3390/ijms24021219
Chicago/Turabian StyleSun, Yuan, Xiaona Jia, and Qing Meng. 2023. "Characteristic Evaluation of Recombinant MiSp/Poly(lactic-co-glycolic) Acid (PLGA) Nanofiber Scaffolds as Potential Scaffolds for Bone Tissue Engineering" International Journal of Molecular Sciences 24, no. 2: 1219. https://doi.org/10.3390/ijms24021219





