Regioselective Cycloaddition of Nitrile Imines to 5-Methylidene-3-phenyl-hydantoin: Synthesis and DFT Calculations
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. DFT Calculations
- Comparison of the energies of the frontier orbitals of the reagents to determine the nature of the interaction between the 1,3-dipole and the dipolarophile.
- Analysis of the global and local reactivity indices of the ground states is necessary to better understand preferable regioselectivity.
- Determination of Fukui functions to predict the most favorable regioisomeric reactive channel.
- Calculation of the minimum energy path (MEP), localization of the transitional states, and determination of the corresponding geometries.
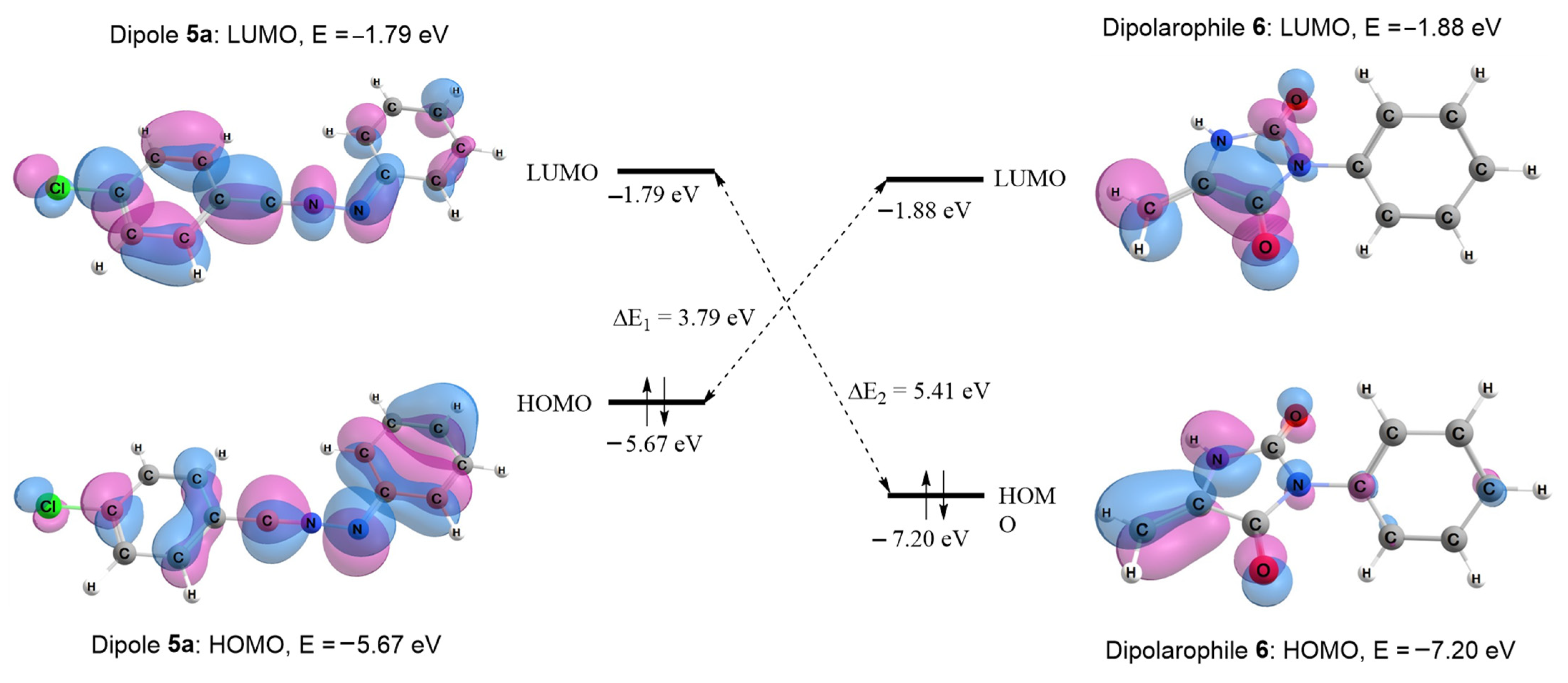
2.2.1. Frontier Molecular Orbital Interaction
2.2.2. Analysis of the Conceptual DFT Reactivity Indices of the Reagents
2.2.3. Regioselectivity of Nitrile Imines Cycloaddition to Methylydene Hydantoin 6
2.2.4. Finding Transition States Using NEB-TS
3. Materials and Methods
3.1. General
3.2. Synthesis
1,3-Diporal Cycloaddition of Nitrile Imines 5 with 5-Methylene-3-Phenylhydantoin 6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chalkha, M.; Akhazzane, M.; Moussaid, F.Z.; Daoui, O.; Nakkabi, A.; Bakhouch, M.; Chtita, S.; Elkhattabi, S.; Housseini, A.I.; El Yazidi, M. Design, synthesis, characterization, in vitro screening, molecular docking, 3D-QSAR, and ADME-Tox investigations of novel pyrazole derivatives as antimicrobial agents. New J. Chem. 2022, 46, 2747–2760. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman. Review: Biologically active pyrazole derivatives. New J. Chem. 2017, 41, 16–41. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.M.; Huang, X.C.; Mao, Z.Y.; Wang, H.S. A novel methodology for synthesis of dihydropyrazole derivatives as potential anticancer agents. Org. Biomol. Chem. 2014, 12, 2028–2032. [Google Scholar] [CrossRef] [PubMed]
- Padwa, A.; Pearson, W.H. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; Taylor, E.C., Wipf, P., Eds.; John Wiley and Sons Inc.: New York, NY, USA, 2002; Volume 59, ISBN 0-471-38726-6. [Google Scholar]
- Yet, L. 4.01—Pyrazoles, 4th ed.; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: New York, NY, USA, 2008; ISBN 9780080449920. [Google Scholar]
- Huisgen, R. 1,3-Dipolar Cycloadditions Past and Future. Angew. Chem. Int. Ed. 1963, 2, 565–632. [Google Scholar] [CrossRef]
- Huisgen, R.; Seidel, M.; Wallbillich, G.; Knupfer, H. Diphenyl-nitrilimin und seine 1.3-dipolaren additionen an alkene und alkine. Tetrahedron 1962, 17, 3–29. [Google Scholar] [CrossRef]
- Huisgen, R. The formation of nitrile imines in the thermal breakdown of 2,5-disubstituted tetrazoles. Communications 1954, 24, 1954–1955. [Google Scholar] [CrossRef]
- Huisgen, R.; Grashey, R.; Seidel, M.; Knupfer, H.; Schmidt, R. 1.3-Dipolare Additionen, III. Umsetzungen des Diphenylnitrilimins mit Carbonyl und Thiocarbonyl-Verbindungen. Justus Liebigs Ann. Chem. 1962, 658, 169–180. [Google Scholar] [CrossRef]
- Miqdad, O.A.; Abunada, N.M.; Hassaneen, H.M. Regioselectivity of Nitrilimines 1,3-Dipolar Cycloaddition: Novel Synthesis of Spiro[4,4]nona-2,8-dien-6-one Derivatives. Heteroat. Chem. 2011, 22, 131–136. [Google Scholar] [CrossRef]
- Utecht, G.; Fruziński, A.; Jasiński, M. Polysubstituted 3-trifluoromethylpyrazoles: Regioselective (3 + 2)-cycloaddition of trifluoroacetonitrile imines with enol ethers and functional group transformations. Org. Biomol. Chem. 2018, 16, 1252–1257. [Google Scholar] [CrossRef]
- Gazzeh, H.; Boudriga, S.; Askri, M.; Khatyr, A.; Knorr, M.; Strohmann, C.; Golz, C.; Rousselin, Y.; Kubicki, M.M. Stoichiometry-controlled cycloaddition of nitrilimines with unsymmetrical exocyclic dienones: Microwave-assisted synthesis of novel mono and dispiropyrazoline derivatives. RSC Adv. 2016, 6, 49868–49875. [Google Scholar] [CrossRef]
- Houk, K.N.; Sims, J.; Watts, C.R.; Luskus, L.J. The origin of reactivity, regioselectivity, and periselectivity in 1,3-dipolar cycloadditions. J. Am. Chem. Soc. 1973, 95, 7301–7315. [Google Scholar] [CrossRef]
- Belskaya, N.P.; Eliseeva, A.I.; Bakulev, V.A. Hydrazones as substrates for cycloaddition reactions. Russ. Chem. Rev. 2015, 84, 1226–1257. [Google Scholar] [CrossRef]
- Fleming, I. Molecular Orbitals and Organic Reactions; John Wiley & Sons, Ltd.: Chichester, UK, 2010; ISBN 9780470746585. [Google Scholar]
- Hassaneen, H.M.; Daboun, H.A.; Abdelhadi, H.A. Site Selectivity and Regiochemistry of Nitrileimines. Cycloadditions to 1,3-diphenyl-2-thiono-4-imidazolidinone and Its 5-phenylmethylene Derivatives. Phosphorus Sulfur Silicon Relat. Elem. 1995, 107, 269–273. [Google Scholar] [CrossRef]
- Yavari, I.; Taheri, Z.; Sheikhi, S.; Bahemmat, S.; Halvagar, M.R. Synthesis of thia- and thioxo-tetraazaspiro[4.4]nonenones from nitrile imines and arylidenethiohydantoins. Mol. Divers. 2020, 25, 777–785. [Google Scholar] [CrossRef]
- Shybanov, D.E.; Filkina, M.E.; Kukushkin, M.E.; Grishin, Y.K.; Roznyatovsky, V.A.; Zyk, N.V.; Beloglazkina, E.K. Diffusion mixing with a volatile tertiary amine as a very efficient technique for 1,3-dipolar cycloaddition reactions proceeding via dehydrohalogenation of stable precursors of reactive dipoles. New J. Chem. 2022, 46, 18575–18586. [Google Scholar] [CrossRef]
- Rouatbi, F.; Mhiri, C.; Askri, M.; Knorr, M.; Rousselin, Y.; Kubicki, M.M. Regioselective Synthesis of Mono- and Dispiropyrazoline Derivatives via 1,3-dipolar Cycloaddition with Nitrilimines. J. Heterocycl. Chem. 2017, 54, 1152–1160. [Google Scholar] [CrossRef]
- Singh, A.; Loomer, A.L.; Roth, G.P. Synthesis of Oxindolyl Pyrazolines and 3-Amino Oxindole Building Blocks via a Nitrile Imine [3+2] Cycloaddition Strategy. Org. Lett. 2012, 14, 5266–5269. [Google Scholar] [CrossRef]
- Strauss, A.; Otto, H. 1,3-Cycloadditions to Highly Substituted, Strained Double Bonds: Spiro-fl-lactams from a-Methylidene-fl-lactams by Reactions with Diphenylnitrilimine, Acetonitrile Oxide, Nitrones, and Diazomethane. Helv. Chim. Acta 1997, 80, 1823–1830. [Google Scholar] [CrossRef]
- Ribeiro, C.J.A.; Nunes, R.C.; Amaral, J.D.; Gonçalves, L.M.; Rodrigues, C.M.P.; Moreira, R.; Santos, M.M.M. Spirotriazoline oxindoles: A novel chemical scaffold with in vitro anticancer properties. Eur. J. Med. Chem. 2017, 140, 494–509. [Google Scholar] [CrossRef]
- Bora, D.; Kaushal, A.; Shankaraiah, N. Anticancer potential of spirocompounds in medicinal chemistry: A pentennial expedition. Eur. J. Med. Chem. 2021, 215, 113263. [Google Scholar] [CrossRef]
- Liu, H.; Jia, H.; Wang, B.; Xiao, Y.; Guo, H. Synthesis of Spirobidihydropyrazole through Double 1,3-Dipolar Cycloaddition of Nitrilimines with Allenoates. Org. Lett. 2017, 19, 4714–4717. [Google Scholar] [CrossRef] [PubMed]
- Areephong, J.; Treat, N.; Kramer, J.W.; Christianson, M.D.; Hawker, C.J.; Collins, H.A. Triazine Mediated Living Radical Controlling Polymerization Background of the Invention. Patent WO 2015/061189 Al, 30 April 2015. [Google Scholar]
- Huisgen, R.; Aufderhaar, E.; Wallbillich, G. 1.3-Dipolare Cycloadditionen, XVI: Zur Bildung von 1.4-Dihydro-tetrazinen aus Nitriliminen; 1.4-Diphenyl-1.4-dihydro-1.2.4.5-tetrazin und isomere Verbindungen. Chem. Ber. 1965, 98, 1476–1486. [Google Scholar] [CrossRef]
- Wang, Y.; Song, W.; Hu, W.J.; Lin, Q. Fast alkene functionalization in vivo by photoclick chemistry: HOMO lifting of nitrile imine dipoles. Angew. Chem. Int. Ed. 2009, 48, 5330–5333. [Google Scholar] [CrossRef] [PubMed]
- Yavari, I.; Taheri, Z.; Naeimabadi, M.; Bahemmat, S.; Halvagar, M.R. A Convenient Synthesis of Tetrasubstituted Pyrazoles from Nitrile Imines and 2-(Thioxothiazolidin-5-ylidene)acetates. Synlett 2018, 29, 918–921. [Google Scholar] [CrossRef]
- Ghasempour, L.; Asghari, S.; Tajbakhsh, M.; Mohseni, M. Preparation of New Spiropyrazole, Pyrazole and Hydantoin Derivatives and Investigation of Their Antioxidant and Antibacterial Activities. Chem. Biodivers. 2021, 18, e2100197. [Google Scholar] [CrossRef]
- Islam, M.S.; Haukka, M.; Soliman, S.M.; Al-Majid, A.M.; Rahman, A.F.M.M.; Bari, A.; Barakat, A. Regio- and stereoselective synthesis of spiro-heterocycles bearing the pyrazole scaffold via [3 + 2] cycloaddition reaction. J. Mol. Struct. 2022, 1250, 131711. [Google Scholar] [CrossRef]
- Molteni, G.; Ponti, A. The Nitrilimine-Alkene Cycloaddition Regioselectivity Rationalized by Density Functional Theory Reactivity Indices. Molecules 2017, 22, 202. [Google Scholar] [CrossRef]
- Conti, P.; Pinto, A.; Tamborini, L.; Rizzo, V.; De Micheli, C. A regioselective route to 5-substituted pyrazole- and pyrazoline-3-phosphonic acids and esters. Tetrahedron 2007, 63, 5554–5560. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Rappoport, D.; Furche, F. Property-optimized Gaussian basis sets for molecular response calculations. J. Chem. Phys. 2010, 133, 134105. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.H. General Methods for Geometry and Wave Function Optimization. J. Phys. Chem. 1992, 96, 9768–9774. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Soleymani, M. DFT study of double 1,3-dipolar cycloaddition of nitrilimines with allenoates. Mon. Fur Chem. 2018, 149, 2183–2193. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ghodsi, F.; Ríos-Gutiérrez, M. A molecular electron density theory study of the synthesis of spirobipyrazolines through the domino reaction of nitrilimines with allenoates. Molecules 2019, 24, 4159. [Google Scholar] [CrossRef] [PubMed]
- Méndez, F.; Gazquez, J.L. Chemical Reactivity of Enolate Ions: The Local Hard and Soft Acids and Bases Principle Viewpoint. J. Am. Chem. Soc. 1994, 116, 9298–9301. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-Atom Fragments for Describing Molecular Charge Densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Sustmann, R.; Trill, H. Substituent Effects in 1,3-Dipolar Cycloadditions of Phenyl Azide. Angew. Chem. Int. Ed. 1972, 11, 838–840. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Arnó, M.; Sáez, J.A. Toward an understanding of the 1,3-dipolar cycloaddition between diphenylnitrone and a maleimide: Bisamide complex. A DFT analysis of the reactivity of symmetrically substituted dipolarophiles. J. Mol. Struct. THEOCHEM 2007, 811, 125–133. [Google Scholar] [CrossRef]
- Jaramillo, P.; Domingo, L.R.; Chamorro, E.; Pérez, P. A further exploration of a nucleophilicity index based on the gas-phase ionization potentials. J. Mol. Struct. THEOCHEM 2008, 865, 68–72. [Google Scholar] [CrossRef]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual density functional theory. Chem. Rev. 2003, 103, 1793–1873. [Google Scholar] [CrossRef] [PubMed]
- Ponti, A.; Molteni, G. DFT-Based Quantitative Prediction of Regioselectivity: Cycloaddition of Nitrilimines to Methyl Propiolate. J. Org. Chem. 2001, 66, 5252–5255. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Aurell, M.J.; Pérez, P.; Contreras, R. Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels-Alder reactions. Tetrahedron 2002, 58, 4417–4423. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef]
- Molteni, G.; Ponti, A. Stereoselective nitrilimine cycloadditions to the C=N bond of enantiopure N-(1-phenylethyl)-1-arylmethanimines. Tetrahedron Asymmetry 2004, 15, 3711–3714. [Google Scholar] [CrossRef]
- Fukui, K. Role of frontier orbitals in chemical reactions. Science 1982, 218, 747–754. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Approach to the Frontier-Electron Theory of Chemical Reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Padmanabhan, J.; Parthasarathi, R.; Sarkar, U.; Subramanian, V.; Chattaraj, P.K. Effect of solvation on the condensed Fukui function and the generalized philicity index. Chem. Phys. Lett. 2004, 383, 122–128. [Google Scholar] [CrossRef]
- Roy, R.K.; Pal, S.; Hirao, K. On non-negativity of Fukui function indices. II. J. Chem. Phys. 1999, 110, 1372. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P.; Sáez, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Eckell, A.; Huisgen, R.; Sustmann, R.; Wallbillich, G.; Grashey, D.; Spindler, E. 1.3-Dipolare Cycloadditionen, XXXI. Dipolarophilen-Aktivitäten gegenüber Diphenylnitrilimin und zahlenmäßige Ermittlung der Substituenteneinflüsse. Chem. Ber. 1967, 100, 2192–2213. [Google Scholar] [CrossRef]
- Tietze, L.F.; Eicher, T. Reaktionen und Synthesen Im Organisch-Chemischen Praktikum und Forschungslaboratorium (2. Auflage), 117th ed.; Thieme Verlag: Stuttgart, Germany; New York, NY, USA, 1991; ISBN 3527308741. [Google Scholar]
- Dascalu, A.E.; Rouleau Billamboz, M.; Guinet, A.; Rigo, B.; Lipka Belloli, E.; Hartkoorn, R.C.; Ple, C. Hydrazide Derivatives and Their Specific Use as Antibacterial Agents by Controlling Acinetobacter Baumannii Bacterium. Patent WO 2020/169682, 27 August 2020. [Google Scholar]
- Wang, W.-J.; Zhang, T.; Duan, L.-J.; Zhang, X.-J.; Yan, M. KOt-Bu promoted homocoupling and decomposition of N′-aryl acylhydrazines: Synthesis of unsymmetric N′,N′-diaryl acylhydrazines. Tetrahedron 2015, 71, 9073–9080. [Google Scholar] [CrossRef]
- Morrill, L.C.; Lebl, T.; Slawin, A.M.Z.; Smith, A.D. Catalytic asymmetric α-amination of carboxylic acids using isothioureas. Chem. Sci. 2012, 3, 2088–2093. [Google Scholar] [CrossRef]
- Tanimori, S.; Kobayashi, Y.; Iesaki, Y.; Ozaki, Y.; Kirihata, M. Copper-catalyzed synthesis of substituted indazoles from 2-chloroarenes at low catalyst-loading. Org. Biomol. Chem. 2011, 10, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Catarzi, D.; Varano, F.; Poli, D.; Squarcialupi, L.; Betti, M.; Trincavelli, L.; Martini, C.; Ben, D.D.; Thomas, A.; Volpini, R.; et al. 1,2,4-Triazolo[1,5-a]quinoxaline derivatives and their simplified analogues as adenosine A3 receptor antagonists. Synthesis, structure–affinity relationships and molecular modeling studies. Bioorganic Med. Chem. 2015, 23, 9–21. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Huang, G.-B.; Weng, J.; Lu, G.; Chan, A.S.C. Copper(ii)-catalyzed coupling reaction: An efficient and regioselective approach to N′,N′-diaryl acylhydrazines. Org. Biomol. Chem. 2014, 13, 2055–2063. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Q.; Luo, M. Reduction of hydrazines to amines with aqueous solution of titanium(iii) trichloride. Org. Biomol. Chem. 2011, 9, 4977–4982. [Google Scholar] [CrossRef]
- Gao, L.; Wang, X.; Wei, Q.; Su, K.; Huang, R.; Guo, J.; Zheng, Y.; Liu, J. [3+3] Cycloadditions of Azomethine Ylides with Nitrile Imines for the Synthesis of 2,3,4,5-Tetrahydro-1,2,4-Triazine-5-Carboxylates. Eur. J. Org. Chem. 2022, 2022, e202200768. [Google Scholar] [CrossRef]
- El-Abadelah, M.M.; Hussein, A.Q.; Kamal, M.R.; Al-Adhami, K.H. Heterocycles from Nitrile Imines. Part 1. 1,2,3,4-Tetrahydro-1,2,4,5-Tetrazines. Heterocycles 1988, 27, 917. [Google Scholar] [CrossRef]
- Dadiboyena, S.; Valente, E.J.; Hamme, A.T. A novel synthesis of 1,3,5-trisubstituted pyrazoles through a spiro-pyrazoline intermediate via a tandem 1,3-dipolar cycloaddition/elimination. Tetrahedron Lett. 2009, 50, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Rector, D.L.; Folz, S.D.; Conklin, R.D.; Nowakowski, L.H.; Kaugars, G. Structure-activity relationships in a broad-spectrum anthelmintic series. Acid chloride phenylhydrazones. I. Aryl substitutions and chloride variations. J. Med. Chem. 1981, 24, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Bonini, B.F.; Franchini, M.C.; Gentili, D.; Locatelli, E.; Ricci, A. ChemInform Abstract: 1,3-Dipolar Cycloaddition of Nitrile Imines with Functionalized Acetylenes: Regiocontrolled Sc(OTf)3-Catalyzed Synthesis of 4- and 5-Substituted Pyrazoles. Cheminform 2009, 41. [Google Scholar] [CrossRef]
- Reinov, M.V.; Yurovskaya, M.A.; Davydov, D.V.; Streletskii, A.V. Heterocyclic Derivatives of Fullerene C60. 1. Synthesis of New Fulleropyrazolines by the 1,3-Dipolar Cycloaddition of Nitrile Imines. Chem. Heterocycl. Compd. 2004, 40, 188–193. [Google Scholar] [CrossRef]
- Tewari, R.S.; Parihar, P. Halogenation of substituted hydrazones. A facile route for the synthesis of some new hydrazidoyl halides. J. Chem. Eng. Data 1981, 26, 418–420. [Google Scholar] [CrossRef]






 | ||||||
|---|---|---|---|---|---|---|
| Entry | 4a, Equiv. | Et3N, Equiv. | Solvent | T, °C | Time, h | Yield a (%) |
| 1 | 1 | 1 | CH2Cl2 | 22 | 24 | 81 |
| 2 | 1,1 | 2,2 | CH2Cl2 | 22 | 24 | 88 |
| 3 | 2 | 4 | CH2Cl2 | 22 | 48 | 58 |
| 4 | 1,1 | 2,2 | CH3CN | 22 | 48 | 99 |
| 5 | 1,1 | 2,2 | PhH | 60 | 12 | 82 |
| 6 | 1,1 | 2,2 | PhMe | 110 | 12 | 44 |
| 7 | 1,1 | 2,2 | EtOAc | 22 | 48 | 74 |
| 8 | 1,1 | 2,2 | CHCl3 | 22 | 24 | 74 |
| LUMO, eV | HOMO, eV | LUMO6–HOMO5, eV | LUMO5–HOMO6, eV | |
|---|---|---|---|---|
| 6 | −1.88 | −7.20 | - | - |
| 5a (R1 = Cl) | −1.79 | −5.67 | 3.79 | 5.41 |
| 5j (R1 = CF3) | −2.03 | −5.79 | 3.90 | 5.17 |
| 5k (R1 = NO2) | −3.09 | −5.93 | 4.05 | 4.11 |
| 5l (R2 = NO2) | −2.64 | −6.11 | 4.22 | 4.56 |
| Compound | R1 | R2 | HOMO | LUMO | μ | η | ω | N |
|---|---|---|---|---|---|---|---|---|
| 6 (gas) | - | - | −6.94 | −1.72 | −4.33 | 5.21 | 1.80 | 2.77 |
| 5a (gas) | Cl | H | −5.63 | −1.90 | −3.77 | 3.74 | 1.90 | 4.08 |
| 5j (gas) | CF3 | H | −5.83 | −2.20 | −4.01 | 3.63 | 2.22 | 3.98 |
| 5k (gas) | NO2 | H | −6.07 | −3.09 | −4.58 | 2.98 | 3.52 | 3.64 |
| 5l (gas) | H | NO2 | −6.19 | −2.51 | −4.35 | 3.68 | 2.57 | 3.52 |
| 6 (CH2Cl2) | - | - | −7.20 | −1.88 | −4.54 | 5.32 | 1.94 | 2.52 |
| 5a (CH2Cl2) | Cl | H | −5.67 | −1.79 | −3.73 | 3.88 | 1.80 | 3.69 |
| 5j (CH2Cl2) | CF3 | H | −5.79 | −2.03 | −3.91 | 3.75 | 2.04 | 3.93 |
| 5k (CH2Cl2) | NO2 | H | −5.93 | −3.09 | −4.51 | 2.85 | 3.57 | 3.79 |
| 5l (CH2Cl2) | H | NO2 | −6.11 | −2.64 | −4.37 | 3.47 | 2.76 | 3.61 |
| Gas | CH2Cl2 | ||||||
|---|---|---|---|---|---|---|---|
| Entry | ωk | Nk | |||||
| 6 | C5 | 0.074 | 0.024 | 0.083 | 0.095 | 0.16 | 0.24 |
| C4 | 0.157 | 0.084 | 0.179 | 0.178 | 0.35 | 0.45 | |
| 5a | N1 | 0.065 | 0.123 | 0.064 | 0.155 | 0.11 | 0.63 |
| N2 | 0.059 | 0.028 | 0.066 | 0.044 | 0.12 | 0.18 | |
| C3 | 0.037 | 0.105 | 0.060 | 0.111 | 0.11 | 0.45 | |
| 5j | N1 | 0.068 | 0.120 | 0.067 | 0.151 | 0.14 | 0.59 |
| N2 | 0.056 | 0.033 | 0.064 | 0.045 | 0.13 | 0.18 | |
| C3 | 0.039 | 0.111 | 0.054 | 0.116 | 0.11 | 0.46 | |
| 5k | N1 | 0.067 | 0.112 | 0.053 | 0.138 | 0.19 | 0.52 |
| N2 | 0.044 | 0.029 | 0.035 | 0.043 | 0.12 | 0.16 | |
| C3 | 0.014 | 0.107 | 0.021 | 0.114 | 0.07 | 0.43 | |
| 5l | N1 | 0.031 | 0.130 | 0.023 | 0.155 | 0.06 | 0.56 |
| N2 | 0.044 | 0.028 | 0.027 | 0.041 | 0.07 | 0.15 | |
| C3 | 0.051 | 0.100 | 0.052 | 0.098 | 0.14 | 0.35 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filkina, M.E.; Baray, D.N.; Beloglazkina, E.K.; Grishin, Y.K.; Roznyatovsky, V.A.; Kukushkin, M.E. Regioselective Cycloaddition of Nitrile Imines to 5-Methylidene-3-phenyl-hydantoin: Synthesis and DFT Calculations. Int. J. Mol. Sci. 2023, 24, 1289. https://doi.org/10.3390/ijms24021289
Filkina ME, Baray DN, Beloglazkina EK, Grishin YK, Roznyatovsky VA, Kukushkin ME. Regioselective Cycloaddition of Nitrile Imines to 5-Methylidene-3-phenyl-hydantoin: Synthesis and DFT Calculations. International Journal of Molecular Sciences. 2023; 24(2):1289. https://doi.org/10.3390/ijms24021289
Chicago/Turabian StyleFilkina, Maria E., Daria N. Baray, Elena K. Beloglazkina, Yuri K. Grishin, Vitaly A. Roznyatovsky, and Maxim E. Kukushkin. 2023. "Regioselective Cycloaddition of Nitrile Imines to 5-Methylidene-3-phenyl-hydantoin: Synthesis and DFT Calculations" International Journal of Molecular Sciences 24, no. 2: 1289. https://doi.org/10.3390/ijms24021289
APA StyleFilkina, M. E., Baray, D. N., Beloglazkina, E. K., Grishin, Y. K., Roznyatovsky, V. A., & Kukushkin, M. E. (2023). Regioselective Cycloaddition of Nitrile Imines to 5-Methylidene-3-phenyl-hydantoin: Synthesis and DFT Calculations. International Journal of Molecular Sciences, 24(2), 1289. https://doi.org/10.3390/ijms24021289







