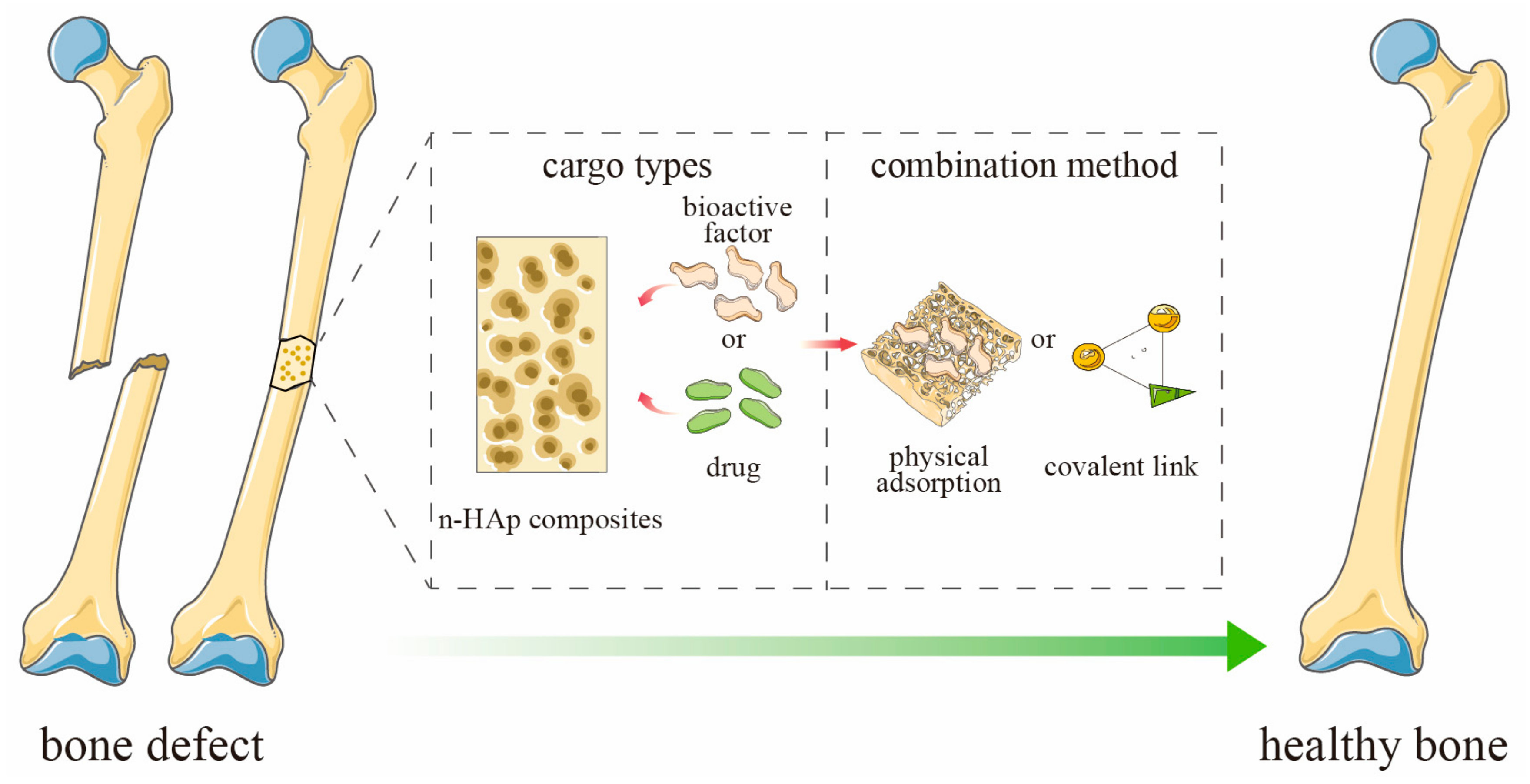
Nano-Hydroxyapatite Composite Scaffolds Loaded with Bioactive Factors and Drugs for Bone Tissue Engineering
Abstract
:1. Introduction
2. Structure and Properties of n-HAp
3. n-HAp Composite Scaffolds for Bone Repair
3.1. n-HAp/Natural Polymers Composite Scaffolds
3.2. n-HAp/Synthetic Polymers Composite Scaffolds
4. Bioactive Factors-Loaded n-HAp Composite Scaffolds
4.1. Growth Factors
4.1.1. BMPs
4.1.2. VEGF
4.1.3. bFGF
4.1.4. Combined Application of Growth Factors
4.2. Polypeptides
4.3. Vitamins
5. Drug-Loaded n-HAp Composite Scaffolds
5.1. Antibacterial Drugs
5.2. Antitumor Drugs
5.3. Anti-Osteoporotic Drugs
5.4. Anti-Tuberculosis Drugs
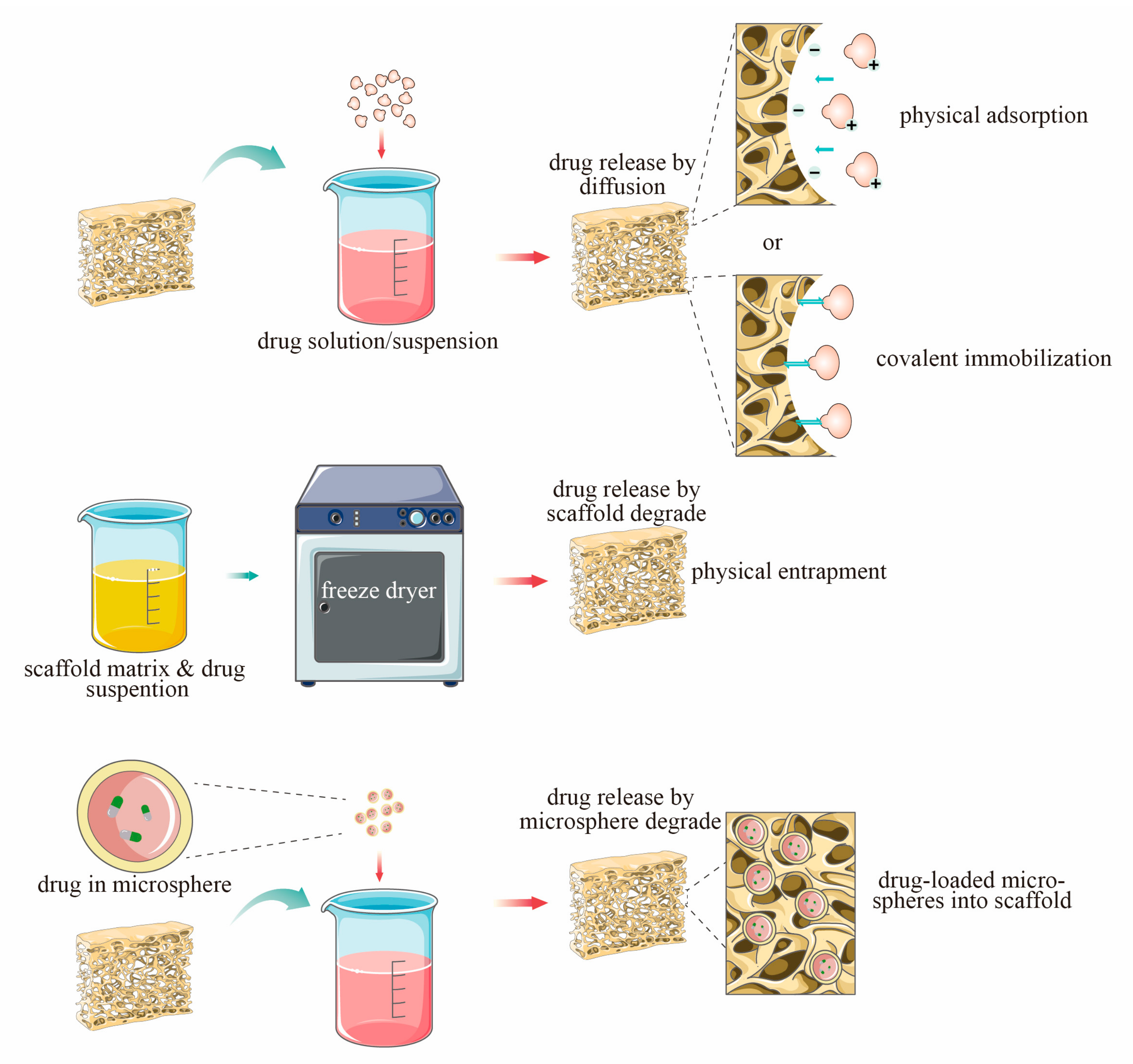
6. Loading Mode of n-HAp Composite Scaffolds
6.1. Physical Combination
6.2. Coating
6.3. Microsphere Loading System
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, W.; Gao, C.; Feng, P.; Liu, Q.; Liu, C.; Wang, Z.; Deng, Y.; Shuai, C. Dual-functional scaffolds of poly(L-lactic acid)/nanohydroxyapatite encapsulated with metformin: Simultaneous enhancement of bone repair and bone tumor inhibition. Mater. Sci. Eng. C 2021, 120, 111592. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zheng, P.; Gao, F.; Wang, W.; Zhang, H.; Zhang, X.; Feng, X.; Liu, W. A Mineralized High Strength and Tough Hydrogel for Skull Bone Regeneration. Adv. Funct. Mater. 2017, 27, 1604327. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Zhao, Z.; Wang, X.; Mikos, A.G.; Qiu, Z.; Song, T.; Sun, X.; Zhao, L.; Zhang, C.; et al. Mineralized Collagen-Based Composite Bone Materials for Cranial Bone Regeneration in Developing Sheep. ACS Biomater. Sci. Eng. 2017, 3, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, M.; Mottaghitalab, F.; Shokrgozar, M.A.; Ou, K.-L.; Mao, C.; Hosseinkhani, H. Importance of dual delivery systems for bone tissue engineering. J. Control. Release 2016, 225, 152–169. [Google Scholar] [CrossRef]
- Norbertczak, H.T.; Fermor, H.L.; Edwards, J.H.; Rooney, P.; Ingham, E.; Herbert, A. Decellularised human bone allograft from different anatomical sites as a basis for functionally stratified repair material for bone defects. J. Mech. Behav. Biomed. Mater. 2022, 125, 104965. [Google Scholar] [CrossRef]
- Al-Nawas, B.; Schiegnitz, E. Augmentation procedures using bone substitute materials or autogenous bone—A systematic review and meta-analysis. Eur. J. Oral Implantol. 2014, 7 (Suppl. S2), S219–S234. [Google Scholar]
- Aslam Khan, M.U.; Mehboob, H.; Abd Razak, S.I.; Yahya, M.Y.; Mohd Yusof, A.H.; Ramlee, M.H.; Anand, T.J.S.; Hassan, R.; Aziz, A.; Amin, R. Development of Polymeric Nanocomposite (Xyloglucan-co-Methacrylic Acid/Hydroxyapatite/SiO(2)) Scaffold for Bone Tissue Engineering Applications-In-Vitro Antibacterial, Cytotoxicity and Cell Culture Evaluation. Polymers 2020, 12, 1238. [Google Scholar] [CrossRef]
- Rider, P.; Kačarević, P.; Alkildani, S.; Retnasingh, S.; Schnettler, R.; Barbeck, M. Additive Manufacturing for Guided Bone Regeneration: A Perspective for Alveolar Ridge Augmentation. Int. J. Mol. Sci. 2018, 19, 3308. [Google Scholar] [CrossRef] [Green Version]
- Monroe, E.; Bass, D.; Votava, W.; Mc Mullen, J. New Calcium Phosphate Ceramic Material for Bone and Tooth Implants. J. Dent. Res. 1971, 50, 860–861. [Google Scholar] [CrossRef]
- Jarcho, M.; Bolen, C.H.; Thomas, M.B.; Bobick, J.; Kay, J.F.; Doremus, R.H. Hydroxylapatite synthesis and characterization in dense polycrystalline form. J. Mater. Sci. 1976, 11, 2027–2035. [Google Scholar] [CrossRef]
- Rodrigues, C.; Serricella, P.; Linhares, A.; Guerdes, R.; Borojevic, R.; Rossi, M.; Duarte, M.; Farina, M. Characterization of a bovine collagen–hydroxyapatite composite scaffold for bone tissue engineering. Biomaterials 2003, 24, 4987–4997. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cao, N.; Zhang, Y.; Cao, G.; Hao, C.; Liu, K.; Li, X.; Wang, W. The Ability and Mechanism of nHAC/CGF in Promoting Osteogenesis and Repairing Mandibular Defects. Nanomaterials 2022, 12, 212. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Feng, X.; Cao, G.; She, Z.; Tan, R.; Aifantis, K.E.; Zhang, R.; Li, X. The effect of carbon nanotubes on osteogenic functions of adipose-derived mesenchymal stem cells in vitro and bone formation in vivo compared with that of nano-hydroxyapatite and the possible mechanism. Bioact. Mater. 2020, 6, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Shahrezaie, M.; Moshiri, A.; Shekarchi, B.; Oryan, A.; Maffulli, N.; Parvizi, J. Effectiveness of tissue engineered three-dimensional bioactive graft on bone healing and regeneration: An in vivo study with significant clinical value. J. Tissue Eng. Regen. Med. 2017, 12, 936–960. [Google Scholar] [CrossRef] [PubMed]
- Kubasiewicz-Ross, P.; Hadzik, J.; Seeliger, J.; Kozak, K.; Jurczyszyn, K.; Gerber, H.; Dominiak, M.; Kunert-Keil, C. New nano-hydroxyapatite in bone defect regeneration: A histological study in rats. Ann. Anat. Anat. Anz. 2017, 213, 83–90. [Google Scholar] [CrossRef]
- Chen, X.; Wu, D.; Xu, J.; Yan, T.; Chen, Q. Gelatin/Gelatin-modified nano hydroxyapatite composite scaffolds with hollow channel arrays prepared by extrusion molding for bone tissue engineering. Mater. Res. Express 2021, 8, 015027. [Google Scholar] [CrossRef]
- Bal, Z.; Korkusuz, F.; Ishiguro, H.; Okada, R.; Kushioka, J.; Chijimatsu, R.; Kodama, J.; Tateiwa, D.; Ukon, Y.; Nakagawa, S.; et al. A novel nano-hydroxyapatite/synthetic polymer/bone morphogenetic protein-2 composite for efficient bone regeneration. Spine J. 2021, 21, 865–873. [Google Scholar] [CrossRef]
- Farrokhi-Rad, M.; Shahrabi, T.; Mahmoodi, S.; Khanmohammadi, S. Electrophoretic deposition of hydroxyapatite-chitosan-CNTs nanocomposite coatings. Ceram. Int. 2017, 43, 4663–4669. [Google Scholar] [CrossRef]
- Safari, B.; Davaran, S.; Aghanejad, A. Osteogenic potential of the growth factors and bioactive molecules in bone regeneration. Int. J. Biol. Macromol. 2021, 175, 544–557. [Google Scholar] [CrossRef]
- Vo, T.N.; Kasper, F.; Mikos, A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Meng, C.-X.; Lv, Z.-Y.; Zhang, Y.-J.; Li, J.; Li, K.-Y.; Liu, F.-Z.; Zhang, B.; Cui, F.-Z. Enhancement of BMP-2 and VEGF carried by mineralized collagen for mandibular bone regeneration. Regen. Biomater. 2020, 7, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Gao, Y.; Lu, G.; Gong, Y.; Zhao, N.; Zhang, X. A study on the bioactivity of chitosan/nano-hydroxyapatite composite scaffolds for bone tissue engineering. Eur. Polym. J. 2006, 42, 3171–3179. [Google Scholar] [CrossRef]
- Jackson, B.K.; Bow, A.; Kannarpady, G.; Biris, A.S.; Anderson, D.E.; Dhar, M.; Bourdo, S.E. Polyurethane/nano-hydroxyapatite composite films as osteogenic platforms. J. Biomater. Sci. Polym. Ed. 2018, 29, 1426–1443. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhang, M.; Hai, Z.; Wu, C.; Lin, J.; Kuang, W.; Tang, H.; Huang, Y.; Chen, X.; Liang, G. Sustained Release of Two Bioactive Factors from Supramolecular Hydrogel Promotes Periodontal Bone Regeneration. ACS Nano 2019, 13, 5616–5622. [Google Scholar] [CrossRef]
- Bretschneider, H.; Quade, M.; Lode, A.; Gelinsky, M.; Rammelt, S.; Vater, C. Chemotactic and Angiogenic Potential of Mineralized Collagen Scaffolds Functionalized with Naturally Occurring Bioactive Factor Mixtures to Stimulate Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 5836. [Google Scholar] [CrossRef]
- Ferracini, R.; Herreros, I.M.; Russo, A.; Casalini, T.; Rossi, F.; Perale, G. Scaffolds as Structural Tools for Bone-Targeted Drug Delivery. Pharmaceutics 2018, 10, 122. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Zhang, D.; Wang, W. Nanoparticle-Based Drug Delivery System—A Target Strategy for Osteoarthritis Treatment. J. Nanomater. 2021, 2021, 4064983. [Google Scholar] [CrossRef]
- Wu, H.; Lei, P.; Liu, G.; Zhang, Y.S.; Yang, J.; Zhang, L.; Xie, J.; Niu, W.; Liu, H.; Ruan, J.; et al. Reconstruction of Large-scale Defects with a Novel Hybrid Scaffold Made from Poly(L-lactic acid)/Nanohydroxyapatite/Alendronate-loaded Chitosan Microsphere: In vitro and in vivo Studies. Sci. Rep. 2017, 7, 359. [Google Scholar] [CrossRef]
- Dorati, R.; DeTrizio, A.; Modena, T.; Conti, B.; Benazzo, F.; Gastaldi, G.; Genta, I. Biodegradable Scaffolds for Bone Regeneration Combined with Drug-Delivery Systems in Osteomyelitis Therapy. Pharmaceuticals 2017, 10, 96. [Google Scholar] [CrossRef] [Green Version]
- Soundrapandian, C.; Datta, S.; Kundu, B.; Basu, D.; Sa, B. Porous Bioactive Glass Scaffolds for Local Drug Delivery in Osteomyelitis: Development and In Vitro Characterization. AAPS PharmSciTech 2010, 11, 1675–1683. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, T.; Ma, H.; Zhai, D.; Deng, C.; Wang, J.; Zhuo, S.; Chang, J.; Wu, C. 3D-printed scaffolds with bioactive elements-induced photothermal effect for bone tumor therapy. Acta Biomater. 2018, 73, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Correa-Piña, B.A.; Gomez-Vazquez, O.M.; Londoño-Restrepo, S.M.; Zubieta-Otero, L.F.; Millan-Malo, B.M.; Rodriguez-García, M.E. Synthesis and characterization of nano-hydroxyapatite added with magnesium obtained by wet chemical precipitation. Prog. Nat. Sci. 2021, 31, 575–582. [Google Scholar] [CrossRef]
- Londoño-Restrepo, S.M.; Jeronimo-Cruz, R.; Millán-Malo, B.M.; Rivera-Muñoz, E.M.; Rodriguez-García, M.E. Effect of the Nano Crystal Size on the X-ray Diffraction Patterns of Biogenic Hydroxyapatite from Human, Bovine, and Porcine Bones. Sci. Rep. 2019, 9, 1–12. [Google Scholar]
- Kumar Balu, S.; Andra, S.; Jeevanandam, J.; Sampath, V. Emerging marine derived nanohydroxyapatite and their composites for implant and biomedical applications. J. Mech. Behav. Biomed. Mater. 2021, 119, 104523. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Gutierrez, C.; Lujan-Cabrera, I.; Valencia-Molina, L.; Castillo-Chamorro, J.; Londoño-Restrepo, S. Thermal behavior of porcine biogenic hydroxyapatite: An in-situ impedance spectroscopy study. Mater. Today Commun. 2022, 33, 104188. [Google Scholar] [CrossRef]
- Swain, S.; Bhaskar, R.; Gupta, M.K.; Sharma, S.; Dasgupta, S.; Kumar, A.; Kumar, P. Mechanical, Electrical, and Biological Properties of Mechanochemically Processed Hydroxyapatite Ceramics. Nanomaterials 2021, 11, 2216. [Google Scholar] [CrossRef]
- Londoño-Restrepo, S.M.; Millán-Malo, B.M.; del Real-López, A.; Rodriguez-García, M.E. In situ study of hydroxyapatite from cattle during a controlled calcination process using HT-XRD. Mater. Sci. Eng. C 2019, 105, 110020. [Google Scholar] [CrossRef]
- Castillo-Paz, A.M.; Londoño-Restrepo, S.M.; Tirado-Mejía, L.; Mondragón, M.; Rodríguez-García, M.E. Nano to micro size transition of hydroxyapatite in porcine bone during heat treatment with low heating rates. Prog. Nat. Sci. 2020, 30, 494–501. [Google Scholar] [CrossRef]
- Horiuchi, N.; Madokoro, K.; Nozaki, K.; Nakamura, M.; Katayama, K.; Nagai, A.; Yamashita, K. Electrical conductivity of polycrystalline hydroxyapatite and its application to electret formation. Solid State Ionics 2018, 315, 19–25. [Google Scholar] [CrossRef]
- Maiti, M.; Sarkar, M.; Liu, D.; Xu, S.; Maiti, S.; Paul, B.K.; Das, S. Tungsten doped hydroxyapatite processed at different temperatures: Dielectric behaviour and anti-microbial properties. New J. Chem. 2018, 42, 16948–16959. [Google Scholar] [CrossRef]
- Gittings, J.; Bowen, C.; Dent, A.; Turner, I.; Baxter, F.; Chaudhuri, J. Electrical characterization of hydroxyapatite-based bioceramics. Acta Biomater. 2009, 5, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Vazquez, O.M.; Correa-Piña, B.A.; Zubieta-Otero, L.F.; Castillo-Paz, A.M.; Londoño-Restrepo, S.M.; Rodriguez-García, M.E. Synthesis and characterization of bioinspired nano-hydroxyapatite by wet chemical precipitation. Ceram. Int. 2021, 47, 32775–32785. [Google Scholar] [CrossRef]
- Ramirez-Gutierrez, C.; Londoño-Restrepo, S.; del Real, A.; Mondragón, M.; Rodriguez-García, M. Effect of the temperature and sintering time on the thermal, structural, morphological, and vibrational properties of hydroxyapatite derived from pig bone. Ceram. Int. 2017, 43, 7552–7559. [Google Scholar] [CrossRef]
- Londoño-Restrepo, S.M.; Jeronimo-Cruz, R.; Rubio-Rosas, E.; Rodriguez-García, M.E. The effect of cyclic heat treatment on the physicochemical properties of bio hydroxyapatite from bovine bone. J. Mater. Sci. Mater. Med. 2018, 29, 52. [Google Scholar] [CrossRef]
- Pal, A.; Nasker, P.; Paul, S.; Chowdhury, A.R.; Sinha, A.; Das, M. Strontium doped hydroxyapatite from Mercenaria clam shells: Synthesis, mechanical and bioactivity study. J. Mech. Behav. Biomed. Mater. 2018, 90, 328–336. [Google Scholar] [CrossRef]
- Wei, K.; Li, Y.; Kim, K.-O.; Nakagawa, Y.; Kim, B.-S.; Abe, K.; Chen, G.-Q.; Kim, I.-S. Fabrication of nano-hydroxyapatite on electrospun silk fibroin nanofiber and their effects in osteoblastic behavior. J. Biomed. Mater. Res. Part A 2011, 97, 272–280. [Google Scholar] [CrossRef]
- Liao, J.; Li, X.; He, W.; Guo, Q.; Fan, Y. A biomimetic triple-layered biocomposite with effective multifunction for dura repair. Acta Biomater. 2021, 130, 248–267. [Google Scholar] [CrossRef]
- El-Meliegy, E.; Mabrouk, M.; El-Sayed, S.A.; Abd El-Hady, B.M.; Shehata, M.R.; Hosny, W.M. Novel Fe2O3-doped glass/chitosan scaffolds for bone tissue replacement. Ceram. Int. 2018, 44, 9140–9151. [Google Scholar] [CrossRef]
- Atak, B.H.; Buyuk, B.; Huysal, M.; Isik, S.; Senel, M.; Metzger, W.; Cetin, G. Preparation and characterization of amine functional nano-hydroxyapatite/chitosan bionanocomposite for bone tissue engineering applications. Carbohydr. Polym. 2017, 164, 200–213. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Kong, M.; An, Y.; Liu, Y.; Li, J.J.; Zhou, X.; Feng, C.; Li, J.; Jiang, S.Y.; Cheng, X.J.; et al. Hydroxybutyl chitosan thermo-sensitive hydrogel: A potential drug delivery system. J. Mater. Sci. 2013, 48, 5614–5623. [Google Scholar] [CrossRef]
- Dinescu, S.; Ionita, M.; Ignat, S.-R.; Costache, M.; Hermenean, A. Graphene Oxide Enhances Chitosan-Based 3D Scaffold Properties for Bone Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 5077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatzipetros, E.; Christopoulos, P.; Donta, C.; Tosios, K.; Tsiambas, E.; Tsiourvas, D.; Kalogirou, E.; Tsiklakis, K. Application of nano-hydroxyapatite/chitosan scaffolds on rat calvarial critical-sized defects: A pilot study. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e625–e632. [Google Scholar] [CrossRef] [PubMed]
- Tsiourvas, D.; Sapalidis, A.; Papadopoulos, T. Hydroxyapatite/chitosan-based porous three-dimensional scaffolds with complex geometries. Mater. Today Commun. 2016, 7, 59–66. [Google Scholar] [CrossRef]
- Chatzipetros, E.; Yfanti, Z.; Christopoulos, P.; Donta, C.; Damaskos, S.; Tsiambas, E.; Tsiourvas, D.; Kalogirou, E.-M.; Tosios, K.I.; Tsiklakis, K. Imaging of nano-hydroxyapatite/chitosan scaffolds using a cone beam computed tomography device on rat calvarial defects with histological verification. Clin. Oral Investig. 2019, 24, 437–446. [Google Scholar] [CrossRef]
- Chatzipetros, E.; Damaskos, S.; Tosios, K.I.; Christopoulos, P.; Donta, C.; Kalogirou, E.-M.; Yfanti, Z.; Tsiourvas, D.; Papavasiliou, A.; Tsiklakis, K. The effect of nano-hydroxyapatite/chitosan scaffolds on rat calvarial defects for bone regeneration. Int. J. Implant. Dent. 2021, 7, 40. [Google Scholar] [CrossRef]
- Collins, A.M.; Skaer, N.J.V.; Gheysens, T.; Knight, D.; Bertram, C.; Roach, H.I.; Oreffo, R.O.C.; Von-Aulock, S.; Baris, T.; Skinner, J.; et al. Bone-like Resorbable Silk-based Scaffolds for Load-bearing Osteoregenerative Applications. Adv. Mater. 2009, 21, 75–78. [Google Scholar] [CrossRef]
- Liu, X.; Baldit, A.; De Brosses, E.; Velard, F.; Cauchois, G.; Chen, Y.; Wang, X.; De Isla, N.; Laurent, C. Characterization of Bone Marrow and Wharton’s Jelly Mesenchymal Stromal Cells Response on Multilayer Braided Silk and Silk/PLCL Scaffolds for Ligament Tissue Engineering. Polymers 2020, 12, 2163. [Google Scholar] [CrossRef]
- Ziadlou, R.; Rotman, S.; Teuschl, A.; Salzer, E.; Barbero, A.; Martin, I.; Alini, M.; Eglin, D.; Grad, S. Optimization of hyaluronic acid-tyramine/silk-fibroin composite hydrogels for cartilage tissue engineering and delivery of anti-inflammatory and anabolic drugs. Mater. Sci. Eng. C 2021, 120, 111701. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, L.; Li, Y.; Lou, X. Osteoblast-derived extracellular matrix coated PLLA/silk fibroin composite nanofibers promote osteogenic differentiation of bone mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2021, 110, 525–534. [Google Scholar] [CrossRef]
- Shao, Y.-F.; Qing, X.; Peng, Y.; Wang, H.; Shao, Z.; Zhang, K.-Q. Enhancement of mechanical and biological performance on hydroxyapatite/silk fibroin scaffolds facilitated by microwave-assisted mineralization strategy. Colloids Surf. B Biointerfaces 2020, 197, 111401. [Google Scholar] [CrossRef]
- Nie, L.; Zhang, H.; Ren, A.; Li, Y.; Fu, G.; Cannon, R.D.; Ji, P.; Wu, X.; Yang, S. Nano-hydroxyapatite mineralized silk fibroin porous scaffold for tooth extraction site preservation. Dent. Mater. 2019, 35, 1397–1407. [Google Scholar] [CrossRef]
- Mobika, J.; Rajkumar, M.; Priya, V.N.; Sibi, S.L. Substantial effect of silk fibroin reinforcement on properties of hydroxyapatite/silk fibroin nanocomposite for bone tissue engineering application. J. Mol. Struct. 2020, 1206, 127739. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Zhao, L.; Li, M.; Han, Y.; Wang, L.; Zhang, Z.; Li, J.; Zhou, C.; Liu, L. Fabrication and properties of PLA/nano-HA composite scaffolds with balanced mechanical properties and biological functions for bone tissue engineering application. Nanotechnol. Rev. 2021, 10, 1359–1373. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Garot, C.; Bettega, G.; Picart, C. Additive Manufacturing of Material Scaffolds for Bone Regeneration: Toward Application in the Clinics. Adv. Funct. Mater. 2020, 31, 2006967. [Google Scholar] [CrossRef]
- Chen, G.; Okamura, A.; Sugiyama, K.; Wozniak, M.J.; Kawazoe, N.; Sato, S.; Tateishi, T. Surface modification of porous scaffolds with nanothick collagen layer by centrifugation and freeze-drying. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 864–872. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.; Jiang, J.; Wu, Y.; Zhu, P.; Chen, G. 3D printed porous PLA/nHA composite scaffolds with enhanced osteogenesis and osteoconductivity in vivo for bone regeneration. Biomed. Mater. 2019, 14, 065003. [Google Scholar] [CrossRef]
- Zimmerling, A.; Yazdanpanah, Z.; Cooper, D.M.L.; Johnston, J.D.; Chen, X. 3D printing PCL/nHA bone scaffolds: Exploring the influence of material synthesis techniques. Biomater. Res. 2021, 25, 3. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Li, M.; Li, J.; Zhang, C.; Han, Y.; Wang, L.; Wang, K.; Zhou, C.; Liu, L.; et al. 3D printing of PLA/n-HA composite scaffolds with customized mechanical properties and biological functions for bone tissue engineering. Compos. Part B Eng. 2021, 224, 109192. [Google Scholar] [CrossRef]
- Hartatiek Fahmi, N.K.; Putra AA, D.; Yudyanto Nasikhudin Utomo, J.; Ahmad, N. Effect of nano-hydroxyapatite (n-HAp)/PLA scaffold composites on porosity and microstructure. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2020. [Google Scholar]
- Yoshimoto, H.; Shin, Y.; Terai, H.; Vacanti, J. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Cipitria, A.; Skelton, A.; Dargaville, T.R.; Dalton, P.D.; Hutmacher, D.W. Design, fabrication and characterization of PCL electrospun scaffolds—A review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Liao, S.; Liu, M.; Zhao, Q.; Zhu, Y. Polymer Composites Reinforced by Nanotubes as Scaffolds for Tissue Engineering. Int. J. Polym. Sci. 2014, 2014, 805634. [Google Scholar] [CrossRef]
- Juan, P.-K.; Fan, F.-Y.; Lin, W.-C.; Liao, P.-B.; Huang, C.-F.; Shen, Y.-K.; Ruslin, M.; Lee, C.-H. Bioactivity and Bone Cell Formation with Poly-ε-Caprolactone/Bioceramic 3D Porous Scaffolds. Polymers 2021, 13, 2718. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, B.; Liu, X.S.; Fields, A.J.; Sanyal, A.; Shi, X.; Adams, M.; Keaveny, T.M.; Guo, X.E. Trabecular plates and rods determine elastic modulus and yield strength of human trabecular bone. Bone 2015, 72, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Doyle, S.; Henry, L.; McGennisken, E.; Onofrillo, C.; Bella, C.; Duchi, S.; O’Connell, C.; Pirogova, E. Characterization of Polycaprolactone Nanohydroxyapatite Composites with Tunable Degradability Suitable for Indirect Printing. Polymers 2021, 13, 295. [Google Scholar] [CrossRef]
- Xue, W.; Chen, P.; Wang, F.; Wang, L. Melt spinning of nano-hydroxyapatite and polycaprolactone composite fibers for bone scaffold application. J. Mater. Sci. 2019, 54, 8602–8612. [Google Scholar] [CrossRef]
- Harikrishnan, P.; Islam, H.; Sivasamy, A. Biocompatibility Studies of Nanoengineered Polycaprolactone and Nanohydroxyapatite Scaffold for Craniomaxillofacial Bone Regeneration. J. Craniofacial Surg. 2019, 30, 265–269. [Google Scholar] [CrossRef]
- Badrossamay, M.R.; Balachandran, K.; Capulli, A.K.; Golecki, H.M.; Agarwal, A.; Goss, J.A.; Kim, H.; Shin, K.; Parker, K.K. Engineering hybrid polymer-protein super-aligned nanofibers via rotary jet spinning. Biomaterials 2014, 35, 3188–3197. [Google Scholar] [CrossRef] [Green Version]
- Andrade, T.M.; Mello, D.C.R.; Elias, C.M.V.; Abdala, J.M.A.; Silva, E.; Vasconcellos, L.M.R.; Tim, C.R.; Marciano, F.R.; Lobo, A.O. In vitro and in vivo evaluation of rotary-jet-spun poly(ɛ-caprolactone) with high loading of nano-hydroxyapatite. J. Mater. Sci. Mater. Med. 2019, 30, 19. [Google Scholar] [CrossRef]
- Vasconcellos, L.M.R.; Elias, C.D.M.V.; Minhoto, G.; Abdala, J.M.A.; Andrade, T.M.; De Araujo, J.C.R.; Gusmão, S.B.S.; Viana, B.; Marciano, F.R.; Lobo, A.O. Rotary-jet spun polycaprolactone/nano-hydroxyapatite scaffolds modified by simulated body fluid influenced the flexural mode of the neoformed bone. J. Mater. Sci. Mater. Med. 2020, 31, 72. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Li, X.; Zhang, R.; Tan, X.; Li, B.; Liu, Y.; Wang, X. Synthesis and Evaluation of BMMSC-seeded BMP-6/nHAG/GMS Scaffolds for Bone Regeneration. Int. J. Med. Sci. 2019, 16, 1007–1017. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, É.R.; Nie, L.; Podstawczyk, D.; Allahbakhsh, A.; Ratnayake, J.; Brasil, D.L.; Shavandi, A. Advances in Growth Factor Delivery for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 903. [Google Scholar] [CrossRef]
- E, L.-L.; Zhang, R.; Li, C.-J.; Zhang, S.; Ma, X.-C.; Xiao, R.; Liu, H.-C. Effects of rhBMP-2 on Bone Formation Capacity of Rat Dental Stem/Progenitor Cells from Dental Follicle and Alveolar Bone Marrow. Stem Cells Dev. 2021, 30, 441–457. [Google Scholar] [CrossRef]
- Herberg, S.; Susin, C.; Pelaez, M.; Howie, R.N.; Moreno de Freitas, R.; Lee, J.; Cray, J.J.; Johnson, M.H.; Elsalanty, M.E.; Hamrick, M.W.; et al. Low-dose bone morphogenetic protein-2/stromal cell-derived factor-1β cotherapy induces bone regeneration in critical-size rat calvarial defects. Tissue Eng. Part A 2014, 20, 1444–1453. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yin, H.-M.; Luo, E.; Zhu, S.; Wang, P.; Zhang, Z.; Liao, G.-Q.; Xu, J.-Z.; Li, Z.-M.; Li, J.-H. Accelerating Bone Healing by Decorating BMP-2 on Porous Composite Scaffolds. ACS Appl. Bio Mater. 2019, 2, 5717–5726. [Google Scholar] [CrossRef]
- Wang, B.; Yuan, S.; Xin, W.; Chen, Y.; Fu, Q.; Li, L.; Jiao, Y. Synergic adhesive chemistry-based fabrication of BMP-2 immobilized silk fibroin hydrogel functionalized with hybrid nanomaterial to augment osteogenic differentiation of rBMSCs for bone defect repair. Int. J. Biol. Macromol. 2021, 192, 407–416. [Google Scholar] [CrossRef]
- Yan, S.; Feng, L.; Zhu, Q.; Yang, W.; Lan, Y.; Li, D.; Liu, Y.; Xue, W.; Guo, R.; Wu, G. Controlled Release of BMP-2 from a Heparin-Conjugated Strontium-Substituted Nanohydroxyapatite/Silk Fibroin Scaffold for Bone Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 3291–3303. [Google Scholar] [CrossRef]
- Lin, Z.-Y.; Duan, Z.-X.; Guo, X.-D.; Li, J.-F.; Lu, H.-W.; Zheng, Q.-X.; Quan, D.-P.; Yang, S.-H. Bone induction by biomimetic PLGA-(PEG-ASP)n copolymer loaded with a novel synthetic BMP-2-related peptide in vitro and in vivo. J. Control. Release 2010, 144, 190–195. [Google Scholar] [CrossRef]
- Ye, K.; Liu, D.; Kuang, H.; Cai, J.; Chen, W.; Sun, B.; Xia, L.; Fang, B.; Morsi, Y.; Mo, X. Three-dimensional electrospun nanofibrous scaffolds displaying bone morphogenetic protein-2-derived peptides for the promotion of osteogenic differentiation of stem cells and bone regeneration. J. Colloid Interface Sci. 2018, 534, 625–636. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, K.; Liu, M.; Guo, X.; Qu, Y.; Cui, W.; Shao, Z.; Zhang, X.; Xu, S. Loading of BMP-2-related peptide onto three-dimensional nano-hydroxyapatite scaffolds accelerates mineralization in critical-sized cranial bone defects. J. Tissue Eng. Regen. Med. 2018, 12, 864–877. [Google Scholar] [CrossRef]
- Sun, T.; Qu, Y.; Cui, W.; Yang, L.; Ji, Y.; Yu, W.; Navinduth, R.; Shao, Z.; Yang, H.; Guo, X. Evaluation of osteogenic inductivity of a novel BMP2-mimicking peptide P28 and P28-containing bone composite. J. Biomed. Mater. Res. Part A 2017, 106, 210–220. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Ma, S.; Gao, Y.; Zuo, Y.; Hu, J. Enhancement of bone formation by BMP-7 transduced MSCs on biomimetic nano-hydroxyapatite/polyamide composite scaffolds in repair of mandibular defects. J. Biomed. Mater. Res. Part A 2010, 95, 973–981. [Google Scholar] [CrossRef]
- Toprak, Ö.; Topuz, B.; Monsef, Y.A.; Oto, Ç.; Orhan, K.; Karakeçili, A. BMP-6 carrying metal organic framework-embedded in bioresorbable electrospun fibers for enhanced bone regeneration. Mater. Sci. Eng. C 2020, 120, 111738. [Google Scholar] [CrossRef]
- Zhang, R.; Li, X.; Liu, Y.; Gao, X.; Zhu, T.; Lu, L. Acceleration of Bone Regeneration in Critical-Size Defect Using BMP-9-Loaded nHA/ColI/MWCNTs Scaffolds Seeded with Bone Marrow Mesenchymal Stem Cells. BioMed. Res. Int. 2019, 2019, 7343957. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, C.C.; Wang, C.Y.; Lee AK, X.; Yeh, C.L.; Lin, C.P. Assessment of the Release of Vascular Endothelial Growth Factor from 3D-Printed Poly-ε-Caprolactone/Hydroxyapatite/Calcium Sulfate Scaffold with Enhanced Osteogenic Capacity. Polymers 2020, 12, 1455. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Yang, G.; Hu, X.; Wang, L.; Liu, B.; Wang, J.; Zhang, S. Delivering Proangiogenic Factors from 3D-Printed Polycaprolactone Scaffolds for Vascularized Bone Regeneration. Adv. Healthc. Mater. 2020, 9, 2000727. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.-Y.; Chu, X.-Y.; Zheng, C.-Y.; Luan, Y.-Y.; He, X.; Yang, K.; Zhang, D.-L. VEGF-Loaded Heparinised Gelatine-Hydroxyapatite-Tricalcium Phosphate Scaffold Accelerates Bone Regeneration via Enhancing Osteogenesis-Angiogenesis Coupling. Front. Bioeng. Biotechnol. 2022, 10, 915181. [Google Scholar] [CrossRef]
- Quinlan, E.; López-Noriega, A.; Thompson, E.M.; Hibbitts, A.; Cryan, S.A.; O’Brien, F.J. Controlled release of vascular endothelial growth factor from spray-dried alginate microparticles in collagen-hydroxyapatite scaffolds for promoting vascularization and bone repair. J. Tissue Eng. Regen. Med. 2015, 11, 1097–1109. [Google Scholar] [CrossRef]
- Piard, C.; Luthcke, R.; Kamalitdinov, T.; Fisher, J. Sustained delivery of vascular endothelial growth factor from mesoporous calcium-deficient hydroxyapatite microparticles promotes in vitro angiogenesis and osteogenesis. J. Biomed. Mater. Res. Part A 2020, 109, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Luong, L.N.; Ramaswamy, J.; Kohn, D.H. Effects of osteogenic growth factors on bone marrow stromal cell differentiation in a mineral-based delivery system. Biomaterials 2012, 33, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Nakamura, O.; Tobiume, S.; Yamamoto, T.; Kaji, Y. Prefabrication of Vascularized Allogenic Bone Graft in a Rat by Implanting a Flow-Through Vascular Pedicle and Basic Fibroblast Growth Factor Containing Hydroxyapatite/Collagen Composite. J. Reconstr. Microsurg. 2017, 33, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, Y.; Zhou, A.; Chen, X.; Li, K.; Chen, S.; Qiao, B.; Jiang, D. Controlled release of basic fibroblast growth factor from a peptide biomaterial for bone regeneration. R. Soc. Open Sci. 2020, 7, 191830. [Google Scholar] [CrossRef]
- He, B.; Zhang, M.; Yin, L.; Quan, Z.; Ou, Y.; Huang, W. bFGF-incorporated composite biomaterial for bone regeneration. Mater. Des. 2022, 215, 110469. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Yao, Z.; Wang, C. Study of a new nano-hydroxyapatite/basic fibroblast growth factor composite promoting periodontal tissue regeneration. Mater. Express 2020, 10, 1802–1807. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kim, J.-J.; Kim, H.-W. Basic fibroblast growth factor-loaded, mineralized biopolymer-nanofiber scaffold improves adhesion and proliferation of rat mesenchymal stem cells. Biotechnol. Lett. 2013, 36, 383–390. [Google Scholar] [CrossRef]
- Cai, Y.; Tan, X.; Zhao, L.; Zhang, R.; Zhu, T.; Du, Y.; Wang, X. Withdrawal: Synthesis of a Novel bFGF/nHAP/COL Bone Tissue Engineering Scaffold for Mandibular Defect Regeneration in a Rabbit Model. J. Hard Tissue Biol. 2018, 27, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Kuttappan, S.; Mathew, D.; Jo, J.-I.; Tanaka, R.; Menon, D.; Ishimoto, T.; Nakano, T.; Nair, S.V.; Nair, M.B.; Tabata, Y. Dual release of growth factor from nanocomposite fibrous scaffold promotes vascularisation and bone regeneration in rat critical sized calvarial defect. Acta Biomater. 2018, 78, 36–47. [Google Scholar] [CrossRef]
- Wang, T.; Guo, S.; Zhang, H. Synergistic Effects of Controlled-Released BMP-2 and VEGF from nHAC/PLGAs Scaffold on Osteogenesis. BioMed Res. Int. 2018, 2018, 3516463. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Li, B.; Chen, L. Controlled dual delivery of low doses of BMP-2 and VEGF in a silk fibroin–nanohydroxyapatite scaffold for vascularized bone regeneration. J. Mater. Chem. B 2017, 5, 6963–6972. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Li, J.; Zhang, Z.; Li, Z.; Chi, H.; Song, C.; Wang, X.; Wang, Y.; Chen, G.; Yan, J. Nanohydroxyapatite/polyamide 66 crosslinked with QK and BMP-2-derived peptide prevented femur nonunion in rats. J. Mater. Chem. B 2021, 9, 2249–2265. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zheng, L.; Zhang, J.; Lin, L.; Shen, Y.; Zhang, X.; Wu, B. Dual delivery of bone morphogenetic protein-2 and basic fibroblast growth factor from nanohydroxyapatite/collagen for bone tissue engineering. Appl. Biol. Chem. 2019, 62, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Liu, Q.; Zheng, S.; Zhu, J.; Qi, Z.; Fu, C.; Yang, X.; Zhao, Y. Synergistic delivery of bFGF and BMP-2 from poly(l-lactic-co-glycolic acid)/graphene oxide/hydroxyapatite nanofibre scaffolds for bone tissue engineering applications. RSC Adv. 2018, 8, 31911–31923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Ren, J.; Ren, T.; Gu, S.; Tan, Q.; Zhang, L.; Lv, K.; Pan, K.; Jiang, X. Bone marrow stromal cells cultured on poly (lactide-co-glycolide)/nano-hydroxyapatite composites with chemical immobilization of Arg-Gly-Asp peptide and preliminary bone regeneration of mandibular defect thereof. J. Biomed. Mater. Res. A 2010, 95, 993–1003. [Google Scholar] [CrossRef]
- Sun, Y.; Deng, Y.; Ye, Z.; Liang, S.; Tang, Z.; Wei, S. Peptide decorated nano-hydroxyapatite with enhanced bioactivity and osteogenic differentiation via polydopamine coating. Colloids Surf. B Biointerfaces 2013, 111, 107–116. [Google Scholar] [CrossRef]
- Zhao, W.; He, B.; Zhou, A.; Li, Y.; Chen, X.; Yang, Q.; Chen, B.; Qiao, B.; Jiang, D. D-RADA16-RGD-Reinforced Nano-Hydroxyapatite/Polyamide 66 Ternary Biomaterial for Bone Formation. Tissue Eng. Regen. Med. 2019, 16, 177–189. [Google Scholar] [CrossRef]
- Teng, N.-C.; Pandey, A.; Hsu, W.-H.; Huang, C.-S.; Lee, W.-F.; Lee, T.-H.; Yang, T.C.-K.; Yang, T.-S.; Yang, J.-C. Rehardening and the Protective Effect of Gamma-Polyglutamic Acid/Nano-Hydroxyapatite Paste on Surface-Etched Enamel. Polymers 2021, 13, 4268. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Zeng, W.-Y. Adsorption of Cu(II) by Poly-γ-glutamate/Apatite Nanoparticles. Polymers 2021, 13, 962. [Google Scholar] [CrossRef]
- Shu, X.; Liao, J.; Wang, L.; Shi, Q.; Xie, X. Osteogenic, Angiogenic, and Antibacterial Bioactive Nano-Hydroxyapatite Co-Synthesized Using γ-Polyglutamic Acid and Copper. ACS BioMater. Sci. Eng. 2020, 6, 1920–1930. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Q.; Bai, B.-L.; Weng, S.-J.; Wu, Z.-Y.; Xie, Z.-J.; Feng, Z.-H.; Cheng, L.; Boodhun, V.; Yang, L. Effects of combined human parathyroid hormone (1–34) and menaquinone-4 treatment on the interface of hydroxyapatite-coated titanium implants in the femur of osteoporotic rats. J. Bone Miner. Metab. 2017, 36, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Wang, L.; Zhou, Z.; Sun, Q.; Liu, D.; Chen, Y.; Hu, H.; Cai, Y.; Lin, S.; Yu, Z.; et al. Simultaneous incorporation of PTH(1–34) and nano-hydroxyapatite into Chitosan/Alginate Hydrogels for efficient bone regeneration. Bioact. Mater. 2020, 6, 1839–1851. [Google Scholar] [CrossRef]
- Mahdavi, R.; Belgheisi, G.; Haghbin-Nazarpak, M.; Omidi, M.; Khojasteh, A.; Solati-Hashjin, M. Bone tissue engineering gelatin-hydroxyapatite/graphene oxide scaffolds with the ability to release vitamin D: Fabrication, characterization, and in vitro study. J. Mater. Sci. Mater. Med. 2020, 31, 97. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, R.K.; Perera, K.D.C.; Perera, W.P.T.D.; Wijesinghe, W.P.S.L.; Unagolla, J.M. Enteric Coated Oral Delivery of Hydroxyapatite Nanoparticle for Modified Release Vitamin D3 Formulation. J. Nanomater. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Sumathra, M.; Munusamy, M.A.; Alarfaj, A.A.; Rajan, M. Osteoblast response to Vitamin D3 loaded cellulose enriched hydroxyapatite Mesoporous silica nanoparticles composite. Biomed. Pharmacother. 2018, 103, 858–868. [Google Scholar] [CrossRef]
- Fayyazbakhsh, F.; Solati-Hashjin, M.; Keshtkar, A.; Shokrgozar, M.A.; Dehghan, M.M.; Larijani, B. Release behavior and signaling effect of vitamin D3 in layered double hydroxides-hydroxyapatite/gelatin bone tissue engineering scaffold: An in vitro evaluation. Colloids Surf. B Biointerfaces 2017, 158, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Ignjatović, N.; Uskoković, V.; Ajduković, Z.; Uskoković, D. Multifunctional hydroxyapatite and poly(d,l-lactide-co-glycolide) nanoparticles for the local delivery of cholecalciferol. Mater. Sci. Eng. C 2013, 33, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, N.; Bose, S. Controlled Delivery of Curcumin and Vitamin K2 from Hydroxyapatite-Coated Titanium Implant for Enhanced in Vitro Chemoprevention, Osteogenesis, and in Vivo Osseointegration. ACS Appl. Mater. Interfaces 2020, 12, 13644–13656. [Google Scholar] [CrossRef] [PubMed]
- Villa, J.K.D.; Diaz, M.A.N.; Pizziolo, V.R.; Martino, H.S.D. Effect of vitamin K in bone metabolism and vascular calcification: A review of mechanisms of action and evidences. Crit. Rev. Food Sci. Nutr. 2016, 57, 3959–3970. [Google Scholar] [CrossRef]
- Bügel, S. Vitamin K and Bone Health in Adult Humans. Vitam. Horm. 2008, 78, 393–416. [Google Scholar]
- Dai, Z.; Dang, M.; Zhang, W.; Murugan, S.; Teh, S.W.; Pan, H. Biomimetic hydroxyapatite/poly xylitol sebacic adibate/vitamin K nanocomposite for enhancing bone regeneration. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1898–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, S.; Lee, S.; Miyajima, T.; Kato, K.; Sugawara-Narutaki, A.; Sakurai, M.; Nagata, F. Evaluation of Drug-Loading Ability of Poly(Lactic Acid)/Hydroxyapatite Core-Shell Particles. Materials 2021, 14, 1959. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Botelho, M.; Lu, W.; Monmaturapoj, N. Development of nanocomposite collagen/HA/β-TCP scaffolds with tailored gradient porosity and permeability using vitamin E. J. Biomed. Mater. Res. Part A 2020, 108, 2379–2394. [Google Scholar] [CrossRef]
- Kremers, H.M.; Nwojo, M.E.; Ransom, J.E.; Wood-Wentz, C.M.; Melton, L.J., III; Huddleston, P.M., III. PM Trends in the epidemiology of osteomyelitis: A population-based study, 1969 to 2009. J. Bone Jt. Surg. Am. 2015, 97, 837–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials 2016, 81, 58–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Chen, C.; Liu, W.; He, X.; Zhou, N.; Zhang, D.; Gu, H.; Li, J.; Jiang, J.; Huang, W. Levofloxacin loaded mesoporous silica microspheres/nano-hydroxyapatite/polyurethane composite scaffold for the treatment of chronic osteomyelitis with bone defects. Sci. Rep. 2017, 7, 41808. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.-D.; Jiang, D.-M.; Yan, L.; Wu, J. Biosafety of the Novel Vancomycin-loaded Bone-like Hydroxyapatite/Poly-amino Acid Bony Scaffold. Chin. Med. J. 2016, 129, 194–199. [Google Scholar] [CrossRef]
- Egawa, S.; Hirai, K.; Matsumoto, R.; Yoshii, T.; Yuasa, M.; Okawa, A.; Sugo, K.; Sotome, S. Efficacy of Antibiotic-Loaded Hydroxyapatite/Collagen Composites Is Dependent on Adsorbability for Treating Staphylococcus aureus Osteomyelitis in Rats. J. Orthop. Res. 2020, 38, 843–851. [Google Scholar] [CrossRef] [Green Version]
- Padrão, T.; Coelho, C.C.; Costa, P.; Alegrete, N.; Monteiro, F.J.; Sousa, S.R. Combining local antibiotic delivery with heparinized nanohydroxyapatite/collagen bone substitute: A novel strategy for osteomyelitis treatment. Mater. Sci. Eng. C 2021, 119, 111329. [Google Scholar] [CrossRef]
- Rajendran, M.; Iraivan, G.; Ghayathri, B.L.; Mohan, P.; Chandran, K.R.; Nagaiah, H.P.; Selvaraj, R.C. Antibiotic Loaded Nano Rod Bone Cement for the Treatment of Osteomyelitis. Recent Patents Nanotechnol. 2021, 15, 70–89. [Google Scholar] [CrossRef]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R., Jr.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.G.; Biswas, R.; Menon, D.; Nair, M.B. Biodegradable nanocomposite fibrous scaffold mediated local delivery of vancomycin for the treatment of MRSA infected experimental osteomyelitis. Biomater. Sci. 2020, 8, 2653–2665. [Google Scholar] [CrossRef] [PubMed]
- Beenken, K.E.; Campbell, M.J.; Ramirez, A.M.; Alghazali, K.; Walker, C.M.; Jackson, B.; Griffin, C.; King, W.; Bourdo, S.E.; Rifkin, R.; et al. Evaluation of a bone filler scaffold for local antibiotic delivery to prevent Staphylococcus aureus infection in a contaminated bone defect. Sci. Rep. 2021, 11, 10254. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.-F.; Zhou, D.-M.; Sun, X.-H.; Zhao, Z. Nano Sized Hydroxyapatite-Polylactic Acid-Vancomycin in Alleviation of Chronic Osteomyelitis. Drug Des. Dev. Ther. 2022, 16, 1983–1993. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Liapikou, A. Levofloxacin for the treatment of respiratory tract infections. Expert Opin. Pharmacother. 2012, 13, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, Y.; Jiang, J.; Yan, Y.; Fan, N.; Yang, X.; Zuo, Y.; Li, Y.; Gu, H.; Li, J. Development of an Antibacterial Bone Graft by Immobilization of Levofloxacin Hydrochloride-Loaded Mesoporous Silica Microspheres on a Porous Scaffold Surface. J. Biomed. Nanotechnol. 2019, 15, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Z.; Dai, G.; Wan, R.; Zhang, D.; Zhao, C.; Chen, C.; Li, J.; Gu, H.; Huang, W. Osteogenic and antibacterial dual functions of a novel levofloxacin loaded mesoporous silica microspheres/nano-hydroxyapatite/polyurethane composite scaffold. Genes Dis. 2019, 8, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Cortini, M.; Baldini, N.; Avnet, S. New Advances in the Study of Bone Tumors: A Lesson From the 3D Environment. Front. Physiol. 2019, 10, 814. [Google Scholar] [CrossRef] [Green Version]
- Biermann, J.S.; Adkins, D.R.; Agulnik, M.; Benjamin, R.S.; Brigman, B.; Butrynski, J.E.; Cheong, D.; Chow, W.; Curry, W.T.; Frassica, D.A.; et al. Bone cancer. J. Natl. Compr. Cancer Netw. 2013, 11, 688–723. [Google Scholar] [CrossRef]
- Bădilă, A.E.; Rădulescu, D.M.; Niculescu, A.-G.; Grumezescu, A.M.; Rădulescu, M.; Rădulescu, A.R. Recent Advances in the Treatment of Bone Metastases and Primary Bone Tumors: An Up-to-Date Review. Cancers 2021, 13, 4229. [Google Scholar] [CrossRef]
- Yang, X.; Zhai, D.; Song, J.; Qing, R.; Wang, B.; Ji, J.; Chen, X.; Hao, S. Rhein-PEG-nHA conjugate as a bone targeted drug delivery vehicle for enhanced cancer chemoradiotherapy. Nanomedicine 2020, 27, 102196. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ghosh, S.; Pramanik, N. Bio-evaluation of doxorubicin (DOX)-incorporated hydroxyapatite (HAp)-chitosan (CS) nanocomposite triggered on osteosarcoma cells. Adv. Compos. Hybrid Mater. 2020, 3, 303–314. [Google Scholar] [CrossRef]
- Rong, Z.-J.; Yang, L.-J.; Cai, B.-T.; Zhu, L.-X.; Cao, Y.-L.; Wu, G.-F.; Zhang, Z.-J. Porous nano-hydroxyapatite/collagen scaffold containing drug-loaded ADM–PLGA microspheres for bone cancer treatment. J. Mater. Sci. Mater. Med. 2016, 27, 89. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, Y.; Yang, Z.; Zuo, G.; Zhang, Q.; Yao, F.; Wan, Y. Encapsulating doxorubicin-intercalated lamellar nanohydroxyapatite into PLGA nanofibers for sustained drug release. Curr. Appl. Phys. 2019, 19, 1204–1210. [Google Scholar] [CrossRef]
- Lu, Y.; Wan, Y.; Gan, D.; Zhang, Q.; Luo, H.; Deng, X.; Li, Z.; Yang, Z. Enwrapping Polydopamine on Doxorubicin-Loaded Lamellar Hydroxyapatite/Poly(lactic-co-glycolic acid) Composite Fibers for Inhibiting Bone Tumor Recurrence and Enhancing Bone Regeneration. ACS Appl. Bio Mater. 2021, 4, 6036–6045. [Google Scholar] [CrossRef]
- Teufelsbauer, M.; Lang, C.; Plangger, A.; Rath, B.; Moser, D.; Staud, C.; Radtke, C.; Neumayer, C.; Hamilton, G. Effects of metformin on human bone-derived mesenchymal stromal cell—Breast cancer cell line interactions. Med. Oncol. 2022, 39, 54. [Google Scholar] [CrossRef]
- Afshari, K.; Dehdashtian, A.; Haddadi, N.-S.; Haj-Mirzaian, A.; Iranmehr, A.; Ebrahimi, M.A.; Tavangar, S.M.; Faghir-Ghanesefat, H.; Mohammadi, F.; Rahimi, N.; et al. Anti-inflammatory effects of Metformin improve the neuropathic pain and locomotor activity in spinal cord injured rats: Introduction of an alternative therapy. Spinal Cord 2018, 56, 1032–1041. [Google Scholar] [CrossRef]
- Conry, R.M.; Rodriguez, M.G.; Pressey, J.G. Zoledronic acid in metastatic osteosarcoma: Encouraging progression free survival in four consecutive patients. Clin. Sarcoma Res. 2016, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Li, M.; Li, L.; Wei, S.; Hu, X.; Wang, X.; Shan, G.; Zhang, Y.; Xia, H.; Yin, Q. High-activity chitosan/nano hydroxyapatite/zoledronic acid scaffolds for simultaneous tumor inhibition, bone repair and infection eradication. Mater. Sci. Eng. C 2018, 82, 225–233. [Google Scholar] [CrossRef]
- Sartori, A.R.; Moreira, J.A.; Santos, A.M.M.; Cintra, D.E.C.; Sartori, L.R.; Baraúna, M.A.; Canto, R.S.T. Bone repair process in normal and osteopenic female rats’ tibiae: A comparative study. Acta Ortopédica Bras. 2008, 16, 37–40. [Google Scholar] [CrossRef]
- Chandran, S.; S, S.B.; Vs, H.K.; Varma, H.; John, A. Osteogenic efficacy of strontium hydroxyapatite micro-granules in osteoporotic rat model. J. Biomater. Appl. 2016, 31, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Tella, S.H.; Gallagher, J.C. Prevention and treatment of postmenopausal osteoporosis. J. Steroid Biochem. Mol. Biol. 2014, 142, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Miladi, K.; Sfar, S.; Fessi, H.; Elaissari, A. Drug carriers in osteoporosis: Preparation, drug encapsulation and applications. Int. J. Pharm. 2013, 445, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.K.; Chandran, S.; Sreelatha, H.V.; John, A.; Parameswaran, R. Pamidronate-Encapsulated Electrospun Polycaprolactone-Based Composite Scaffolds for Osteoporotic Bone Defect Repair. ACS Appl. Bio Mater. 2020, 3, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, R.; Xie, C.; Liang, Q.; Xiao, X. Biomimetic mineralization of nanocrystalline hydroxyapatites on aminated modified polylactic acid microspheres to develop a novel drug delivery system for alendronate. Mater. Sci. Eng. C 2020, 110, 110655. [Google Scholar] [CrossRef] [PubMed]
- Inada, M.; Tsukamoto, K.; Hirata, M.; Takita, M.; Nagasawa, K.; Miyaura, C. Novel vitamin D3 analogs, 1α, 25(OH)2D3-26, 23-lactam (DLAMs), antagonize bone resorption via suppressing RANKL expression in osteoblasts. Biochem. Biophys. Res. Commun. 2008, 372, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tang, Q.; Yan, D.; Zheng, G.; Gu, M.; Luo, Z.; Mao, C.; Qian, Z.; Ni, W.; Shen, L. A multi-functionalized calcitriol sustainable delivery system for promoting osteoporotic bone regeneration both in vitro and in vivo. Appl. Mater. Today 2020, 22, 100906. [Google Scholar] [CrossRef]
- Murgia, D.; Mauceri, R.; Campisi, G.; De Caro, V. Advance on Resveratrol Application in Bone Regeneration: Progress and Perspectives for Use in Oral and Maxillofacial Surgery. Biomolecules 2019, 9, 94. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Zhou, H.; Wang, X.; Mai, K.; He, G. Resveratrol attenuates oxidative stress and inflammatory response in turbot fed with soybean meal based diet. Fish Shellfish. Immunol. 2019, 91, 130–135. [Google Scholar] [CrossRef]
- Li, L.; Yu, M.; Li, Y.; Li, Q.; Yang, H.; Zheng, M.; Han, Y.; Lu, D.; Lu, S.; Gui, L. Synergistic anti-inflammatory and osteogenic n-HA/resveratrol/chitosan composite microspheres for osteoporotic bone regeneration. Bioact. Mater. 2020, 6, 1255–1266. [Google Scholar] [CrossRef]
- Pang, W.-Y.; Wang, X.-L.; Mok, S.-K.; Lai, W.-P.; Chow, H.K.D.; Leung, P.-C.; Yao, X.-S.; Wong, M.S. Naringin improves bone properties in ovariectomized mice and exerts oestrogen-like activities in rat osteoblast-like (UMR-106) cells. Br. J. Pharmacol. 2010, 159, 1693–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Shen, G.; Shang, Q.; Zhang, Z.; Zhao, W.; Zhang, P.; Liang, D.; Ren, H.; Jiang, X. A Naringin-loaded gelatin-microsphere/nano-hydroxyapatite/silk fibroin composite scaffold promoted healing of critical-size vertebral defects in ovariectomised rat. Int. J. Biol. Macromol. 2021, 193, 510–518. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Ma, Y.; Tang, D.; Chen, Y.; Luo, F.; Li, D.; Hou, T.; Zhou, Q.; Dai, F.; et al. Surgical strategy and management outcomes for adjacent multisegmental spinal tuberculosis: A retrospective study of forty-eight patients. Spine 2014, 39, E40–E48. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.K.; Blumberg, H. Musculoskeletal Tuberculosis. Microbiol. Spectr. 2017, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, K.; Zhu, Y.; Zhang, J.; Ye, X. 3D-printed hierarchical scaffold for localized isoniazid/rifampin drug delivery and osteoarticular tuberculosis therapy. Acta Biomater. 2015, 16, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Broderick, C.; Hopkins, S.; Mack, D.J.F.; Aston, W.; Pollock, R.; Skinner, J.A.; Warren, S. Delays in the diagnosis and treatment of bone and joint tuberculosis in the United Kingdom. Bone Jt. J. 2018, 100, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Qian, H.; Lei, T.; Liu, W.; He, X.; Zhang, Y.; Lei, P.; Hu, Y. Anti-tuberculosis drug delivery for tuberculous bone defects. Expert Opin. Drug Deliv. 2022, 18, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- Mulla, J.A.; Mabrouk, M.; Choonara, Y.E.; Kumar, P.; Chejara, D.R.; du Toit, L.C.; Pillay, V. Development of respirable rifampicin-loaded nano-lipomer composites by microemulsion-spray drying for pulmonary delivery. J. Drug Deliv. Sci. Technol. 2017, 41, 13–19. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, H.; Wu, F.; Gan, X. Synthesis and characterization of an injectable rifampicin-loaded chitosan/hydroxyapatite bone cement for drug delivery. J. Mater. Res. 2021, 36, 487–498. [Google Scholar] [CrossRef]
- Xie, T.; Song, Y.; Peng, H.; Dai, Z.; Kang, Y.; Xiu, P.; Wang, L.; Li, H.; Yang, X. A bioactive implant combining isoniazid with nanohydroxyapatite/polyamide 66 for the treatment of osteoarticular tuberculosis. Mater. Des. 2021, 210, 110064. [Google Scholar] [CrossRef]
- QQayoom, I.; Verma, R.; Murugan, P.A.; Raina, D.B.; Teotia, A.K.; Matheshwaran, S.; Nair, N.N.; Tägil, M.; Lidgren, L.; Kumar, A. A biphasic nanohydroxyapatite/calcium sulphate carrier containing Rifampicin and Isoniazid for local delivery gives sustained and effective antibiotic release and prevents biofilm formation. Sci. Rep. 2020, 10, 14128. [Google Scholar] [CrossRef] [PubMed]
- Praphakar, R.A.; Sumathra, M.; Ebenezer, R.S.; Vignesh, S.; Shakila, H.; Rajan, M. Fabrication of bioactive rifampicin loaded κ-Car-MA-INH/Nano hydroxyapatite composite for tuberculosis osteomyelitis infected tissue regeneration. Int. J. Pharm. 2019, 565, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, L.; Ruan, Q.; Gao, A.; Liao, Q.; Mo, S.; Lv, Y.; Tong, L.; Wang, H.; Chu, P.K.; et al. Diamond-like carbon coating and surface grafting of osteoprotegerin and alendronate on polyetheretherketone to ameliorate the mechanical performance and osseointegration simultaneously. Compos. Part B Eng. 2022, 236, 109815. [Google Scholar] [CrossRef]
- Zhou, L.; Du, B.; Liu, W.; Deng, Y.; Li, S.; Liu, X.; Gao, Y. Angiogenesis and bone regeneration of porous nano-hydroxyapatite/coralline blocks coated with rhVEGF165 in critical-size alveolar bone defects in vivo. Int. J. Nanomed. 2015, 10, 2555–2565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, H.; Zheng, Q.; Yang, S.; Guo, X. Effects of functionalization of PLGA-[Asp-PEG] n copolymer surfaces with Arg-Gly-Asp peptides, hydroxyapatite nanoparticles, and BMP-2-derived peptides on cell behavior in vitro. J. Biomed. Mater. Res. Part A 2014, 102, 4526–4535. [Google Scholar]
- Chi, M.; Li, N.; Cui, J.; Karlin, S.; Rohr, N.; Sharma, N.; Thieringer, F.M. Biomimetic, mussel-inspired surface modification of 3D-printed biodegradable polylactic acid scaffolds with nano-hydroxyapatite for bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 989729. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, C.; Lin, K.; Wang, X. Mussel-Inspired Polydopamine-Based Multilayered Coatings for Enhanced Bone Formation. Front. Bioeng. Biotechnol. 2022, 10, 952500. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, X.; Zheng, Q.; Wu, Y.; Cui, F.; Wu, B. Improving osteogenesis of three-dimensional porous scaffold based on mineralized recombinant human-like collagen via mussel-inspired polydopamine and effective immobilization of BMP-2-derived peptide. Colloids Surf. B Biointerfaces 2017, 152, 124–132. [Google Scholar] [CrossRef]
- Zhao, X.; Han, Y.; Li, J.; Cai, B.; Gao, H.; Feng, W.; Li, S.; Liu, J.; Li, D. BMP-2 immobilized PLGA/hydroxyapatite fibrous scaffold via polydopamine stimulates osteoblast growth. Mater. Sci. Eng. C 2017, 78, 658–666. [Google Scholar] [CrossRef]
- Yu, W.-L.; Sun, T.-W.; Qi, C.; Zhao, H.-K.; Ding, Z.-Y.; Zhang, Z.-W.; Sun, B.-B.; Shen, J.; Chen, F.; Zhu, Y.-J.; et al. Enhanced osteogenesis and angiogenesis by mesoporous hydroxyapatite microspheres-derived simvastatin sustained release system for superior bone regeneration. Sci. Rep. 2017, 7, 44129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, Z.; Zhang, Z. Porous Chitosan/Nano-Hydroxyapatite Composite Scaffolds Incorporating Simvastatin-Loaded PLGA Microspheres for Bone Repair. Cells Tissues Organs 2018, 205, 20–31. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Gu, Y.; Xu, Y.; Liu, Y.; Li, B.; Chen, L. Sequential and sustained release of SDF-1 and BMP-2 from silk fibroin-nanohydroxyapatite scaffold for the enhancement of bone regeneration. Biomaterials 2016, 106, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Zhi, W.; Wang, J.; Feng, B.; Qu, S.; Mu, Y.; Weng, J. Immobilization of salvianolic acid B-loaded chitosan microspheres distributed three-dimensionally and homogeneously on the porous surface of hydroxyapatite scaffolds. Biomed. Mater. 2016, 11, 055014. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Feng, Q.; Wang, M.; Guo, X.; Zheng, Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J. Control. Release 2009, 134, 111–117. [Google Scholar] [CrossRef] [PubMed]

| Material | Scaffold | Feature | Reference |
|---|---|---|---|
| Chitosan | CS/n-HAp composite scaffold | Scaffolds with an n-HAp/CS concentration of 75/25 w/w have better physicomechanical characteristics An increased HAp concentration exceeding 80% has been reported to result in friable scaffolds in vitro | [52,54,55] |
| Silk fibroin | SF/n-HAp composite scaffold | The compressive strength increases with an increase in n-HAp concentration | [58] [60] |
| Polylactic acid | PLA/n-HAp composite scaffold | n-HAp can buffer the acidity of PLA degradation With an increase in PLA concentration, the porosity of the scaffold decreases | [70] [69] [63] |
| Polycaprolactone | PCL/n-HAp composite scaffold | The degradation rate of scaffold increases with an increase in n-HAp content It has antibacterial activity | [74] [77] [81] |
| Growth Factor | Scaffold | Release Profile | Outcome | Reference |
|---|---|---|---|---|
| BMP-2 | n-HAp/PLA-PEG | BMP-2 sustained release for 21 days | Promotes bone regeneration in spinal fusion models | [17] |
| BMP-2 | PHB-PDA | BMP-2 can be released continuously for 30 days | Cell attachment, proliferation and differentiation were significantly upregulated in vitro In vivo promotion of bone healing in rabbit calvarial defects | [88] |
| BMP-2 | n-HG-SFD hydrogel | BMP-2 sustained release for more than 24 days | Promoted in vitro osteogenic differentiation and compatibility of rBMSCs Skull defect in mice repaired | [89] |
| BMP-2 | Sr-n-HAp-SF | The BMP-2 from 10% Sr-n-HAp/SF-BMP-2 scaffold released approximately 80% by the 14th day | Osteogenesis-related genes upregulated in BMSCs Increased bone density in skull defects, new bone formation | [90] |
| BMP-2-derived peptides | n-HAp/PLA/Gel nanofibers | The release of the polypeptide continued until 21d | Promotes osteogenic differentiation of BMSCs in vitro and bone regeneration in a rat calvarial defect model | [92] |
| BMP2-mimicking peptide P28 | nHAC/PLA | The release rate of P28 in 14 days is about 72 ± 3% | Promoted MC3T3-E1 proliferation, recruitment and differentiation in vitro Rabbit femoral condyle defect was repaired in vivo | [94] |
| BMP-6 | n-HAp/Gel/GMS | BMP-6 was released for 20 consecutive days, and the cumulative release amount was about 95% | The composites can induce osteogenic differentiation of BMMSCs in vitro and promote formation of new bone in vivo | [84] |
| BMP-9 | n-HAp/ColI/MWCNT | – | The 1%MWCNT group was the most suitable for bone tissue engineering BMP-9-loaded nHACM scaffolds exhibit good biocompatibility and osteogenic capacity | [97] |
| VEGF | HACS | The amount of VEGF released by the HACS scaffolds on days 1 and 14 was 1.42 μg/mL and 3.04 μg/mL | Promote proliferation of hBMSCs and HUVECs; improved regeneration and remineralization of bone tissue in rabbit femoral defect | [98] |
| VEGF | PCL/HAp | VEGF was released rapidly in the first 3 days and slowed down after 3 days | Enhanced osteogenesis of stem cells and preeminent vascularized bone regeneration | [99] |
| VEGF | HG/HAp/TCP | – | Increased expression of genes related to osteogenesis and angiogenesis and regeneration of new bone | [100] |
| BMP-2 and VEGF | SF/n-HAp | BMP-2 released 6.7% in the first day and 15.5% in 28 days VEGF released 48.7% in the first day and 66.4% in 10 days | Promoted adhesion and proliferation of MC3T3-E1 cells and accelerated formation of vascularized bone in rats with calvarial defects | [112] |
| BMP-2-derived peptide and QK | n-HAp/PA66 | n-HAp/PA66-B1+Q1 released about 81.07 ± 3.27% of QK and 78.56 ± 3.66% of BMP-2-derived peptides within 15 days | Promoted proliferation and differentiation of rBMSCs and HUVECs and bone formation after severe femoral fracture (periosteal scraping) in SD2 rats | [113] |
| bFGF | n-HAp/PA66 | The n-HAp/PA66/D-RADA16/bFGF scaffold released approximately 50% of bFGF within 48 h with no apparent burst release | Promoted proliferation, adhesion and osteogenesis of bone marrow mesenchymal stem cells and bone repair of rat femoral defects | [105] |
| bFGF | CaSO4/n-HAp | bFGF is released suddenly within 8 days, with a slow and sustainable release over 32 days | Improved in vitro osteogenic differentiation of MC3T3 cells and in vivo bone formation in femoral bone defects | [106] |
| bFGF | nHAC/bFGF-GBG | – | Promoted HPDLC cell growth and attachment and growth of new alveolar bone Increased ALP expression and calcium nodule formation by the BMSCs | [107] |
| bFGF and BMP-2 | n-HAp/COL | The cumulative release days of bFGF and BMP-2 were 17d and 19d, respectively, and the amount of factor released was 91.05 ± 3.38% and 90.05 ± 2.08%, respectively | Promotes BMSCs adhesion, proliferation and ALP expression | [114] |
| bFGF and BMP-2 | PLGA/HAp | – | Promoted adhesion, proliferation and osteogenic differentiation of MC3T3-E1 cells, and increased ALP activity and osteogenesis-related gene expression levels | [115] |
| Polypeptide | Scaffold | Feature | Function | Reference |
|---|---|---|---|---|
| RADA16-RGD | n-HAp/PA66 | Self-assembled nanofibers by intermolecular hydrogen bonding | Promote cell proliferation, differentiation, adhesion and migration | [118] |
| γ-PGA | γ-PGA/CuxHAp | Forming network complex to control the release of metal ions | Control the release of copper ions and have angiogenesis, osteogenesis and antibacterial activities | [121] |
| PTH (1–34) | PTH (1–34)/n-HAp /CS/SA | With an increase in PTH (1–34), the nanofibers of the scaffold become thinner and more conducive to osteogenesis | Promote osteogenic differentiation of rBMSCs and enhance cell adhesion | [123] |
| Vitamin | Scaffold | Load Method | In Vitro Drug Release | Reference |
|---|---|---|---|---|
| VD3 | C/HAp/MSNS-3 | Freeze drying | The release amount reached 75.32% within 20 days | [126] |
| LDH-HAp/gelatin | Gelatin coating | Bursting release within the first 2 h, stable release within 48 h, slow release until 16 days | [127] | |
| HAp/PLGA | Microsphere loading | – | [128] | |
| VK | HAp/PXSA | Freeze drying | The release amount reaches 50.87% within 10 days | [132] |
| VK1 | PLA/HAp | Microsphere loading | The release amount increases with the decrease in pH value of phosphate buffer solution | [133] |
| Drug | Scaffold | Cell/Animal | Function | Reference |
|---|---|---|---|---|
| Vancomycin | Silica-coated n-HAp/gelatin reinforced with PLLA yarns | MSCs/Wistar rats | Remove bacteria, promote new bone formation | [143] |
| HAp/PU/ decellularized bovine bone particles | Rabbit model of post-surgical OM | Promotes bone formation, prevents infection in bone defects | [144] | |
| Levofloxacin | MSNs/n-HAp/PU | Rabbit model of chronic osteomyelitis | Repair bone defect, inflammation controlled | [137] |
| L929 cells | Perfect antibacterial activity, no cytotoxicity, biosafety | [147] | ||
| BMSCs/MC3T3-E1 | Promote osteogenic differentiation of MSCs, proliferation of MC3T3-E1 cells and inhibit apoptosis | [148] | ||
| Doxorubicin | n-HAp/collagen | MG63 cells/New Zealand rabbits/ Sprague–Dawley rats/tumor-bearing nude mice model | Sustained release drug properties, inhibited tumor cell growth, bone repair | [155] |
| LHAp/PLGA | MG-63 cells/ MC3T3-E1 cells/mice | Inhibits tumor cell growth, improves osteoblast adhesion and proliferation and promotes new bone formation | [157] | |
| Metformin | PLLA/n-HAp | BMSCs/Saos-2 cells | Induces apoptosis of osteosarcoma cells, promotes osteogenic differentiation of cells | [1] |
| Zoledronic acid | CS/n-HAp | GCTBs/hBMSCs | Upregulates pro-apoptotic genes, downregulates osteoclast genes, induces osteogenesis, inhibits bacterial growth | [136] |
| Pamidronate | PCH | Osteoporosis rat animal model | Accelerates healing of osteoporotic bone defects | [166] |
| Alendronate | PLLA/n-HAp | ASCs/Rabbit radius defect model | Improves ALP activity and calcium deposition, repairs large bone defects | [28] |
| Calcitriol | Gel/HA-D/M | BMSCs/Rat critical-size femoral defect model | Promotes cell proliferation, migration and differentiation, healing of femoral defects | [169] |
| Resveratrol | n-HAp/CS | RAW264.7 cells/BMSCs/SD rats | Anti-inflammatory, promotes cell osteogenic differentiation, bone regeneration and endochondral growth | [172] |
| Naringin | GMs/n-HAp/SF | BMSCs/SD rats | Promotes BMSCs adhesion, proliferation and calcium nodule formation, new bone formation | [174] |
| Rifampicin | CS/n-HAp | MC3T3-E1 cells | Long-term controlled drug release, anti-tuberculosis, no cytotoxicity | [181] |
| Isoniazid | n-HAp/PA66 | BMSCs/New Zealand rabbits | Inhibition of mycobacterium tuberculosis activity, good osteoconduction and osseointegration properties | [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, X.; Zhang, D.; Liu, K.; Zhao, X.; Li, X.; Wang, W. Nano-Hydroxyapatite Composite Scaffolds Loaded with Bioactive Factors and Drugs for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 1291. https://doi.org/10.3390/ijms24021291
Mo X, Zhang D, Liu K, Zhao X, Li X, Wang W. Nano-Hydroxyapatite Composite Scaffolds Loaded with Bioactive Factors and Drugs for Bone Tissue Engineering. International Journal of Molecular Sciences. 2023; 24(2):1291. https://doi.org/10.3390/ijms24021291
Chicago/Turabian StyleMo, Xiaojing, Dianjian Zhang, Keda Liu, Xiaoxi Zhao, Xiaoming Li, and Wei Wang. 2023. "Nano-Hydroxyapatite Composite Scaffolds Loaded with Bioactive Factors and Drugs for Bone Tissue Engineering" International Journal of Molecular Sciences 24, no. 2: 1291. https://doi.org/10.3390/ijms24021291
APA StyleMo, X., Zhang, D., Liu, K., Zhao, X., Li, X., & Wang, W. (2023). Nano-Hydroxyapatite Composite Scaffolds Loaded with Bioactive Factors and Drugs for Bone Tissue Engineering. International Journal of Molecular Sciences, 24(2), 1291. https://doi.org/10.3390/ijms24021291






