Folic-Acid-Conjugated Thermoresponsive Polymeric Particles for Targeted Delivery of 5-Fluorouracil to CRC Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of PHEA and PHEA-b-PNIPAAm Polymers
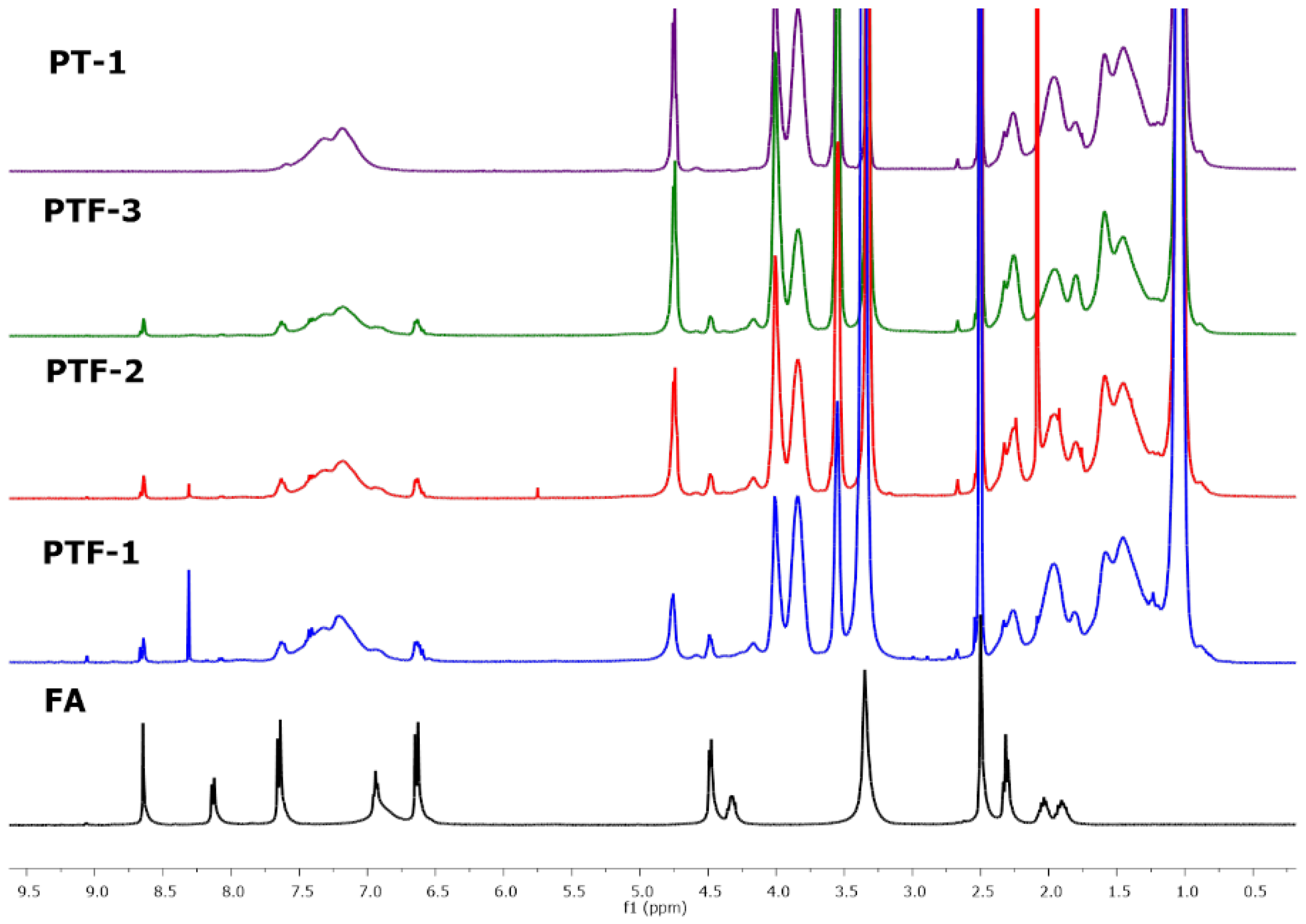
2.2. Conjugation of Folic Acid
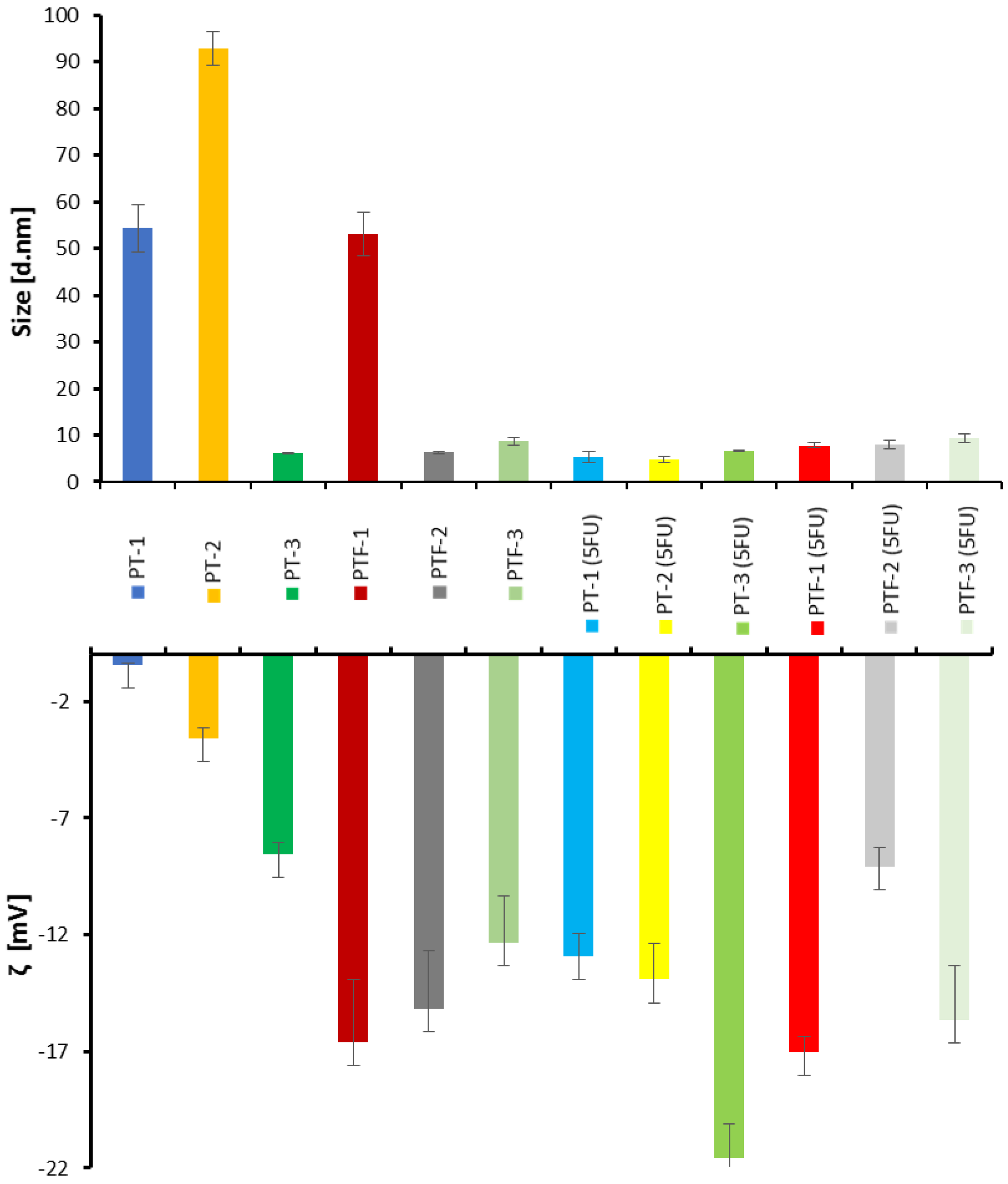
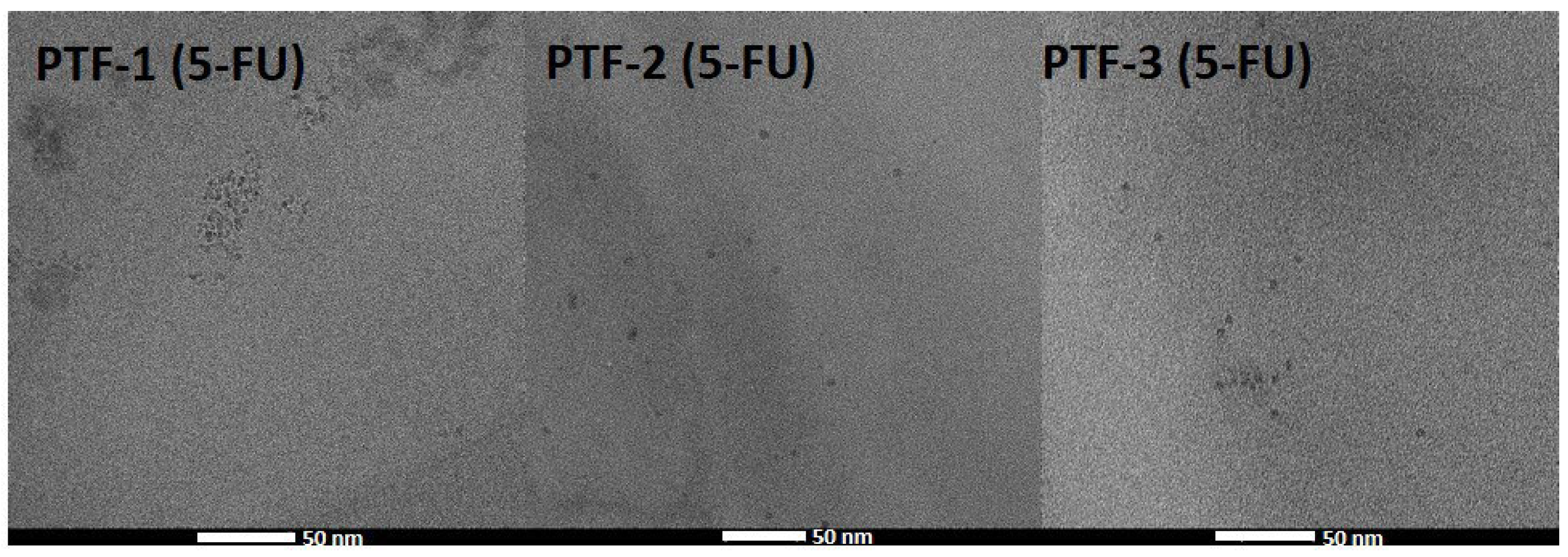
2.3. Hydrodynamic Diameter and Colloidal Stability Measurements
2.4. Thermoresponsive Behavior
2.5. Drug Encapsulation and Release
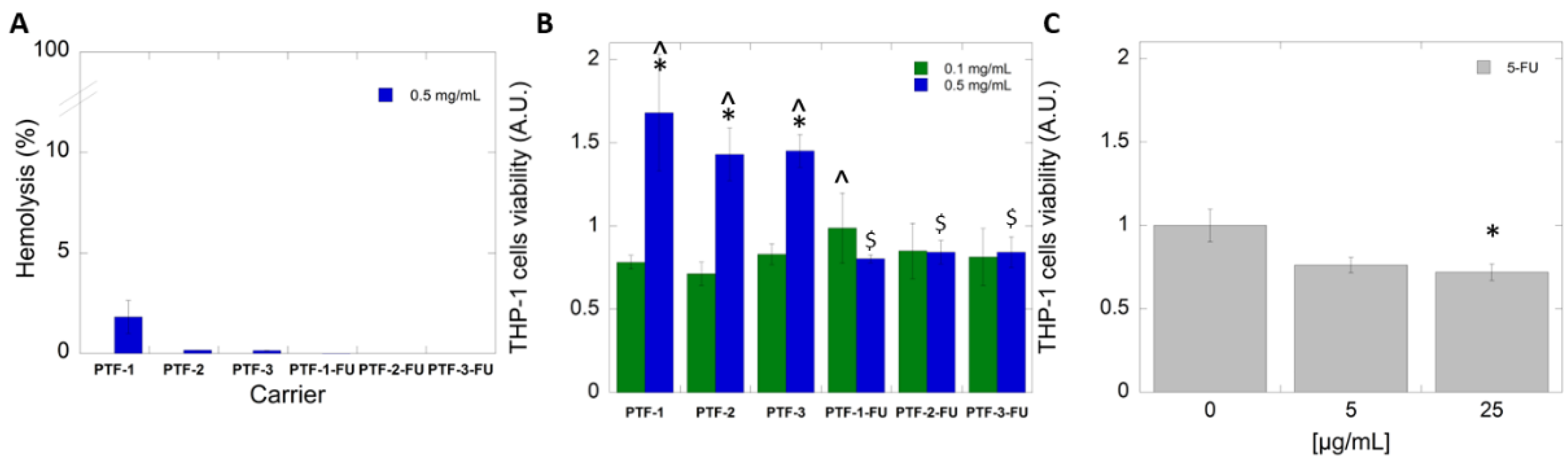
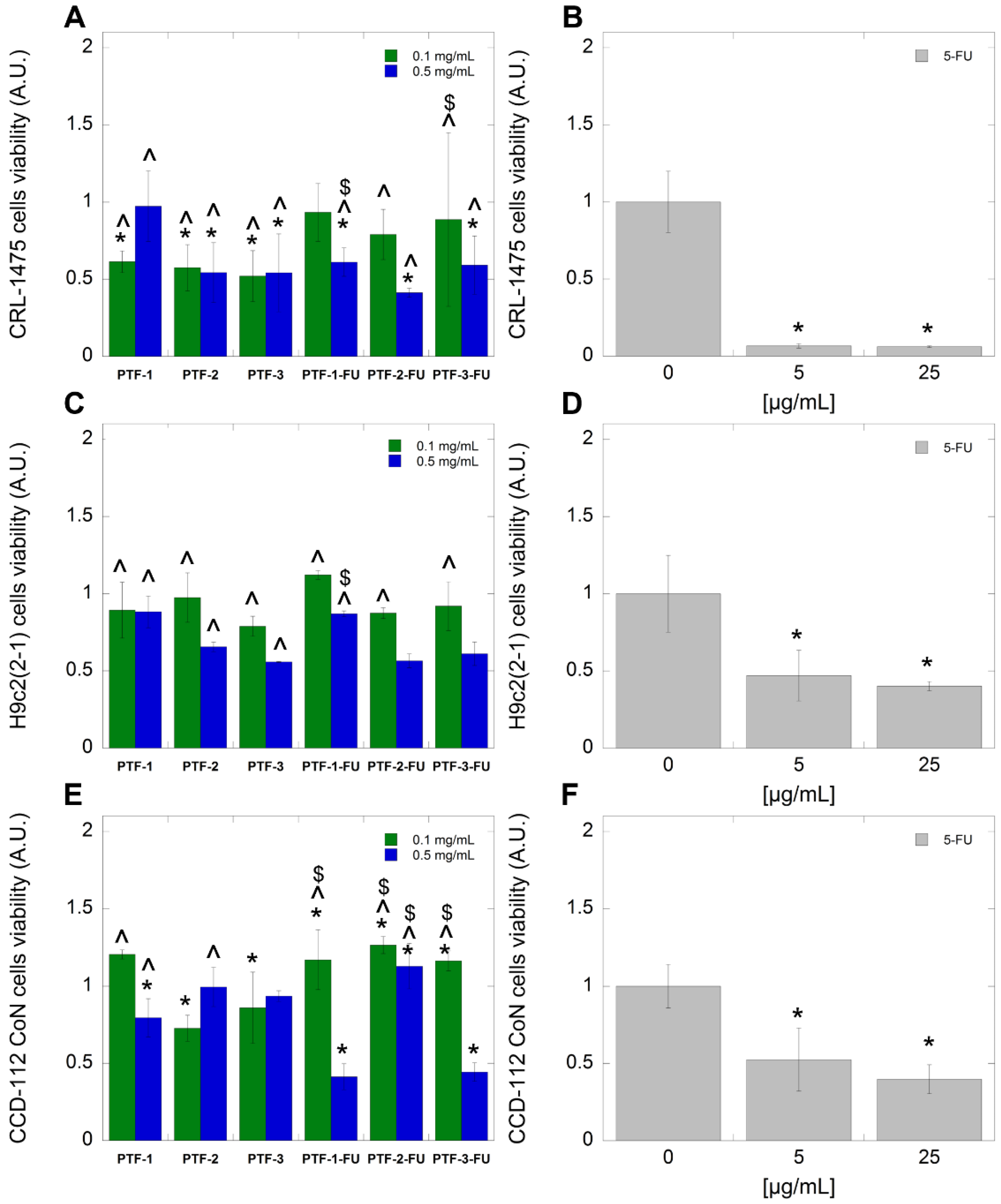
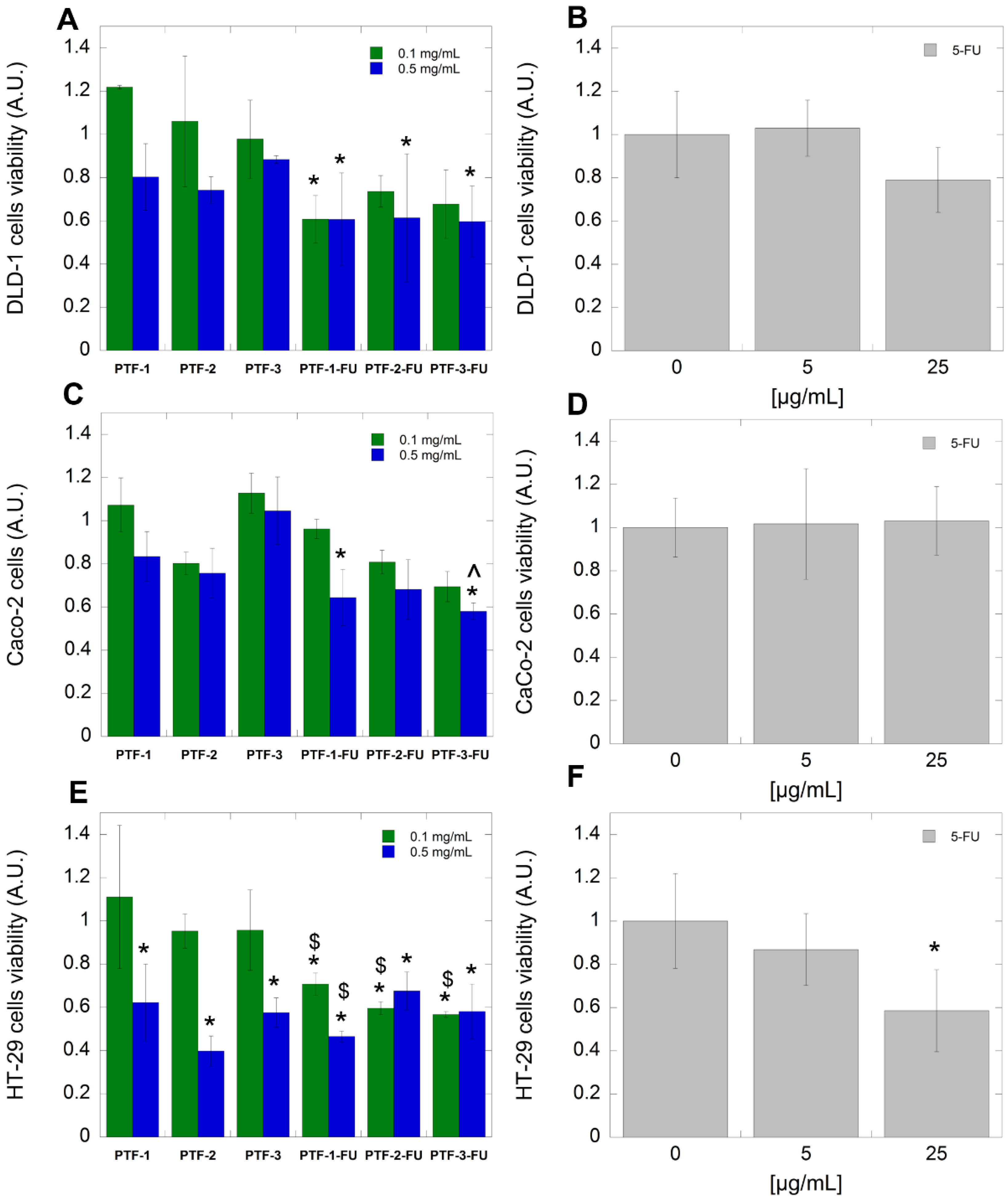
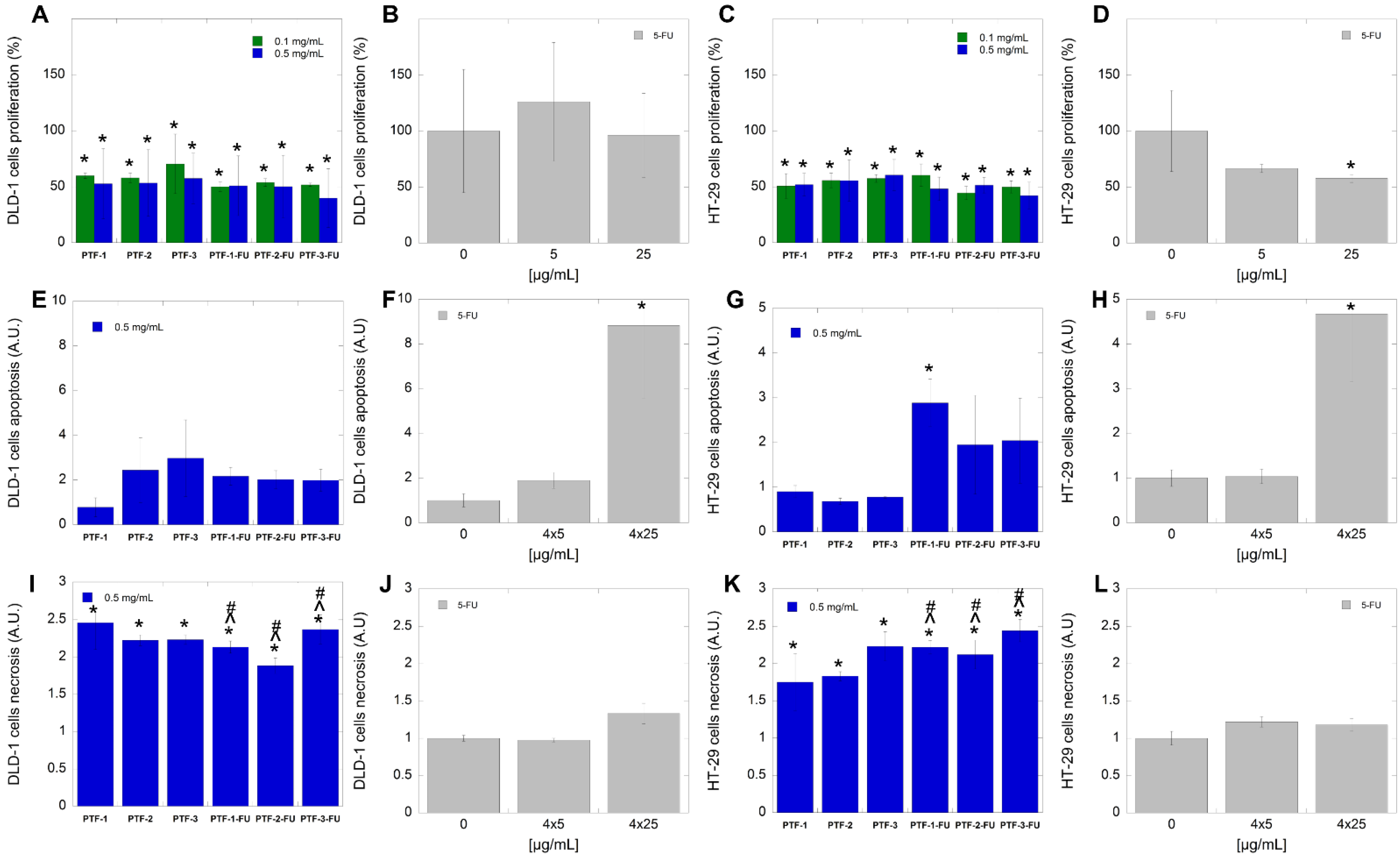
2.6. Biological Studies
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Nuclear Magnetic Resonance (NMR)
3.2.2. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3.2.3. Size Exclusion Chromatography (SEC)
3.2.4. Dynamic Light Scattering (DLS) and ζ Potential
3.2.5. Ultraviolet–Visible Spectroscopy (UV–Vis)
3.2.6. Turbidimetry
3.2.7. Thermogravimetric Analysis (TGA)
3.2.8. Differential Scanning Calorimetry (DSC)
3.2.9. Transmission Electron Microscope
3.2.10. Freeze-Drying
3.2.11. Dialysis
3.3. Synthetic Procedures
3.3.1. General Procedure for RAFT Polymerization of HEA (P-(1-3))
3.3.2. General Procedure for RAFT Polymerization of PHEA with NIPAAm (PT-(1-3))
3.3.3. General Procedure for Conjugation of PHEA-b-PNIPAAm with Folic Acid (PTF-(1-3))
3.3.4. Polymeric Micelles Formation
3.3.5. Drug Encapsulation
3.3.6. Drug Release
3.4. Biological Studies
3.4.1. Hemolysis Assay
3.4.2. Cell Culture
3.4.3. Cytotoxicity Assay
3.4.4. Mode of Action–Apoptosis and Necrosis Detection
4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Przeglad Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Lewandowska, A.; Rudzki, G.; Lewandowski, T.; Stryjkowska-Góra, A.; Rudzki, S. Title: Risk Factors for the Diagnosis of Colorectal Cancer. Cancer Control. 2022, 29, 10732748211056692. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef] [PubMed]
- Francipane, M.G.; Bulanin, D.; Lagasse, E. Establishment and Characterization of 5-Fluorouracil-Resistant Human Colorectal Cancer Stem-Like Cells: Tumor Dynamics under Selection Pressure. Int. J. Mol. Sci. 2019, 20, 1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blondy, S.; David, V.; Verdier, M.; Mathonnet, M.; Perraud, A.; Christou, N. 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci. 2020, 111, 3142–3154. [Google Scholar] [CrossRef]
- Chandran, S.P.; Natarajan, S.B.; Chandraseharan, S.; Mohd Shahimi, M.S.B. Nano Drug Delivery Strategy of 5-Fluorouracil for the Treatment of Colorectal Cancer. J. Cancer Res. Pract. 2017, 4, 45–48. [Google Scholar] [CrossRef]
- Entezar-Almahdi, E.; Mohammadi-Samani, S.; Tayebi, L.; Farjadian, F. Recent Advances in Designing 5-Fluorouracil Delivery Systems: A Stepping Stone in the Safe Treatment of Colorectal Cancer. Int. J. Nanomed. 2020, 15, 5445–5458. [Google Scholar] [CrossRef] [PubMed]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.; Sarwar, S.; Hu, Y.; Munir, M.U.; Nisar, M.F.; Ikram, F.; Asif, A.; Rahman, S.U.; Chaudhry, A.A.; Rehman, I.U. Surface-modified polymeric nanoparticles for drug delivery to cancer cells. Expert Opin. Drug Deliv. 2021, 18, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Grabnar, P.A.; Kristl, J. The manufacturing techniques of drug-loaded polymeric nanoparticles from preformed polymers. J. Microencapsul. 2011, 28, 323–335. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef]
- Ashford, M.B.; England, R.M.; Akhtar, N. Highway to Success—Developing Advanced Polymer Therapeutics. Adv. Ther. 2021, 4, 2000285. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Acevedo, A.; Davidoff, E.J.; Timmins, L.M.; Marrero-Berrios, I.; Patel, M.; White, C.; Lowe, C.; Sherba, J.J.; Hartmanshenn, C.; et al. The growing role of precision and personalized medicine for cancer treatment. Technology 2018, 6, 79–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef] [Green Version]
- Jurczyk, M.; Jelonek, K.; Musiał-Kulik, M.; Beberok, A.; Wrześniok, D.; Kasperczyk, J. Single- versus Dual-Targeted Nanoparticles with Folic Acid and Biotin for Anticancer Drug Delivery. Pharmaceutics 2021, 13, 326. [Google Scholar] [CrossRef]
- Siemiaszko, G.; Niemirowicz-Laskowska, K.; Markiewicz, K.H.; Misztalewska-Turkowicz, I.; Dudź, E.; Milewska, S.; Misiak, P.; Kurowska, I.; Sadowska, A.; Car, H.; et al. Synergistic effect of folate-conjugated polymers and 5-fluorouracil in the treatment of colon cancer. Cancer Nanotechnol. 2021, 12, 1–24. [Google Scholar] [CrossRef]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv. Drug Deliv. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive polymers and their biomedical application in tissue engineering—A review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Misiak, P.; Niemirowicz-Laskowska, K.; Markiewicz, K.H.; Misztalewska-Turkowicz, I.; Wielgat, P.; Kurowska, I.; Siemiaszko, G.; Destarac, M.; Car, H.; Wilczewska, A.Z. Evaluation of Cytotoxic Effect of Cholesterol End-Capped Poly(N-Isopropylacrylamide)s on Selected Normal and Neoplastic Cells. Int. J. Nanomed. 2020, 15, 7263–7278. [Google Scholar] [CrossRef] [PubMed]
- Pavia, F.C.; Carrubba, V.L.; Palumbo, F.; Giammona, G.; Brucato, V. PHEA-PLLA: A New Polymer Blend for Tissue Engineering Applications. AIP Conf. Proc. 2011, 1353, 809–814. [Google Scholar] [CrossRef]
- Panja, S.; Dey, G.; Bharti, R.; Kumari, K.; Maiti, T.K.; Mandal, M.; Chattopadhyay, S. Tailor-Made Temperature-Sensitive Micelle for Targeted and On-Demand Release of Anticancer Drugs. ACS Appl. Mater. Interfaces 2016, 8, 12063–12074. [Google Scholar] [CrossRef]
- Liu, S.Q.; Wiradharma, N.; Gao, S.J.; Tong, Y.W.; Yang, Y.Y. Bio-functional micelles self-assembled from a folate-conjugated block copolymer for targeted intracellular delivery of anticancer drugs. Biomaterials 2007, 28, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.J.; Nabid, M.R.; Niknejad, H.; Entezami, A.A. Folate-decorated thermoresponsive micelles based on star-shaped amphiphilic block copolymers for efficient intracellular release of anticancer drugs. Int. J. Pharm. 2012, 437, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Zangabad, P.S.; Aghanejad, A.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Folate-conjugated thermosensitive O-maleoyl modified chitosan micellar nanoparticles for targeted delivery of erlotinib. Carbohydr. Polym. 2017, 172, 130–141. [Google Scholar] [CrossRef] [PubMed]
- John, J.V.; Jeong, Y.I.; Johnson, R.P.; Chung, C.W.; Park, H.; Kang, D.H.; Cho, J.K.; Kim, Y.; Kim, I. Folic acid-tethered poly(N-isopropylacrylamide)-phospholipid hybrid nanocarriers for targeted drug delivery. J. Mater. Chem. B 2015, 3, 8268–8278. [Google Scholar] [CrossRef]
- De, P.; Gondi, S.R.; Sumerlin, B.S. Folate-conjugated thermoresponsive block copolymers: Highly efficient conjugation and solution self-assembly. Biomacromolecules 2008, 9, 1064–1070. [Google Scholar] [CrossRef]
- Yang, W.J.; Zhao, T.; Zhou, P.; Chen, S.; Gao, Y.; Liang, L.; Wang, X.; Wang, L. “Click” Functionalization of Dual Stimuli-Responsive Polymer Nanocapsules for Drug Delivery Systems. Polym. Chem. 2017, 8, 3056–3065. [Google Scholar] [CrossRef]
- Li, R.; Feng, F.; Wang, Y.; Yang, X.; Yang, V.C. Folic acid-conjugated pH/temperature/redox multi-stimuli responsive polymer microspheres for delivery of anti-cancer drug. J. Colloid Interface Sci. 2014, 429, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jo, A.; Baek, S.; Lim, D.; Park, S.Y.; Cho, S.K.; Chung, J.W.; Yoon, J. Synergistically enhanced selective intracellular uptake of anticancer drug carrier comprising folic acid-conjugated hydrogels containing magnetite nanoparticles. Sci. Rep. 2017, 7, 41090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, R.; Gulati, N.; Kotnala, R.K.; Sharma, U.; Jayasundar, R.; Koul, V. Evaluation of folate conjugated pegylated thermosensitive magnetic nanocomposites for tumor imaging and therapy. Colloids Surf. B Biointerfaces 2011, 82, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Torkpur-Biglarianzadeh, M.; Salami-Kalajahi, M. Multilayer Fluorescent Magnetic Nanoparticles with Dual Thermoresponsive and PH-Sensitive Polymeric Nanolayers as Anti-Cancer Drug Carriers. RSC Adv. 2015, 5, 29653–29662. [Google Scholar] [CrossRef]
- Yata, V.K.; Banerjee, S.; Ghosh, S.S. Folic Acid Conjugated-Bio Polymeric Nanocarriers: Synthesis, Characterization and In Vitro Delivery of Prodrug Converting Enzyme. Adv. Sci. Eng. Med. 2014, 6, 388–392. [Google Scholar] [CrossRef]
- Dubé, D.; Francis, M.; Leroux, J.C.; Winnik, F.M. Preparation and tumor cell uptake of poly(N-isopropylacrylamide) folate conjugates. Bioconjugate Chem. 2002, 13, 685–692. [Google Scholar] [CrossRef]
- Shin, H.H.; Choi, H.W.; Lim, J.H.; Kim, J.W.; Chung, B.G. Near-Infrared Light-Triggered Thermo-responsive Poly(N-Isopropylacrylamide)-Pyrrole Nanocomposites for Chemo-photothermal Cancer Therapy. Nanoscale Res. Lett. 2020, 15, 214. [Google Scholar] [CrossRef]
- Turkevich, N.M.; Zimenkovskii, B.S. Some characteristic properties of 3-alkyl-2-thiothiazon-4-ones and of intermediates in their synthesis. Chem. Heterocycl. Compd. 1967, 3, 667–671. [Google Scholar] [CrossRef]
- Sugihara, Y.; O’Connor, P.; Zetterlund, P.B.; Aldabbagh, F. Chain transfer to solvent in the radical polymerization of N-isopropylacrylamide. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1856–1864. [Google Scholar] [CrossRef]
- Yi, M.; Li, J.; Ha, H. Radiation preparation of the water-soluble, temperature sensitive polymers in organic solvents. Radiat. Phys. Chem. 1995, 46, 855–858. [Google Scholar] [CrossRef]
- Eliezar, J.; Scarano, W.; Boase, N.R.; Thurecht, K.J.; Stenzel, M.H. In vivo evaluation of folate decorated cross-linked micelles for the delivery of platinum anticancer drugs. Biomacromolecules 2015, 16, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Biju, S.; Gallo, J.; Bañobre-López, M.; Manshian, B.B.; Soenen, S.J.; Himmelreich, U.; Vander Elst, L.; Parac-Vogt, T.N. A Magnetic Chameleon: Biocompatible Lanthanide Fluoride Nanoparticles with Magnetic Field Dependent Tunable Contrast Properties as a Versatile Contrast Agent for Low to Ultrahigh Field MRI and Optical Imaging in Biological Window. Chemistry 2018, 24, 7388–7397. [Google Scholar] [CrossRef]
- Off, M.K.; Steindal, A.E.; Porojnicu, A.C.; Juzeniene, A.; Vorobey, A.; Johnsson, A.; Moan, J. Ultraviolet photodegradation of folic acid. J. Photochem. Photobiol. B Biol. 2005, 80, 47–55. [Google Scholar] [CrossRef]
- Schild, H.G. Poly(N-Isopropylacrylamide): Experiment, Theory and Application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Bansal, M.; Singh, N.; Pal, S.; Dev, I.; Ansari, K.M. Chemopreventive Role of Dietary Phytochemicals in Colorectal Cancer. Adv. Mol. Toxicol. 2018, 12, 69–121. [Google Scholar] [CrossRef]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High drug-loading nanomedicines: Progress, current status, and prospects. Int. J. Nanomed. 2017, 12, 4085–4109. [Google Scholar] [CrossRef] [Green Version]
- McQuade, R.M.; Stojanovska, V.; Bornstein, J.C.; Nurgali, K. Colorectal Cancer Chemotherapy: The Evolution of Treatment and New Approaches. Curr. Med. Chem. 2017, 24, 1537–1557. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef] [PubMed]
- Radu, I.C.; Hudita, A.; Zaharia, C.; Galateanu, B.; Iovu, H.; Tanasa, E.V.; Georgiana Nitu, S.; Ginghina, O.; Negrei, C.; Tsatsakis, A.; et al. Poly(3-hydroxybutyrate-CO-3-hydroxyvalerate) PHBHV biocompatible nanocarriers for 5-FU delivery targeting colorectal cancer. Drug Deliv. 2019, 26, 318–327. [Google Scholar] [CrossRef] [Green Version]
- de la Harpe, K.M.; Kondiah, P.P.D.; Choonara, Y.E.; Marimuthu, T.; du Toit, L.C.; Pillay, V. The Hemocompatibility of Nanoparticles: A Review of Cell-Nanoparticle Interactions and Hemostasis. Cells 2019, 8, 1209. [Google Scholar] [CrossRef]
- Mehrizi, T.Z. Hemocompatibility and Hemolytic Effects of Functionalized Nanoparticles on Red Blood Cells: A Recent Review Study. Nano 2021, 16, 21300073. [Google Scholar] [CrossRef]
- Amin, K.; Dannenfelser, R.M. In vitro hemolysis: Guidance for the pharmaceutical scientist. J. Pharm. Sci. 2006, 95, 1173–1176. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vĕtvicka, V.; Bilej, M.; Kincade, P.W. Resistance of macrophages to 5-fluorouracil treatment. Immunopharmacology 1990, 19, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Bosshart, H.; Heinzelmann, M. THP-1 cells as a model for human monocytes. Ann. Transl. Med. 2016, 4, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, M.; Oshita, F.; Kato, Y.; Yamada, K.; Nomura, I.; Noda, K. Early monocytopenia after chemotherapy as a risk factor for neutropenia. Am. J. Clin. Oncol. 1999, 22, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Nygren, P. What is cancer chemotherapy? Acta Oncol. 2001, 40, 166–174. [Google Scholar] [CrossRef]
- Ragnhammar, P.; Hafström, L.; Nygren, P.; Glimelius, B. A systematic overview of chemotherapy effects in colorectal cancer. Acta Oncol. 2001, 40, 282–308. [Google Scholar] [CrossRef]
- Sara, J.D.; Kaur, J.; Khodadadi, R.; Rehman, M.; Lobo, R.; Chakrabarti, S.; Herrmann, J.; Lerman, A.; Grothey, A. 5-fluorouracil and cardiotoxicity: A review. Ther. Adv. Med. Oncol. 2018, 10, 1758835918780140. [Google Scholar] [CrossRef] [Green Version]
- Ophir, A. Effects of 5-fluorouracil on proliferating fibroblasts in vivo. Exp. Eye Res. 1991, 53, 799–803. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Li, F.; Wang, J.; Zhang, Z. Preparation and in vitro studies of MRI-specific superparamagnetic iron oxide antiGPC3 probe for hepatocellular carcinoma. Int. J. Nanomed. 2012, 7, 4593–4611. [Google Scholar] [CrossRef] [Green Version]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef] [PubMed]
- Doucette, M.M.; Stevens, V.L. Folate receptor function is regulated in response to different cellular growth rates in cultured mammalian cells. J. Nutr. 2001, 131, 2819–2825. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Wang, Y.; Zeng, F.; Chen, L.; Peng, Z.; Kong, L.X. Synthesis and characterization of folate conjugated chitosan and cellular uptake of its nanoparticles in HT-29 cells. Carbohydr. Res. 2011, 346, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Bracht, K.; Nicholls, A.M.; Liu, Y.; Bodmer, W.F. 5-Fluorouracil response in a large panel of colorectal cancer cell lines is associated with mismatch repair deficiency. Br. J. Cancer 2010, 103, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Moodley, T.; Singh, M. Polymeric Mesoporous Silica Nanoparticles for Enhanced Delivery of 5-Fluorouracil In Vitro. Pharmaceutics 2019, 11, 288. [Google Scholar] [CrossRef] [Green Version]
- Öztürk, K.; Mashal, A.R.; Yegin, B.A.; Çalış, S. Preparation and in vitro evaluation of 5-fluorouracil-loaded PCL nanoparticles for colon cancer treatment. Pharm. Dev. Technol. 2017, 22, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Chen, L.; Gao, W.; Zeng, F.; Kong, L.X. Targeted delivery of 5-fluorouracil to HT-29 cells using high efficient folic acid-conjugated nanoparticles. Drug Deliv. 2015, 22, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Guo, J.; Xie, Z.; Shan, D.; Gerhard, E.; Qian, G.; Yang, J. Fluorescence imaging enabled poly(lactide-co-glycolide). Acta Biomater. 2016, 29, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Wong, E.H.H.; Fu, Q.; Ren, J.M.; Sulistio, A.; Ladewig, K.; Blencowe, A.; Qiao, G.G. Azobenzene-Functionalised Core Cross-Linked Star Polymers and their Host–Guest Interactions. Aust. J. Chem. 2013, 67, 173–178. [Google Scholar] [CrossRef]
- Despax, L.; Fitremann, J.; Destarac, M.; Harrisson, S. Low concentration thermoresponsive hydrogels from readily accessible triblock copolymers. Polym. Chem. 2016, 7, 3375–3377. [Google Scholar] [CrossRef]


| Polymer | wt% FAth a | wt% FA b | Mn,th (g·mol−1) c | Mn,NMR (g·mol−1) d | Mn,SEC (g·mol−1) e | Ð e |
|---|---|---|---|---|---|---|
| P-1 | - | - | 2710 | 3500 | 2740 | 1.87 |
| P-2 | - | - | 5220 | 5800 | 6640 | 1.55 |
| P-3 | - | - | 10,210 | 8700 | 8300 | 1.97 |
| PT-1 | - | - | 9210 | - | 9070 | 1.40 |
| PT-2 | - | - | 12,600 | - | 9780 | 1.42 |
| PT-3 | - | - | 19,610 | - | 11,040 | 1.55 |
| PTF-1 | 33 | 10 | 13,700 | - | 13,040 | 1.65 |
| PTF-2 | 29 | 9 | 17,840 | - | 14,700 | 1.51 |
| PTF-3 | 26 | 8 | 26,500 | - | 17,040 | 1.57 |
| Polymer | Parameters | |
|---|---|---|
| Size (nm) | ζ [mV] | |
| PT-1 | 54.33 ± 4.98 | −0.44 ± 0.07 |
| PT-2 | 92.83 ± 3.66 | −3.56 ± 0.45 |
| PT-3 | 6.21 ± 0.11 | −8.56 ± 0.54 |
| PTF-1 | 53.10 ± 4.60 | −16.61 ± 2.67 |
| PTF-2 | 6.37 ± 0.28 | −15.18 ± 2.51 |
| PTF-3 | 8.69 ± 0.70 | −12.34 ± 2.02 |
| PT-1 (5FU) | 5.33 ± 1.27 | −12.94 ± 1.02 |
| PT-2 (5FU) | 4.73 ± 0.63 | −13.91 ± 1.52 |
| PT-3 (5FU) | 6.66 ± 0.11 | −21.55 ± 1.47 |
| PTF-1 (5FU) | 7.77 ± 0.54 | −17.02 ± 0.65 |
| PTF-2 (5FU) | 8.02 ± 0.91 | −9.08 ± 0.84 |
| PTF-3 (5FU) | 9.4 ± 0.96 | −15.64 ± 2.3 |
| Polymer | TCP [°C] | |
|---|---|---|
| Without 5-FU | With 5-FU | |
| PT-1 | 35.7 | 36.0 |
| PT-2 | 35.5 | 36.0 |
| PT-3 | 35.4 | 36.0 |
| PTF-1 | 36.1 | 36.5 |
| PTF-2 | 36.0 | 36.6 |
| PTF-3 | 35.6 | 36.5 |
| Polymer | LE [%] a | LC [%] b | Release after 24 h [%] | |
|---|---|---|---|---|
| PBS | H2O | |||
| PT-1 | 16.8 | 3.3 | n.d. | - |
| PT-2 | 29.5 | 5.6 | n.d. | - |
| PT-3 | 89.4 | 15.2 | n.d. | 1.5 |
| PTF-1 | 69.2 | 12.2 | 4.0 | 2.0 |
| PTF-2 | 45.5 | 8.3 | n.d. | - |
| PTF-3 | 41.7 | 7.7 | n.d. | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milewska, S.; Siemiaszko, G.; Wilczewska, A.Z.; Misztalewska-Turkowicz, I.; Markiewicz, K.H.; Szymczuk, D.; Sawicka, D.; Car, H.; Lazny, R.; Niemirowicz-Laskowska, K. Folic-Acid-Conjugated Thermoresponsive Polymeric Particles for Targeted Delivery of 5-Fluorouracil to CRC Cells. Int. J. Mol. Sci. 2023, 24, 1364. https://doi.org/10.3390/ijms24021364
Milewska S, Siemiaszko G, Wilczewska AZ, Misztalewska-Turkowicz I, Markiewicz KH, Szymczuk D, Sawicka D, Car H, Lazny R, Niemirowicz-Laskowska K. Folic-Acid-Conjugated Thermoresponsive Polymeric Particles for Targeted Delivery of 5-Fluorouracil to CRC Cells. International Journal of Molecular Sciences. 2023; 24(2):1364. https://doi.org/10.3390/ijms24021364
Chicago/Turabian StyleMilewska, Sylwia, Gabriela Siemiaszko, Agnieszka Zofia Wilczewska, Iwona Misztalewska-Turkowicz, Karolina Halina Markiewicz, Dawid Szymczuk, Diana Sawicka, Halina Car, Ryszard Lazny, and Katarzyna Niemirowicz-Laskowska. 2023. "Folic-Acid-Conjugated Thermoresponsive Polymeric Particles for Targeted Delivery of 5-Fluorouracil to CRC Cells" International Journal of Molecular Sciences 24, no. 2: 1364. https://doi.org/10.3390/ijms24021364






