Dapagliflozin Treatment Augments Bioactive Phosphatidylethanolamine Concentrations in Kidney Cortex Membrane Fractions of Hypertensive Diabetic db/db Mice and Alters the Density of Lipid Rafts in Mouse Proximal Tubule Cells
Abstract
1. Introduction
2. Results
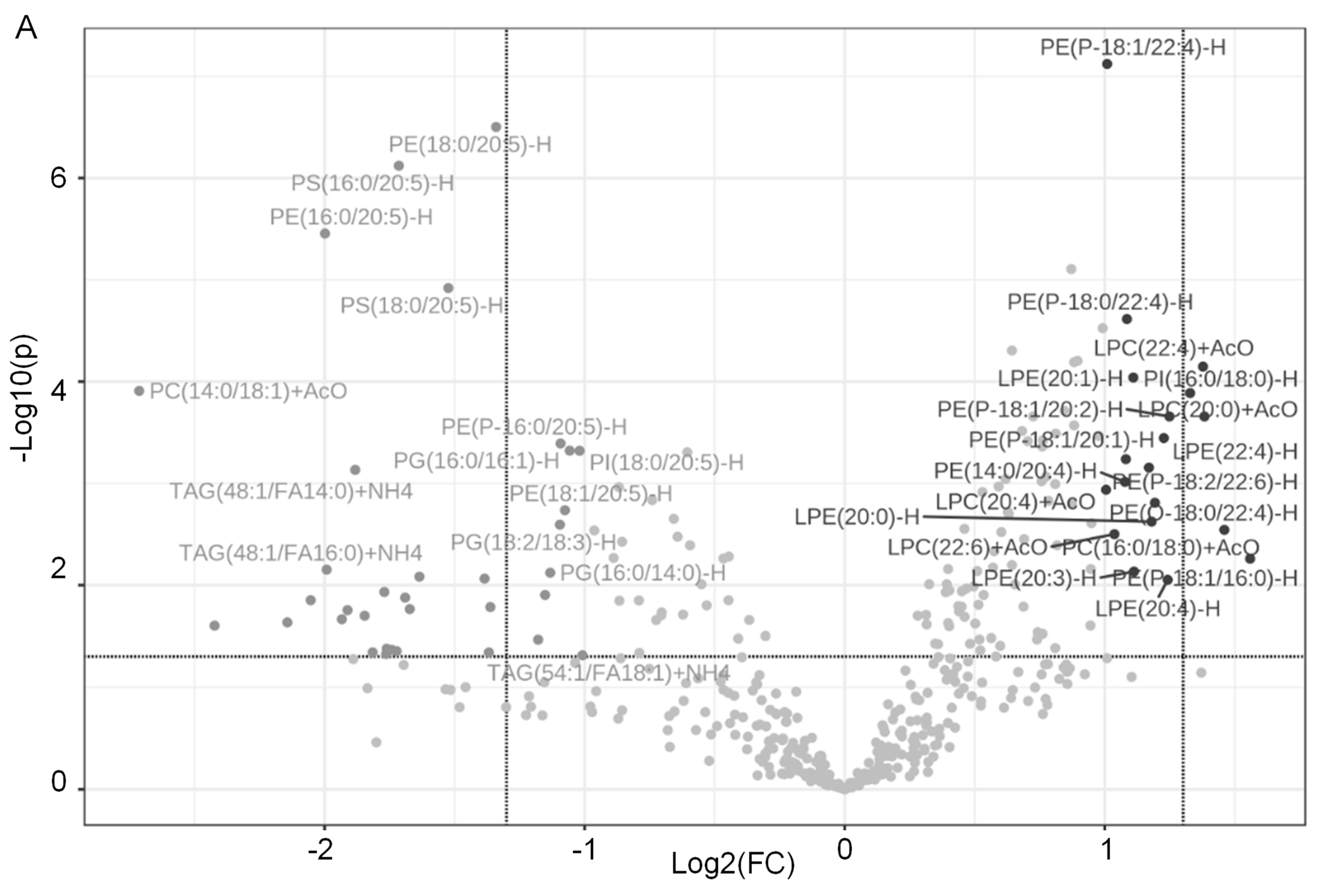
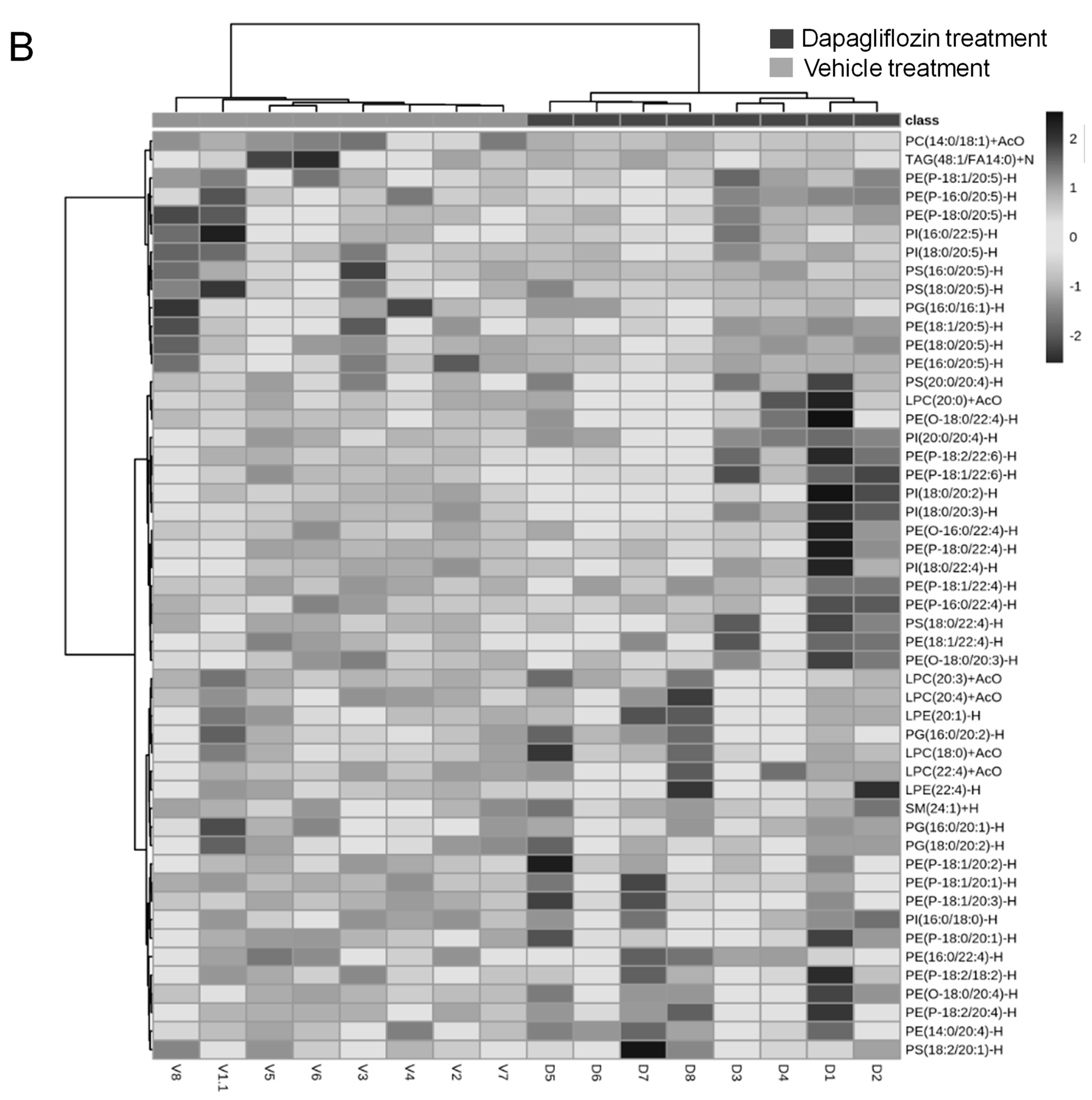
2.1. Dapagliflozin Treatment Alters Membrane Lipid Composition in the Kidneys of Salt-Loaded Hypertensive Diabetic db/db Mice
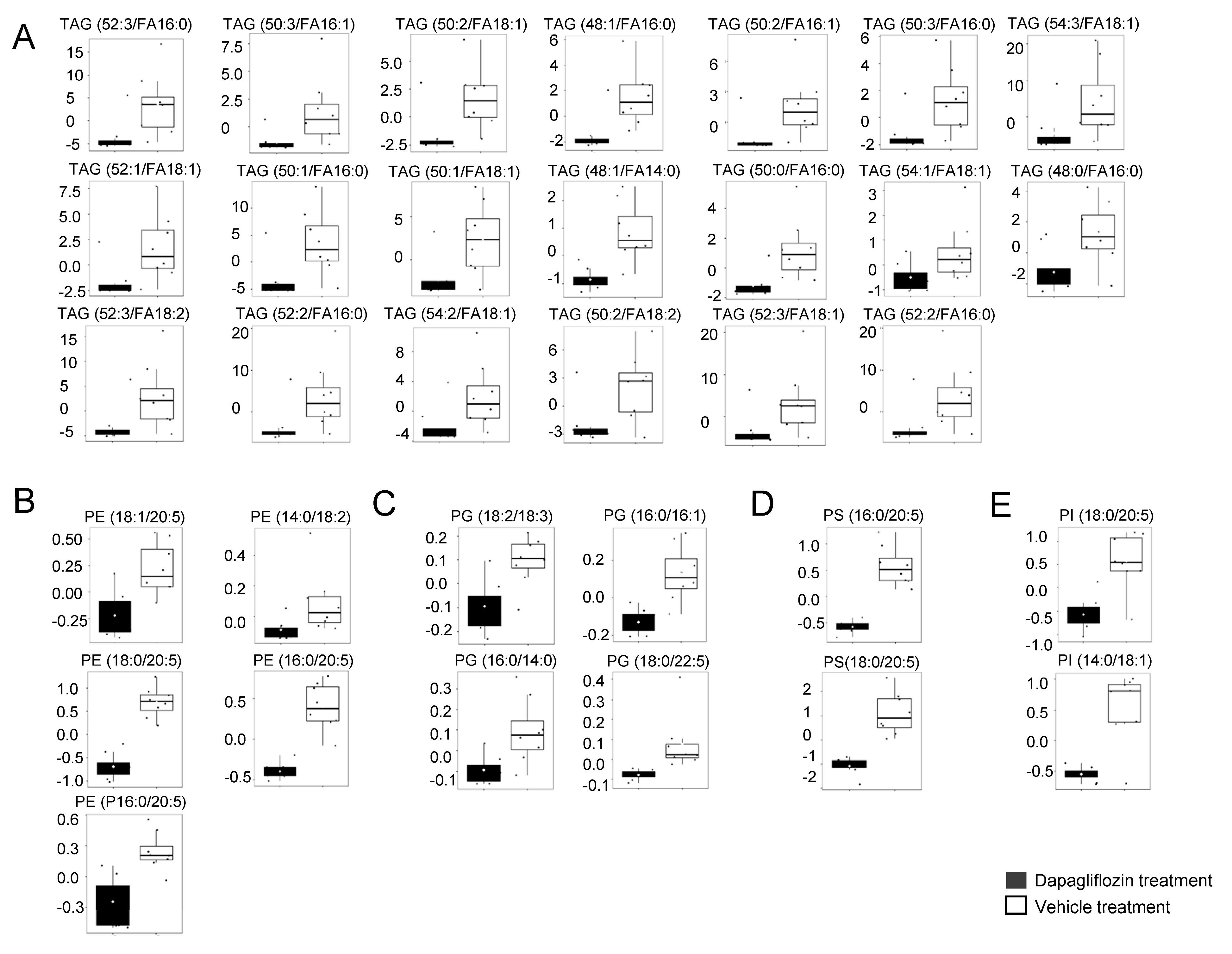
2.2. Decrease in TAGs in Kidney Cortex Membrane Fractions of db/db Mice Treated with Dapagliflozin
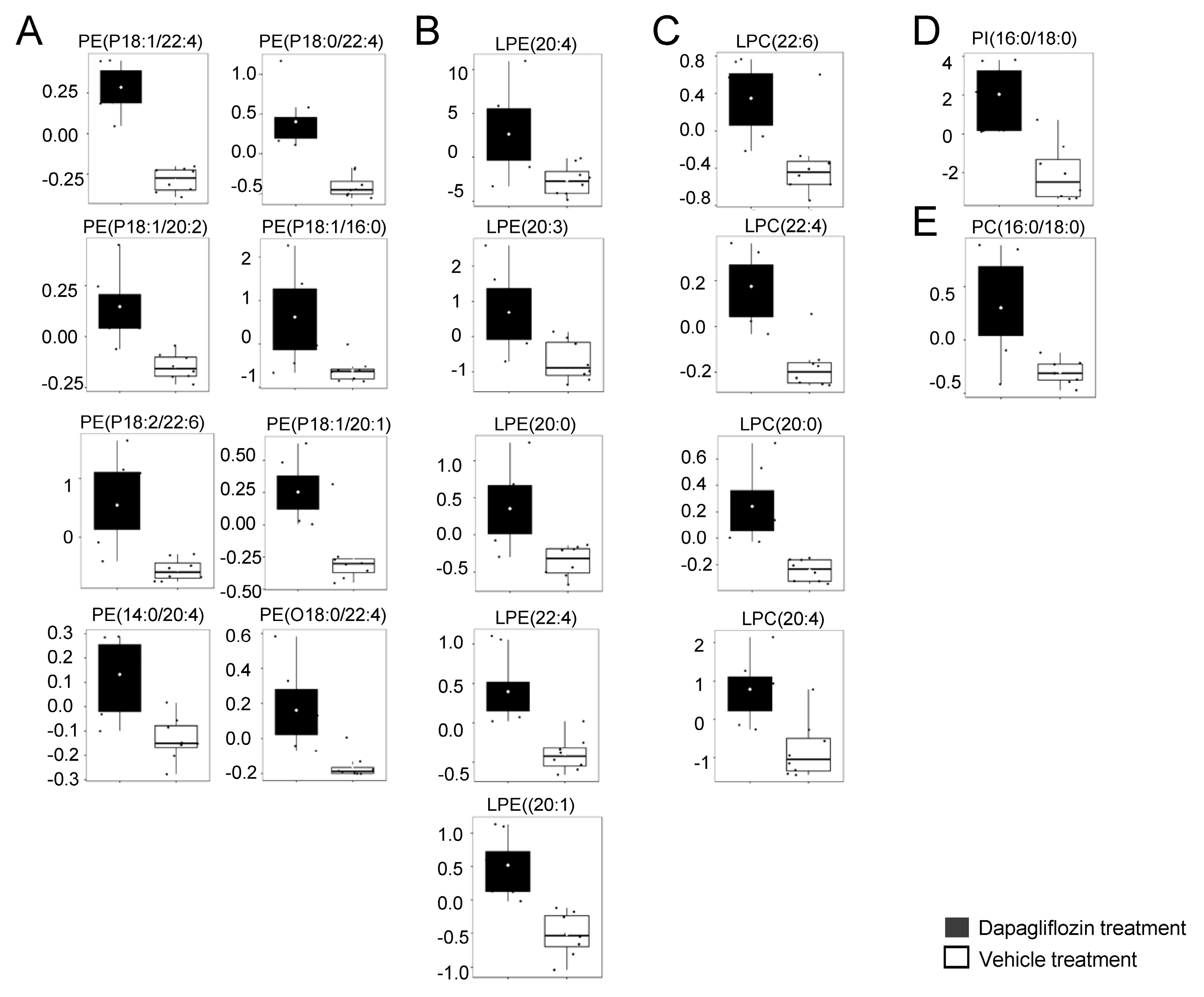
2.3. Increase in LPCs in Kidney Cortex Membrane Fractions of db/db Mice Treated with Dapagliflozin
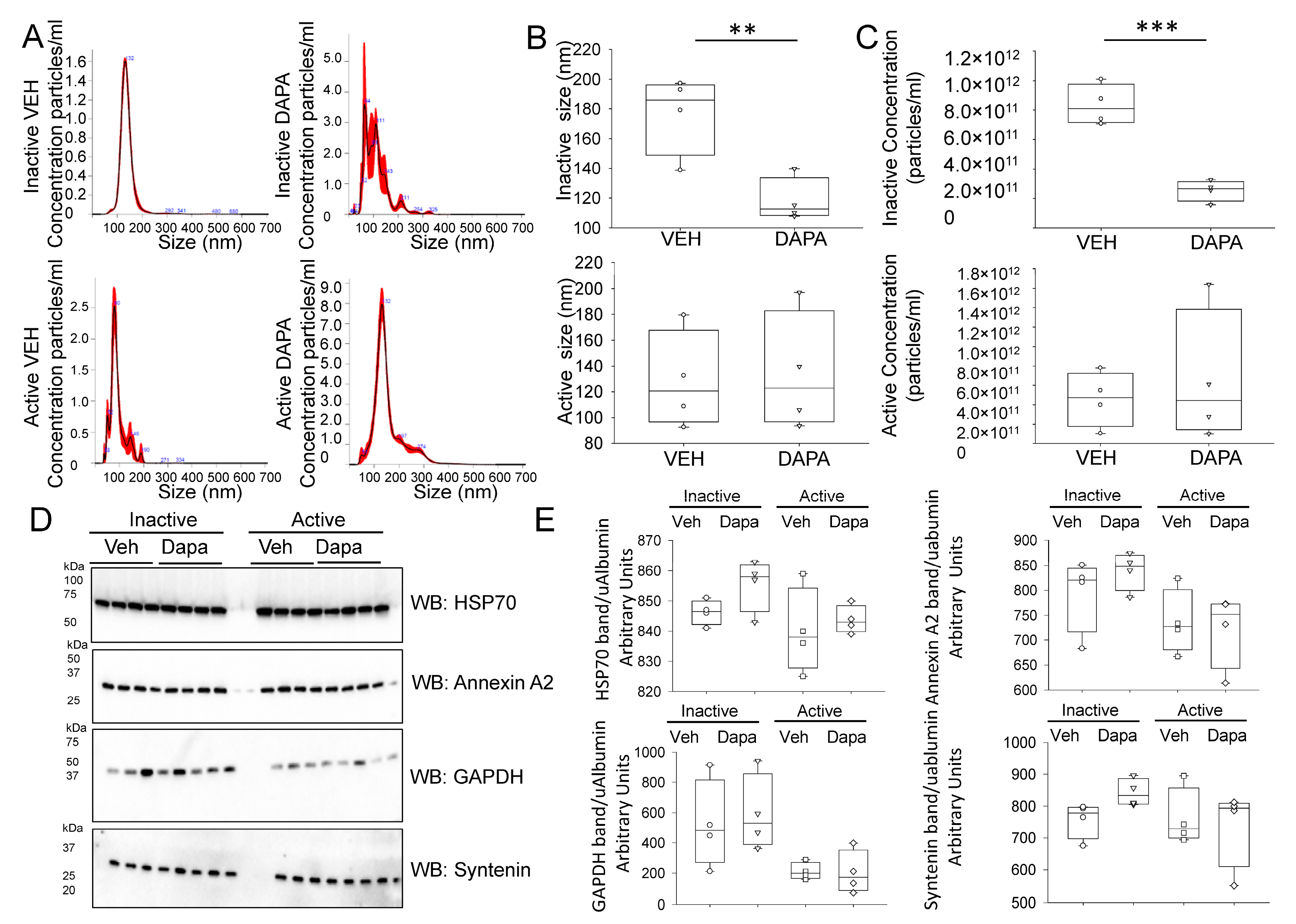
2.4. Dapagliflozin Treatment Decreases EV Release and Excretion into the Urine of db/db Mice during the Inactive Phase
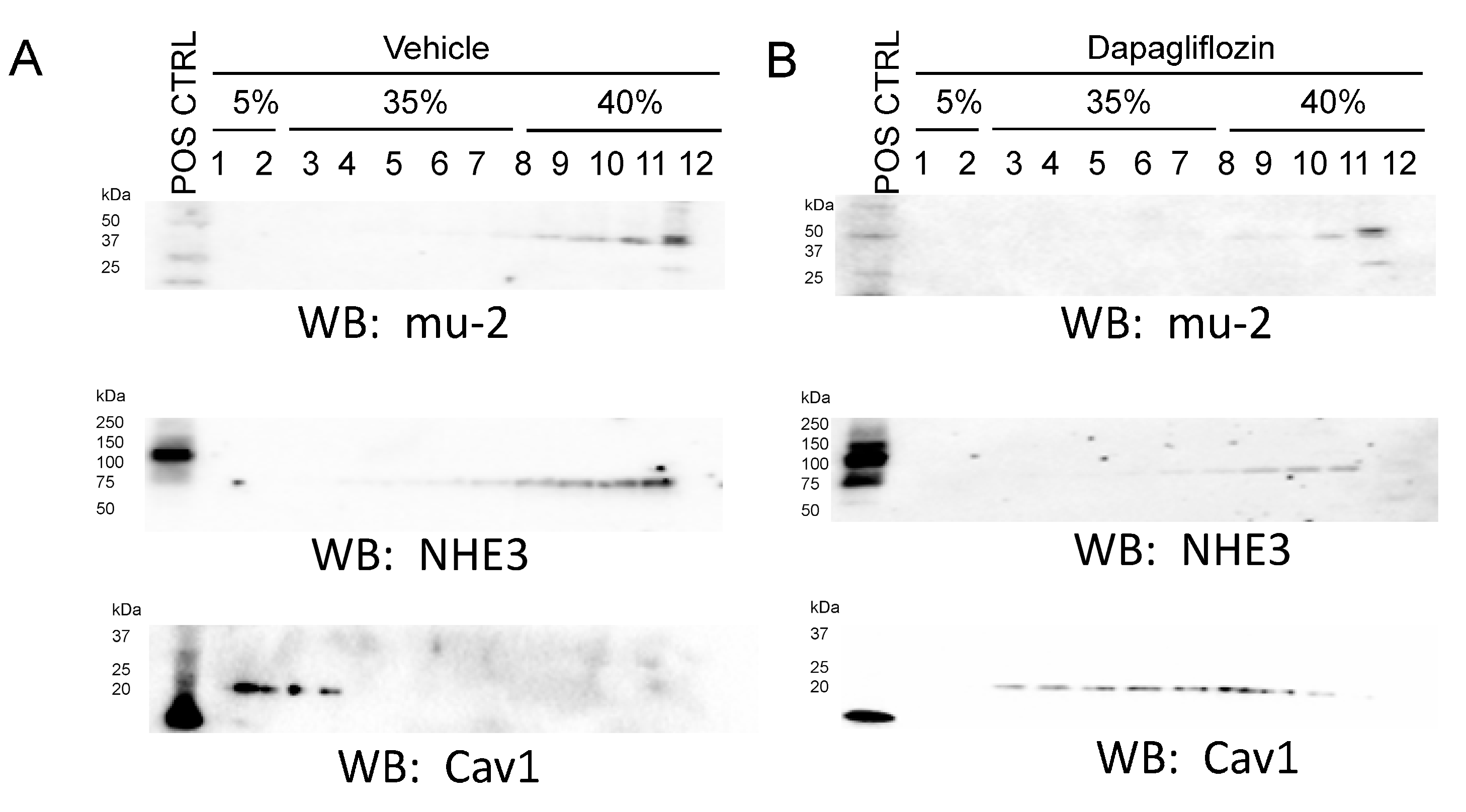
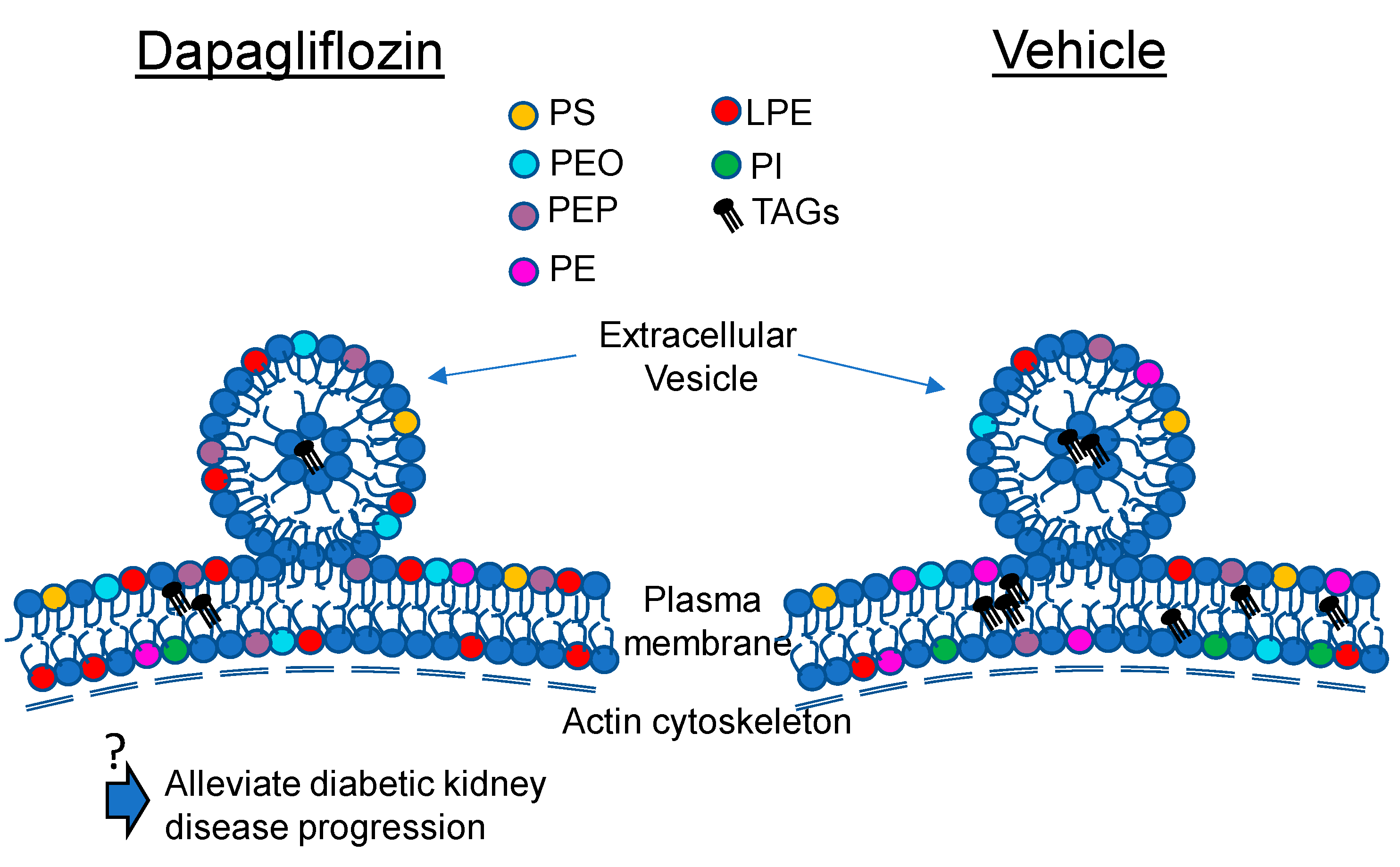
2.5. Dapagliflozin Treatment Disrupts Lipid Rafts in Mouse Proximal Tubule Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Animal Diet
4.3. Metabolic Cage Studies and Administration of Vehicle or Dapagliflozin
4.4. Tissue Lysate Preparation
4.5. Lipid Extraction from Kidney Cortex Membrane Fractions
4.6. Liquid Chromatography Tandem Mass Spectrometry
4.7. Urinary Extracellular Vesicle Isolation
4.8. Nanoparticle Tracking Analysis
4.9. Urinary Albumin Measurements
4.10. Western Blot Analysis
4.11. Culture of Mouse Proximal Tubule Cells and Lipid Raft Isolation
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrannini, E. Sodium-Glucose Co-transporters and Their Inhibition: Clinical Physiology. Cell Metab. 2017, 26, 27–38. [Google Scholar] [CrossRef]
- Fioretto, P.; Zambon, A.; Rossato, M.; Busetto, L.; Vettor, R. SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care 2016, 39, S165–S171. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Sabatine, M.S. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 1880–1882. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, K.; Wang, Q.; Li, J.; Xu, N.; Nemoto, Y.; Morimoto, C.; Fujii, W.; Tamura, Y.; Fujigaki, Y.; Tsukamoto, K.; et al. Inhibition of Sodium Glucose Cotransporter 2 Attenuates the Dysregulation of Kelch-Like 3 and NaCl Cotransporter in Obese Diabetic Mice. J. Am. Soc. Nephrol. 2019, 30, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Varzideh, F.; Kansakar, U.; Santulli, G. SGLT2 inhibitors in cardiovascular medicine. Eur. Hear. J.-Cardiovasc. Pharmacother. 2021, 7, e67–e68. [Google Scholar] [CrossRef] [PubMed]
- Bellido, V.; Martínez, J.; Calvo, F.; Villarroel, A.; Lecumberri, E.; Moreno, J.; Morillas, C.; Rodrigo, S.; Izarra, A.; Lecube, A. Beyond the Glycaemic Control of Dapagliflozin: Microangiopathy and Non-classical Complications. Diabetes Ther. 2022, 13, 873–888. [Google Scholar] [CrossRef]
- Euh, W.; Lim, S.; Kim, J.-W. Sodium-Glucose Cotransporter-2 Inhibitors Ameliorate Liver Enzyme Abnormalities in Korean Patients With Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease. Front. Endocrinol. 2021, 12, 34177796. [Google Scholar] [CrossRef]
- Ma, C.; De Baaij, J.H.; Millar, P.J.; Gault, V.A.; De Galan, B.E.; Bindels, R.J.; Hoenderop, J.G. Effect of Dapagliflozin Treatment on the Expression of Renal Sodium Transporters/Channels on High-Fat Diet Diabetic Mice. Nephron 2019, 142, 51–60. [Google Scholar] [CrossRef]
- Park, S.-H.; Belcastro, E.; Hasan, H.; Matsushita, K.; Marchandot, B.; Abbas, M.; Toti, F.; Auger, C.; Jesel, L.; Ohlmann, P.; et al. Angiotensin II-induced upregulation of SGLT1 and 2 contributes to human microparticle-stimulated endothelial senescence and dysfunction: Protective effect of gliflozins. Cardiovasc. Diabetol. 2021, 20, 1–17. [Google Scholar] [CrossRef]
- Seijas, M.C.; Agra-Bermejo, R.M.; Fernández, A.L.; Martínez-Cereijo, J.M.; Sierra, J.; Soto-Pérez, M.; Rozados-Luis, A.; Juanatey, J.R.G.; Eiras, S. High released lactate by epicardial fat from coronary artery disease patients is reduced by dapagliflozin treatment. Atherosclerosis 2019, 292, 60–69. [Google Scholar] [CrossRef]
- Al-Sharea, A.; Murphy, A.J.; Huggins, L.; Hu, Y.; Goldberg, I.J.; Nagareddy, P.R. SGLT2 inhibition reduces atherosclerosis by enhancing lipoprotein clearance in Ldlr type 1 diabetic mice. Atherosclerosis 2018, 271, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Rau, M.; Thiele, K.; Hartmann, N.-U.K.; Möllmann, J.; Wied, S.; Böhm, M.; Scharnagl, H.; März, W.; Marx, N.; Lehrke, M. Effects of empagliflozin on lipoprotein subfractions in patients with type 2 diabetes–Data from a randomized, placebo-controlled study. Atherosclerosis 2021, 330, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Le, L.; Xiang, J.; Jiang, B.; Chen, S.; Xiao, P. Liver Transcriptomic Reveals Novel Pathways of Empagliflozin Associated With Type 2 Diabetic Rats. Front. Endocrinol. 2020, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Mone, P.; Varzideh, F.; Jankauskas, S.S.; Pansini, A.; Lombardi, A.; Frullone, S.; Santulli, G. SGLT2 Inhibition via Empagliflozin Improves Endothelial Function and Reduces Mitochondrial Oxidative Stress: Insights From Frail Hypertensive and Diabetic Patients. Hypertension 2022, 79, 1633–1643. [Google Scholar] [CrossRef]
- Chen, L.; LaRocque, L.M.; Efe, O.; Wang, J.; Sands, J.M.; Klein, J.D. Effect of Dapagliflozin Treatment on Fluid and Electrolyte Balance in Diabetic Rats. Am. J. Med. Sci. 2016, 352, 517–523. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Paneni, F.; Beckman, J.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Hear. J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef]
- Fryar, C.D.; Ostchega, Y.; Hales, C.; Zhang, G.; Kruszon-Moran, D. Hypertension Prevalence and Control Among Adults: United States, 2015-2016. NCHS Data Brief. 2017, 289, 1–8. [Google Scholar]
- Filippone, E.J.; Ruzieh, M.; Foy, A. Thiazide-Associated Hyponatremia: Clinical Manifestations and Pathophysiology. Am. J. Kidney Dis. 2020, 75, 256–264. [Google Scholar] [CrossRef]
- Richards, J.; Gumz, M.L. Mechanism of the circadian clock in physiology. Am. J. Physiol. Integr. Comp. Physiol. 2013, 304, R1053–R1064. [Google Scholar] [CrossRef]
- van Balkom, B.W.; Pisitkun, T.; Verhaar, M.C.; Knepper, M.A. Exosomes and the kidney: Prospects for diagnosis and therapy of renal diseases. Kidney Int. 2011, 80, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.C.; Bond, D.T.; McKee, M.; Skog, J.; Păunescu, T.G.; Da Silva, N.; Brown, D.; Russo, L.M. Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int. 2010, 78, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Jella, K.K.; Yu, L.; Yue, Q.; Friedman, D.; Duke, B.J.; Alli, A.A. Exosomal GAPDH from Proximal Tubule Cells Regulate ENaC Activity. PLoS ONE 2016, 11, e0165763. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wang, Q.; Zhang, Y.; Jiang, D. Extracellular vesicles produced by bone marrow mesenchymal stem cells attenuate renal fibrosis, in part by inhibiting the RhoA/ROCK pathway, in a UUO rat model. Stem Cell Res. Ther. 2020, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhu, Y.; Sun, L.; Zhu, W.; Lu, Y.; Zhang, J.; Mao, Y.; Chen, Q.; Zhang, F. Circulating Exosomal miR-1-3p from Rats with Myocardial Infarction Plays a Protective Effect on Contrast-Induced Nephropathy via Targeting ATG13 and activating the AKT Signaling Pathway. Int. J. Biol. Sci. 2021, 17, 972–985. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Sun, X.; Qi, X.; Xia, L.; Wu, Y. Exosomes from high glucose-treated macrophages activate macrophages and induce inflammatory responses via NF-κB signaling pathway in vitro and in vivo. Int. Immunopharmacol. 2020, 84, 106551. [Google Scholar] [CrossRef]
- Pekkucuksen, N.T.; Liu, L.P.; Aly, R.; Shoemaker, L.R.; Alli, A.A. Extracellular vesicles from focal segmental glomerulosclerosis pediatric patients induce STAT3 activation and mesangial cell proliferation. PLoS ONE 2022, 17, e0274598. [Google Scholar] [CrossRef]
- Hirohama, D.; Nishimoto, M.; Ayuzawa, N.; Kawarazaki, W.; Fujii, W.; Oba, S.; Shibata, S.; Marumo, T.; Fujita, T. Activation of Rac1-Mineralocorticoid Receptor Pathway Contributes to Renal Injury in Salt-Loaded db/db Mice. Hypertension 2021, 78, 82–93. [Google Scholar] [CrossRef]
- Sas, K.M.; Lin, J.; Wang, C.-H.; Zhang, H.; Saha, J.; Rajendiran, T.M.; Soni, T.; Nair, V.; Eichinger, F.; Kretzler, M.; et al. Renin-angiotensin system inhibition reverses the altered triacylglycerol metabolic network in diabetic kidney disease. Metabolomics 2021, 17, 1–12. [Google Scholar] [CrossRef]
- Yoshioka, K.; Hirakawa, Y.; Kurano, M.; Ube, Y.; Ono, Y.; Kojima, K.; Iwama, T.; Kano, K.; Hasegawa, S.; Inoue, T.; et al. Lysophosphatidylcholine mediates fast decline in kidney function in diabetic kidney disease. Kidney Int. 2021, 101, 510–526. [Google Scholar] [CrossRef]
- Wiggenhauser, L.M.; Metzger, L.; Bennewitz, K.; Soleymani, S.; Boger, M.; Tabler, C.T.; Hausser, I.; Sticht, C.; Wohlfart, P.; Volk, N.; et al. pdx1 Knockout Leads to a Diabetic Nephropathy– Like Phenotype in Zebrafish and Identifies Phosphatidylethanolamine as Metabolite Promoting Early Diabetic Kidney Damage. Diabetes 2022, 71, 1073–1080. [Google Scholar] [CrossRef]
- Wallner, S.; Schmitz, G. Plasmalogens the neglected regulatory and scavenging lipid species. Chem. Phys. Lipids 2011, 164, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Sindelar, P.J.; Guan, Z.; Dallner, G.; Ernster, L. The protective role of plasmalogens in iron-induced lipid peroxidation. Free. Radic. Biol. Med. 1999, 26, 318–324. [Google Scholar] [CrossRef]
- Bozelli, J.C.J.; Azher, S.; Epand, R.M. Plasmalogens and Chronic Inflammatory Diseases. Front. Physiol. 2021, 12, 730829. [Google Scholar] [CrossRef] [PubMed]
- Ares, G.R.; Ortiz, P.A. Dynamin2, Clathrin, and Lipid Rafts Mediate Endocytosis of the Apical Na/K/2Cl Cotransporter NKCC2 in Thick Ascending Limbs. J. Biol. Chem. 2012, 287, 37824–37834. [Google Scholar] [CrossRef]
- Welker, P.; Böhlick, A.; Mutig, K.; Salanova, M.; Kahl, T.; Schlüter, H.; Blottner, D.; Ponce-Coria, J.; Gamba, G.; Bachmann, S. Renal Na+-K+-Cl−cotransporter activity and vasopressin-induced trafficking are lipid raft-dependent. Am. J. Physiol. Physiol. 2008, 295, F789–F802. [Google Scholar] [CrossRef] [PubMed]
- Hill, W.G.; Butterworth, M.B.; Wang, H.; Edinger, R.S.; Lebowitz, J.; Peters, K.W.; Frizzell, R.A.; Johnson, J.P. The Epithelial Sodium Channel (ENaC) Traffics to Apical Membrane in Lipid Rafts in Mouse Cortical Collecting Duct Cells. J. Biol. Chem. 2007, 282, 37402–37411. [Google Scholar] [CrossRef]
- Lee, I.-H.; Campbell, C.; Song, S.-H.; Day, M.L.; Kumar, S.; Cook, D.I.; Dinudom, A. The Activity of the Epithelial Sodium Channels Is Regulated by Caveolin-1 via a Nedd4-2-dependent Mechanism. J. Biol. Chem. 2009, 284, 12663–12669. [Google Scholar] [CrossRef] [PubMed]
- Tuna, K.M.; Liu, B.-C.; Yue, Q.; Ghazi, Z.M.; Ma, H.-P.; Eaton, U.C.; Alli, A.A. Mal protein stabilizes luminal membrane PLC-β3 and negatively regulates ENaC in mouse cortical collecting duct cells. Am. J. Physiol. Physiol. 2019, 317, F986–F995. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Glover, S.C.; Nouri, M.; Tuna, K.M.; Alvarez, L.B.M.; Ryan, L.K.; Shirley, J.F.; Tang, Y.; Denslow, N.D.; Alli, A.A. Lipidomic analysis of urinary exosomes from hereditary α-tryptasemia patients and healthy volunteers. FASEB BioAdv. 2019, 1, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Chacko, K.M.; Nouri, M.-Z.; Schramm, W.C.; Malik, Z.; Liu, L.P.; Denslow, N.D.; Alli, A.A. Tempol Alters Urinary Extracellular Vesicle Lipid Content and Release While Reducing Blood Pressure during the Development of Salt-Sensitive Hypertension. Biomolecules 2021, 11, 1804. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gholam, M.F.; Liu, L.P.; Searcy, L.A.; Denslow, N.D.; Alli, A.A. Dapagliflozin Treatment Augments Bioactive Phosphatidylethanolamine Concentrations in Kidney Cortex Membrane Fractions of Hypertensive Diabetic db/db Mice and Alters the Density of Lipid Rafts in Mouse Proximal Tubule Cells. Int. J. Mol. Sci. 2023, 24, 1408. https://doi.org/10.3390/ijms24021408
Gholam MF, Liu LP, Searcy LA, Denslow ND, Alli AA. Dapagliflozin Treatment Augments Bioactive Phosphatidylethanolamine Concentrations in Kidney Cortex Membrane Fractions of Hypertensive Diabetic db/db Mice and Alters the Density of Lipid Rafts in Mouse Proximal Tubule Cells. International Journal of Molecular Sciences. 2023; 24(2):1408. https://doi.org/10.3390/ijms24021408
Chicago/Turabian StyleGholam, Mohammed F., Lauren P. Liu, Louis A. Searcy, Nancy D. Denslow, and Abdel A. Alli. 2023. "Dapagliflozin Treatment Augments Bioactive Phosphatidylethanolamine Concentrations in Kidney Cortex Membrane Fractions of Hypertensive Diabetic db/db Mice and Alters the Density of Lipid Rafts in Mouse Proximal Tubule Cells" International Journal of Molecular Sciences 24, no. 2: 1408. https://doi.org/10.3390/ijms24021408
APA StyleGholam, M. F., Liu, L. P., Searcy, L. A., Denslow, N. D., & Alli, A. A. (2023). Dapagliflozin Treatment Augments Bioactive Phosphatidylethanolamine Concentrations in Kidney Cortex Membrane Fractions of Hypertensive Diabetic db/db Mice and Alters the Density of Lipid Rafts in Mouse Proximal Tubule Cells. International Journal of Molecular Sciences, 24(2), 1408. https://doi.org/10.3390/ijms24021408




