Sex-Dependent Effect of Chronic Piromelatine Treatment on Prenatal Stress-Induced Memory Deficits in Rats
Abstract
1. Introduction
2. Results
2.1. The Chronic Piromelatine Treatment Corrected Impairments of Associative Memory in Male and Female Offspring with a History of Prenatal Stress
2.2. The Chronic Piromelatine Treatment Corrected Impairments of Hippocampus-Dependent Spatial Memory in Male Offspring with a History of Prenatal Stress
2.3. The Chronic Piromelatine Treatment Tended to Increase Melatonin Levels in Male Rats with a History of Prenatal Stress
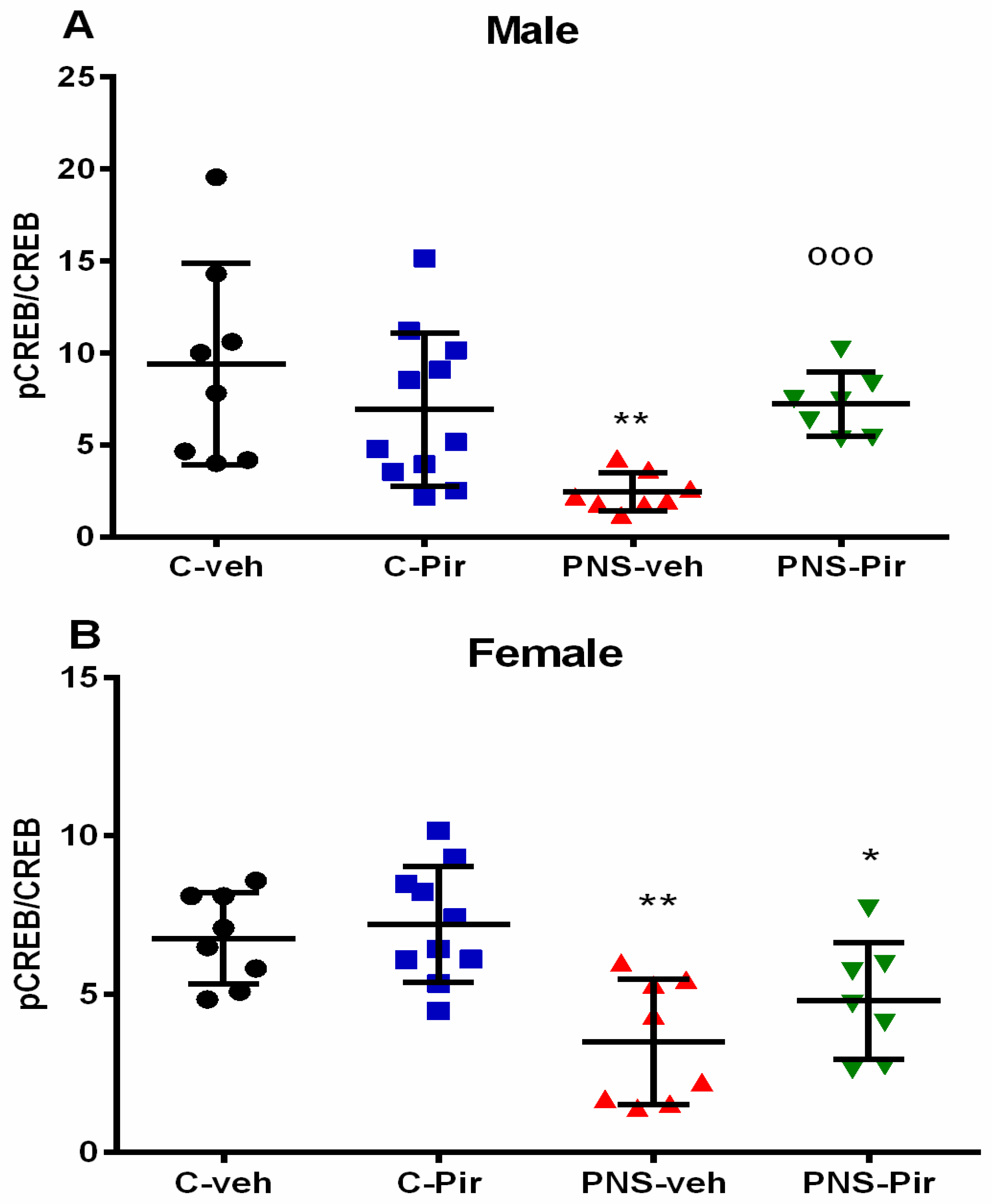
2.4. The Chronic Piromelatine Treatment Exerted a Sex-Dependent Elevation of Phosphorylated and Nonphosphorylated Adenosine Monophosphate Response Element Binding Protein (pCREB/CREB) Ratio in the Hippocampus in a PNS Model
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Prenatal Stress Procedure
4.3. Drug Administration and Protocol Design
4.4. Behavioral Tests
4.4.1. Object Recognition Test (ORT)
4.4.2. Radial Arm Maze
4.5. Biochemical Methods
4.5.1. Measurement of Melatonin Levels in the Plasma
4.5.2. Measurement of CREB1 and Phosphorylated CREB in the Hippocampus
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Y.-L.; Wang, S. Prenatal Lipopolysaccharide Exposure Increases Depression-like Behaviors and Reduces Hippocampal Neurogenesis in Adult Rats. Behav. Brain Res. 2014, 259, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Dunn, E.; Kostaki, A.; Andrews, M.H.; Matthews, S.G. Fetal Programming of Hypothalamo-Pituitary-Adrenal Function: Prenatal Stress and Glucocorticoids: Fetal Programming of HPA Function. J. Physiol. 2006, 572, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Buss, C.; Davis, E.P.; Muftuler, L.T.; Head, K.; Sandman, C.A. High Pregnancy Anxiety during Mid-Gestation Is Associated with Decreased Gray Matter Density in 6–9-Year-Old Children. Psychoneuroendocrinology 2010, 35, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Glover, V. Maternal Depression, Anxiety and Stress during Pregnancy and Child Outcome; What Needs to Be Done. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 25–35. [Google Scholar] [CrossRef] [PubMed]
- La Marca-Ghaemmaghami, P.; Dainese, S.M.; Stalla, G.; Haller, M.; Zimmermann, R.; Ehlert, U. Second-Trimester Amniotic Fluid Corticotropin-Releasing Hormone and Urocortin in Relation to Maternal Stress and Fetal Growth in Human Pregnancy. Stress 2017, 20, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, N.; Nenchovska, Z.; Atanasova, M.; Laudon, M.; Mitreva, R.; Tchekalarova, J. Chronic Piromelatine Treatment Alleviates Anxiety, Depressive Responses and Abnormal Hypothalamic–Pituitary–Adrenal Axis Activity in Prenatally Stressed Male and Female Rats. Cell Mol. Neurobiol. 2021, 42, 2257–2272. [Google Scholar] [CrossRef]
- Ivanova, N.; Nenchovska, Z.; Atanasova, M.; Mitreva, R.; Stoynova, T.; Kortenska, L.; Tchekalarova, J. Sex-Dependent Differences of Emotional Status in a Rat Model of Prenatal Stress. C. R. Acad. Bulg. Sci. 2022, 75, 1082–1088. [Google Scholar] [CrossRef]
- Laplante, D.P.; Brunet, A.; Schmitz, N.; Ciampi, A.; King, S. Project Ice Storm: Prenatal Maternal Stress Affects Cognitive and Linguistic Functioning in 5½-Year-Old Children. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 1063–1072. [Google Scholar] [CrossRef]
- Moura, C.A.; Oliveira, M.C.; Costa, L.F.; Tiago, P.R.F.; Holanda, V.A.D.; Lima, R.H.; Cagni, F.C.; Lobão-Soares, B.; Bolaños-Jiménez, F.; Gavioli, E.C. Prenatal Restraint Stress Impairs Recognition Memory in Adult Male and Female Offspring. Acta Neuropsychiatr. 2020, 32, 122–127. [Google Scholar] [CrossRef]
- Mueller, B.R.; Bale, T.L. Early Prenatal Stress Impact on Coping Strategies and Learning Performance Is Sex Dependent. Physiol. Behav. 2007, 91, 55–65. [Google Scholar] [CrossRef]
- Zuena, A.R.; Mairesse, J.; Casolini, P.; Cinque, C.; Alemà, G.S.; Morley-Fletcher, S.; Chiodi, V.; Spagnoli, L.G.; Gradini, R.; Catalani, A.; et al. Prenatal Restraint Stress Generates Two Distinct Behavioral and Neurochemical Profiles in Male and Female Rats. PLoS ONE 2008, 3, e2170. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R. The Molecular Biology of Memory Storage: A Dialogue Between Genes and Synapses. Science 2001, 294, 1030–1038. [Google Scholar] [CrossRef]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF Val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-G.; Son, H. Adult Hippocampal Neurogenesis and Related Neurotrophic Factors. BMB Rep. 2009, 42, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Tchekalarova, J.; Kortenska, L.; Marinov, P.; Ivanova, N. Sex-Dependent Effects of Piromelatine Treatment on Sleep-Wake Cycle and Sleep Structure of Prenatally Stressed Rats. IJMS 2022, 23, 10349. [Google Scholar] [CrossRef] [PubMed]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.J.O.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef]
- Bourtchuladze, R.; Frenguelli, B.; Blendy, J.; Cioffi, D.; Schutz, G.; Silva, A.J. Deficient Long-Term Memory in Mice with a Targeted Mutation of the CAMP-Responsive Element-Binding Protein. Cell 1994, 79, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.C.P.; Wallach, J.S.; Del Vecchio, M.; Wilder, E.L.; Zhou, H.; Quinn, W.G.; Tully, T. Induction of a Dominant Negative CREB Transgene Specifically Blocks Long-Term Memory in Drosophila. Cell 1994, 79, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Masaki, T.; Hori, K.; Masuo, Y.; Miyamoto, M.; Tsubokawa, H.; Noguchi, H.; Nomura, M.; Takamatsu, K. Hippocalcin-Deficient Mice Display a Defect in CAMP Response Element-Binding Protein Activation Associated with Impaired Spatial and Associative Memory. Neuroscience 2005, 133, 471–484. [Google Scholar] [CrossRef]
- Hong, E.J.; McCord, A.E.; Greenberg, M.E. A Biological Function for the Neuronal Activity-Dependent Component of Bdnf Transcription in the Development of Cortical Inhibition. Neuron 2008, 60, 610–624. [Google Scholar] [CrossRef]
- Simonetti, M.; Giniatullin, R.; Fabbretti, E. Mechanisms Mediating the Enhanced Gene Transcription of P2X3 Receptor by Calcitonin Gene-Related Peptide in Trigeminal Sensory Neurons. J. Biol. Chem. 2008, 283, 18743–18752. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, H.; Liu, J.; Wen, J.; Zhu, Z.; Li, H. Prenatal Stress Impairs Spatial Learning and Memory Associated with Lower MRNA Level of the CAMKII and CREB in the Adult Female Rat Hippocampus. Neurochem. Res. 2017, 42, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Peck, J.S. Cognitive Effects of Exogenous Melatonin Administration in Elderly Persons: A Pilot Study. Am. J. Geriatr. Psychiatry 2004, 12, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Savaskan, E.; Olivieri, G.; Brydon, L.; Jockers, R.; Kräuchi, K.; Wirz-Justice, A.; Müller-Spahn, F. Cerebrovascular Melatonin MT1-Receptor Alterations in Patients with Alzheimer’s Disease. Neurosci. Lett. 2001, 308, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Savaskan, E.; Ayoub, M.A.; Ravid, R.; Angeloni, D.; Fraschini, F.; Meier, F.; Eckert, A.; Muller-Spahn, F.; Jockers, R. Reduced Hippocampal MT2 Melatonin Receptor Expression in Alzheimer’s Disease. J. Pineal. Res. 2005, 38, 10–16. [Google Scholar] [CrossRef] [PubMed]
- McNulty, S.; Rosst, A.W.; Barrettt, P.; Hastings, M.H.; Morgan, P.J. Melatonin Regulates the Phosphorylation of CREB in Ovine Pars Tuberalis. J. Neuroendocr. 1994, 6, 523–532. [Google Scholar] [CrossRef]
- Maronde, E.; Schomerus, C.; Stehle, J.H.; Korf, H.-W. Control of CREB Phosphorylation and Its Role for Induction of Melatonin Synthesis in Rat Pinealocytes*. Biol. Cell 1997, 89, 505–511. [Google Scholar] [CrossRef]
- Laudon, M.; Nir, T.; Zisapel, N. Development of Piromelatine, a Novel Multimodal Sleep Medicine. Eur. Neuropsychopharmacol. 2014, 24, S145. [Google Scholar] [CrossRef]
- Tian, S.; Laudon, M.; Han, L.; Gao, J.; Huang, F.; Yang, Y.; Deng, H. Antidepressant- and Anxiolytic Effects of the Novel Melatonin Agonist Neu-P11 in Rodent Models. Acta. Pharm. Sin. 2010, 31, 775–783. [Google Scholar] [CrossRef]
- She, M.; Hu, X.; Su, Z.; Zhang, C.; Yang, S.; Ding, L.; Laudon, M.; Yin, W. Piromelatine, a Novel Melatonin Receptor Agonist, Stabilizes Metabolic Profiles and Ameliorates Insulin Resistance in Chronic Sleep Restricted Rats. Eur. J. Pharmacol. 2014, 727, 60–65. [Google Scholar] [CrossRef]
- He, P.; Ouyang, X.; Zhou, S.; Yin, W.; Tang, C.; Laudon, M.; Tian, S. A Novel Melatonin Agonist Neu-P11 Facilitates Memory Performance and Improves Cognitive Impairment in a Rat Model of Alzheimer’ Disease. Horm. Behav. 2013, 64, 1–7. [Google Scholar] [CrossRef]
- King, M.; Marsden, C.; Fone, K. A Role for the 5-HT1A, 5-HT4 and 5-HT6 Receptors in Learning and Memory. Trends Pharmacol. Sci. 2008, 29, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Ögren, S.O.; Eriksson, T.M.; Elvander-Tottie, E.; D’Addario, C.; Ekström, J.C.; Svenningsson, P.; Meister, B.; Kehr, J.; Stiedl, O. The Role of 5-HT1A Receptors in Learning and Memory. Behav. Brain Res. 2008, 195, 54–77. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Begni, V.; Malpighi, C.; Cattane, N.; Luoni, A.; Pariante, C.; Riva, M.A. Transcriptional Signatures of Cognitive Impairment in Rat Exposed to Prenatal Stress. Mol. Neurobiol. 2019, 56, 6251–6260. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Xie, H.; Laudon, M.; Zhou, S.; Tian, S.; You, Y. Piromelatine Ameliorates Memory Deficits Associated with Chronic Mild Stress-Induced Anhedonia in Rats. Psychopharmacology 2016, 233, 2229–2239. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.R.; Bale, T.L. Sex-Specific Programming of Offspring Emotionality after Stress Early in Pregnancy. J. Neurosci. 2008, 28, 9055–9065. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, X.; Li, F.; Yin, T.; Zhang, J.; Zhang, T. RTMS Ameliorates Prenatal Stress–Induced Cognitive Deficits in Male-Offspring Rats Associated With BDNF/TrkB Signaling Pathway. Neurorehabil. Neural. Repair. 2019, 33, 271–283. [Google Scholar] [CrossRef]


| Group | Median ± SD | Range |
|---|---|---|
| Male | ||
| C-veh | 0.14 ± 0.09 | (0.075 ± 0.24) |
| PNS-veh | 0.058 ± 0.038 | (0.05 ± 0.14) |
| PNS-Pir | 0.85 ± 0.44 | (0.092 ± 1.608) |
| Female | ||
| C-veh | 0.12 ± 0.79 | (0.1–4.0) |
| PNS-veh | 0.096 ± 0.083 | (0.06 ± 0.42) |
| PNS-Pir | 0.095 ± 0.03 | (0.06 ± 1.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, N.; Atanasova, M.; Nenchovska, Z.; Tchekalarova, J. Sex-Dependent Effect of Chronic Piromelatine Treatment on Prenatal Stress-Induced Memory Deficits in Rats. Int. J. Mol. Sci. 2023, 24, 1271. https://doi.org/10.3390/ijms24021271
Ivanova N, Atanasova M, Nenchovska Z, Tchekalarova J. Sex-Dependent Effect of Chronic Piromelatine Treatment on Prenatal Stress-Induced Memory Deficits in Rats. International Journal of Molecular Sciences. 2023; 24(2):1271. https://doi.org/10.3390/ijms24021271
Chicago/Turabian StyleIvanova, Natasha, Milena Atanasova, Zlatina Nenchovska, and Jana Tchekalarova. 2023. "Sex-Dependent Effect of Chronic Piromelatine Treatment on Prenatal Stress-Induced Memory Deficits in Rats" International Journal of Molecular Sciences 24, no. 2: 1271. https://doi.org/10.3390/ijms24021271
APA StyleIvanova, N., Atanasova, M., Nenchovska, Z., & Tchekalarova, J. (2023). Sex-Dependent Effect of Chronic Piromelatine Treatment on Prenatal Stress-Induced Memory Deficits in Rats. International Journal of Molecular Sciences, 24(2), 1271. https://doi.org/10.3390/ijms24021271






