Trichosanthin Promotes Anti-Tumor Immunity through Mediating Chemokines and Granzyme B Secretion in Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Results
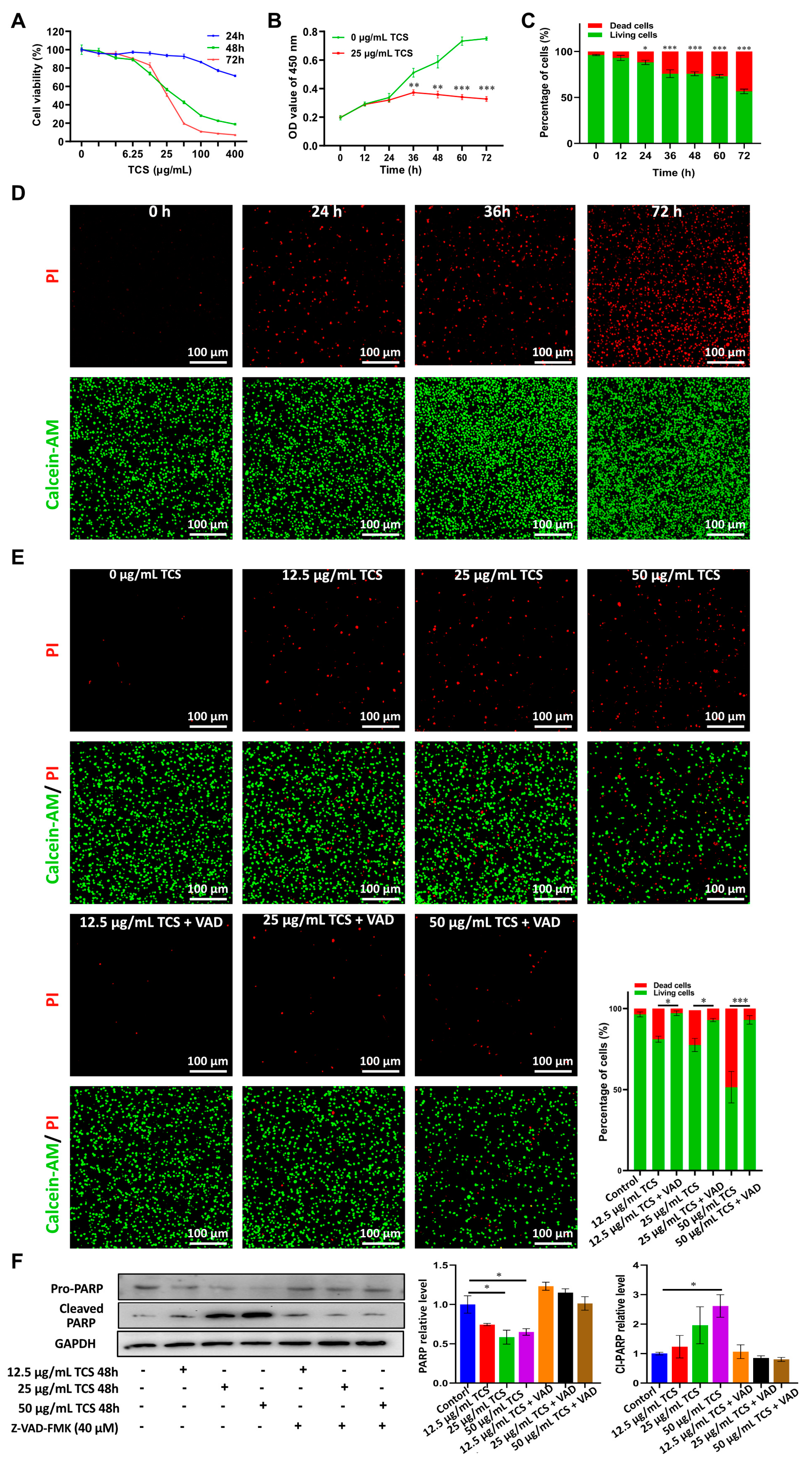
2.1. TCS Reduces the Viability of HCC Cells in Culture
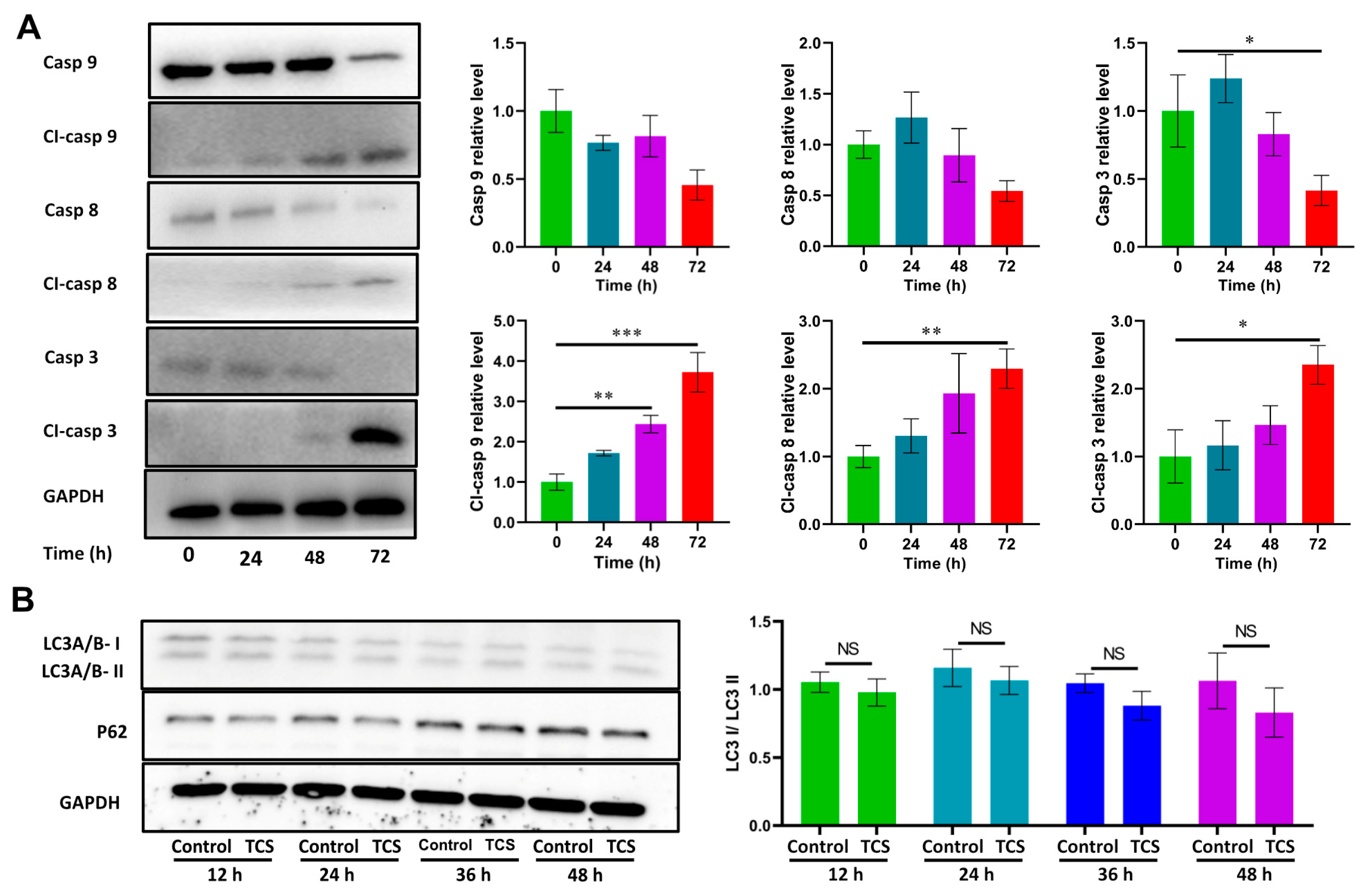
2.2. TCS Promoted HCC Cell Death via Apoptosis
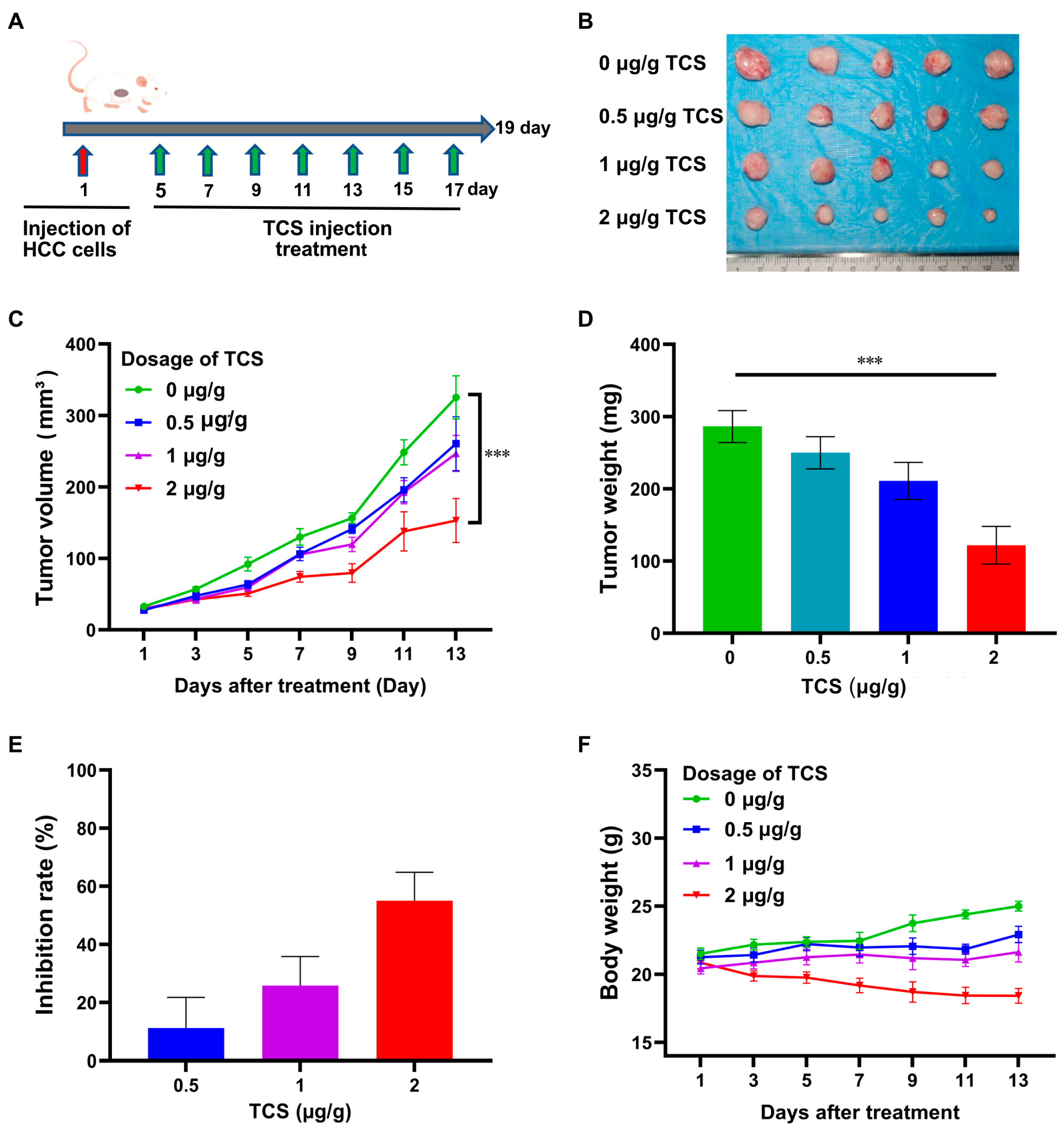
2.3. TCS Inhibits HCC Tumor Growth In Vivo
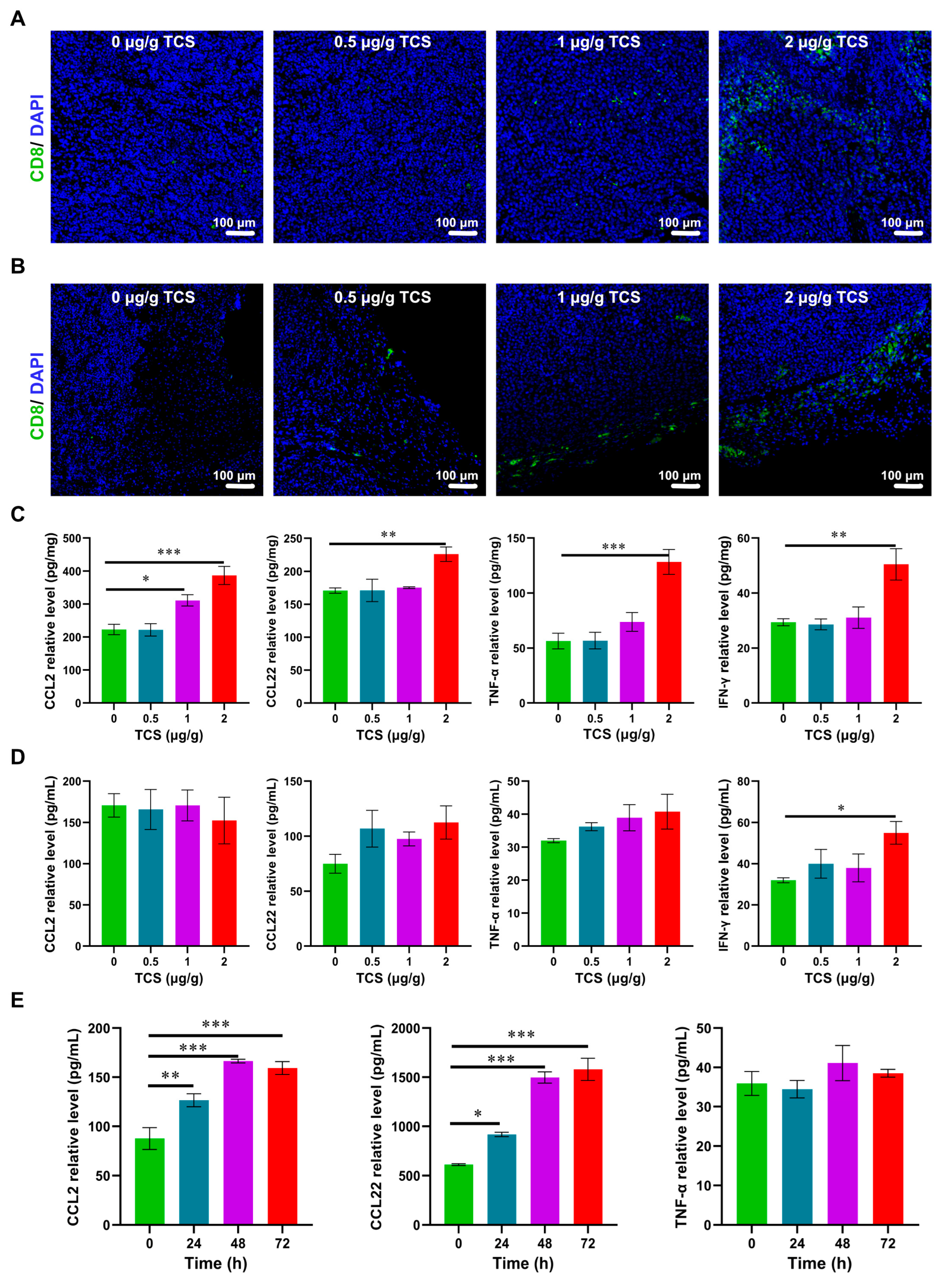
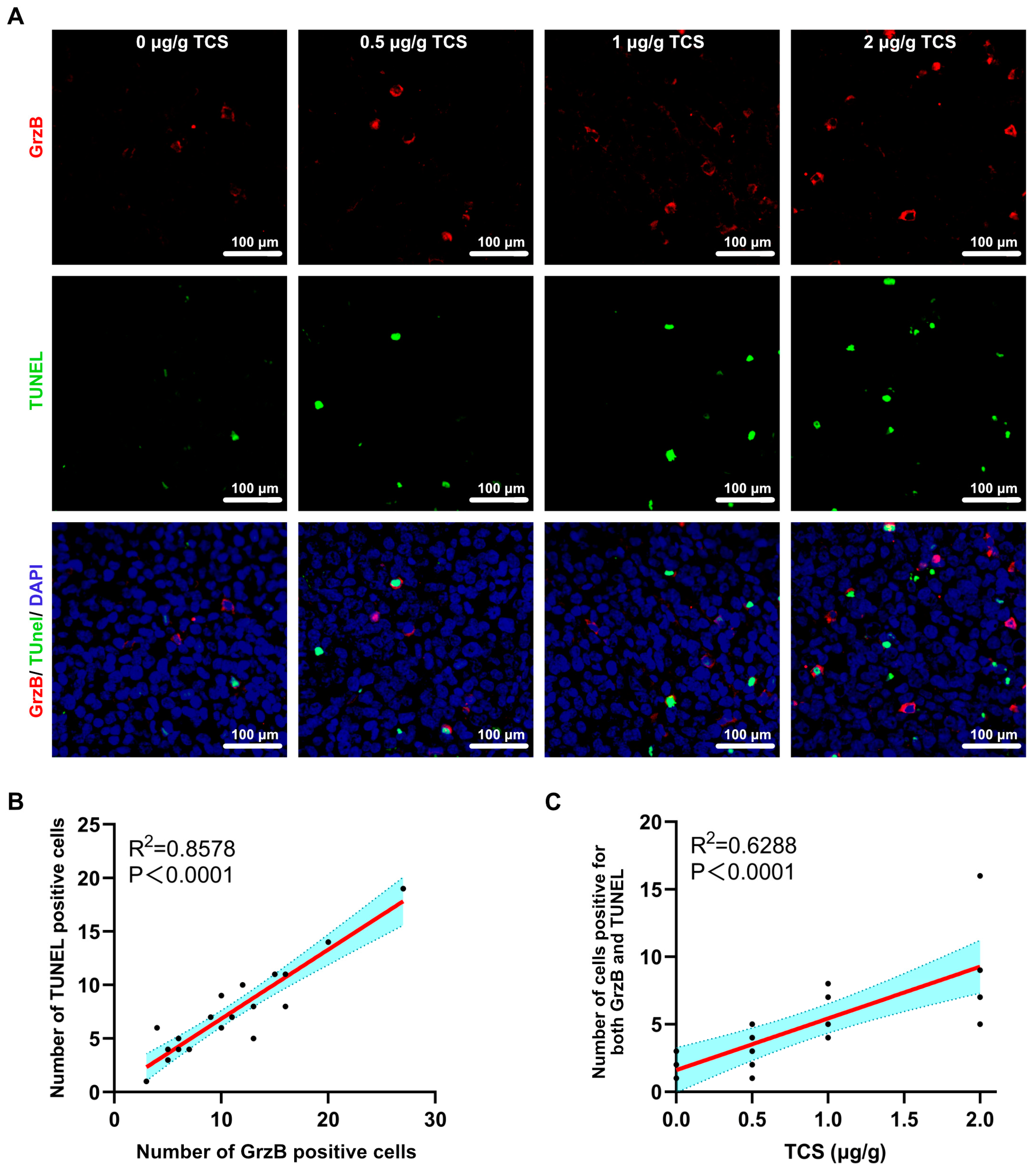
2.4. TCS Promotes Infiltration of CD8+ T Cells into HCC
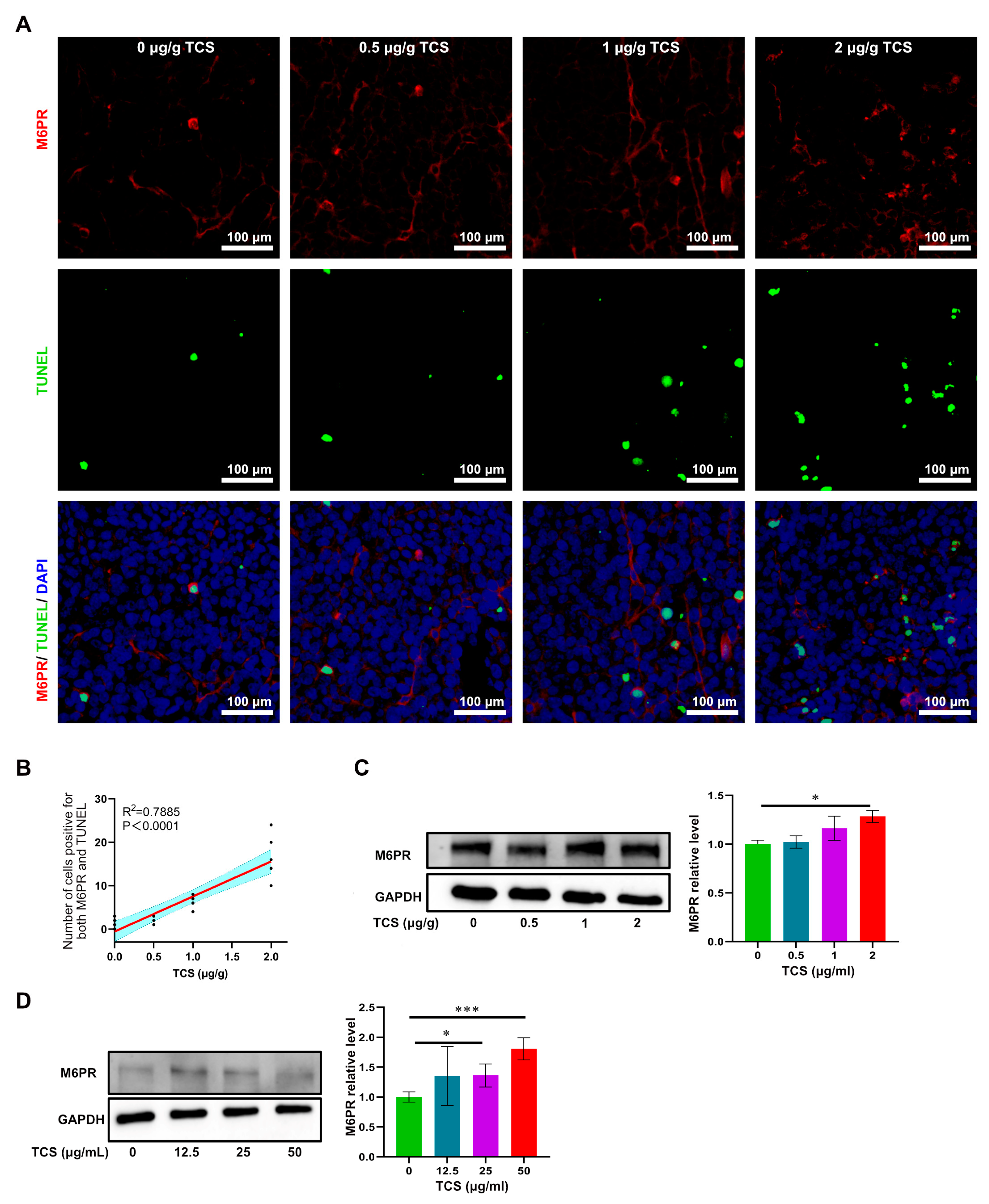
2.5. TCS Enhances the Expression of Granzyme B and M6PR
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Maki, H.; Hasegawa, K. Advances in the surgical treatment of liver cancer. Biosci. Trends 2022, 16, 178–188. [Google Scholar] [CrossRef]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. et Biophys. Acta (BBA)-Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, W.; Goel, A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin. Cancer Biol. 2022, 80, 73–86. [Google Scholar] [CrossRef]
- Shen, L.-W.; Jiang, X.-X.; Li, Z.-Q.; Li, J.; Wang, M.; Jia, G.-F.; Ding, X.; Lei, L.; Gong, Q.-H.; Gao, N. Cepharanthine sensitizes human triple negative breast cancer cells to chemotherapeutic agent epirubicin via inducing cofilin oxidation-mediated mitochondrial fission and apoptosis. Acta Pharmacol. Sin. 2022, 43, 177–193. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, C.; Cui, X.; Wu, J.; Cui, Z.; Shen, X. Trichosanthin inhibits cervical cancer by regulating oxidative stress-induced apoptosis. Bioengineered 2021, 12, 2779–2790. [Google Scholar] [CrossRef]
- Lu, J.-Q.; Wong, K.-B.; Shaw, P.-C. A Sixty-Year Research and Development of Trichosanthin, a Ribosome-Inactivating Protein. Toxins 2022, 14, 178. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ying, Z.; Zeng, M.; Zhang, Q.; Liao, G.; Liang, Y.; Li, C.; Zhang, C.; Wang, X.; Jiang, W.; et al. Trichosanthin cooperates with Granzyme B to restrain tumor formation in tongue squamous cell carcinoma. BMC Complement. Med. Ther. 2021, 21, 88. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, B.; Zhou, J.; Zhou, L.; Li, Q.; Liu, F.; Chou, K.-Y.; Tao, L.; Lü, L.-M. Low concentrations of trichosanthin induce apoptosis and cell cycle arrest via c-Jun N-terminal protein kinase/mitogen-activated protein kinase activation. Mol. Med. Rep. 2015, 11, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Han, L.; Bai, L.; Tang, H.; Zheng, A. Trichosanthin inhibits the proliferation of cervical cancer cells and downregulates STAT-5/C-myc signaling pathway. Pathol.-Res. Pract. 2019, 215, 632–638. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Li, J.-C. Possible Mechanisms of Trichosanthin-Induced Apoptosis of Tumor Cells. Anat. Rec. 2010, 293, 986–992. [Google Scholar] [CrossRef]
- You, C.; Sun, Y.; Zhang, S.; Tang, G.; Zhang, N.; Li, C.; Tian, X.; Ma, S.; Luo, Y.; Sun, W.; et al. Trichosanthin enhances sensitivity of non-small cell lung cancer (NSCLC) TRAIL-resistance cells. Int. J. Biol. Sci. 2018, 14, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Xiang, J.; Huang, Z.; Wang, L.; Huang, Y. Trichosanthin inhibits cell growth and metastasis by promoting pyroptosis in non-small cell lung cancer. J. Thorac. Dis. 2022, 14, 1193–1202. [Google Scholar] [CrossRef]
- Cai, Y.; Xiong, S.; Zheng, Y.; Luo, F.; Jiang, P.; Chu, Y. Trichosanthin enhances anti-tumor immune response in a murine Lewis lung cancer model by boosting the interaction between TSLC1 and CRTAM. Cell. Mol. Immunol. 2011, 8, 359–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Xu, J.; Huang, X.; Wu, L.; Wen, C.; Hu, Y.; Su, Y.; Chen, Y.; Zhang, Z. Trichosanthin down-regulated p210Bcr-Abl and enhanced imatinib-induced growth arrest in chronic myelogenous leukemia cell line K562. Cancer Chemother. Pharmacol. 2007, 60, 581–587. [Google Scholar] [CrossRef]
- Wu, A.; Chen, Y.; Wang, H.; Chang, Y.; Zhang, M.; Zhao, P.; Tang, Y.; Xu, Q.; Zhu, Z.; Cao, Y.; et al. Genetically-engineered “all-in-one” vaccine platform for cancer immunotherapy. Acta Pharm. Sin. B 2021, 11, 3622–3635. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yao, S.; Chen, Y.; Huang, J.; Wu, A.; Zhang, M.; Xu, F.; Li, F.; Huang, Y. Genetically-engineered protein prodrug-like nanoconjugates for tumor-targeting biomimetic delivery via a SHEATH strategy. Nanoscale 2019, 11, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, M.; Jin, H.; Tang, Y.; Wu, A.; Xu, Q.; Huang, Y. Prodrug-Like, PEGylated Protein Toxin Trichosanthin for Reversal of Chemoresistance. Mol. Pharm. 2017, 14, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Hiroyasu, S.; Zeglinski, M.R.; Zhao, H.; Pawluk, M.A.; Turner, C.T.; Kasprick, A.; Tateishi, C.; Nishie, W.; Burleigh, A.; Lennox, P.A.; et al. Granzyme B inhibition reduces disease severity in autoimmune blistering diseases. Nat. Commun. 2021, 12, 302. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, Y.; Chen, X.; Wang, Z.; Liang, X.; Zhang, T.; Liu, M.; Zhou, N.; Lv, J.; Tang, K.; et al. Gasdermin E–mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci. Immunol. 2020, 5, eaax7969. [Google Scholar] [CrossRef]
- Arias, M.; Martínez-Lostao, L.; Santiago, L.; Ferrandez, A.; Granville, D.J.; Pardo, J. The Untold Story of Granzymes in Oncoimmunology: Novel Opportunities with Old Acquaintances. Trends Cancer 2017, 3, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zeng, M.; Chi, H.; Shen, J.; Ng, T.-B.; Jin, G.; Lu, D.; Fan, X.; Xiong, B.; Xiao, Z.; et al. Trichosanthin increases Granzyme B penetration into tumor cells by upregulation of CI-MPR on the cell surface. Oncotarget 2017, 8, 26460–26470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galia, A.; Calogero, A.; Condorelli, R.A.; Fraggetta, F.; La Corte, A.; Ridolfo, F.; Bosco, P.; Castiglione, R.; Salemi, M. PARP-1 protein expression in glioblastoma multiforme. Eur. J. Histochem. 2012, 56, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Z.; Yang, Z.; Xu, Y.; Chen, Y.; Yu, Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer 2015, 14, 48. [Google Scholar] [CrossRef] [Green Version]
- Russo, M.; Russo, G.L. Autophagy inducers in cancer. Biochem. Pharmacol. 2018, 153, 51–61. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, B.; Huang, Q.; Huang, S.; Mai, W.; Zhong, X. Trichosanthin-induced autophagy in gastric cancer cell MKN-45 is dependent on reactive oxygen species (ROS) and NF-κB/p53 pathway. J. Pharmacol. Sci. 2016, 131, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Sirohi, K.; Swarup, G. Defects in autophagy caused by glaucoma-associated mutations in optineurin. Exp. Eye Res. 2016, 144, 54–63. [Google Scholar] [CrossRef]
- Kałuza, J.; Adamek, D.; Pyrich, M. Ki-67 as a marker of proliferation activity in tumor progression of recurrent gliomas of supratentorial localization. Immunocytochemical quantitative studies. Pol. J. Pathol 1997, 48, 31–36. [Google Scholar]
- Strazza, M.; Mor, A. Consider the Chemokines: A Review of the Interplay Between Chemokines and T Cell Subset Function. Discov. Med. 2017, 24, 31–39. [Google Scholar] [PubMed]
- Opal, S.M.; DePalo, V.A. Anti-Inflammatory Cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sucker, A.; Zhao, F.; Pieper, N.; Heeke, C.; Maltaner, R.; Stadtler, N.; Real, B.; Bielefeld, N.; Howe, S.; Weide, B.; et al. Acquired IFNγ resistance impairs anti-tumor immunity and gives rise to T-cell-resistant melanoma lesions. Nat. Commun. 2017, 8, 15440. [Google Scholar] [CrossRef] [Green Version]
- Turner, C.T.; Hiroyasu, S.; Granville, D.J. Granzyme B as a therapeutic target for wound healing. Expert Opin. Ther. Targets 2019, 23, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Krepela, E. Granzyme B-induced apoptosis in cancer cells and its regulation (Review). Int. J. Oncol. 2010, 37, 1361–1378. [Google Scholar] [CrossRef] [Green Version]
- Mondal, A. A novel extraction of trichosanthin from Trichosanthes kirilowii roots using three-phase partitioning and its in vitro anticancer activity. Pharm. Biol. 2014, 52, 677–680. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Y.; Cai, Y.; Sha, O.; Jiang, W. Trichosanthin reduces the viability of SU-DHL-2 cells via the activation of the extrinsic and intrinsic apoptotic pathways. Mol. Med. Rep. 2016, 13, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Huang, S.; Wang, F.; Ren, Y.; Hehir, M.; Wang, X.; Cai, J. Cyclic AMP-Response Element Regulated Cell Cycle Arrests in Cancer Cells. PLoS ONE 2013, 8, e65661. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef]
- Gao, W.; Wang, X.; Zhou, Y.; Wang, X.; Yu, Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 196. [Google Scholar] [CrossRef]
- Asrorov, A.M.; Muhitdinov, B.; Tu, B.; Mirzaakhmedov, S.; Wang, H.; Huang, Y. Advances on Delivery of Cytotoxic Enzymes as Anticancer Agents. Molecules 2022, 27, 3836. [Google Scholar] [CrossRef]
- Tummers, B.; Green, D.R. Caspase-8: Regulating life and death. Immunol. Rev. 2017, 277, 76–89. [Google Scholar] [CrossRef] [Green Version]
- Avrutsky, M.I.; Troy, C.M. Caspase-9: A Multimodal Therapeutic Target With Diverse Cellular Expression in Human Disease. Front. Pharmacol. 2021, 12, 701301. [Google Scholar] [CrossRef] [PubMed]
- Boulares, A.H.; Yakovlev, A.G.; Ivanova, V.; Stoica, B.A.; Wang, G.; Iyer, S.; Smulson, M. Role of poly(adp-ribose) polymerase (parp) cleavage in apoptosis. Caspase 3-resistant parp mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999, 274, 22932–22940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creagh, E.; Conroy, H.; Martin, S.J. Caspase-activation pathways in apoptosis and immunity. Immunol. Rev. 2003, 193, 10–21. [Google Scholar] [CrossRef]
- Jiandong, L.; Yang, Y.; Peng, J.; Xiang, M.; Wang, D.; Xiong, G.; Li, S. Trichosanthes kirilowii lectin ameliorates streptozocin-induced kidney injury via modulation of the balance between M1/M2 phenotype macrophage. Biomed. Pharmacother. 2019, 109, 93–102. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, N.; Lu, L.; Ding, Q.; Jiao, Z.; Zhou, Y.; Chou, K.-Y. Up-regulation of IL-10 expression in dendritic cells is involved in Trichosanthin-induced immunosuppression. Immunol. Lett. 2007, 110, 74–81. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Wei, H. Trichosanthin induced Th2 polarization status. Cell. Mol. Immunol. 2006, 3, 297–301. [Google Scholar]
- Zhao, J.; Ben, L.-H.; Wu, Y.-L.; Hu, W.; Ling, K.; Xin, S.-M.; Nie, H.-L.; Ma, L.; Pei, G. Anti-HIV Agent Trichosanthin Enhances the Capabilities of Chemokines to Stimulate Chemotaxis and G Protein Activation, and This Is Mediated through Interaction of Trichosanthin and Chemokine Receptors. J. Exp. Med. 1999, 190, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Sangro, B.; Chan, S.L.; Meyer, T.; Reig, M.; El-Khoueiry, A.; Galle, P.R. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J. Hepatol. 2020, 72, 320–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Cai, S.F.; Fehniger, T.A.; Song, J.; Collins, L.I.; Piwnica-Worms, D.R.; Ley, T.J. Granzyme B and Perforin Are Important for Regulatory T Cell-Mediated Suppression of Tumor Clearance. Immunity 2007, 27, 635–646. [Google Scholar] [CrossRef] [Green Version]
- Han, N.-R.; Kim, K.-C.; Kim, J.-S.; Ko, S.-G.; Park, H.-J.; Moon, P.-D. The immune-enhancing effects of a mixture of Astragalus membranaceus (Fisch.) Bunge, Angelica gigas Nakai, and Trichosanthes Kirilowii (Maxim.) or its active constituent nodakenin. J. Ethnopharmacol. 2022, 285, 114893. [Google Scholar] [CrossRef] [PubMed]
- Gocher, A.M.; Workman, C.J.; Vignali, D.A.A. Interferon-γ: Teammate or opponent in the tumour microenvironment? Nat. Rev. Immunol. 2022, 22, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Polesso, F.; Wang, C.; Sehrawat, A.; Hawkins, R.M.; Murray, S.E.; Thomas, G.V.; Caruso, B.; Thompson, R.F.; Wood, M.A.; et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature 2022, 606, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Shi, L.Z.; Zhao, H.; Chen, J.; Xiong, L.; He, Q.; Chen, T.; Roszik, J.; Bernatchez, C.; Woodman, S.E.; et al. Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell 2016, 167, 397–404.e9. [Google Scholar] [CrossRef] [Green Version]
- Angeli, J.P.F.; da Silva, T.N.X.; Schilling, B. CD8+ T cells PUF(A)ing the flames of cancer ferroptotic cell death. Cancer Cell 2022, 40, 346–348. [Google Scholar] [CrossRef]
- Shen, J.; Xiao, Z.; Zhao, Q.; Li, M.; Wu, X.; Zhang, L.; Hu, W.; Cho, C.H. Anti-cancer therapy with TNFα and IFNγ: A comprehensive review. Cell Prolif. 2018, 51, e12441. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Reza, I.; Díaz, L.; García-Becerra, R. Preclinical and clinical aspects of TNF-α and its receptors TNFR1 and TNFR2 in breast cancer. J. Biomed. Sci. 2017, 24, 90. [Google Scholar] [CrossRef] [Green Version]
- Henry, C.J.; Ornelles, D.A.; Mitchell, L.M.; Brzoza-Lewis, K.L.; Hiltbold, E.M. IL-12 Produced by Dendritic Cells Augments CD8+ T Cell Activation through the Production of the Chemokines CCL1 and CCL17. J. Immunol. 2008, 181, 8576–8584. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, H.; Hiroi, M.; Mori, K.; Ushio, R.; Matsumoto, A.; Yamamoto, N.; Shimada, J.; Ohmori, Y. Simultaneous Expression of Th1- and Treg-Associated Chemokine Genes and CD4+, CD8+, and Foxp3+ Cells in the Premalignant Lesions of 4NQO-Induced Mouse Tongue Tumorigenesis. Cancers 2021, 13, 1835. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y.; Xia, R.; Wei, Y.; Wei, X. Role of the CCL2-CCR2 signalling axis in cancer: Mechanisms and therapeutic targeting. Cell Prolif. 2021, 54, e13115. [Google Scholar] [CrossRef]
- Lança, T.; Silva-Santos, B. Recruitment of γδ T lymphocytes to tumors. Oncoimmunology 2013, 2, e25461. [Google Scholar] [CrossRef]
- Lança, T.; Costa, M.F.; Gonçalves-Sousa, N.; Rei, M.; Grosso, A.R.; Penido, C.; Silva-Santos, B. Protective Role of the Inflammatory CCR2/CCL2 Chemokine Pathway through Recruitment of Type 1 Cytotoxic γδ T Lymphocytes to Tumor Beds. J. Immunol. 2013, 190, 6673–6680. [Google Scholar] [CrossRef] [Green Version]
- Molon, B.; Ugel, S.; Del Pozzo, F.; Soldani, C.; Zilio, S.; Avella, D.; De Palma, A.; Mauri, P.; Monegal, A.; Rescigno, M.; et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 2011, 208, 1949–1962. [Google Scholar] [CrossRef]
- Rapp, M.; Grassmann, S.; Chaloupka, M.; Layritz, P.; Kruger, S.; Ormanns, S.; Rataj, F.; Janssen, K.-P.; Endres, S.; Anz, D.; et al. C-C chemokine receptor type-4 transduction of T cells enhances interaction with dendritic cells, tumor infiltration and therapeutic efficacy of adoptive T cell transfer. Oncoimmunology 2016, 5, e1105428. [Google Scholar] [CrossRef] [Green Version]
- Fialová, A.; Partlová, S.; Sojka, L.; Hromádková, H.; Brtnický, T.; Fučíková, J.; Kocián, P.; Rob, L.; Bartůňková, J.; Špíšek, R. Dynamics of T-cell infiltration during the course of ovarian cancer: The gradual shift from a Th17 effector cell response to a predominant infiltration by regulatory T-cells. Int. J. Cancer 2012, 132, 1070–1079. [Google Scholar] [CrossRef]
- Afonina, I.S.; Cullen, S.P.; Martin, S.J. Cytotoxic and non-cytotoxic roles of the CTL/NK protease granzyme B. Immunol. Rev. 2010, 235, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.A.; Xiang, J. mTORC1 regulates mannose-6-phosphate receptor transport and T-cell vulnerability to regulatory T cells by controlling kinesin KIF13A. Cell Discov. 2017, 3, 17011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, T.; Marchetti, P.; Susin, S.A.; Dallaporta, B.; Zamzami, N.; Marzo, I.; Geuskens, M.; Kroemer, G. The apoptosis-necrosis paradox. Apoptogenic proteases activated after mitochondrial permeability transition determine the mode of cell death. Oncogene 1997, 15, 1573–1581. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Wang, X.; Zhang, M.; Ying, Z.; Zhu, Z.; Tam, K.Y.; Li, C.; Zhou, G.; Gao, F.; Zeng, M.; et al. Trichosanthin Promotes Anti-Tumor Immunity through Mediating Chemokines and Granzyme B Secretion in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 1416. https://doi.org/10.3390/ijms24021416
Wang K, Wang X, Zhang M, Ying Z, Zhu Z, Tam KY, Li C, Zhou G, Gao F, Zeng M, et al. Trichosanthin Promotes Anti-Tumor Immunity through Mediating Chemokines and Granzyme B Secretion in Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2023; 24(2):1416. https://doi.org/10.3390/ijms24021416
Chicago/Turabian StyleWang, Kaifang, Xiaona Wang, Minghuan Zhang, Zhenguang Ying, Zeyao Zhu, Kin Yip Tam, Chunman Li, Guowei Zhou, Feng Gao, Meiqi Zeng, and et al. 2023. "Trichosanthin Promotes Anti-Tumor Immunity through Mediating Chemokines and Granzyme B Secretion in Hepatocellular Carcinoma" International Journal of Molecular Sciences 24, no. 2: 1416. https://doi.org/10.3390/ijms24021416






