siRNA-Mediated MELK Knockdown Induces Accelerated Wound Healing with Increased Collagen Deposition
Abstract
:1. Introduction
Innovation
2. Results
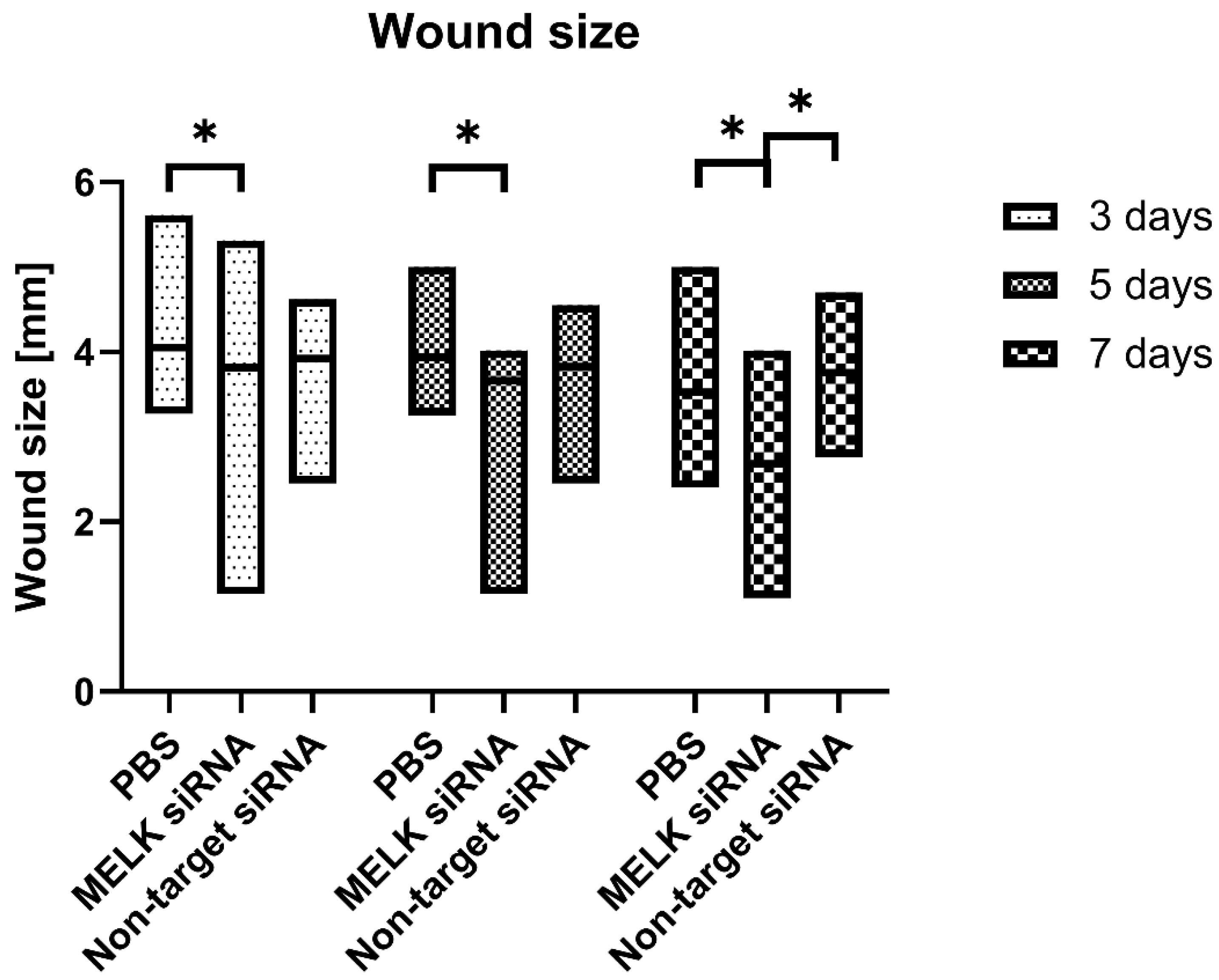
2.1. Wound Size
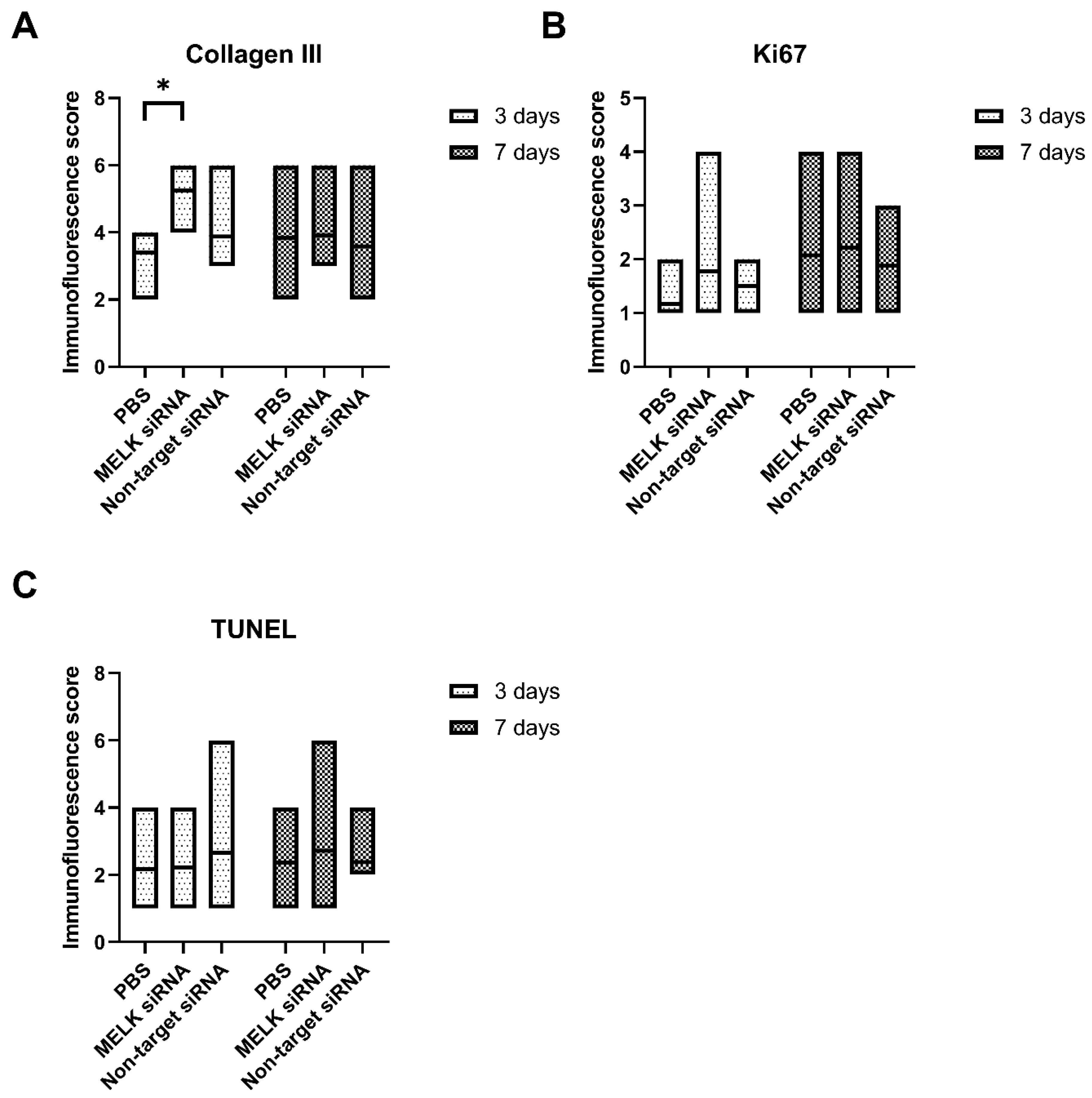
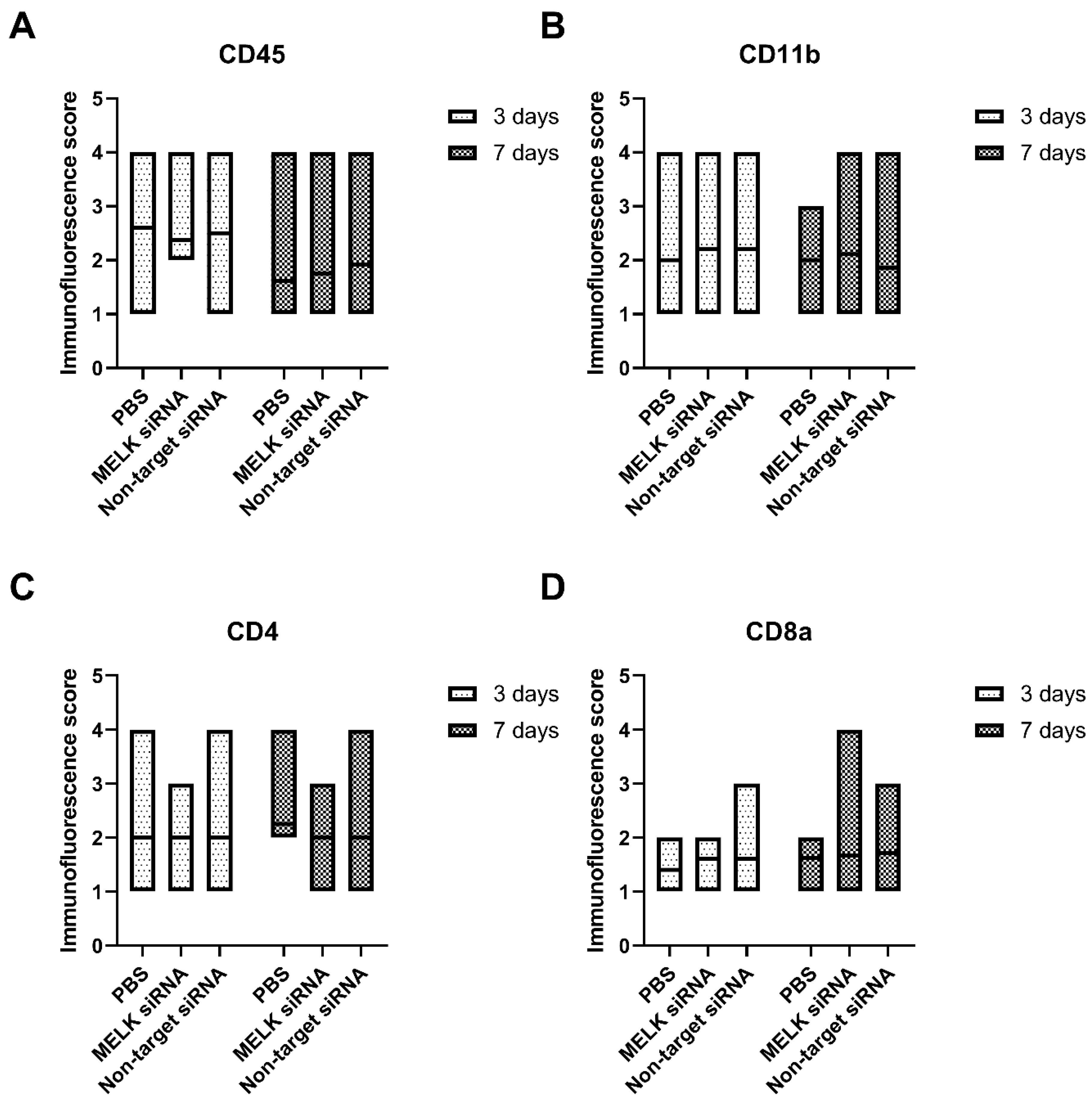
2.2. Immunohistological Staining
2.3. Immunofluorescence Staining
3. Discussion
Key Findings
- -
- siRNA-mediated MELK knockdown leads to accelerated wound healing in mice;
- -
- siRNA-mediated MELK knockdown leads to increased collagen deposition in the wound area;
- -
- MELK siRNA treatment does not affect the balance of immune cells in the wound area;
- -
- MELK siRNA treatment does not affect the long-term apoptosis or proliferation rate of the cells in the wound, indicating a low carcinogenicity risk.
4. Material and Methods
4.1. siRNA
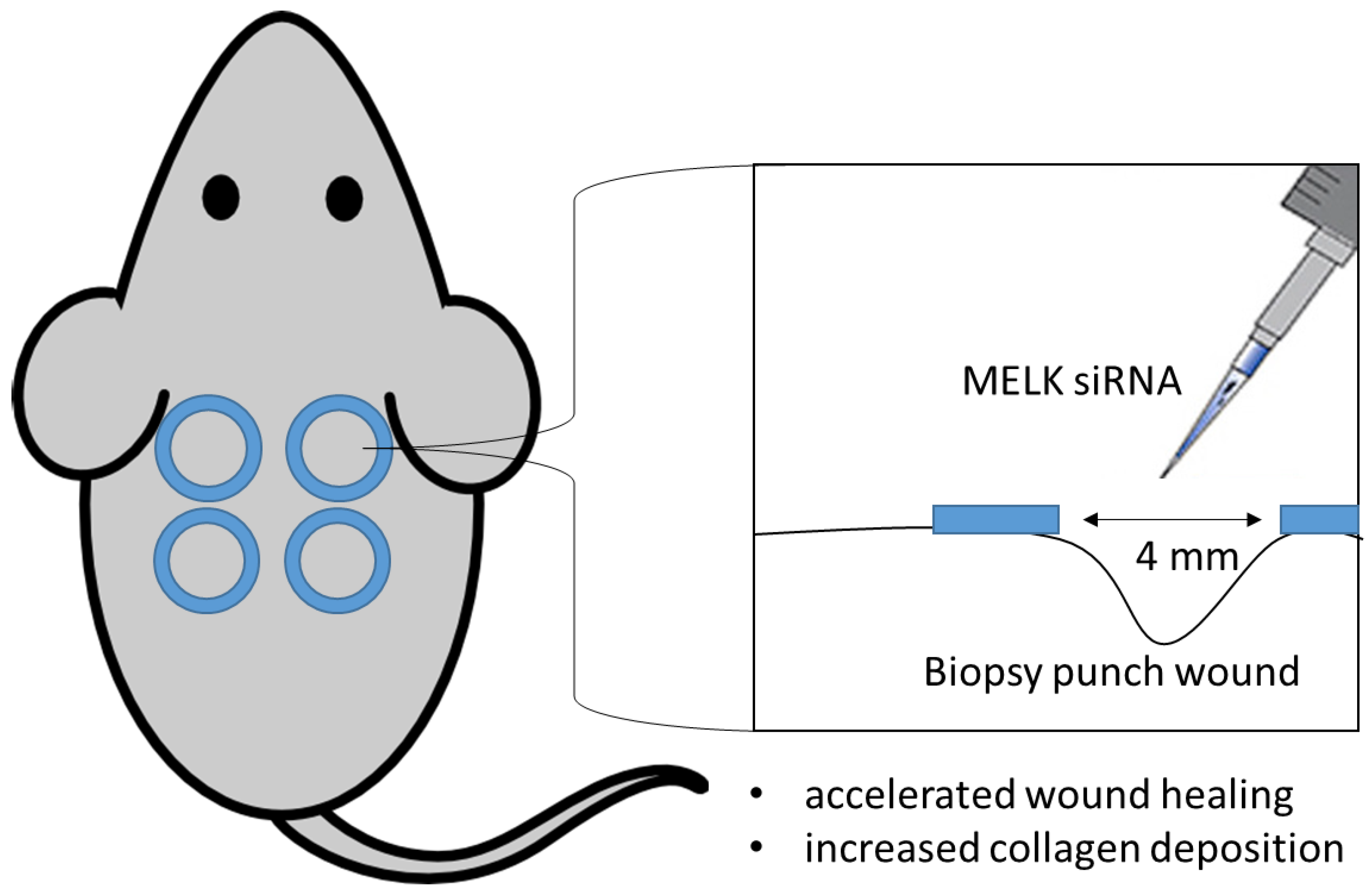
4.2. Animal Experiments
4.3. Immunohistological Staining
4.4. Immunofluorescence Staining
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ozgok Kangal, M.K.; Regan, J.-P. Wound Healing. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bowden, L.G.; Byrne, H.M.; Maini, P.K.; Moulton, D.E. A Morphoelastic Model for Dermal Wound Closure. Biomech. Model. Mechanobiol. 2016, 15, 663–681. [Google Scholar] [CrossRef]
- Coger, V.; Million, N.; Rehbock, C.; Sures, B.; Nachev, M.; Barcikowski, S.; Wistuba, N.; Strauß, S.; Vogt, P.M. Tissue Concentrations of Zinc, Iron, Copper, and Magnesium during the Phases of Full Thickness Wound Healing in a Rodent Model. Biol. Trace Elem. Res. 2019, 191, 167–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- MacCarthy-Morrogh, L.; Martin, P. The Hallmarks of Cancer Are also the Hallmarks of Wound Healing. Sci. Signal. 2020, 13, eaay8690. [Google Scholar] [CrossRef] [PubMed]
- Janostiak, R.; Rauniyar, N.; Lam, T.T.; Ou, J.; Zhu, L.J.; Green, M.R.; Wajapeyee, N. MELK Promotes Melanoma Growth by Stimulating the NF-ΚB Pathway. Cell Rep. 2017, 21, 2829–2841. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, P.; Lv, W.; Han, X.; Yang, J.; Qin, S.; Zhang, Y. MELK Is Upregulated in Advanced Clear Cell Renal Cell Carcinoma and Promotes Disease Progression by Phosphorylating PRAS40. Cell Transplant. 2019, 28, 37S. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lee, Y.M.; Baitsch, L.; Huang, A.; Xiang, Y.; Tong, H.; Lako, A.; Von, T.; Choi, C.; Lim, E.; et al. MELK Is an Oncogenic Kinase Essential for Mitotic Progression in Basal-like Breast Cancer Cells. Elife 2014, 3, e01763. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Giuliano, C.J.; Sayles, N.M.; Sheltzer, J.M. CRISPR/Cas9 Mutagenesis Invalidates a Putative Cancer Dependency Targeted in on-Going Clinical Trials. Elife 2017, 6, e24179. [Google Scholar] [CrossRef]
- Marcotte, R.; Brown, K.R.; Suarez, F.; Sayad, A.; Karamboulas, K.; Krzyzanowski, P.M.; Sircoulomb, F.; Medrano, M.; Fedyshyn, Y.; Koh, J.L.Y.; et al. Essential Gene Profiles in Breast, Pancreatic, and Ovarian Cancer Cells. Cancer Discov. 2012, 2, 172–189. [Google Scholar] [CrossRef] [Green Version]
- Marcotte, R.; Sayad, A.; Brown, K.R.; Sanchez-Garcia, F.; Reimand, J.; Haider, M.; Virtanen, C.; Bradner, J.E.; Bader, G.D.; Mills, G.B.; et al. Functional Genomic Landscape of Human Breast Cancer Drivers, Vulnerabilities, and Resistance. Cell 2016, 164, 293–309. [Google Scholar] [CrossRef]
- Hart, T.; Chandrashekhar, M.; Aregger, M.; Steinhart, Z.; Brown, K.R.; MacLeod, G.; Mis, M.; Zimmermann, M.; Fradet-Turcotte, A.; Sun, S.; et al. High-Resolution CRISPR Screens Reveal Fitness Genes and Genotype-Specific Cancer Liabilities. Cell 2015, 163, 1515–1526. [Google Scholar] [CrossRef] [Green Version]
- Giuliano, C.J.; Lin, A.; Smith, J.C.; Palladino, A.C.; Sheltzer, J.M. MELK Expression Correlates with Tumor Mitotic Activity but Is Not Required for Cancer Growth. Elife 2018, 7, e32838. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, A.C.D.O.; Costa, T.F.; Andrade, Z.D.A.; Medrado, A.R.A.P. Wound Healing—A Literature Review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Kloc, M.; Ghobrial, R.M.; Wosik, J.; Lewicka, A.; Lewicki, S.; Kubiak, J.Z. Macrophage Functions in Wound Healing. J. Tissue Eng. Regen. Med. 2019, 13, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Spear, M. Acute or Chronic? What’s the Difference? Plast. Surg. Nurs. Off. J. Am. Soc. Plast. Reconstr. Surg. Nurses 2013, 33, 98–100. [Google Scholar] [CrossRef]
- Le Page, Y.; Chartrain, I.; Badouel, C.; Tassan, J.-P. A Functional Analysis of MELK in Cell Division Reveals a Transition in the Mode of Cytokinesis during Xenopus Development. J. Cell Sci. 2011, 124, 958–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, J.; Bolomsky, A.; Dubois, S.; Duray, E.; Stangelberger, K.; Plougonven, E.; Lejeune, M.; Léonard, A.; Marty, C.; Hempel, U.; et al. Maternal Embryonic Leucine Zipper Kinase Inhibitor OTSSP167 Has Preclinical Activity in Multiple Myeloma Bone Disease. Haematologica 2018, 103, 1359–1368. [Google Scholar] [CrossRef]
- Risteli, L.; Koivula, M.-K.; Risteli, J. Chapter Three-Procollagen Assays in Cancer. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 66, pp. 79–100. [Google Scholar]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Das, A.; Abas, M.; Biswas, N.; Banerjee, P.; Ghosh, N.; Rawat, A.; Khanna, S.; Roy, S.; Sen, C.K. A Modified Collagen Dressing Induces Transition of Inflammatory to Reparative Phenotype of Wound Macrophages. Sci. Rep. 2019, 9, 14293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, S.; Storm, G.; Schiffelers, R.M. Targeted Delivery of SiRNA. J. Biomed. Biotechnol. 2006, 2006, 63675. [Google Scholar] [CrossRef] [PubMed]
- Gethin, G. Understanding the Inflammatory Process in Wound Healing. Br. J. Community Nurs. 2012, 7 (Suppl. S3), S17–S22. [Google Scholar] [CrossRef] [PubMed]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from Inflammation to Proliferation: A Critical Step during Wound Healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, S.K.; Sharma, A.K.; Gupta, V.; Yashavarddhan, M.H. Pharmacological Control of Inflammation in Wound Healing. J. Tissue Viability 2019, 28, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.-J. Therapeutic SiRNA: State of the Art. Signal Transduct. Target. Ther. 2020, 5, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Andersson, P. Preclinical Safety Assessment of Therapeutic Oligonucleotides. In Antisense RNA Design, Delivery, and Analysis; Arechavala-Gomeza, V., Garanto, A., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2022; pp. 355–370. ISBN 978-1-07-162010-6. [Google Scholar]
- McDonald, I.M.; Graves, L.M. Enigmatic MELK: The Controversy Surrounding Its Complex Role in Cancer. J. Biol. Chem. 2020, 295, 8195–8203. [Google Scholar] [CrossRef]
- Sundberg, J.P. Chapter 12—Skin and Adnexa of the Laboratory Mouse. In The Laboratory Mouse; Hedrich, H.J., Bullock, G., Eds.; Academic Press: London, UK, 2004; pp. 195–206. ISBN 978-0-12-336425-8. [Google Scholar]
- Layliev, J.; Wilson, S.; Warren, S.M.; Saadeh, P.B. Improving Wound Healing with Topical Gene Therapy. Adv. Wound Care 2012, 1, 218–223. [Google Scholar] [CrossRef] [Green Version]
- Hickerson, R.P.; Flores, M.A.; Leake, D.; Lara, M.F.; Contag, C.H.; Leachman, S.A.; Kaspar, R.L. Use of Self-Delivery SiRNAs to Inhibit Gene Expression in an Organotypic Pachyonychia Congenita Model. J. Investig. Dermatol. 2011, 131, 1037–1044. [Google Scholar] [CrossRef]
- Weidner, N.; Semple, J.P.; Welch, W.R.; Folkman, J. Tumor Angiogenesis and Metastasis—Correlation in Invasive Breast Carcinoma. N. Engl. J. Med. 1991, 324, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zaqout, S.; Becker, L.-L.; Kaindl, A.M. Immunofluorescence Staining of Paraffin Sections Step by Step. Front. Neuroanat. 2020, 14, 582218. [Google Scholar] [CrossRef] [PubMed]
- Fedchenko, N.; Reifenrath, J. Different Approaches for Interpretation and Reporting of Immunohistochemistry Analysis Results in the Bone Tissue—A Review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymanski, L.; Lewicki, S.; Markiewicz, T.; Cierniak, S.; Tassan, J.-P.; Kubiak, J.Z. siRNA-Mediated MELK Knockdown Induces Accelerated Wound Healing with Increased Collagen Deposition. Int. J. Mol. Sci. 2023, 24, 1326. https://doi.org/10.3390/ijms24021326
Szymanski L, Lewicki S, Markiewicz T, Cierniak S, Tassan J-P, Kubiak JZ. siRNA-Mediated MELK Knockdown Induces Accelerated Wound Healing with Increased Collagen Deposition. International Journal of Molecular Sciences. 2023; 24(2):1326. https://doi.org/10.3390/ijms24021326
Chicago/Turabian StyleSzymanski, Lukasz, Sławomir Lewicki, Tomasz Markiewicz, Szczepan Cierniak, Jean-Pierre Tassan, and Jacek Z. Kubiak. 2023. "siRNA-Mediated MELK Knockdown Induces Accelerated Wound Healing with Increased Collagen Deposition" International Journal of Molecular Sciences 24, no. 2: 1326. https://doi.org/10.3390/ijms24021326




