Concern about the Effectiveness of mRNA Vaccination Technology and Its Long-Term Safety: Potential Interference on miRNA Machinery
Abstract
1. Introduction
2. Different SARS-CoV-2 Variant of Concern and Effectiveness of Vaccination
3. Immune Response and Circulating Extracellular Vesicles Containing miRNAs
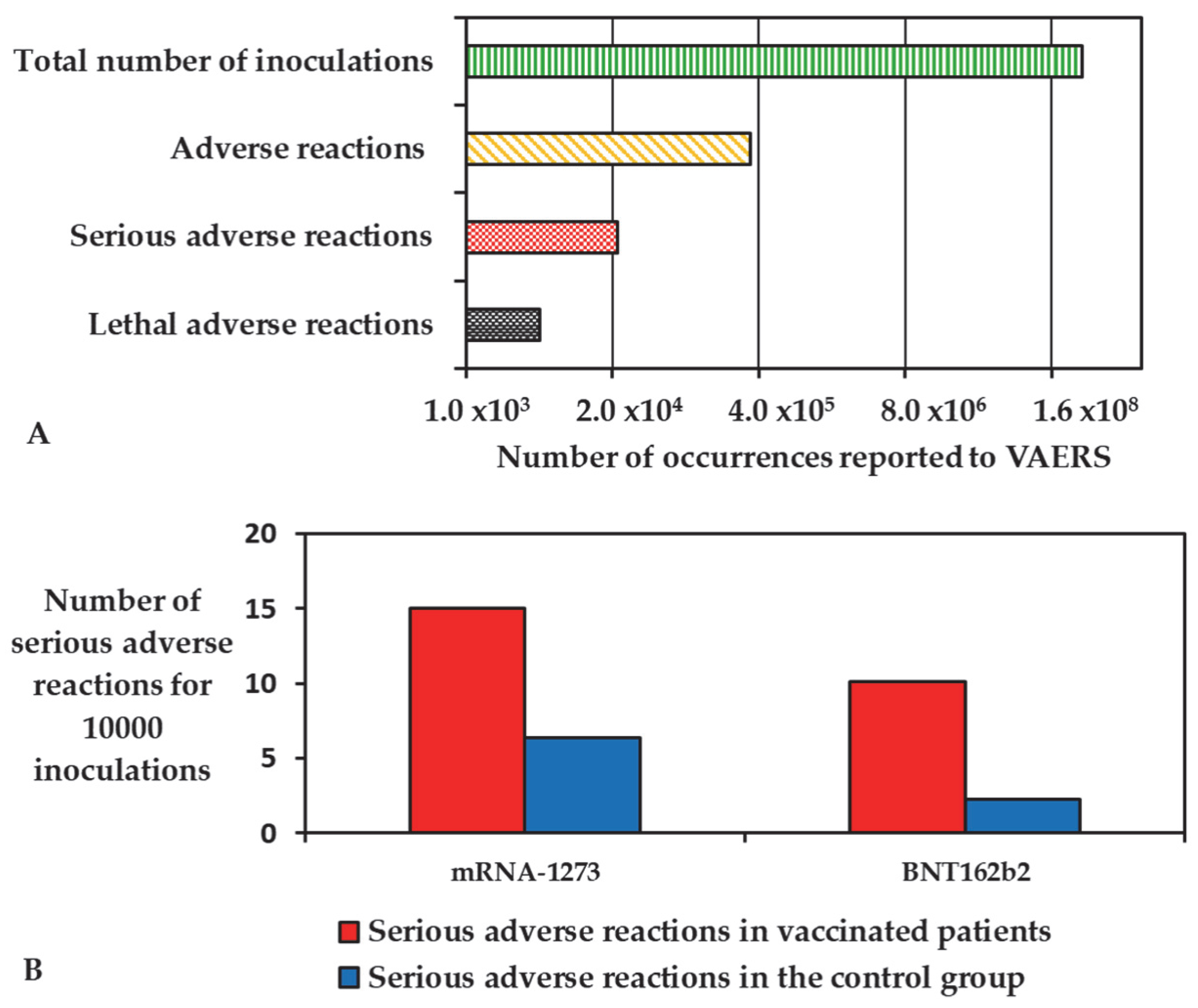
4. Known Adverse Reactions of mRNA Vaccines
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. SARS-CoV-2 immunity: Review and applications to phase 3 vaccine candidates. Lancet 2020, 396, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Joffe, S. Evaluating SARS-CoV-2 vaccines after emergency use authorization or licensing of initial candidate vaccines. JAMA 2021, 325, 221–222. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Dudley, M.Z.; Chen, X.; Bai, X.; Dong, K.; Zhuang, T.; Salmon, D.; Yu, H. Evaluation of the safety profile of COVID-19 vaccines: A rapid review. BMC Med. 2021, 19, 173. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez, M.G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Di Pietro, R.; Basile, M.; Antolini, L.; Alberti, S. Genetic Drift Versus Climate Region Spreading Dynamics of COVID-19. Front. Genet. 2021, 12, 663371. [Google Scholar] [CrossRef]
- Desingu, P.A.; Nagarajan, K.; Dhama, K. Emergence of Omicron third lineage BA.3 and its importance. J. Med. Virol. 2022, 94, 1808–1810. [Google Scholar] [CrossRef]
- Callaway, E. What Omicron’s BA.4 and BA.5 variants mean for the pandemic. Nature 2022, 606, 848–849. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Jian, M.J.; Chang, C.K.; Lin, J.C.; Yeh, K.M.; Chen, C.W.; Hsieh, S.S.; Hung, K.S.; Tang, S.H.; Perng, C.L.; et al. Emergency SARS-CoV-2 Variants of Concern: Novel Multiplex Real-Time RT-PCR Assay for Rapid Detection and Surveillance. Microbiol. Spectr. 2022, 10, e0251321. [Google Scholar] [CrossRef]
- Chen, J.; Wei, G.W. Omicron BA.2 (B.1.1.529.2): High potential to becoming the next dominating variant. arXiv 2022, arXiv:2202.05031v1. [Google Scholar]
- Cao, Y.; Yisimayi, A.; Jian, F.; Song, W.; Xiao, T.; Wang, L.; Du, S.; Wang, J.; Li, Q.; Chen, X.; et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 2022, 608, 593–602. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Country Overview Report: Week 21 2022. June 2022. Available online: https://www.ecdc.europa.eu/en/covid-19/country-overviews (accessed on 14 July 2022).
- Dhawan, M.; Saied, A.A.; Emran, T.B.; Choudhary, O.P. Emergence of omicron variant’s sublineages BA.4 and BA.5: Risks assessment and possible countermeasures. New Microbes New Infect. 2022, 48, 100997. [Google Scholar] [CrossRef]
- Cagigi, A.; Yu, M.; Österberg, B.; Svensson, J.; Falck-Jones, S.; Vangeti, S.; Åhlberg, E.; Azizmohammadi, L.; Warnqvist, A.; Falck-Jones, R. Airway antibodies emerge according to COVID-19 severity and wane rapidly but reappear after SARS-CoV-2 vaccination. JCI Insight 2021, 6, e151463. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. Will ‘Centaurus’ be the next global coronavirus variant? Indian cases offers clues. Nature 2022, 608, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Evans, J.P.; Zheng, Y.M.; Carlin, C.; Saif, L.J.; Oltz, E.M.; Xu, K.; Gumina, R.J.; Liu, S.L. Evasion of Neutralizing Antibody Response by the SARS-CoV-2 BA.2.75 Variant. Cell Host Microbe 2022, 30, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Regev-Yochay, G.; Gonen, T.; Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Amit, S.; Meltzer, L.; Asraf, K.; Cohen, C.; Fluss, R.; et al. Efficacy of a Fourth Dose of COVID-19 mRNA Vaccine against Omicron. N. Engl. J. Med. 2022, 386, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, Y.; Yoshida, T.; Takagi, Y.; Tsukamoto, H.; Takashima, K.; Kouwaki, T.; Makino, K.; Fukushima, S.; Nakamura, K.; Oshiumi, H. Circulating extracellular vesicle microRNAs associated with adverse reactions, proinflammatory cytokine, and antibody production after COVID-19 vaccination. NPJ Vaccines 2022, 7, 16. [Google Scholar] [CrossRef]
- Naranbhai, V.; Garcia-Beltran, W.F.; Chang, C.C.; Berrios Mairena, C.; Thierauf, J.C.; Kirkpatrick, G.; Onozato, M.L.; Cheng, J.; St Denis, K.J.; Lam, E.C. Comparative Immunogenicity and Effectiveness of mRNA-1273, BNT162b2, and Ad26.COV2.S COVID-19 Vaccines. J. Infect. Dis. 2022, 225, 1141–1150. [Google Scholar] [CrossRef]
- Huang, H.Y.; Lin, Y.C.; Li, J.; Huang, K.Y.; Shrestha, S.; Hong, H.C.; Tang, Y.; Chen, Y.G.; Jin, C.N.; Yu, Y. miRTarBase 2020: Updates to the experimentally validated microRNA-target interaction database. Nucleic Acids Res. 2020, 48, D148–D154. [Google Scholar] [CrossRef]
- Gromak, N. Intronic microRNAs: A crossroad in gene regulation. Biochem. Soc. Trans. 2012, 40, 759–761. [Google Scholar] [CrossRef]
- Berindan-Neagoe, I.; Monroig, P.d.C.; Pasculli, B.; Calin, G.A. MicroRNAome genome: A treasure for cancer diagnosis and therapy. CA Cancer J. Clin. 2014, 64, 311–336. [Google Scholar] [CrossRef]
- Han, J.; LaVigne, C.A.; Jones, B.T.; Zhang, H.; Gillett, F.; Mendell, J.T. A ubiquitin ligase mediates target-directed microRNA decay independently of tailing and trimming. Science 2020, 370, eabc9546. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.B.; Zhang, L.; Bhushan, A.; Swanson, B.; Zhang, L.; Mamede, J.I.; Voigt, R.M.; Shaikh, M.; Engen, P.A.; Keshavarzian, A. The SARS-CoV-2 S1 Spike Protein Promotes MAPK and NF-kB Activation in Human Lung Cells and Inflammatory Cytokine Production in Human Lung and Intestinal Epithelial Cells. Microorganisms 2022, 10, 1996. [Google Scholar] [CrossRef] [PubMed]
- Kircheis, R.; Planz, O. Could a Lower Toll-like Receptor (TLR) and NF-κB Activation Due to a Changed Charge Distribution in the Spike Protein Be the Reason for the Lower Pathogenicity of Omicron? Int. J. Mol. Sci. 2022, 23, 5966. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, H.G.; Gee, J.; Liu, R.; Marquez, P.L.; Zhang, B.; Strid, P.; Abara, W.E.; McNeil, M.M.; Myers, T.R.; Hause, A.M.; et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: An observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. Lancet Infect. Dis. 2022, 22, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#appendix-f (accessed on 18 November 2022).
- Fraiman, J.; Erviti, J.; Jones, M.; Greenland, S.; Whelan, P.; Kaplan, R.M.; Doshi, P. Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults. Vaccines 2022, 40, 5798. [Google Scholar] [CrossRef]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA 2022, 327, 331. [Google Scholar] [CrossRef]
- Wong, H.L.; Hu, M.; Zhou, C.K.; Lloyd, P.C.; Amend, K.L.; Beacher, D.C.; Secora, A.; McMahill-Walraven, C.N.; Lu, Y.; Wu, Y.; et al. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: A cohort study in claims databases. Lancet 2022, 399, 2191. [Google Scholar] [CrossRef]
- Tu, T.M.; Yi, S.J.; Koh, J.S. Incidence of Cerebral Venous Thrombosis Following SARS-CoV-2 Infection vs mRNA SARS-CoV-2 Vaccination in Singapore. JAMA Netw. Open 2022, 5, e222940. [Google Scholar] [CrossRef]
- Jaiswal, V.; Nepal, G.; Dijamco, P.; Ishak, A.; Dagar, M.; Sarfraz, Z.; Shama, N.; Sarfraz, A.; Lnu, K.; Mitra, S.; et al. Cerebral venous sinus thrombosis following COVID-19 vaccination: A systematic review. J. Prim. Care Community Health 2022, 13, 1–10. [Google Scholar] [CrossRef]
- Cardoza-Ochoa, D.; Hernandez-Villa, D.; Vazquez-Cardoso, G.; Trejo, C.; Rivera-Bravo, B. Vaccine-associated hypermetabolic lymphadenopathy on 18F-FDG PET/CT: Experience from a single center in Mexico. J. Nucl. Med. 2022, 63, 2328. [Google Scholar]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021, 144, 471. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, M.S.; Kim, H.J.; Song, T.J. Association of cerebral venous thrombosis with mRNA COVID-19 vaccines: A disproportionality analysis of the world health organization pharmacovigilance database. Vaccines 2022, 10, 799. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Krauthammer, S.H.; Wolf, I.; Even-Sapir, E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA COVID-19 vaccine: Incidence assessed by [18F]FDG PET-CT and relevance to study interpretation. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1854. [Google Scholar] [CrossRef]
- Cohen, D.; Krauthammer, S.H.; Wolf, I.; Even-Sapir, E. A sigh of relief: Vaccine-associated hypermetabolic lymphadenopathy following the third COVID-19 vaccine dose is short in duration and uncommonly interferes with the interpretation of [18F]FDG PET-CT studies performed in oncologic patients. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1338. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Terpos, E.; Alexopoulos, H.; Politou, M.; Paraskevis, D.; Scorilas, A.; Kastritis, E.; Andreakos, E.; Dimopoulos, M. Adverse effects of COVID-19 mRNA vaccines: The spike hypothesis. Trends Mol. Med. 2022, 48, 542. [Google Scholar] [CrossRef]
- Mortazavi-Jahromi, S.S.; Aslani, M. Dysregulated miRNAs network in the critical COVID-19: An important clue for uncontrolled immunothrombosis/thromboinflammation. Int. Immunopharmacol. 2022, 110, 109040. [Google Scholar] [CrossRef]
- Narożna, M.; Rubiś, B. Anti-SARS-CoV-2 Strategies and the Potential Role of miRNA in the Assessment of COVID-19 Morbidity, Recurrence, and Therapy. Int. J. Mol. Sci. 2022, 22, 8663. [Google Scholar] [CrossRef]
- Mohammadi-Dehcheshmeh, M.; Molaei Moghbeli, S.; Rahimirad, S.; Alanazi, I.O.; Saad Al Shehri, Z.; Ebrahimie, E. A Transcription Regulatory Sequence in the 5′ Untranslated Region of SARS-CoV-2 Is Vital for Virus Replication with an Altered Evolutionary Pattern against Human Inhibitory MicroRNAs. Cells 2021, 10, 319. [Google Scholar] [CrossRef]
- Khan, M.A.; Sany, M.R.U.; Islam, M.S.; Islam, A.B.M.M.K. Epigenetic Regulator miRNA Pattern Differences Among SARS-CoV, SARS-CoV-2, and SARS-CoV-2 World-Wide Isolates Delineated the Mystery Behind the Epic Pathogenicity and Distinct Clinical Characteristics of Pandemic COVID-19. Front. Genet. 2020, 11, 765. [Google Scholar] [CrossRef]
- Natesh, N.S.; White, B.M.; Bennett, M.M.C.; Uz, M.; Kalari Kandy, R.R.; Batra, S.K.; Mallapragada, S.K.; Rachagani, S. Emerging Role of miR-345 and Its Effective Delivery as a Potential Therapeutic Candidate in Pancreatic Cancer and Other Cancers. Pharmaceutics 2021, 13, 1987. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, E.; Yendamuri, S.; Shimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M.; et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Wu, S. miR-451: A Novel Biomarker and Potential Therapeutic Target for Cancer. Onco Targets Ther. 2019, 12, 11069–11082. [Google Scholar] [CrossRef] [PubMed]
- Baptista, B.; Carapito, R.; Laroui, N.; Pichon, C.; Sousa, F. mRNA, a Revolution in Biomedicine. Pharmaceutics 2021, 13, 2090. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stati, G.; Amerio, P.; Nubile, M.; Sancilio, S.; Rossi, F.; Di Pietro, R. Concern about the Effectiveness of mRNA Vaccination Technology and Its Long-Term Safety: Potential Interference on miRNA Machinery. Int. J. Mol. Sci. 2023, 24, 1404. https://doi.org/10.3390/ijms24021404
Stati G, Amerio P, Nubile M, Sancilio S, Rossi F, Di Pietro R. Concern about the Effectiveness of mRNA Vaccination Technology and Its Long-Term Safety: Potential Interference on miRNA Machinery. International Journal of Molecular Sciences. 2023; 24(2):1404. https://doi.org/10.3390/ijms24021404
Chicago/Turabian StyleStati, Gianmarco, Paolo Amerio, Mario Nubile, Silvia Sancilio, Francesco Rossi, and Roberta Di Pietro. 2023. "Concern about the Effectiveness of mRNA Vaccination Technology and Its Long-Term Safety: Potential Interference on miRNA Machinery" International Journal of Molecular Sciences 24, no. 2: 1404. https://doi.org/10.3390/ijms24021404
APA StyleStati, G., Amerio, P., Nubile, M., Sancilio, S., Rossi, F., & Di Pietro, R. (2023). Concern about the Effectiveness of mRNA Vaccination Technology and Its Long-Term Safety: Potential Interference on miRNA Machinery. International Journal of Molecular Sciences, 24(2), 1404. https://doi.org/10.3390/ijms24021404






