Macrophages and Intervertebral Disc Degeneration
Abstract
1. Introduction
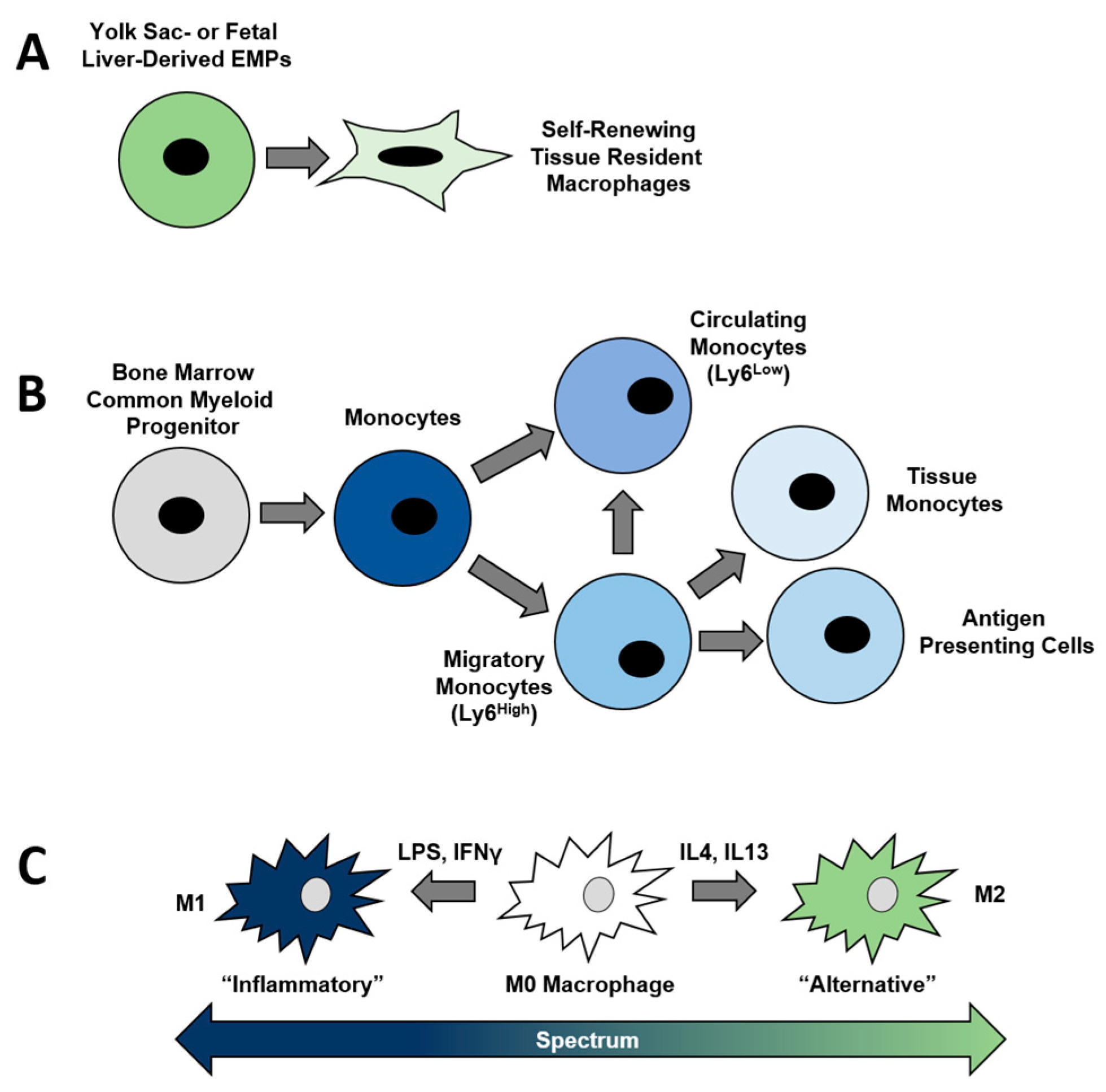
2. Myeloid Lineage Cells and Their Derivatives
3. Macrophage Function and Polarization
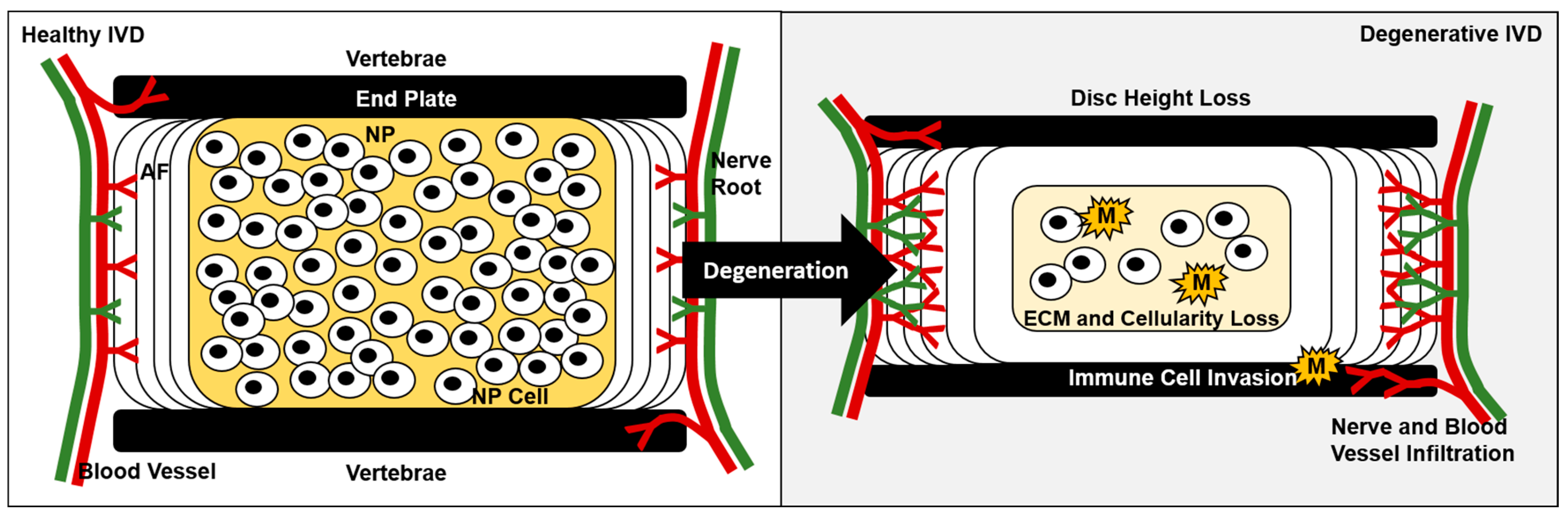
4. Roles of Macrophages in Disc Degeneration
4.1. Increased Vascularization May Facilitate Immune Cell Invasion during Disc Degeneration
4.2. Macrophage Levels Associate with Degenerative Disc Disease Severity
4.3. Intervertebral Disc-Bone Crosstalk
4.4. Regulation of Pain Mediation by Macrophages within Degenerated Discs
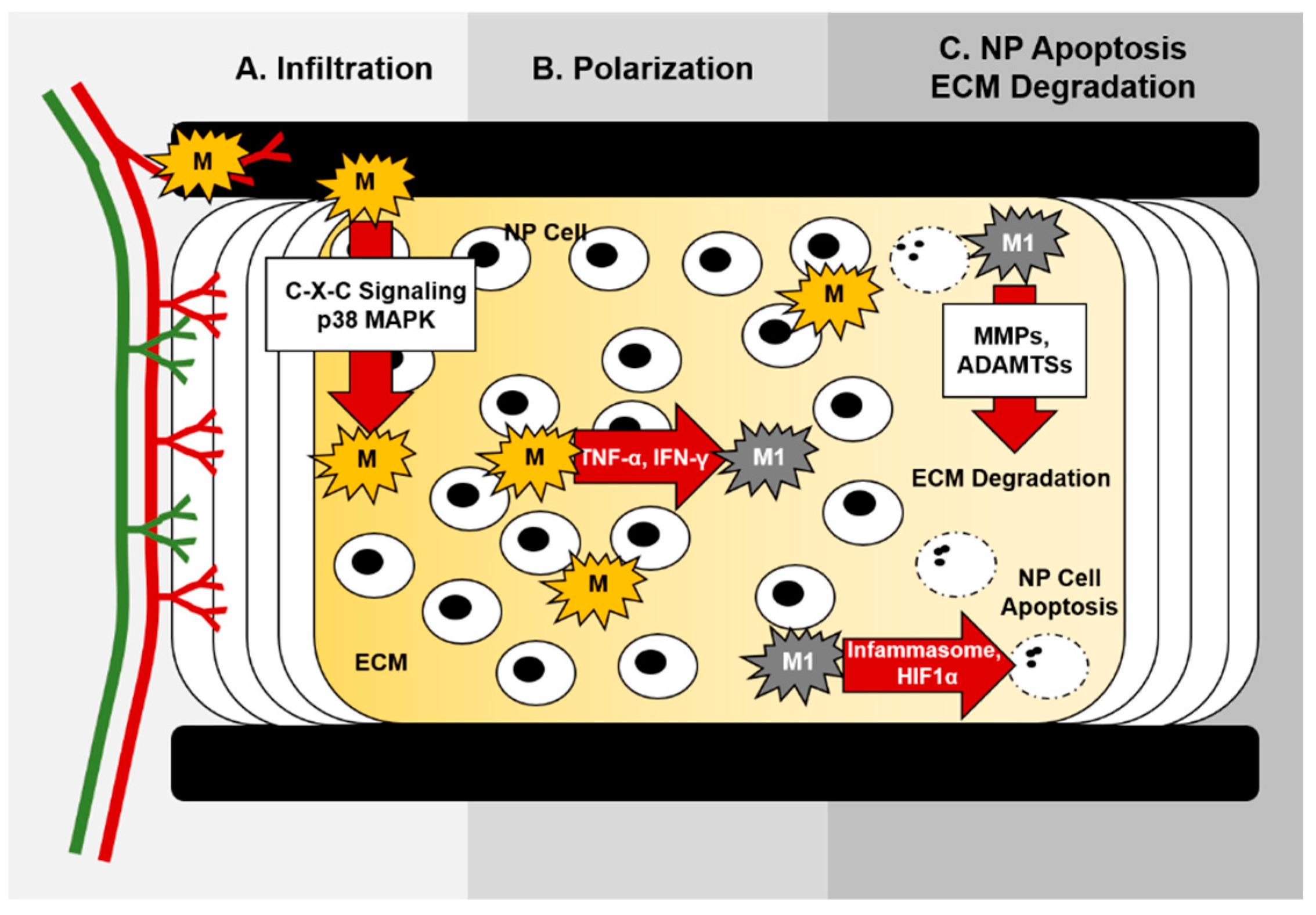
4.5. Extracellular Matrix Degradation
5. Molecular Control of Macrophage Function during IVD Degeneration
5.1. Chemokine Signaling and Macrophage Migration
5.2. Macrophage Inflammasome Activation during IVD Degeneration
5.3. Tgfβ Signaling
5.4. Hypoxia and Oxidative Stress
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersson, G.B. Epidemiological features of chronic low-back pain. Lancet 1999, 354, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.I.; Deyo, R.A.; Mirza, S.K.; Turner, J.A.; Comstock, B.A.; Hollingworth, W.; Sullivan, S.D. Expenditures and Health Status among Adults with Back and Neck Problems. JAMA 2008, 299, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Manchikanti, L.; Soin, A.; Benyamin, R.M.; Singh, V.; Falco, F.J.; Calodney, A.K.; Grami, V.; Hirsch, J.A. An Update of the Systematic Appraisal of the Accuracy and Utility of Discography in Chronic Spinal Pain. Pain Physician 2018, 21, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Teraguchi, M.; Yoshimura, N.; Hashizume, H.; Muraki, S.; Yamada, H.; Minamide, A.; Oka, H.; Ishimoto, Y.; Nagata, K.; Kagotani, R.; et al. Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population-based cohort: The Wakayama Spine Study. Osteoarthr. Cartil. 2014, 22, 104–110. [Google Scholar] [CrossRef]
- Brinjikji, W.; Luetmer, P.; Comstock, B.; Bresnahan, B.; Chen, L.; Deyo, R.; Halabi, S.; Turner, J.; Avins, A.; James, K.; et al. Systematic Literature Review of Imaging Features of Spinal Degeneration in Asymptomatic Populations. Am. J. Neuroradiol. 2015, 36, 811–816. [Google Scholar] [CrossRef]
- Brinjikji, W.; Diehn, F.E.; Jarvik, J.G.; Carr, C.M.; Kallmes, D.F.; Murad, M.H.; Luetmer, P.H. MRI Findings of Disc Degeneration are More Prevalent in Adults with Low Back Pain than in Asymptomatic Controls: A Systematic Review and Meta-Analysis. Am. J. Neuroradiol. 2015, 36, 2394–2399. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, C.J.; Dave, A.; Charytonowicz, D.; Sebra, R.; Iatridis, J.C. Single-cell RNA-sequencing atlas of bovine caudal intervertebral discs: Discovery of heterogeneous cell populations with distinct roles in homeostasis. FASEB J. 2021, 35, e21919. [Google Scholar] [CrossRef]
- Cherif, H.; Mannarino, M.; Pacis, A.S.; Ragoussis, J.; Rabau, O.; Ouellet, J.A.; Haglund, L. Single-Cell RNA-Seq Analysis of Cells from Degenerating and Non-Degenerating Intervertebral Discs from the Same Individual Reveals New Biomarkers for Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2022, 23, 3993. [Google Scholar] [CrossRef]
- Jiang, W.; Glaeser, J.D.; Salehi, K.; Kaneda, G.; Mathkar, P.; Wagner, A.; Ho, R.; Sheyn, D. Single-cell atlas unveils cellular heterogeneity and novel markers in human neonatal and adult intervertebral discs. iScience 2022, 25, 104504. [Google Scholar] [CrossRef]
- Eyre, D.R.; Muir, H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim. Biophys. Acta 1977, 492, 29–42. [Google Scholar] [CrossRef]
- Johnstone, B.; Bayliss, M.T. The Large Proteoglycans of the Human Intervertebral Disc. Changes in their biosynthesis and structure with age, topography, and pathology. Spine 1995, 20, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Fournier, D.; Kiser, P.K.; Shoemaker, J.K.; Battié, M.C.; Séguin, C.A. Vascularization of the human intervertebral disc: A scoping review. JOR Spine 2020, 3, e1123. [Google Scholar] [CrossRef] [PubMed]
- Kauppila, L.I. Ingrowth of blood vessels in disc degeneration. Angiographic and histological studies of cadaveric spines. J. Bone Jt. Surg. 1995, 77, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Nerlich, A.G.; Schaaf, R.; Wälchli, B.; Boos, N. Temporo-spatial distribution of blood vessels in human lumbar intervertebral discs. Eur. Spine J. Am. 2007, 16, 547–555. [Google Scholar] [CrossRef]
- Ellingson, A.M.; Mehta, H.; Polly, D.W.; Ellermann, J.; Nuckley, D.J. Disc Degeneration Assessed by Quantitative T2* (T2 Star) Correlated With Functional Lumbar Mechanics. Spine 2013, 38, E1533–E1540. [Google Scholar] [CrossRef]
- Ellingson, A.M.; Nagel, T.M.; Polly, D.W.; Ellermann, J.; Nuckley, D.J. Quantitative T2* (T2 star) relaxation times predict site specific proteoglycan content and residual mechanics of the intervertebral disc throughout degeneration. J. Orthop. Res. 2014, 32, 1083–1089. [Google Scholar] [CrossRef]
- Lyons, G.; Eisenstein, S.; Sweet, M. Biochemical changes in intervertebral disc degeneration. Biochim. Biophys. Acta BBA Gen. Subj. 1981, 673, 443–453. [Google Scholar] [CrossRef]
- Antoniou, J.; Steffen, T.; Nelson, F.; Winterbottom, N.; Hollander, A.P.; Poole, R.A.; Aebi, M.; Alini, M. The human lumbar intervertebral disc: Evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J. Clin. Investig. 1996, 98, 996–1003. [Google Scholar] [CrossRef]
- Hollander, A.P.; Heathfield, T.F.; Liu, J.J.; Pidoux, I.; Roughley, P.J.; Mort, J.S.; Poole, A.R. Enhanced denaturation of the alpha (II) chains of type-II collagen in normal adult human intervertebral discs compared with femoral articular cartilage. J. Orthop. Res. 1996, 14, 61–66. [Google Scholar] [CrossRef]
- Adams, M.A.; Roughley, P.J. What is Intervertebral Disc Degeneration, and What Causes It? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef]
- Castro, A.L.; Ribeiro-Machado, C.; Oliveira, C.M.; Teixeira, G.Q.; Neidlinger-Wilke, C.; Pereira, P.; Vaz, R.; Barbosa, M.A.; Gonçalves, R.M. Fibrotic alterations in human annulus fibrosus correlate with progression of intervertebral disc herniation. Arthritis Res. Ther. 2022, 24, 25. [Google Scholar] [CrossRef]
- Francisco, V.; Pino, J.; González-Gay, M.; Lago, F.; Karppinen, J.; Tervonen, O.; Mobasheri, A.; Gualillo, O. A new immunometabolic perspective of intervertebral disc degeneration. Nat. Rev. Rheumatol. 2022, 18, 47–60. [Google Scholar] [CrossRef]
- McDonnell, E.E.; Buckley, C.T. Consolidating and re-evaluating the human disc nutrient microenvironment. JOR Spine 2022, 5, e1192. [Google Scholar] [CrossRef] [PubMed]
- Liyew, W.A. Clinical Presentations of Lumbar Disc Degeneration and Lumbosacral Nerve Lesions. Int. J. Rheumatol. 2020, 2020, 2919625. [Google Scholar] [CrossRef] [PubMed]
- Krock, E.; Rosenzweig, D.H.; Chabot-Doré, A.; Jarzem, P.; Weber, M.H.; Ouellet, J.A.; Stone, L.S.; Haglund, L. Painful, degenerating intervertebral discs up-regulate neurite sprouting andCGRPthrough nociceptive factors. J. Cell. Mol. Med. 2014, 18, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Purmessur, D.; Baird, P.; Probyn, B.; Freemont, A.J.; Hoyland, J.A. Degenerate Human Nucleus Pulposus Cells Promote Neurite Outgrowth in Neural Cells. PLoS ONE 2012, 7, e47735. [Google Scholar] [CrossRef]
- Freemont, A.J.; Watkins, A.; Le Maitre, C.; Baird, P.; Jeziorska, M.; Knight, M.T.N.; Ross, E.R.S.; O’Brien, J.P.; Hoyland, J.A. Nerve growth factor expression and innervation of the painful intervertebral disc. J. Pathol. 2002, 197, 286–292. [Google Scholar] [CrossRef]
- Freemont, A.; Peacock, T.; Goupille, P.; Hoyland, J.; O’Brien, J.; Jayson, M. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 1997, 350, 178–181. [Google Scholar] [CrossRef]
- Ohtori, S.; Inoue, G.; Miyagi, M.; Takahashi, K. Pathomechanisms of discogenic low back pain in humans and animal models. Spine J. 2015, 15, 1347–1355. [Google Scholar] [CrossRef]
- Raj, P.P. Intervertebral Disc: Anatomy-Physiology-Pathophysiology-Treatment. Pain Pract. 2008, 8, 18–44. [Google Scholar] [CrossRef]
- James, G.; Sluka, K.A.; Blomster, L.; Hall, L.; Schmid, A.B.; Shu, C.C.; Little, C.B.; Melrose, J.; Hodges, P.W. Macrophage polarization contributes to local inflammation and structural change in the multifidus muscle after intervertebral disc injury. Eur. Spine J. 2018, 27, 1744–1756. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.E.B.; Caterson, B.; Eisenstein, S.M.; Hynds, D.; Snow, D.M.; Roberts, S. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis Rheum. 2002, 46, 2658–2664. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Roberts, S.; Smith, S.; Menage, J.; Ghosh, P. Increased Nerve and Blood Vessel Ingrowth Associated With Proteoglycan Depletion in an Ovine Anular Lesion Model of Experimental Disc Degeneration. Spine 2002, 27, 1278–1285. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Jakubzick, C.V.; Randolph, G.J.; Henson, C.V.J.P.M. Monocyte differentiation and antigen-presenting functions. Nat. Rev. Immunol. 2017, 17, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, B.; Caolo, V.; Peacock, H.M.; Lehoux, S.; Gomez-Perdiguero, E.; Luttun, A.; Jones, E.A.V. Erythro-myeloid progenitors can differentiate from endothelial cells and modulate embryonic vascular remodeling. Sci. Rep. 2017, 7, 43817. [Google Scholar] [CrossRef]
- Frame, J.M.; McGrath, K.E.; Palis, J. Erythro-myeloid progenitors: “Definitive” hematopoiesis in the conceptus prior to the emergence of hematopoietic stem cells. Blood Cells Mol. Dis. 2013, 51, 220–225. [Google Scholar] [CrossRef]
- Yadav, S.; Priya, A.; Borade, D.R.; Agrawal-Rajput, R. Macrophage subsets and their role: Co-relation with colony-stimulating factor-1 receptor and clinical relevance. Immunol. Res. 2022, 1–23. [Google Scholar] [CrossRef]
- Tauber, A.I. Metchnikoff and the phagocytosis theory. Nat. Rev. Mol. Cell Biol. 2003, 4, 897–901. [Google Scholar] [CrossRef]
- Nich, C.; Takakubo, Y.; Pajarinen, J.; Ainola, M.; Salem, A.; Sillat, T.; Rao, A.J.; Raska, M.; Tamaki, Y.; Takagi, M.; et al. Macrophages-Key cells in the response to wear debris from joint replacements. J. Biomed. Mater. Res. Part A 2013, 101, 3033–3045. [Google Scholar] [CrossRef]
- Prattichizzo, F.; Bonafè, M.; Olivieri, F.; Franceschi, C. Senescence associated macrophages and “macroph-aging”: Are they pieces of the same puzzle? Aging 2016, 8, 3159–3160. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front. Oncol. 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Thorseth, M.-L.; Carretta, M.; Jensen, C.; Mølgaard, K.; Jürgensen, H.J.; Engelholm, L.H.; Behrendt, N.; Willumsen, N.; Madsen, D.H. Uncovering mediators of collagen degradation in the tumor microenvironment. Matrix Biol. Plus 2022, 13, 100101. [Google Scholar] [CrossRef] [PubMed]
- Aurora, A.B.; Porrello, E.; Tan, W.; Mahmoud, A.I.; Hill, J.A.; Bassel-Duby, R.; Sadek, H.A.; Olson, E.N. Macrophages are required for neonatal heart regeneration. J. Clin. Investig. 2014, 124, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.W.; Pinto, A.R.; Rosenthal, N.A. Macrophages are required for adult salamander limb regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 9415–9420. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Yang, C.-C.; Chen, I.-H.; Liu, Y.-M.L.; Chang, S.-J.; Chuang, Y.-J. Treatment of Glucocorticoids Inhibited Early Immune Responses and Impaired Cardiac Repair in Adult Zebrafish. PLoS ONE 2013, 8, e66613. [Google Scholar] [CrossRef] [PubMed]
- Otero, K.; Turnbull, I.R.; Poliani, P.L.; Vermi, W.; Cerutti, E.; Aoshi, T.; Tassi, I.; Takai, T.; Stanley, S.L.; Miller, M.; et al. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and β-catenin. Nat. Immunol. 2009, 10, 734–743. [Google Scholar] [CrossRef]
- Boulakirba, S.; Pfeifer, A.; Mhaidly, R.; Obba, S.; Goulard, M.; Schmitt, T.; Chaintreuil, P.; Calleja, A.; Furstoss, N.; Orange, F.; et al. IL-34 and CSF-1 display an equivalent macrophage differentiation ability but a different polarization potential. Sci. Rep. 2018, 8, 256. [Google Scholar] [CrossRef]
- Himes, S.R.; Cronau, S.; Mulford, C.; Hume, D.A. The Runx1 transcription factor controls CSF-1-dependent and -independent growth and survival of macrophages. Oncogene 2005, 24, 5278–5286. [Google Scholar] [CrossRef]
- Hume, D.A.; Summers, K.M.; Rehli, M. Transcriptional Regulation and Macrophage Differentiation. Microbiol. Spectr. 2016, 4, 117–139. [Google Scholar] [CrossRef]
- Sima, C.; Viniegra, A.; Glogauer, M. Macrophage immunomodulation in chronic osteolytic diseases—The case of periodontitis. J. Leukoc. Biol. 2019, 105, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Boniakowski, A.E.; Kimball, A.S.; Jacobs, B.N.; Kunkel, S.L.; Gallagher, K.A. Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing. J. Immunol. 2017, 199, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef] [PubMed]
- Dimitroulas, T.; Nikas, S.N.; Trontzas, P.; Kitas, G.D. Biologic therapies and systemic bone loss in rheumatoid arthritis. Autoimmun. Rev. 2013, 12, 958–966. [Google Scholar] [CrossRef]
- Duplomb, L.; Baud’Huin, M.; Charrier, C.; Berreur, M.; Trichet, V.; Blanchard, F.; Heymann, D. Interleukin-6 Inhibits Receptor Activator of Nuclear Factor κB Ligand-Induced Osteoclastogenesis by Diverting Cells into the Macrophage Lineage: Key Role of Serine727 Phosphorylation of Signal Transducer and Activator of Transcription 3. Endocrinology 2008, 149, 3688–3697. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.-H.; Ha Kim, J.; Choi, Y.; Kim, N. NFATc1 Induces Osteoclast Fusion Via Up-Regulation of Atp6v0d2 and the Dendritic Cell-Specific Transmembrane Protein (DC-STAMP). Mol. Endocrinol. 2008, 22, 176–185. [Google Scholar] [CrossRef]
- Lam, J.; Takeshita, S.; Barker, J.E.; Kanagawa, O.; Ross, F.P.; Teitelbaum, S.L. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Investig. 2000, 106, 1481–1488. [Google Scholar] [CrossRef]
- Tamura, T.; Udagawa, N.; Takahashi, N.; Miyaura, C.; Tanaka, S.; Yamada, Y.; Koishihara, Y.; Ohsugi, Y.; Kumaki, K.; Taga, T. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc. Natl. Acad. Sci. USA 1993, 90, 11924–11928. [Google Scholar] [CrossRef]
- Vogel, D.Y.S.; Heijnen, P.D.A.M.; Breur, M.; De Vries, H.E.; Tool, A.T.J.; Amor, S.; Dijkstra, C.D. Macrophages migrate in an activation-dependent manner to chemokines involved in neuroinflammation. J. NeuroInflamm. 2014, 11, 23. [Google Scholar] [CrossRef]
- Yao, Z.; Li, P.; Zhang, Q.; Schwarz, E.M.; Keng, P.; Arbini, A.; Boyce, B.F.; Xing, L. Tumor Necrosis Factor-α Increases Circulating Osteoclast Precursor Numbers by Promoting Their Proliferation and Differentiation in the Bone Marrow through Up-regulation of c-Fms Expression. J. Biol. Chem. 2006, 281, 11846–11855. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Sato, K.; Miyazaki, T.; Kitaura, H.; Kayama, H.; Miyoshi, F.; Araki, Y.; Akiyama, Y.; Takeda, K.; Mimura, T. Combination of Tumor Necrosis Factor α and Interleukin-6 Induces Mouse Osteoclast-like Cells With Bone Resorption Activity Both In Vitro and In Vivo. Arthritis Rheumatol. 2014, 66, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.-C.; Smith, A.M.; Everts, B.; Colonna, M.; Pearce, E.L.; Schilling, J.D.; Pearce, E.J. Metabolic Reprogramming Mediated by the mTORC2-IRF4 Signaling Axis Is Essential for Macrophage Alternative Activation. Immunity 2016, 45, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Tugal, D.; Liao, X.; Jain, M.K. Transcriptional Control of Macrophage Polarization. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Assoian, R.K.; Fleurdelys, B.E.; Stevenson, H.C.; Miller, P.J.; Madtes, D.K.; Raines, E.W.; Ross, R.; Sporn, M.B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc. Natl. Acad. Sci. USA 1987, 84, 6020–6024. [Google Scholar] [CrossRef]
- Muñoz, J.; Akhavan, N.; Mullins, A.; Arjmandi, B. Macrophage Polarization and Osteoporosis: A Review. Nutrients 2020, 12, 2999. [Google Scholar] [CrossRef]
- Takahashi, N.; Ejiri, S.; Yanagisawa, S.; Ozawa, H. Regulation of osteoclast polarization. Odontology 2007, 95, 1–9. [Google Scholar] [CrossRef]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef]
- Sakai, D.; Grad, S. Advancing the cellular and molecular therapy for intervertebral disc disease. Adv. Drug Deliv. Rev. 2015, 84, 159–171. [Google Scholar] [CrossRef]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef]
- Willems, N.; Tellegen, A.R.; Bergknut, N.; Creemers, L.B.; Wolfswinkel, J.; Freudigmann, C.; Benz, K.; Grinwis, G.C.M.; Tryfonidou, M.A.; Meij, B.P. Inflammatory profiles in canine intervertebral disc degeneration. BMC Vet. Res. 2016, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, K.R.; Walter, B.A.; Laudier, D.M.; Krishnamoorthy, D.; Mosley, G.E.; Spiller, K.L.; Iatridis, J.C. Accumulation and localization of macrophage phenotypes with human intervertebral disc degeneration. Spine J. 2018, 18, 343–356. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, P.; Hu, B.; Liu, W.; Lv, X.; Chen, S.; Shao, Z. HSP90 Inhibitor 17-AAG Attenuates Nucleus Pulposus Inflammation and Catabolism Induced by M1-Polarized Macrophages. Front. Cell Dev. Biol. 2021, 9, 796974. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; An, R.; Xiang, Q.; Li, G.; Wang, K.; Song, Y.; Liao, Z.; Li, S.; Hua, W.; Feng, X.; et al. Acid-sensing ion channels regulate nucleus pulposus cell inflammation and pyroptosis via the NLRP3 inflammasome in intervertebral disc degeneration. Cell Prolif. 2021, 54, e12941. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Millecamps, M.; Foster, D.Z.; Stone, L.S. Long-term histological analysis of innervation and macrophage infiltration in a mouse model of intervertebral disc injury–induced low back pain. J. Orthop. Res. 2020, 38, 1238–1247. [Google Scholar] [CrossRef]
- Nakawaki, M.; Uchida, K.; Miyagi, M.; Inoue, G.; Kawakubo, A.; Satoh, M.; Takaso, M. Changes in Nerve Growth Factor Expression and Macrophage Phenotype Following Intervertebral Disc Injury in Mice. J. Orthop. Res. 2019, 37, 1798–1804. [Google Scholar] [CrossRef]
- Silva, A.J.; Ferreira, J.R.; Cunha, C.; Côrte-Real, J.V.; Gonçalves, M.; Barbosa, M.; Santos, S.; Gonçalves, R.M. Macrophages Down-Regulate Gene Expression of Intervertebral Disc Degenerative Markers Under a Pro-inflammatory Microenvironment. Front. Immunol. 2019, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jang, S.; Suzuki-Narita, M.; Gregoire, S.; Millecamps, M.; Stone, L. Voluntary running attenuates behavioural signs of low back pain: Dimorphic regulation of intervertebral disc inflammation in male and female SPARC-null mice. Osteoarthr. Cartil. 2022, 30, 110–123. [Google Scholar] [CrossRef]
- Miyagi, M.; Uchida, K.; Takano, S.; Fujimaki, H.; Aikawa, J.; Sekiguchi, H.; Nagura, N.; Ohtori, S.; Inoue, G.; Takaso, M. Macrophage-derived inflammatory cytokines regulate growth factors and pain-related molecules in mice with intervertebral disc injury. J. Orthop. Res. 2018, 36, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhang, Y.; Zhang, X.; Zhang, H.; Meng, S.; Kong, M.; Liu, X.; Ma, X. Single-Cell RNA Sequencing of the Nucleus Pulposus Reveals Chondrocyte Differentiation and Regulation in Intervertebral Disc Degeneration. Front. Cell Dev. Biol. 2022, 10, 824771. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Liu, Y.; Wang, Z.; Zhang, Z.; Chen, B.; Yang, J.; Zeng, B.; Gao, Y.; Jiang, C.; Huang, Y.; et al. Single-Cell RNA-Seq Analysis Reveals Macrophage Involved in the Progression of Human Intervertebral Disc Degeneration. Front. Cell Dev. Biol. 2021, 9, 833420. [Google Scholar] [CrossRef] [PubMed]
- Rutges, J.; Oner, F.; Verbout, A.; Castelein, R.; Kummer, J.; Weinans, H.; Creemers, L.; Dhert, W. Micro-CT quantification of subchondral endplate changes in intervertebral disc degeneration. Osteoarthr. Cartil. 2011, 19, 89–95. [Google Scholar] [CrossRef]
- Nguyen, C.; Poiraudeau, S.; Rannou, F. Vertebral subchondral bone. Osteoporos. Int. 2012, 23 (Suppl. S8), S857–S860. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, T.; Dydyk, A.M. Spinal Osteoarthritis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wang, J.; Zheng, Z.; Huang, B.; Wu, H.; Zhang, X.; Chen, Y.; Liu, J.; Shan, Z.; Fan, S.; Chen, J.; et al. Osteal Tissue Macrophages Are Involved in Endplate Osteosclerosis through the OSM-STAT3/YAP1 Signaling Axis in Modic Changes. J. Immunol. 2020, 205, 968–980. [Google Scholar] [CrossRef]
- Chen, K.; Jiao, Y.; Liu, L.; Huang, M.; He, C.; He, W.; Hou, J.; Yang, M.; Luo, X.; Li, C. Communications Between Bone Marrow Macrophages and Bone Cells in Bone Remodeling. Front. Cell Dev. Biol. 2020, 8, 598263. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Xie, X.; Gu, F.; Sui, Z.; Zhang, K.; Yu, T. Macrophage-Osteoclast Associations: Origin, Polarization, and Subgroups. Front. Immunol. 2021, 12, 778078. [Google Scholar] [CrossRef]
- Edgar, M.A. The nerve supply of the lumbar intervertebral disc. J. Bone Jt. Surg. Br. 2007, 89, 1135–1139. [Google Scholar] [CrossRef]
- Grönblad, M.; Weinstein, J.N.; Santavirta, S. Immunohistochemical observations on spinal tissue innervation: A review of hypothetical mechanisms of back pain. Acta Orthop. Scand. 1991, 62, 614–622. [Google Scholar] [CrossRef]
- Richardson, S.M.; Doyle, P.; Minogue, B.M.; Gnanalingham, K.; Hoyland, J.A. Increased expression of matrix metalloproteinase-10, nerve growth factor and substance P in the painful degenerate intervertebral disc. Arthritis Res. Ther. 2009, 11, R126–R128. [Google Scholar] [CrossRef]
- Arnbak, B.; Jensen, R.K.; Manniche, C.; Hendricks, O.; Kent, P.; Jurik, A.G.; Jensen, T.S. Identification of subgroups of inflammatory and degenerative MRI findings in the spine and sacroiliac joints: A latent class analysis of 1037 patients with persistent low back pain. Arthritis Res. Ther. 2016, 18, 237. [Google Scholar] [CrossRef]
- Arnbak, B.; Jensen, T.S.; Egund, N.; Zejden, A.; Hørslev-Petersen, K.; Manniche, C.; Jurik, A.G. Prevalence of degenerative and spondyloarthritis-related magnetic resonance imaging findings in the spine and sacroiliac joints in patients with persistent low back pain. Eur. Radiol. 2016, 26, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Middendorp, M.; Vogl, T.J.; Kollias, K.; Kafchitsas, K.; Khan, M.F.; Maataoui, A. Association between intervertebral disc degeneration and the Oswestry Disability Index. J. Back Musculoskelet. Rehabil. 2017, 30, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Hitselberger, W.E.; Witten, R.M. Abnormal Myelograms in Asymptomatic Patients. J. Neurosurg. 1968, 28, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.C.; Brant-Zawadzki, M.N.; Obuchowski, N.; Modic, M.T.; Malkasian, D.; Ross, J. Magnetic Resonance Imaging of the Lumbar Spine in People without Back Pain. N. Engl. J. Med. 1994, 331, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Van Der Windt, D.A.; Simons, E.; Riphagen, I.I.; Ammendolia, C.; Verhagen, A.P.; Laslett, M.; Devillé, W.; Deyo, R.A.; Bouter, L.; De Vet, H.C.; et al. Physical examination for lumbar radiculopathy due to disc herniation in patients with low-back pain. Cochrane Database Syst. Rev. 2010, 2, CD007431. [Google Scholar] [CrossRef]
- Wiesel, S.W.; Tsourmas, N.; Feffer, H.L.; Citrin, C.M.; Patronas, N. A study of computer-assisted tomography. I. The incidence of positive CAT scans in an asymptomatic group of patients. Spine 1984, 9, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Domoto, R.; Sekiguchi, F.; Tsubota, M.; Kawabata, A. Macrophage as a Peripheral Pain Regulator. Cells 2021, 10, 1881. [Google Scholar] [CrossRef]
- Verri, W.A., Jr.; Cunha, T.M.; Parada, C.A.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. Hypernociceptive role of cytokines and chemokines: Targets for analgesic drug development? Pharmacol. Ther. 2006, 112, 116–138. [Google Scholar] [CrossRef]
- Kang, J.D.; Georgescu, H.I.; McIntyre-Larkin, L.; Stefanovic-Racic, M.; Donaldson, W.F., 3rd; Evans, C.H. Herniated Lumbar Intervertebral Discs Spontaneously Produce Matrix Metalloproteinases, Nitric Oxide, Interleukin-6, and Prostaglandin E2. Spine 1996, 21, 271–277. [Google Scholar] [CrossRef]
- Khan, A.N.; Jacobsen, H.E.; Khan, J.; Filippi, C.G.; Levine, M.; Lehman, R.A., Jr.; Riew, K.D.; Lenke, L.G.; Chahine, N.O. Inflammatory biomarkers of low back pain and disc degeneration: A review. Ann. N. Y. Acad. Sci. 2017, 1410, 68–84. [Google Scholar] [CrossRef]
- Takada, T.; Nishida, K.; Maeno, K.; Kakutani, K.; Yurube, T.; Doita, M.; Kurosaka, M. Intervertebral disc and macrophage interaction induces mechanical hyperalgesia and cytokine production in a herniated disc model in rats. Arthritis Rheum. 2012, 64, 2601–2610. [Google Scholar] [CrossRef]
- Krock, E.; Millecamps, M.; Anderson, K.M.; Srivastava, A.; Reihsen, T.E.; Hari, P.; Sun, Y.R.; Jang, S.H.; Wilcox, G.L.; Belani, K.G.; et al. Interleukin-8 as a therapeutic target for chronic low back pain: Upregulation in human cerebrospinal fluid and pre-clinical validation with chronic reparixin in the SPARC-null mouse model. EBioMedicine 2019, 43, 487–500. [Google Scholar] [CrossRef]
- Miyagi, M.; Uchida, K.; Takano, S.; Nakawaki, M.; Sekiguchi, H.; Nakazawa, T.; Imura, T.; Saito, W.; Shirasawa, E.; Kawakubo, A.; et al. Role of CD14-positive cells in inflammatory cytokine and pain-related molecule expression in human degenerated intervertebral discs. J. Orthop. Res. 2021, 39, 1755–1762. [Google Scholar] [CrossRef]
- Kim, C.F.; Moalem-Taylor, G. Detailed characterization of neuro-immune responses following neuropathic injury in mice. Brain Res. 2011, 1405, 95–108. [Google Scholar] [CrossRef]
- Annunen, S.; Paassilta, P.; Lohiniva, J.; Perälä, M.; Pihlajamaa, T.; Karppinen, J.; Tervonen, O.; Kröger, H.; Lähde, S.; Vanharanta, H.; et al. An Allele of COL9A2 Associated with Intervertebral Disc Disease. Science 1999, 285, 409–412. [Google Scholar] [CrossRef]
- Mio, F.; Chiba, K.; Hirose, Y.; Kawaguchi, Y.; Mikami, Y.; Oya, T.; Mori, M.; Kamata, M.; Matsumoto, M.; Ozaki, K.; et al. A Functional Polymorphism in COL11A1, Which Encodes the α1 Chain of Type XI Collagen, Is Associated with Susceptibility to Lumbar Disc Herniation. Am. J. Hum. Genet. 2007, 81, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Paassilta, P.; Lohiniva, J.; Göring, H.H.H.; Perälä, M.; Räinä, S.S.; Karppinen, J.; Hakala, M.; Palm, T.; Kröger, H.; Kaitila, I.; et al. Identification of a Novel Common Genetic Risk Factor for Lumbar Disk Disease. JAMA 2001, 285, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Kawaguchi, Y.; Chiba, K.; Mikami, Y.; Kizawa, H.; Oya, T.; Mio, F.; Mori, M.; Miyamoto, Y.; Masuda, I.; et al. A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc disease. Nat. Genet. 2005, 37, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, I.M.; Song, Y.Q.; Cheung, K.M.C.; Ala-Kokko, L.; Karppinen, J.; Ho, D.W.H.; Luk, K.D.K.; Yip, S.P.; Leong, J.C.Y.; Cheah, K.S.E.; et al. Phenotypic and population differences in the association between CILP and lumbar disc disease. J. Med. Genet. 2007, 44, 285–288. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Osada, R.; Kanamori, M.; Ishihara, H.; Ohmori, K.; Matsui, H.; Kimura, T. Association between an Aggrecan Gene Polymorphism and Lumbar Disc Degeneration. Spine 1999, 24, 2456–2460. [Google Scholar] [CrossRef]
- Song, Y.-Q.; Cheung, K.; Ho, D.W.H.; Poon, S.C.; Chiba, K.; Kawaguchi, Y.; Hirose, Y.; Alini, M.; Grad, S.; Yee, A.F.; et al. Association of the Asporin D14 Allele with Lumbar-Disc Degeneration in Asians. Am. J. Hum. Genet. 2008, 82, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Hirose, Y.; Chiba, K.; Karasugi, T.; Nakajima, M.; Kawaguchi, Y.; Mikami, Y.; Furuichi, T.; Mio, F.; Miyake, A.; Miyamoto, T.; et al. A Functional Polymorphism in THBS2 that Affects Alternative Splicing and MMP Binding Is Associated with Lumbar-Disc Herniation. Am. J. Hum. Genet. 2008, 82, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, N.; Liu, J.; Liu, H.; Su, X.; Liu, Z.; Zuo, Y.; Chen, W.; Liu, G.; Chen, Y.; et al. Association between ADAMTS-4 gene polymorphism and lumbar disc degeneration in Chinese Han population. J. Orthop. Res. 2016, 34, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Chen, J.; Liu, H.; Zhao, L.; Liu, S.; Liu, J.; Su, X.; Wu, W.; Cong, J.; Qiu, G.; et al. The involvement of ADAMTS-5 genetic polymorphisms in predisposition and diffusion tensor imaging alterations of lumbar disc degeneration. J. Orthop. Res. 2014, 32, 686–694. [Google Scholar] [CrossRef]
- Haro, H.; Crawford, H.C.; Fingleton, B.; MacDougall, J.R.; Shinomiya, K.; Spengler, D.M.; Matrisian, L.M. Matrix metalloproteinase-3–dependent generation of a macrophage chemoattractant in a model of herniated disc resorption. J. Clin. Investig. 2000, 105, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Luo, S.; Fan, W.; Zhou, T.; Huang, C.; Wang, M. M2 macrophage-conditioned medium inhibits intervertebral disc degeneration in a tumor necrosis factor-α-rich environment. J. Orthop. Res. 2022, 40, 2488–2501. [Google Scholar] [CrossRef]
- Gorth, D.J.; Shapiro, I.M.; Risbud, M.V. Transgenic mice overexpressing human TNF-α experience early onset spontaneous intervertebral disc herniation in the absence of overt degeneration. Cell Death Dis. 2018, 10, 7. [Google Scholar] [CrossRef]
- Li, L.; Wei, K.; Ding, Y.; Ahati, P.; Xu, H.; Fang, H.; Wang, H. M2a Macrophage-Secreted CHI3L1 Promotes Extracellular Matrix Metabolic Imbalances via Activation of IL-13Rα2/MAPK Pathway in Rat Intervertebral Disc Degeneration. Front. Immunol. 2021, 12, 666361. [Google Scholar] [CrossRef]
- Phillips, K.L.E.; Chiverton, N.; Michael, A.L.; Cole, A.A.; Breakwell, L.M.; Haddock, G.; Bunning, R.A.; Cross, A.K.; Le Maitre, C.L. The cytokine and chemokine expression profile of nucleus pulposus cells: Implications for degeneration and regeneration of the intervertebral disc. Arthritis Res. Ther. 2013, 15, R213. [Google Scholar] [CrossRef]
- Hiyama, A.; Suyama, K.; Sakai, D.; Tanaka, M.; Watanabe, M. Correlational analysis of chemokine and inflammatory cytokine expression in the intervertebral disc and blood in patients with lumbar disc disease. J. Orthop. Res. 2022, 40, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Le Maitre, C.L.; Freemont, A.J.; Hoyland, J.A. The role of interleukin-1 in the pathogenesis of human Intervertebral disc degeneration. Arthritis Res. Ther. 2005, 7, R732–R745. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, Y.; Phillips, K.L.E.; Chiverton, N.; Haddock, G.; Bunning, R.A.; Cross, A.K.; Shapiro, I.M.; Le Maitre, C.L.; Risbud, M.V. Tumor necrosis factor α- and interleukin-1β-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis Rheum. 2013, 65, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.-H.; Chee, A.; Shi, P.; Lin, C.-L.; Zhao, Y.; Zhang, L.; An, H.S. Small molecule antagonist of C-C chemokine receptor 1 (CCR1) reduces disc inflammation in the rabbit model. Spine J. 2020, 20, 2025–2036. [Google Scholar] [CrossRef]
- Nakawaki, M.; Uchida, K.; Miyagi, M.; Inoue, G.; Kawakubo, A.; Kuroda, A.; Satoh, M.; Takaso, M. Sequential CCL2 Expression Profile After Disc Injury in Mice. J. Orthop. Res. 2020, 38, 895–901. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Pan, H.; Yang, H.; Li, X.; Zhang, K.; Wang, H.; Zheng, Z.; Liu, H.; Wang, J. Resistin promotes CCL4 expression through toll-like receptor-4 and activation of the p38-MAPK and NF-κB signaling pathways: Implications for intervertebral disc degeneration. Osteoarthr. Cartil. 2017, 25, 341–350. [Google Scholar] [CrossRef]
- Park, J.J.; Moon, H.J.; Park, J.H.; Kwon, T.H.; Park, Y.-K.; Kim, J.H. Induction of proinflammatory cytokine production in intervertebral disc cells by macrophage-like THP-1 cells requires mitogen-activated protein kinase activity. J. Neurosurg. Spine 2016, 24, 167–175. [Google Scholar] [CrossRef]
- Yang, C.; Cao, P.; Gao, Y.; Wu, M.; Lin, Y.; Tian, Y.; Yuan, W. Differential expression of p38 MAPK α, β, γ, δ isoforms in nucleus pulposus modulates macrophage polarization in intervertebral disc degeneration. Sci. Rep. 2016, 6, 22182. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Bai, Z.; Liu, W.; He, D.; Wang, Y.; Yi, W.; Luo, C.; Shen, J.; Hu, Z. Protective effects of autophagy and NFE2L2 on reactive oxygen species-induced pyroptosis of human nucleus pulposus cells. Aging 2020, 12, 7534–7548. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, S.; Wang, M.; Jin, X.; Lv, C.; Deng, Y.; Wang, J. Enhanced NLRP3, Caspase-1, and IL- 1β Levels in Degenerate Human Intervertebral Disc and Their Association with the Grades of Disc Degeneration. Anat. Rec. 2015, 298, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018, 17, 688. [Google Scholar] [CrossRef]
- Chao-Yang, G.; Peng, C.; Hai-Hong, Z. Roles of NLRP3 inflammasome in intervertebral disc degeneration. Osteoarthr. Cartil. 2021, 29, 793–801. [Google Scholar] [CrossRef]
- Komada, T.; Muruve, D.A. The role of inflammasomes in kidney disease. Nat. Rev. Nephrol. 2019, 15, 501–520. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, G.; Liu, H.; Li, Z.; Pei, Y.; Wang, H.; Pan, H.; Cui, H.; Long, J.; Wang, J.; et al. Melatonin alleviates intervertebral disc degeneration by disrupting the IL-1β/NF-κB-NLRP3 inflammasome positive feedback loop. Bone Res. 2020, 8, 10. [Google Scholar] [CrossRef]
- Tang, P.; Zhu, R.; Ji, W.-P.; Wang, J.-Y.; Chen, S.; Fan, S.-W.; Hu, Z.-J. The NLRP3/Caspase-1/Interleukin-1β Axis Is Active in Human Lumbar Cartilaginous Endplate Degeneration. Clin. Orthop. Relat. Res. 2016, 474, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Bjornsdottir, G.; Benonisdottir, S.; Sveinbjornsson, G.; Styrkarsdottir, U.; Thorleifsson, G.; Walters, G.B.; Bjornsson, A.; Olafsson, I.H.; Ulfarsson, E.; Vikingsson, A.; et al. Sequence variant at 8q24.21 associates with sciatica caused by lumbar disc herniation. Nat. Commun. 2017, 8, 14265. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Moro, A.; Liu, Y.; Wang, J.; Meng, D.; Zhan, X.; Wei, Q. Two GWAS-identified variants are associated with lumbar spinal stenosis and Gasdermin-C expression in Chinese population. Sci. Rep. 2020, 10, 21069. [Google Scholar] [CrossRef]
- Molinos, M.; Almeida, C.R.; Caldeira, J.; Cunha, C.; Gonçalves, R.M.; Barbosa, M.A. Inflammation in intervertebral disc degeneration and regeneration. J. R. Soc. Interface 2015, 12, 20150429. [Google Scholar] [CrossRef]
- Liao, Z.; Luo, R.; Li, G.; Song, Y.; Zhan, S.; Zhao, K.; Hua, W.; Zhang, Y.; Wu, X.; Yang, C. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics 2019, 9, 4084–4100. [Google Scholar] [CrossRef]
- Shahzad, K.; Bock, F.; Dong, W.; Wang, H.; Kopf, S.; Kohli, S.; Al-Dabet, M.M.; Ranjan, S.; Wolter, J.; Wacker, C.; et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 2015, 87, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, Y.; Zhang, Y.; Geng, W.; Liu, W.; Gao, Y.; Li, S.; Wang, K.; Wu, X.; Kang, L.; et al. Advanced glycation end products regulate anabolic and catabolic activities via NLRP3-inflammasome activation in human nucleus pulposus cells. J. Cell. Mol. Med. 2017, 21, 1373–1387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qiu, C.; Wang, W.; Peng, J.; Cheng, X.; Shangguan, Y.; Xu, M.; Li, J.; Qu, R.; Chen, X.; et al. Cortistatin protects against intervertebral disc degeneration through targeting mitochondrial ROS-dependent NLRP3 inflammasome activation. Theranostics 2020, 10, 7015–7033. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Y.; Fan, Q.; Xu, J.; Shan, H.; Gao, X.; Ma, Q.; Sheng, L.; Zheng, X.; Cheng, W.; et al. Reactive Oxygen Species-Scavenging Scaffold with Rapamycin for Treatment of Intervertebral Disk Degeneration. Adv. Healthc. Mater. 2020, 9, e1901186. [Google Scholar] [CrossRef]
- Van De Laar, I.M.B.H.; Oldenburg, R.A.; Pals, G.; Roos-Hesselink, J.W.; De Graaf, B.M.; Verhagen, J.M.A.; Hoedemaekers, Y.M.; Willemsen, R.; Severijnen, L.-A.; Venselaar, H.; et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat. Genet. 2011, 43, 121–126. [Google Scholar] [CrossRef]
- Zhang, W.; Zeng, Q.; Xu, Y.; Ying, H.; Zhou, W.; Cao, Q.; Zhou, W. Exome sequencing identified a novel SMAD2 mutation in a Chinese family with early onset aortic aneurysms. Clin. Chim. Acta 2017, 468, 211–214. [Google Scholar] [CrossRef]
- Murakami, H.; Yoon, S.T.; Attallah-Wasif, E.S.; Tsai, K.-J.; Fei, Q.; Hutton, W.C. The Expression of Anabolic Cytokines in Intervertebral Discs in Age-Related Degeneration. Spine 2006, 31, 1770–1774. [Google Scholar] [CrossRef]
- Nerlich, A.G.; Bachmeier, B.; Boos, N. Expression of fibronectin and TGF-ß1 mRNA and protein suggest altered regulation of extracellular matrix in degenerated disc tissue. Eur. Spine J. 2005, 14, 17–26. [Google Scholar] [CrossRef]
- Peng, B.; Hao, J.; Hou, S.; Wu, W.; Jiang, D.; Fu, X.; Yang, Y. Possible Pathogenesis of Painful Intervertebral Disc Degeneration. Spine 2006, 31, 560–566. [Google Scholar] [CrossRef]
- Sobajima, S.; Shimer, A.L.; Chadderdon, R.C.; Kompel, J.F.; Kim, J.S.; Gilbertson, L.G.; Kang, J.D. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005, 5, 14–23. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Shi, W.; Yi, S.-J.; Chen, H.; Groffen, J.; Heisterkamp, N. TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 2012, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, A.; Miyagi, M.; Yokozeki, Y.; Nakawaki, M.; Takano, S.; Satoh, M.; Itakura, M.; Inoue, G.; Takaso, M.; Uchida, K. Origin of M2 Mϕ and its macrophage polarization by TGF-β in a mice intervertebral injury model. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221103792. [Google Scholar] [CrossRef] [PubMed]
- Yokozeki, Y.; Kawakubo, A.; Miyagi, M.; Kuroda, A.; Sekiguchi, H.; Inoue, G.; Takaso, M.; Uchida, K. Reduced TGF-β Expression and CD206-Positive Resident Macrophages in the Intervertebral Discs of Aged Mice. BioMed Res. Int. 2021, 2021, 7988320. [Google Scholar] [CrossRef]
- Matsunaga, S.; Nagano, S.; Onishi, T.; Morimoto, N.; Suzuki, S.; Komiya, S. Age-related changes in expression of transforming growth factor-β and receptors in cells of intervertebral discs. J. Neurosurg. Spine 2003, 98, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Greijer, A.; Van Der Groep, P.; Kemming, D.; Shvarts, A.; Semenza, G.; Meijer, G.; Van De Wiel, M.; Belien, J.; Van Diest, P.J.; Van Der Wall, E. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1). J. Pathol. 2005, 206, 291–304. [Google Scholar] [CrossRef]
- McGettrick, A.F.; O’Neill, L.A. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef]
- Blouin, C.C.; Pagé, E.L.; Soucy, G.M.; Richard, D. Hypoxic gene activation by lipopolysaccharide in macrophages: Implication of hypoxia-inducible factor 1α. Blood 2004, 103, 1124–1130. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Meng, X.; Zhuang, L.; Wang, J.; Liu, Z.; Wang, Y.; Xiao, D.; Zhang, X. Hypoxia-inducible factor (HIF)-1alpha knockout accelerates intervertebral disc degeneration in mice. Int. J. Clin. Exp. Pathol. 2018, 11, 548–557. [Google Scholar]
- Kim, J.-W.; An, H.-J.; Yeo, H.; Jeong, Y.; Lee, H.; Lee, J.; Nam, K.; Lee, J.; Shin, D.-E.; Lee, S. Activation of Hypoxia-Inducible Factor-1α Signaling Pathway Has the Protective Effect of Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2021, 22, 11355. [Google Scholar] [CrossRef]
- Johnston, S.N.; Madhu, V.; Shapiro, I.M.; Risbud, M.V. Conditional Deletion of HIF -2α in Mouse Nucleus Pulposus Reduces Fibrosis and Provides Mild and Transient Protection From Age-Dependent Structural Changes in Intervertebral Disc. J. Bone Miner. Res. 2022, 37, 2512–2530. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-H.; Lin, R.-M.; Chiu, Y.-S.; Liu, W.-L.; Huang, K.-Y. Effects of IL-1β, IL-20, and BMP-2 on Intervertebral Disc Inflammation under Hypoxia. J. Clin. Med. 2020, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.-K.; Moon, H.J.; Kwon, T.-H.; Park, Y.-K.; Kim, J.H. The Role of Hypoxia in Angiogenesis and Extracellular Matrix Regulation of Intervertebral Disc Cells During Inflammatory Reactions. Neurosurgery 2017, 81, 867–875. [Google Scholar] [CrossRef] [PubMed]
| Gene | OMIM | Clinical Disease |
|---|---|---|
| ACAN | 155760 | IVD Degeneration |
| ASPN | 608135 | IVD Degeneration |
| CHAD | 602178 | IVD Degeneration |
| CHST3 | 603799 | SEDCJD |
| CILP | 603489 | IVD Degeneration |
| COL9A3 | 120270 | IVD Degeneration |
| COL11A1 | 120280 | IVD Degeneration |
| SMAD2 | 601366 | LDS6 |
| SMAD3 | 603109 | LDS3 |
| THBS2 | 188061 | IVD Degeneration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koroth, J.; Buko, E.O.; Abbott, R.; Johnson, C.P.; Ogle, B.M.; Stone, L.S.; Ellingson, A.M.; Bradley, E.W. Macrophages and Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2023, 24, 1367. https://doi.org/10.3390/ijms24021367
Koroth J, Buko EO, Abbott R, Johnson CP, Ogle BM, Stone LS, Ellingson AM, Bradley EW. Macrophages and Intervertebral Disc Degeneration. International Journal of Molecular Sciences. 2023; 24(2):1367. https://doi.org/10.3390/ijms24021367
Chicago/Turabian StyleKoroth, Jinsha, Erick O. Buko, Rebecca Abbott, Casey P. Johnson, Brenda M. Ogle, Laura S. Stone, Arin M. Ellingson, and Elizabeth W. Bradley. 2023. "Macrophages and Intervertebral Disc Degeneration" International Journal of Molecular Sciences 24, no. 2: 1367. https://doi.org/10.3390/ijms24021367
APA StyleKoroth, J., Buko, E. O., Abbott, R., Johnson, C. P., Ogle, B. M., Stone, L. S., Ellingson, A. M., & Bradley, E. W. (2023). Macrophages and Intervertebral Disc Degeneration. International Journal of Molecular Sciences, 24(2), 1367. https://doi.org/10.3390/ijms24021367







