Both LTA and LTB Subunits Are Equally Important to Heat-Labile Enterotoxin (LT)-Enhanced Bacterial Adherence
Abstract
:1. Introduction
2. Results
2.1. Characterization of LT and LT Derivatives
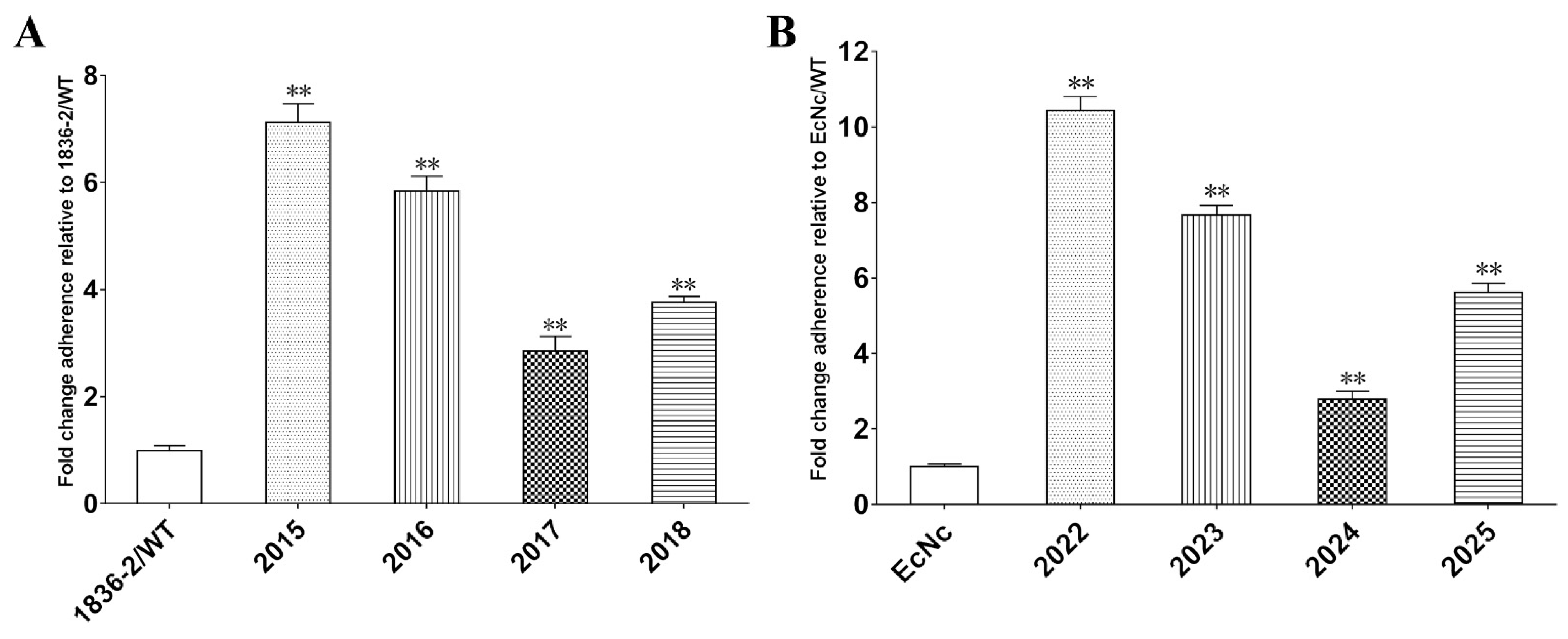
2.2. LT Promotes E. coli Strains Adhesion to Intestinal Epithelial Cells
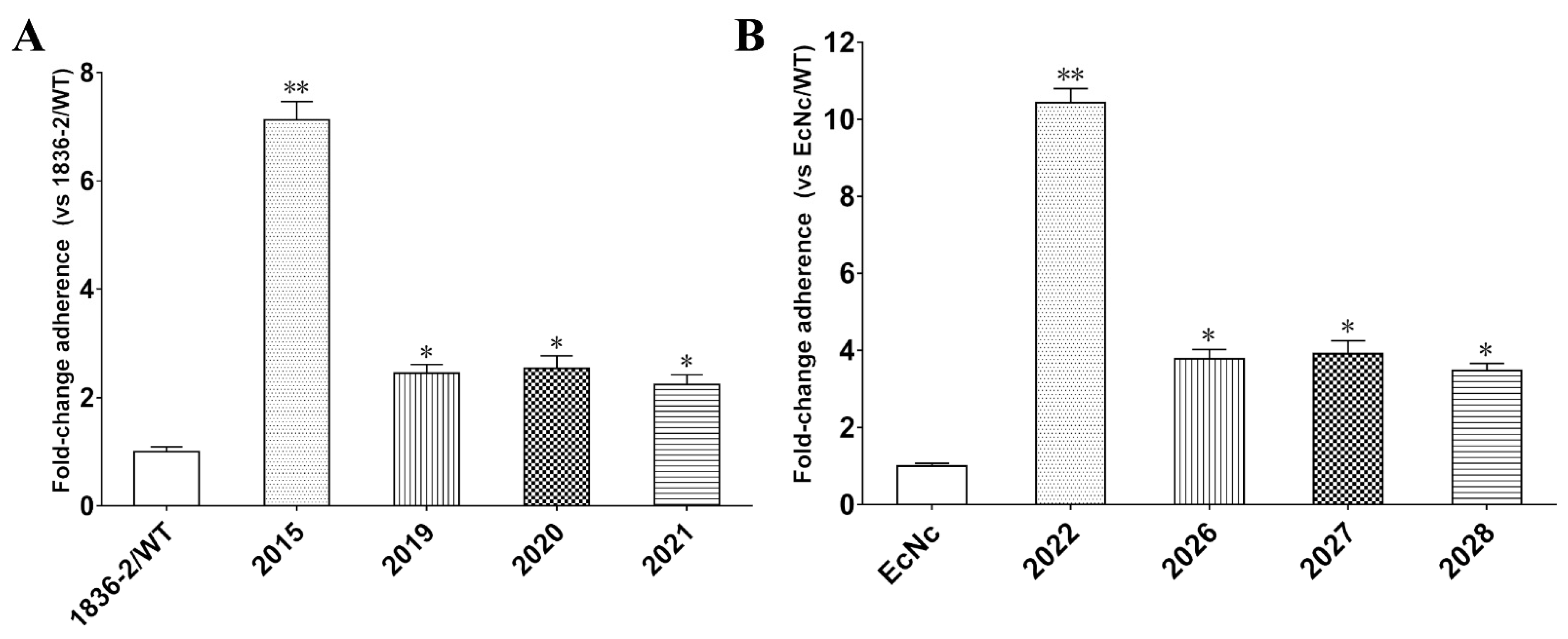
2.3. The ADP-Ribosylation Activity of LTA Subunit Is Required for LT-Enhanced Bacterial Adherence
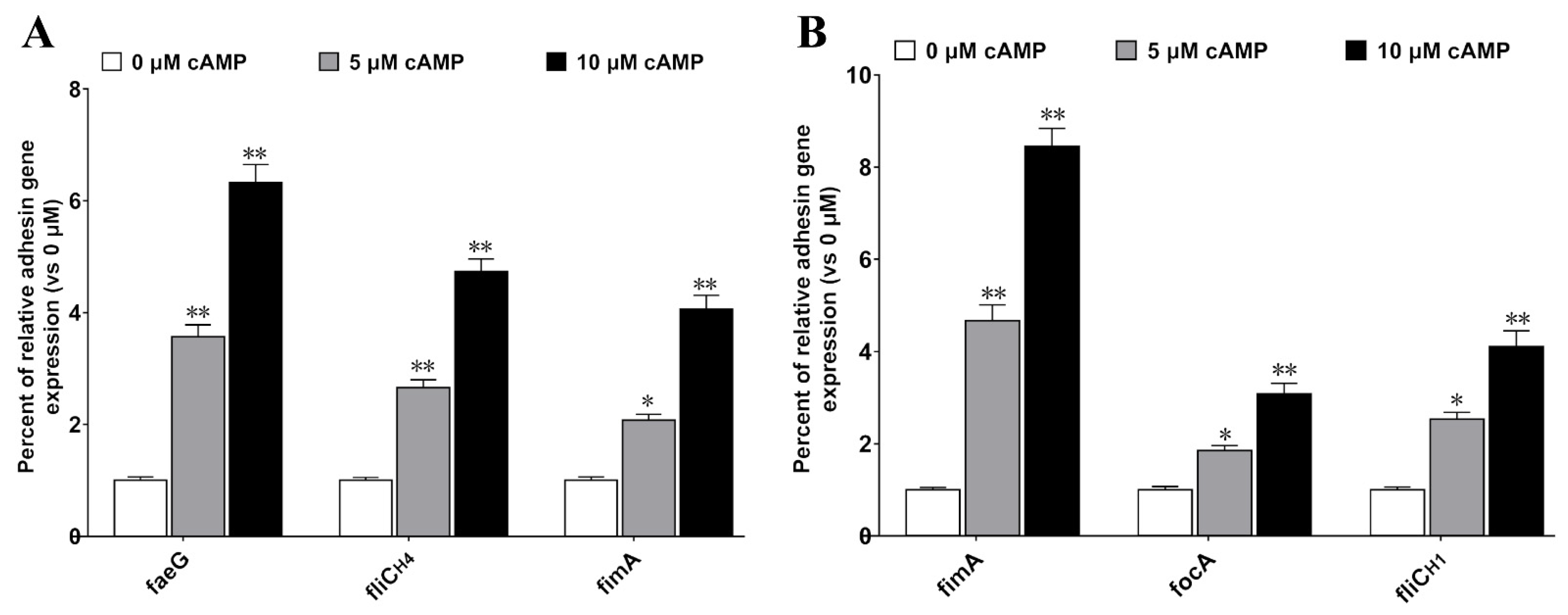
2.4. Exogenously Supplied cAMP Improves Bacterial Adherence
2.5. cAMP Increases the Expression of Bacterial Adhesion Molecules
2.6. Both GM1 and LPS Binding Abilities Are Required for LTB-Enhanced Bacterial Adherence
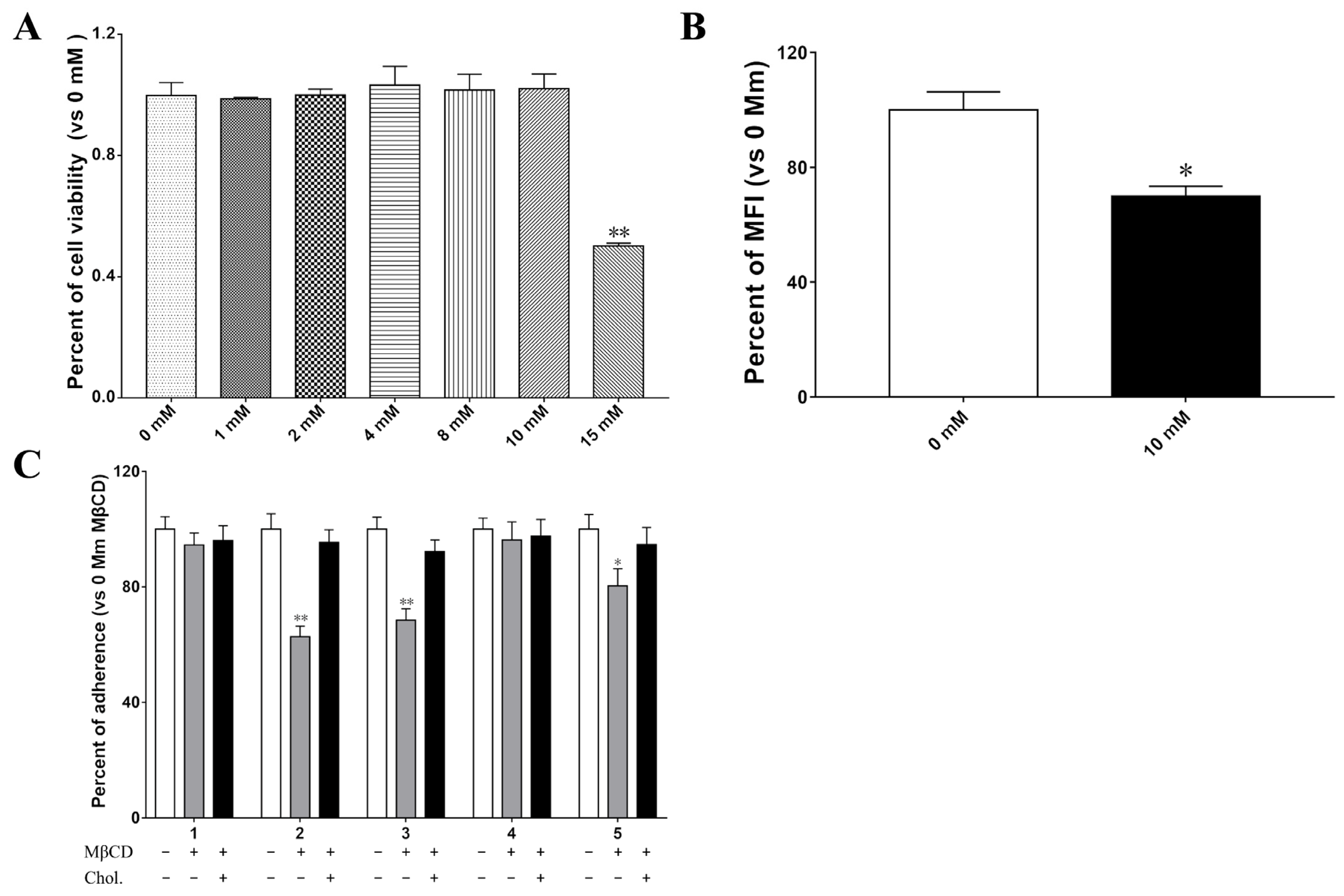
2.7. Membrane Cholesrerol Is Important for LTB–Mediated Bacterial Increased Adherence
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Plasmids, and Cell Line
4.2. Construction of LT and LT Derivatives
4.3. Quantitative Measurement of cAMP Concentration
4.4. GM1-ELISA Assay
4.5. Bacterial Growth Curve Determination
4.6. Western Blotting Analysis
4.7. Generation of eltAB Isogenic Mutants
4.8. Bacterial Adherence Assay
4.9. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
4.10. MβCD Cytotoxicity
4.11. Flow Cytometry Analysis of Efficiency of Cholesterol Removal
4.12. Effect of Lipid Rafts on LTB-Enhanced Bacterial Adherence
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collaborators GBDDD. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 909–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosangadi, D.; Smith, P.G.; Kaslow, D.C.; Giersing, B.K. WHO consultation on ETEC and Shigella burden of disease, Geneva, 6–7th April 2017: Meeting report. Vaccine 2019, 37, 7381–7390. [Google Scholar] [CrossRef]
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal Enterotoxigenic Escherichia coli. EcoSal Plus 2016, 7, 1–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, B.; Fekete, P.Z. Enterotoxigenic Escherichia coli in veterinary medicine. Int. J. Med. Microbiol. 2005, 295, 443–454. [Google Scholar] [CrossRef]
- Mirhosseini, A.; Amani, J.; Nazarian, S. Review on pathogenicity mechanism of enterotoxigenic Escherichia coli and vaccines against it. Microb. Pathog. 2018, 117, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Croxen, M.; Finlay, B.B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Genet. 2009, 8, 26–38. [Google Scholar] [CrossRef]
- Odumosu, O.; Nicholas, D.; Yano, H.; Langridge, W. AB Toxins: A Paradigm Switch from Deadly to Desirable. Toxins 2010, 2, 1612–1645. [Google Scholar] [CrossRef] [Green Version]
- Hardy, S.J.; Holmgren, J.; Johansson, S.; Sanchez, J.; Hirst, T.R. Coordinated assembly of multisubunit proteins: Oligomerization of bacterial enterotoxins in vivo and in vitro. Proc. Natl. Acad. Sci. USA 1988, 85, 7109–7113. [Google Scholar] [CrossRef] [Green Version]
- Mudrak, B.; Kuehn, M.J. Heat-Labile Enterotoxin: Beyond G M1 Binding. Toxins 2010, 2, 1445–1470. [Google Scholar] [CrossRef] [Green Version]
- Merritt, E.A.; Sixma, T.K.; Kalk, K.H.; Zanten, B.A.M.; Hol, W.G.J. Galactose-binding site in Escherichia coli heat-labile enterotoxin (LT) and cholera toxin (CT). Mol. Microbiol. 1994, 13, 745–753. [Google Scholar] [CrossRef]
- Horstman, A.L.; Kuehn, M.J. Bacterial Surface Association of Heat-labile Enterotoxin through Lipopolysaccharide after Secretion via the General Secretory Pathway. J. Biol. Chem. 2002, 277, 32538–32545. [Google Scholar] [CrossRef] [Green Version]
- Holmner; Askarieh, G.; Ökvist, M.; Krengel, U. Blood Group Antigen Recognition by Escherichia coli Heat-labile Enterotoxin. J. Mol. Biol. 2007, 371, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Sixma, T.K.; Pronk, S.E.; Kalk, K.H.; Wartna, E.S.; Van Zanten, B.A.M.; Witholt, B.; Hoi, W.G.J. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature 1991, 351, 371–377. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, P.; Zhao, Y.; Ma, X. Enterotoxigenic Escherichia coli: Intestinal pathogenesis mechanisms and colonization resistance by gut microbiota. Gut Microbes 2022, 14, 2055943. [Google Scholar] [CrossRef] [PubMed]
- Mudrak, B.; Rodriguez, D.L.; Kuehn, M.J. Residues of Heat-Labile Enterotoxin Involved in Bacterial Cell Surface Binding. J. Bacteriol. 2009, 191, 2917–2925. [Google Scholar] [CrossRef] [Green Version]
- Joffré, E.; von Mentzer, A.; El Ghany, M.A.; Oezguen, N.; Savidge, T.; Dougan, G.; Svennerholm, A.-M.; Sjöling. Allele Variants of Enterotoxigenic Escherichia coli Heat-Labile Toxin Are Globally Transmitted and Associated with Colonization Factors. J. Bacteriol. 2014, 197, 392–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berberov, E.M.; Zhou, Y.; Francis, D.H.; Scott, M.A.; Kachman, S.D.; Moxley, R.A. Relative Importance of Heat-Labile Enterotoxin in the Causation of Severe Diarrheal Disease in the Gnotobiotic Piglet Model by a Strain of Enterotoxigenic Escherichia coli That Produces Multiple Enterotoxins. Infect. Immun. 2004, 72, 3914–3924. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Berberov, E.M.; Freeling, J.; He, D.; Moxley, R.; Francis, D.H. Significance of Heat-Stable and Heat-Labile Enterotoxins in Porcine Colibacillosis in an Additive Model for Pathogenicity Studies. Infect. Immun. 2006, 74, 3107–3114. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.M.; Kaushik, R.S.; Francis, D.H.; Fleckenstein, J.M.; Hardwidge, P.R. Heat-Labile Enterotoxin Promotes Escherichia coli Adherence to Intestinal Epithelial Cells. J. Bacteriol. 2009, 191, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Verbrugghe, E.; Van Parys, A.; Leyman, B.; Boyen, F.; Arnouts, S.; Lundberg, U.; Ducatelle, R.; Van den Broeck, W.; Yekta, M.A.; Cox, E.; et al. Heat-labile enterotoxin of Escherichia coli promotes intestinal colonization of Salmonella enterica. Comp. Immunol. Microbiol. Infect. Dis. 2015, 43, 1–7. [Google Scholar] [CrossRef]
- Wang, X.; Gao, X.; Hardwidge, P.R. Heat-labile enterotoxin-induced activation of NF-kappaB and MAPK pathways in intestinal epithelial cells impacts enterotoxigenic Escherichia coli (ETEC) adherence. Cell. Microbiol. 2012, 14, 1231–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fekete, P.Z.; Mateo, K.S.; Zhang, W.; Moxley, R.A.; Kaushik, R.S.; Francis, D.H. Both enzymatic and non-enzymatic properties of heat-labile enterotoxin are responsible for LT-enhanced adherence of enterotoxigenic Escherichia coli to porcine IPEC-J2 cells. Veter. Microbiol. 2013, 164, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Pizza, M.; Domenighini, M.; Hol, W.; Giannelli, V.; Fontana, M.R.; Giuliani, M.M.; Magagnoli, C.; Peppoloni, S.; Manetti, R.; Rappuoli, R. Probing the structure-activity relationship of Escherichia coli LT-A by site-directed mutagenesis. Mol. Microbiol. 1994, 14, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.L.; Clements, J.D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect. Immun. 1995, 63, 1617–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norton, E.B.; Lawson, L.; Freytag, L.C.; Clements, J.D. Characterization of a Mutant Escherichia coli Heat-Labile Toxin, LT(R192G/L211A), as a Safe and Effective Oral Adjuvant. Clin. Vaccine Immunol. 2011, 18, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Byrd, W.; Boedeker, E.C. Attenuated Escherichia coli strains expressing the colonization factor antigen I (CFA/I) and a detoxified heat-labile enterotoxin (LThK63) enhance clearance of ETEC from the lungs of mice and protect mice from intestinal ETEC colonization and LT-induced fluid accumulation. Veter- Immunol. Immunopathol. 2013, 152, 57–67. [Google Scholar] [CrossRef]
- Kline, K.A.; Fälker, S.; Dahlberg, S.; Normark, S.; Henriques-Normark, B. Bacterial Adhesins in Host-Microbe Interactions. Cell Host Microbe 2009, 5, 580–592. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, A.; Rashu, R.; Begum, Y.A.; Kuhlman, F.M.; Ciorba, M.A.; Hultgren, S.J.; Qadri, F.; Fleckenstein, J.M. Highly conserved type 1 pili promote enterotoxigenic E. coli pathogen-host interactions. PLOS Negl. Trop. Dis. 2017, 11, e0005586. [Google Scholar] [CrossRef] [Green Version]
- Field, M.; Rao, M.C.; Chang, E.B. Intestinal electrolyte transport and diarrheal disease (2). N. Engl. J. Med. 1989, 321, 879–883. [Google Scholar]
- Edwards, R.A.; Schifferli, D.M. Differential regulation of fasA and fasH expression of Escherichia coli 987P fimbriae by environmental cues. Mol. Microbiol. 1997, 25, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.M.; Åberg, A.; Straseviçiene, J.; Emődy, L.; Uhlin, B.E.; Balsalobre, C. Type 1 Fimbriae, a Colonization Factor of Uropathogenic Escherichia coli, Are Controlled by the Metabolic Sensor CRP-cAMP. PLOS Pathog. 2009, 5, e1000303. [Google Scholar] [CrossRef] [PubMed]
- Pierrat, X.; Wong, J.P.H.; Al-Mayyah, Z.; Persat, A. The Mammalian Membrane Microenvironment Regulates the Sequential Attachment of Bacteria to Host Cells. mBio 2021, 12, e0139221. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, H.; Lemaire, L.; Acket, S.; Prost, E.; Duma, L.; Erhardt, M.; Čechová, P.; Trouillas, P.; Mohareb, F.; Rossi, C.; et al. The Impact of Plasma Membrane Lipid Composition on Flagellum-Mediated Adhesion of Enterohemorrhagic Escherichia coli. Msphere 2020, 5, e00702-20. [Google Scholar] [CrossRef]
- Zhou, M.; Duan, Q.; Zhu, X.; Guo, Z.; Li, Y.; Hardwidge, P.R.; Zhu, G. Both flagella and F4 fimbriae from F4ac+ enterotoxigenic Escherichia coli contribute to attachment to IPEC-J2 cells in vitro. Veter- Res. 2013, 44, 30. [Google Scholar] [CrossRef] [Green Version]
- Evans, D.J.; Evans, D.G.; DuPont, H.L.; Orskov, F.; Orskov, I. Patterns of loss of enterotoxigenicity by Escherichia coli isolated from adults with diarrhea: Suggestive evidence for an interrelationship with serotype. Infect. Immun. 1977, 17, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yang, Y.; Ou, B.; Xia, P.; Zhou, M.; Li, L.; Zhu, G. The flagellin hypervariable region is a potential flagella display domain in probiotic Escherichia coli strain Nissle 1917. Arch. Microbiol. 2016, 198, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Duan, Q.; Zhang, W. Significance of Enterotoxigenic Escherichia coli (ETEC) Heat-Labile Toxin (LT) Enzymatic Subunit Epitopes in LT Enterotoxicity and Immunogenicity. Appl. Environ. Microbiol. 2018, 84, e00849-18. [Google Scholar] [CrossRef] [Green Version]
- Duan, Q.; Pang, S.; Feng, L.; Liu, J.; Lv, L.; Li, B.; Liang, Y.; Zhu, G. Heat-labile enterotoxin enhances F4-producing enterotoxigenic E. coli adhesion to porcine intestinal epithelial cells by upregulating bacterial adhesins and STb enterotoxin. Vet. Res. 2022, 53, 88. [Google Scholar] [CrossRef]
- Duan, Q.; Zhou, M.; Zhu, X.; Yang, Y.; Zhu, J.; Bao, W.; Wu, S.; Ruan, X.; Zhang, W.; Zhu, G. Flagella from F18+ Escherichia coli play a role in adhesion to pig epithelial cell lines. Microb. Pathog. 2013, 55, 32–38. [Google Scholar] [CrossRef]
- Zhou, M.; Duan, Q.; Li, Y.; Yang, Y.; Hardwidge, P.R.; Zhu, G. Membrane cholesterol plays an important role in enteropathogen adhesion and the activation of innate immunity via flagellin–TLR5 signaling. Arch. Microbiol. 2015, 197, 797–803. [Google Scholar] [CrossRef]



| Strains/Plasmids | Relevant Characteristic | Source or Reference |
|---|---|---|
| C83902 | wild-type ETEC K88ac LT+,STa+, STb+ | [34] |
| H10407 | wild-type ETEC, CFA/I LT+,ST+ | [35] |
| 1836-2 | wild-type ETEC K88ac EAST1+ | [18] |
| EcNc | Wild-type Nissle 1917 deletion of plasmids pMUT1 and pMUT2 | [36] |
| 2015 | 1836-2 carrying pBR-peltAB | This study |
| 2016 | 1836-2 carrying pBR-pLTBQ3K | This study |
| 2017 | 1836-2 carrying pBR-pLTBG33S | This study |
| 2018 | 1836-2 carrying pBR-pLTBT47A | This study |
| 2019 | 1836-2 carrying pBR-pLTAR192G | This study |
| 2020 | 1836-2 carrying pBR-pLTAL211A | This study |
| 2021 | 1836-2 carrying pBR-pLTAR192G/L211A | This study |
| 2022 | EcNc carrying pBR-heltAB | This study |
| 2023 | EcNc carrying pBR-hLTBQ3K | This study |
| 2024 | EcNc carrying pBR-hLTBG33S | This study |
| 2025 | EcNc carrying pBR-hLTBT47A | This study |
| 2026 | EcNc carrying pBR-hLTAR192G | This study |
| 2027 | EcNc carrying pBR-hLTAL211A | This study |
| 2028 | EcNc carrying pBR-hLTAR192G/L211A | This study |
| Plasmid | ||
| p2001 | estAB from gene E. coli H10407 in pBR322 | This study |
| p2002 | estAB from gene E. coli C83902 in pBR322 | This study |
| p2003 | hLTBQ3K in pBR322 | This study |
| p2004 | pLTBQ3K in pBR322 | This study |
| p2005 | hLTBG33S in pBR322 | This study |
| p2006 | pLTBG33S in pBR322 | This study |
| p2007 | hLTBT47A in pBR322 | This study |
| p2008 | pLTBT47A in pBR322 | This study |
| p2009 | hLTAR192G in pBR322 | This study |
| p2010 | pLTAR192G in pBR322 | This study |
| p2011 | hLTAL211A in pBR322 | This study |
| p2012 | pLTAL211A in pBR322 | This study |
| p2013 | hLTAR192G/L211A in pBR322 | This study |
| p2014 | pLTAR192G/L211A in pBR322 | This study |
| Name | Primer Sequences (5′-3′) |
|---|---|
| ΔeltAB-F | ATGAAAAATATAGCTTTCATTTTTTTTATTTTATTAGCATCGCCATTATATGCAAATTGTGTAGGCTGGAGCTGCTTCG |
| ΔeltAB-R | TGTTATATAGGTTCCTAGCATTAGACATGCTTTTAAAGCAAACTAGTTTTTCATACTCATATGAATATCCTCCTTAG |
| pBR-LT-F | CGGATTGTCTTCTTGTATGAT |
| pBR-LT-R | GATCGGTATTGCCTCCTCTAC |
| LTBQ3K-F | CACGGAGCTCCCAAAACTATTACAGAA |
| LTBQ3K-R | TTCTGTAATAGTTTTGGGAGCTCCGTG |
| LTBG33S-F | GAATCGATGGCATCCAAAAGAGAAATG |
| LTBG33S-R | CATTTCTCTTTTGGATGCCATCGATTC |
| LTBT47A-F | AAGAGCGGCGAAGCATTTCAGGTCGAA |
| LTBT47A-R | TTCGACCTGAAATGCTTCGCCGCTCTT |
| LTAR192G-F | GGAAATTCATCAGGAACAATTACAGG |
| LTAR192G-R | CCTGTAATTGTTCCTGATGAATTTCC |
| LTAL211A-F | AGCACAATATATGCCAGGGAATATCAA |
| LTAL211A-R | TTGATATTCCCTGGCATATATTGTGCT |
| FaeG-F | GGGAGCTGCTTTCGCTTTTT |
| FaeG-R | CCTCGGCAAACCACCATAAA |
| fimA-F | TGAATAACGGAACCAACACCATT |
| fimA-R | CGGCACCGGTTGCAA |
| FosA-F | CAGGCGGTTTACTACGCAACT |
| FosA-R | CGTCGGCGTTGGCAATA |
| fliC-F | CGCGGTCACCAACCTGAACAAC |
| fliC-R | CCTGCTGGATGATCTGCGCTT |
| GAPDH-F | CGTTAAAGGCGCTAACTTCG |
| GAPDH-R | ACGGTGGTCATCAGACCTTC |
| CysG-F | GGCAAGGGACGTTTGAAGAC |
| CysG-R | CGGCGATGACCAACCAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Q.; Pang, S.; Feng, L.; Li, B.; Lv, L.; Liang, Y.; Zhu, G. Both LTA and LTB Subunits Are Equally Important to Heat-Labile Enterotoxin (LT)-Enhanced Bacterial Adherence. Int. J. Mol. Sci. 2023, 24, 1245. https://doi.org/10.3390/ijms24021245
Duan Q, Pang S, Feng L, Li B, Lv L, Liang Y, Zhu G. Both LTA and LTB Subunits Are Equally Important to Heat-Labile Enterotoxin (LT)-Enhanced Bacterial Adherence. International Journal of Molecular Sciences. 2023; 24(2):1245. https://doi.org/10.3390/ijms24021245
Chicago/Turabian StyleDuan, Qiangde, Shengmei Pang, Lili Feng, Baoliang Li, Linfen Lv, Yuxuan Liang, and Guoqiang Zhu. 2023. "Both LTA and LTB Subunits Are Equally Important to Heat-Labile Enterotoxin (LT)-Enhanced Bacterial Adherence" International Journal of Molecular Sciences 24, no. 2: 1245. https://doi.org/10.3390/ijms24021245
APA StyleDuan, Q., Pang, S., Feng, L., Li, B., Lv, L., Liang, Y., & Zhu, G. (2023). Both LTA and LTB Subunits Are Equally Important to Heat-Labile Enterotoxin (LT)-Enhanced Bacterial Adherence. International Journal of Molecular Sciences, 24(2), 1245. https://doi.org/10.3390/ijms24021245






