Transcriptional and Physiological Analysis Reveal New Insights into the Regulation of Fertilization (N, P, K) on the Growth and Synthesis of Medicinal Components of Dendrobium denneanum
Abstract
:1. Introduction
2. Results
2.1. Effects of N, P, and K on the Morphological Characteristics
2.2. Effects of N, P, and K on the Physiological Characteristics of D. denneanum
2.3. Effects of N, P, and K on the Main Medicinal Compounds
2.4. Correlation Analysis
2.5. Principal Component Analysis
2.6. Transcriptome Sequencing Results and Data Assembly
2.7. Differential Gene Analysis Expression of D. denneanum
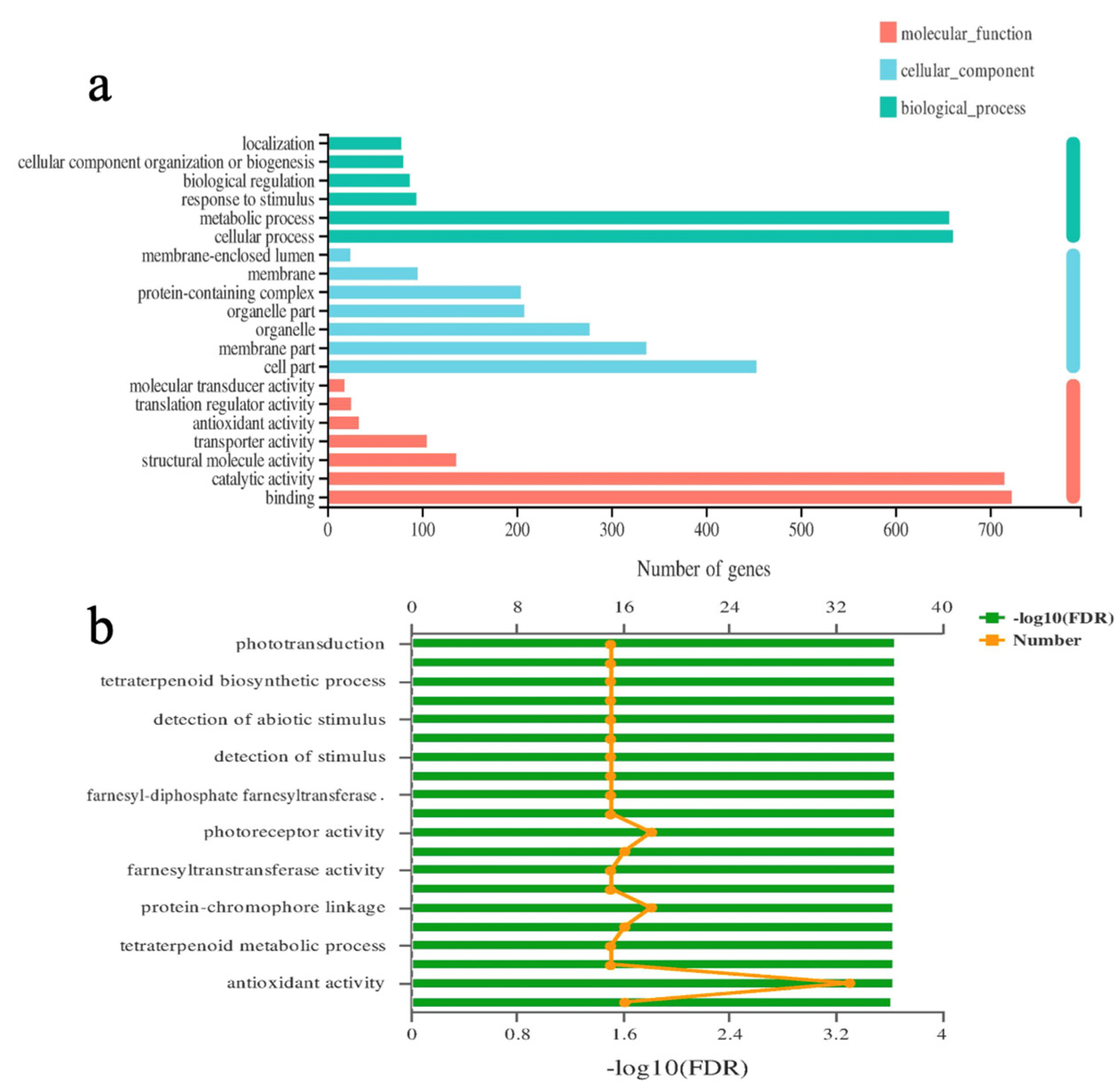
2.8. GO Functional Enrichment Analysis
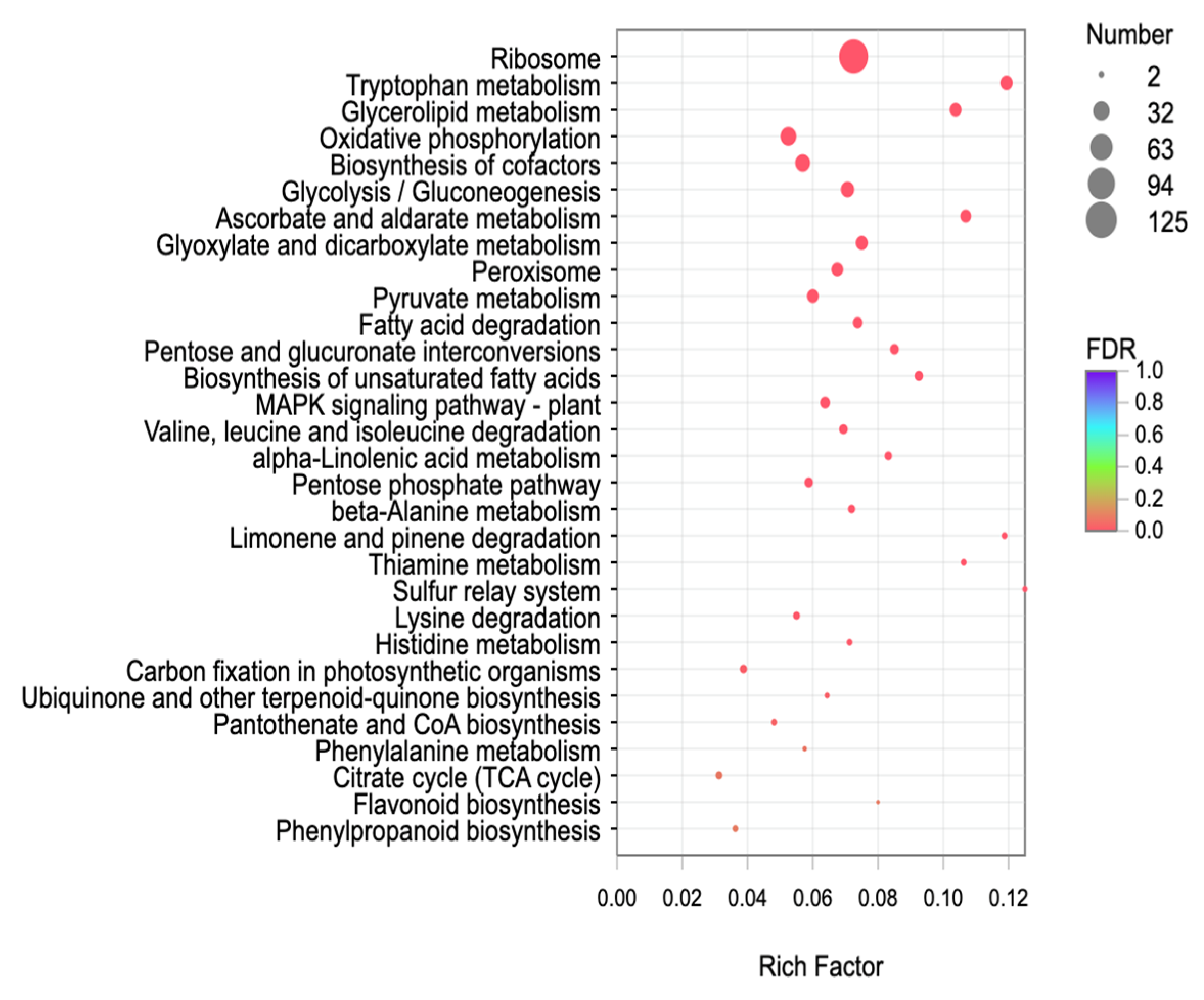
2.9. KEGG Functional Enrichment Analysis
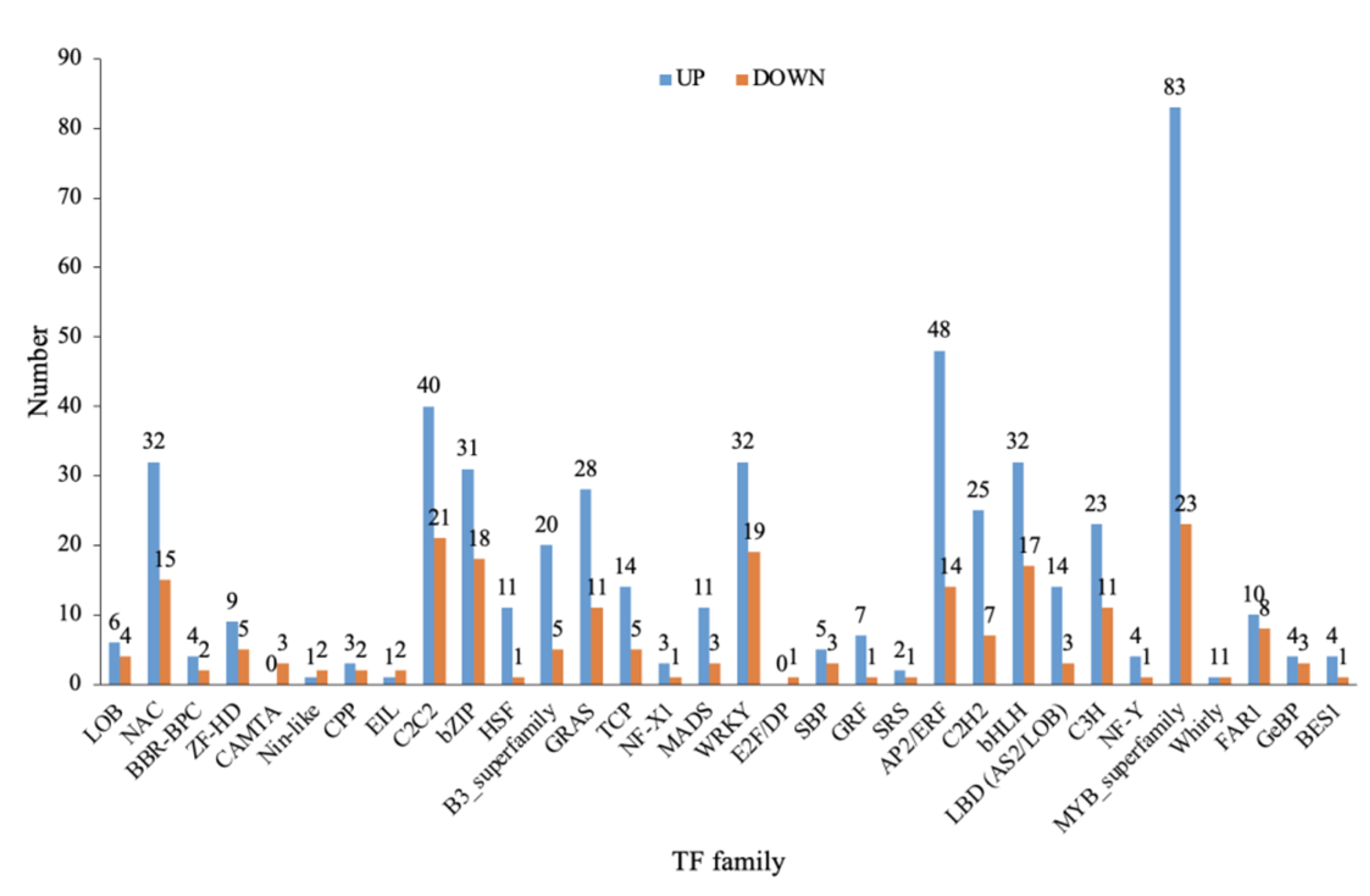
2.10. Transcription Factor Classification of Differentially Expressed Genes
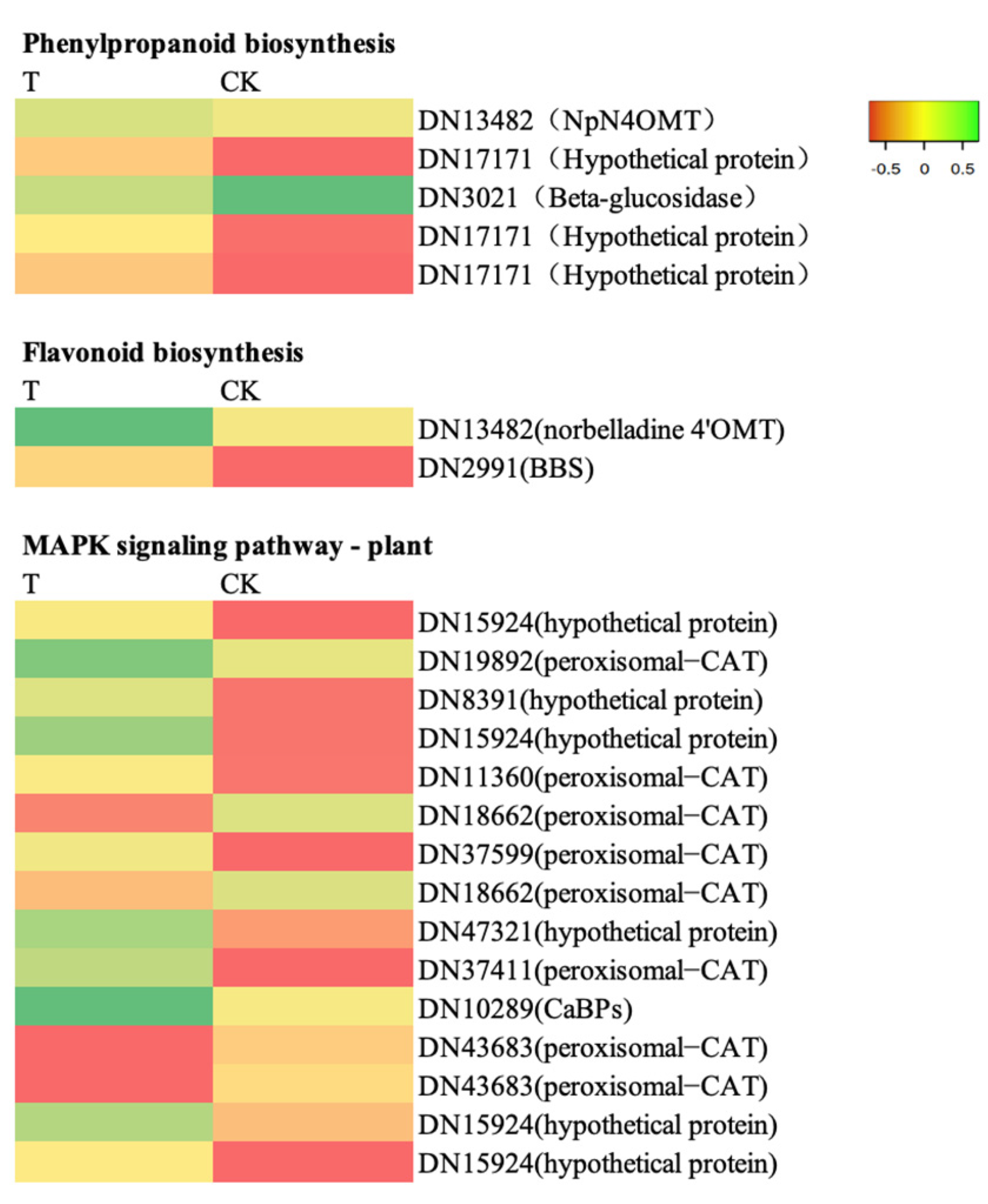
2.11. Key Gene Analysis
2.12. RT-qPCR Analysis
3. Discussion
3.1. Effects of N, P, and K on the Growth and Main Medicinal Compounds of D. denneanum
3.2. Effects of T9 Treatment on Gene Expression and Biosynthesis Pathways
3.3. Effect of T9 Treatment on Transcription Factors
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Determination of Morphological Indices
4.3. Determination of Physiological Indices and the Contents of the Main Medicinal Compounds
4.4. Illumina Sequencing
4.5. Transcriptome Data Processing and Analysis
4.6. qRT-PCR
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, D.Y.; Wang, X.J.; Li, T.Q.; Li, N.Q.; Gao, J.Y. In situ seedling baiting to isolate plant growth-promoting fungi from Dendrobium officinale, an over-collected medicinal orchid in China. Glob. Ecol. Conserv. 2021, 28, e01659. [Google Scholar] [CrossRef]
- He, L.; Su, Q.; Bai, L.; Li, M.F.; Liu, J.R.; Liu, X.M.; Zhang, C.Y.; Jiang, Z.L.; He, J.; Shi, J.Y.; et al. Recent research progress on natural small molecule bibenzyls and its derivatives in Dendrobium species. Eur. J. Med. Chem. 2020, 204, 112530. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, N.; Wang, P.; Liu, J.; Sun, J.; Ye, W.; Fan, B. Flavonoid biosynthesis in four Dendrobium species based on transcriptome sequencing and metabolite analysis. Mol. Biol. Rep. 2022, 49, 2047–2057. [Google Scholar] [CrossRef]
- Luo, A.X.; Ge, Z.F.; Fan, Y.J.; Luo, A.S.; Chun, Z.; He, X.J. In Vitro and In Vivo Antioxidant Activity of a Water-Soluble Polysaccharide from Dendrobium denneanum. Molecules 2011, 16, 1579–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Pan, H.M.; Liu, X.; Chen, B.; Tang, Y.X.; Xi, X.J.; Wang, M.K. New phenanthrene glycosides from Dendrobium denneanum and their cytotoxic activity. Phytochem. Lett. 2013, 6, 640–644. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, F.; Yang, L.J.; Chun, Z.; Bao, J.K.; Zhang, G.L. Anti-inflammatory phenanthrene derivatives from stems of Dendrobium denneanum. Phytochemistry 2013, 95, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Z.T.; Xu, L.S. Phenols and a triterpene from Dendrobium aurantiacum var. denneanum (Orchidaceae). Biochem. Syst. Ecol. 2006, 34, 658–660. [Google Scholar] [CrossRef]
- Fan, Y.J.; Chun, Z.; Luo, A.X.; Luo, A.S.; He, T.; He, X.J. In Vivo Immunomodulatory Activities of Neutral Polysaccharide (DDP1-1) from Dendrobium denneanum. Chin. J. Appl. Environ. Biol. 2010, 16, 376–379. [Google Scholar] [CrossRef]
- Luo, A.S.; Chun, Z.; Ge, S.R.; Luo, A.X.; Fan, Y.J.; Liu, P.; Peng, W.J. Study on hypoglycemic effect of Dendrobium Denneanum polysaccharide. Chin. J. Appl. Environ. Biol. 2006, 12, 334–337. [Google Scholar] [CrossRef]
- Fan, Y.J.; Tan, X.Y.; Zhao, J.; Yang, K.G.; Wu, S.D.; Zhang, J.; Pu, S.R.; Wang, G.; Luo, A.X. Screening and characterization of an acid polysaccharide with antioxidant activity In Vitro and In Vivo from Dendrobium aurantiacum var. denneanum (Kerr). Phcog. Mag. 2022, 18, 44–51. [Google Scholar]
- Yang, L.; Han, H.; Nakamura, N.; Hattori, M.; Wang, Z.; Xu, L. Bio-guided isolation of antioxidants from the stems of Dendrobium aurantiacum var. denneanum. Phytother. Res. 2007, 21, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Li, Y.; Liu, J.; Zhao, T.M.; Zhao, R.X.; Zheng, S.G.; Chun, Z.; Hu, Y.D. Quantitative proteomics of inhibitory mechanism of Dendrobium denneanum ether extract on lung cancer cells. Zhongguo Zhong Yao Za Zhi 2019, 44, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Hu, X.H.; Wang, M.L.; Wan, W.C.; Yang, Q.H.; Sun, X.S.; Gu, Q.; Gao, X.X.; Wang, Z.T.; Gu, L.Q.; et al. Anti-osmotic and antioxidant activities of gigantol from Dendrobium aurantiacum var. denneanum against cataractogenesis in galactosemic rats. J. Ethnopharmacol. 2015, 172, 238–246. [Google Scholar] [PubMed]
- Li, X.B.; Fan, Y.J.; Ma, J.; Gao, X.Y.; Wang, G.; Wu, S.D.; Liu, Y.Y.; Yang, K.G.; Xu, E.Y.; Pu, S.R.; et al. Cerium improves the physiology and medicinal components of Dendrobium nobile Lindl. under copper stress. J. Plant Physiol. 2023, 280, 153896. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.J.; Yang, K.G.; Miao, R.S.; Wang, G.; Chun, Z.; Wu, S.D.; Pu, S.R.; Luo, A.X. Transcriptome analysis reveals the effects of red and blue light on the physiological and medicinal components of Dendrobium denneanum. Ind. Crop Prod. 2022, 180, 114798. [Google Scholar] [CrossRef]
- Ye, T.H.; Li, Y.W.; Zhang, J.L.; Hou, W.F.; Zhou, W.F.; Lu, J.W.; Xing, Y.Z.; Li, X.K. Nitrogen, phosphorus, and potassium fertilization affects the flowering time of rice (Oryza sativa L.). Glob. Ecol. Conserv. 2019, 20, e00753. [Google Scholar] [CrossRef]
- Viola, M.; Christa, L.; Benno, F.Z.; Georg, N.; Mauricio, H. Centelloside accumulation in leaves of Centella asiatica is determined by resource partitioning between primary and secondary metabolism while influenced by supply levels of either nitrogen, phosphorus or potassium. J. Plant Physiol. 2013, 170, 1165–1175. [Google Scholar]
- Sung, J.; Lee, S.; Lee, Y.; Ha, S.; Song, B.; Kim, T.; Waters, B.M.; Krishnan, H.B. Metabolomic profiling from leaves and roots of tomato (Solanum lycopersicum L.) plants grown under nitrogen, phosphorus or potassium-deficient condition. Plant Sci. 2015, 241, 55–64. [Google Scholar] [CrossRef]
- Deng, Q.L.; Li, S.J.; Wen, Q.S.; Lei, F.Y.; Cheng, Y.; Zhou, J.; Liu, Z.; Cheng, X.F. Optimal combination ratio of NPK and organic fertilizers for highest saponin and flavonoid contents in Ophiopogon japonicas. J. Plant Nutr. Fertil. 2021, 27, 1477–1486. [Google Scholar] [CrossRef]
- Tang, S.; Qi, Z. Study on the nutrition properties of dendrobium and the fertilization technique. Acta Hortic. Sin. 1999, 26, 184–187. [Google Scholar] [CrossRef]
- Yan, C.S.; Li, D.Q.; Zhang, J.H. Plant leaf senescence and oxidative stress. Bull. Bot. 1999, 4, 398–404. [Google Scholar]
- Li, T.T.; Chun, Z.; Luo, A.X.; Xiang, H.B.; Guo, L.; Zhang, L.; Chen, S.T.; Fan, Y.J. Effects of LED light quality on physiology and quality of Dendrobium denneanum. Chin. J. Appl. Environ. Biol. 2017, 23, 934–938. [Google Scholar] [CrossRef]
- Wang, Y.L.; Huang, L.Q.; Yuan, Y.; Cha, L.P. Research advances on analysis of medicinal plants transcriptome. China J. Chin. Mater. Med. 2015, 40, 2055–2061. [Google Scholar]
- Li, Q.; Ding, G.; Li, B.; Guo, S.X. Transcriptome analysis of genes involved in dendrobine biosynthesis in Dendrobium nobilelindl infected with mycorrhizal fungus MF23 (Mycenasp.). Sci. Rep. 2017, 7, 316. [Google Scholar] [CrossRef]
- Wen, Z.Z.; Guo, W.Z.; Li, J.C.; Lin, H.S.; He, C.M.; Liu, Y.G.; Zhang, Q.Y.; Liu, W. Comparative transcriptomic analysis of vernalization- and cytokinin-induced floral transition in Dendrobium nobile. Sci. Rep. 2017, 7, 45748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franssen, S.U.; Shrestha, R.P.; Bräutigam, A.; Bornberg-Bauer, E.; Weber, A.P. Comprehensive transcriptome analysis of the highly complex Pisum sativum genome using next generation sequencing. BMC Genom. 2011, 12, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Shao, Y.; Ge, S.; Zhang, M.; Zhang, T.; Hu, X.; Liu, Y.; Walker, J.; Zhang, S.; Xu, J. A MAPK cascade downstream of IDA-HAE/HSL2 ligand-receptor pair in lateral root emergence. Nat. Plants 2019, 5, 414–423. [Google Scholar] [CrossRef]
- Luo, J.; Butelli, E.; Hill, L.; Parr, A.; Niggeweg, R.; Bailey, P.; Weisshaar, B.; Martin, C. AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: Expression in fruit results in very high levels of both types of polyphenol. Plant J. 2008, 56, 316–326. [Google Scholar] [CrossRef]
- Yamagishi, M. A novel R2R3-MYB transcription factor regulates light-mediated floral and vegetative anthocyanin pigmentation patterns in lilium regale. Mol. Breed 2016, 36, 3. [Google Scholar] [CrossRef]
- Ma, R.F.; Xiao, Y.; Lv, Z.Y.; Tan, H.X.; Chen, R.B.; Li, Q.; Chen, J.; Wang, Y.; Yin, J.; Zhang, L.; et al. AP2/ERF transcription factor, li049, positively regulates lignan biosynthesis in Isatis indigotica through activating salicylic acid signaling and lignan/lignin pathway genes. Front. Plant Sci. 2017, 8, 1361–1377. [Google Scholar] [CrossRef] [Green Version]
- Li, J.J.; Wang, L.; Javed, H.U.; Zahid, M.S.; Wu, Z.S.; Ma, C.; Jiu, S.T.; Xu, W.P.; Zhang, C.X.; Wang, S.P. Nutrient solution with high nitrogen content, a suitable facilitator of growth and berry quality in hydroponic ‘Shine Muscat’ grapevine (Vitis vinifera × V. labrusca). Sci. Hortic. 2023, 310, 111749. [Google Scholar] [CrossRef]
- Liu, H.; Fu, Y.M.; Hu, D.W.; Yu, J.; Liu, H. Effect of green, yellow and purple radiation on biomass, photosynthesis, morphology and soluble sugar content of leafy lettuce via spectral wavebands “knock out”. Sci. Hortic. 2018, 236, 10–17. [Google Scholar] [CrossRef]
- Senuma, M.; Dobashi, S.; Bando, Y.; Ko, S.; Shiota, H. Overexpression of eelgrass Rare Cold Inducible 2 (RCI2) maintains chlorophyll content in Arabidopsis subjected to high salinity and dehydration. Plant Stress 2022, 6, 100116. [Google Scholar] [CrossRef]
- Milosevic, N.; Slusarenko, A.J. Active oxygen metabolism and lignification in the hypersensitive response in bean. Physiol. Mol. Plant Pathol. 1996, 49, 143–158. [Google Scholar] [CrossRef]
- Gao, S.S.; Wang, Y.L.; Yu, S.; Huang, Y.Q.; Liu, H.C.; Chen, W.; He, X.Y. Effects of drought stress on growth, physiology and secondary metabolites of Two Adonis species in Northeast China. Sci. Hortic. 2020, 259, 108795. [Google Scholar] [CrossRef]
- Willekens, H.; Chamnongpol, S.; Davey, M.; Schraudner, M.; Langebartels, C.; Van-Montagu, M.; Inze, D.; VanCamp, W. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J. 1997, 16, 4806–4816. [Google Scholar] [CrossRef] [Green Version]
- Quy, D.D.; Artik, E.A.; Phuong, L.T.; Lien, H.H.; Felycia, E.S.; Suryadi, I.; Yi, H.J. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar]
- Luo, A.X.; He, X.J.; Zhou, S.D.; Fan, Y.J.; Luo, A.S.; Chun, Z. Purification, composition analysis and antioxidant activity of the polysaccharides from Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 79, 1014–1019. [Google Scholar] [CrossRef]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.; Terol, J.; Talon, M.; Robles, M. Blast2go: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Quevillon, E.; Silventoinen, V.; Pillai, S.; Harte, N.; Mulder, N.; Apweiler, R.; Lopez, R. InterProScan: Protein domains identifier. Nucleic Acids Res. 2005, 33, 116–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using diamond. Nat Methods 2015, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
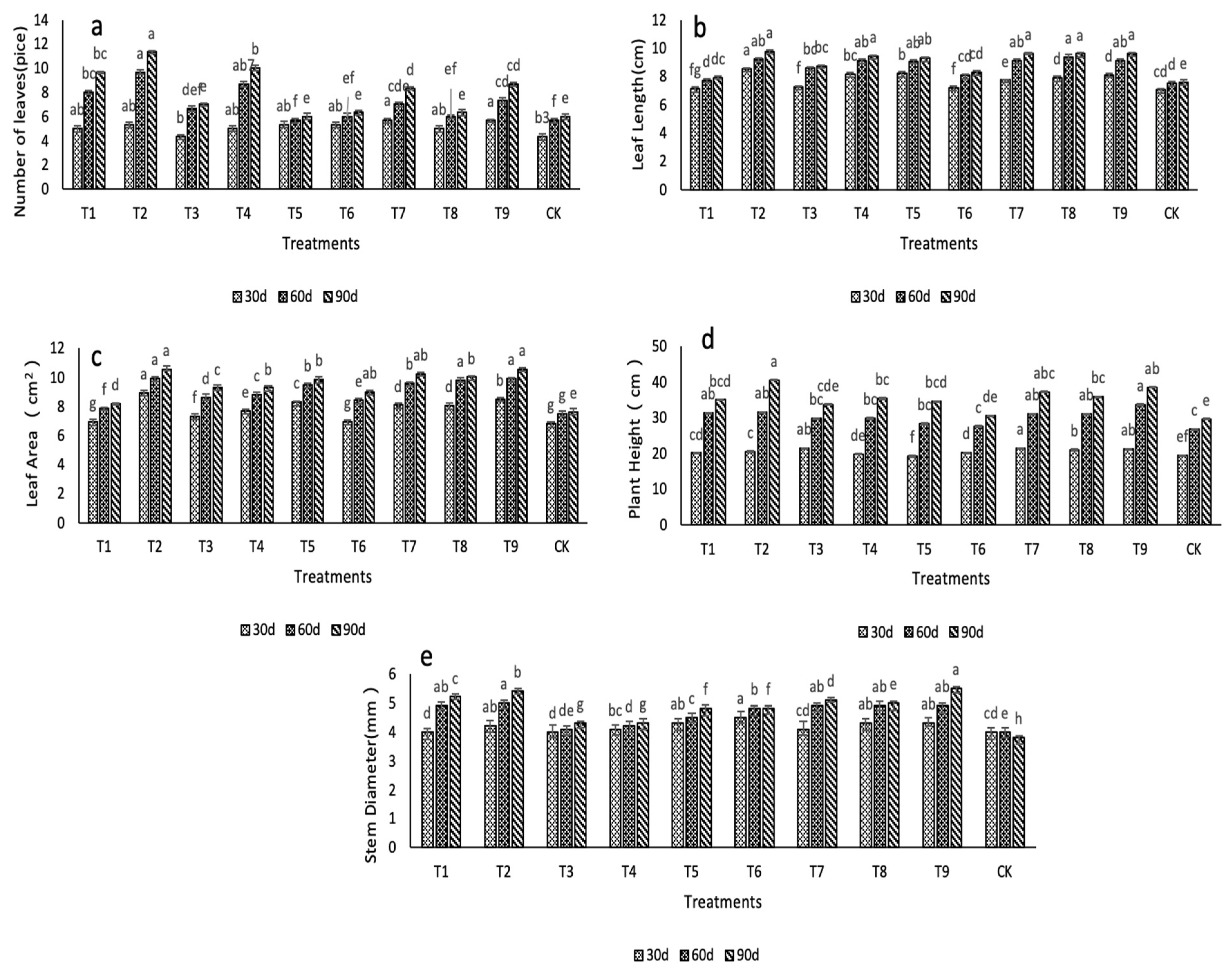
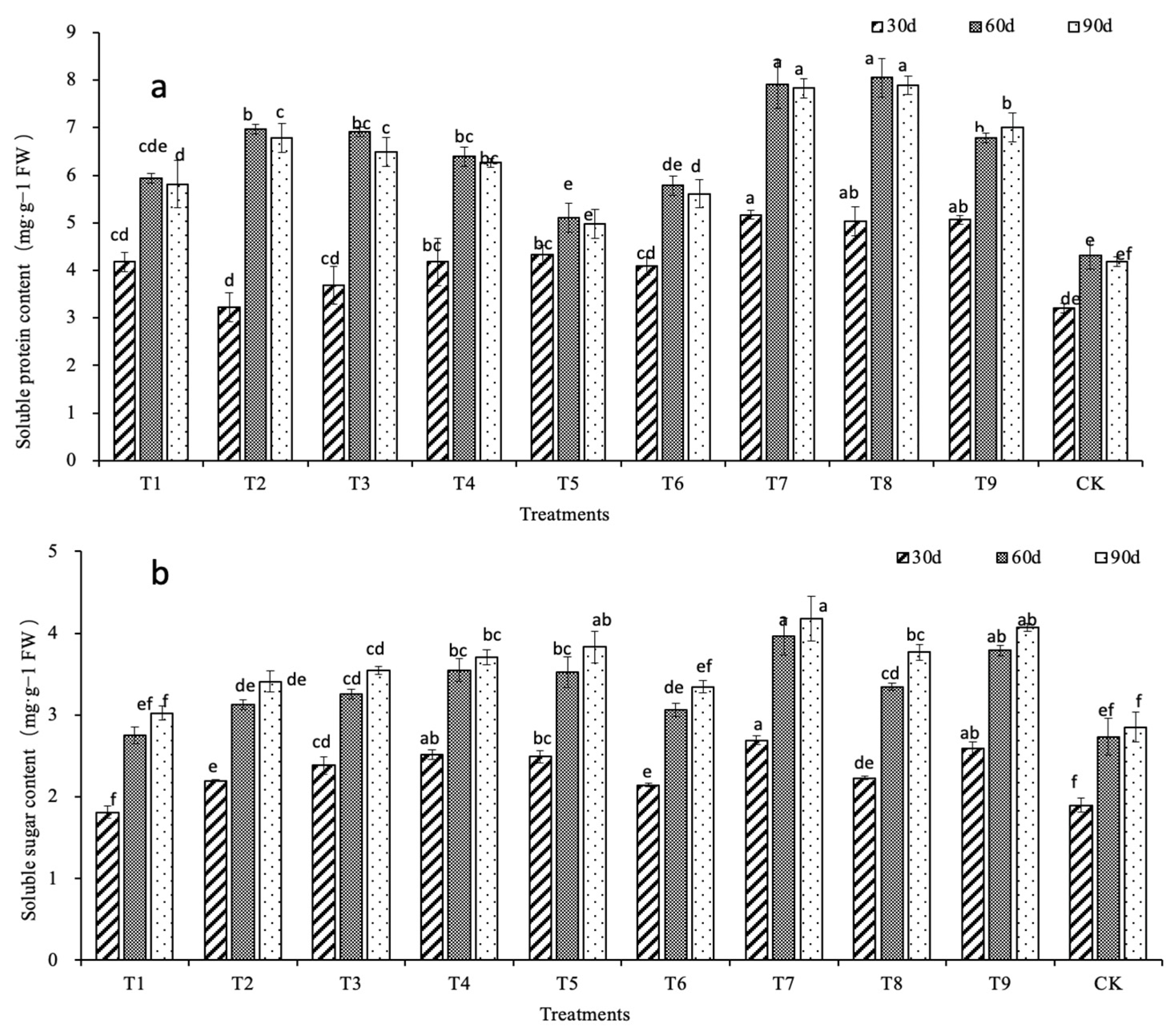
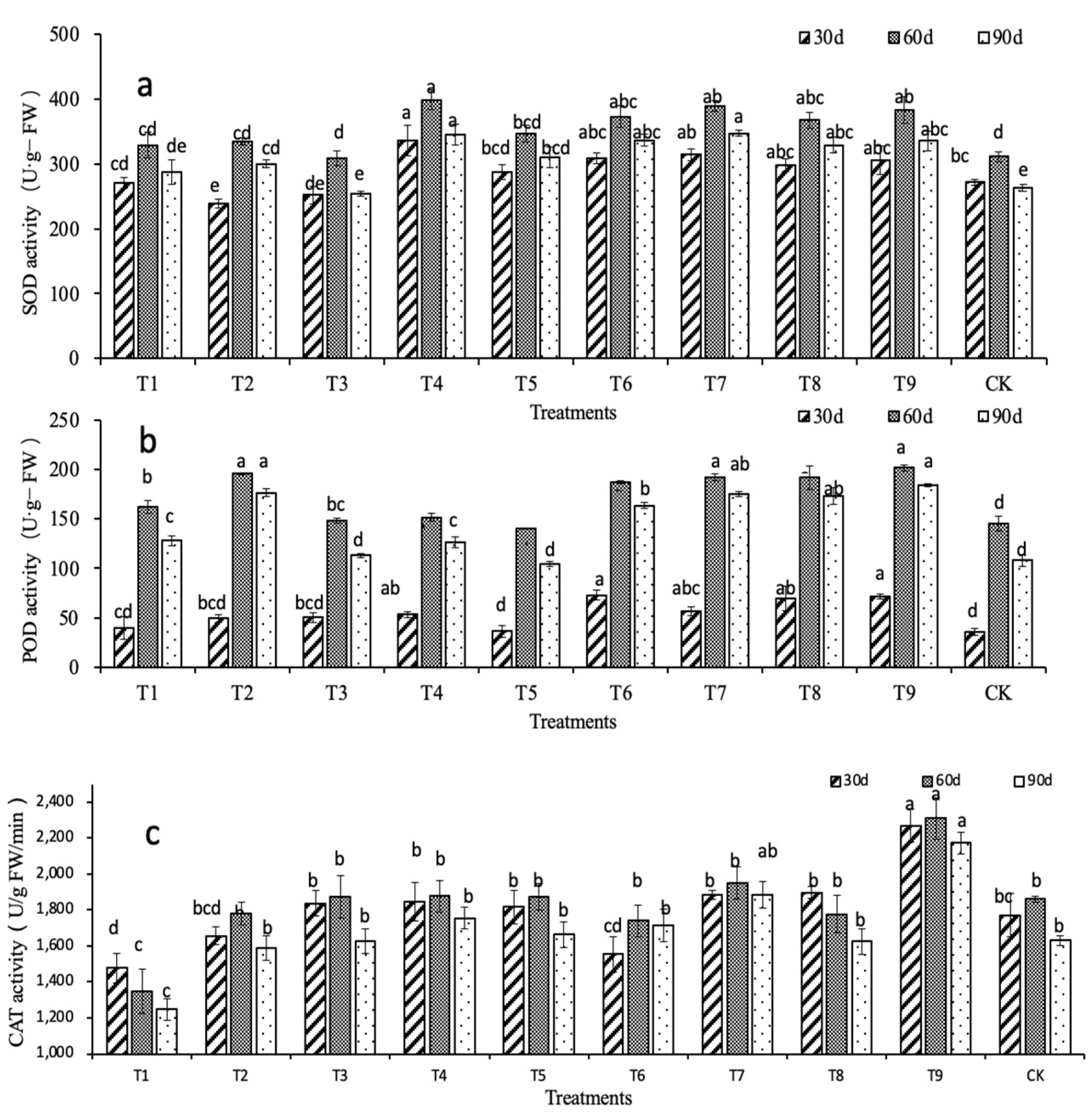
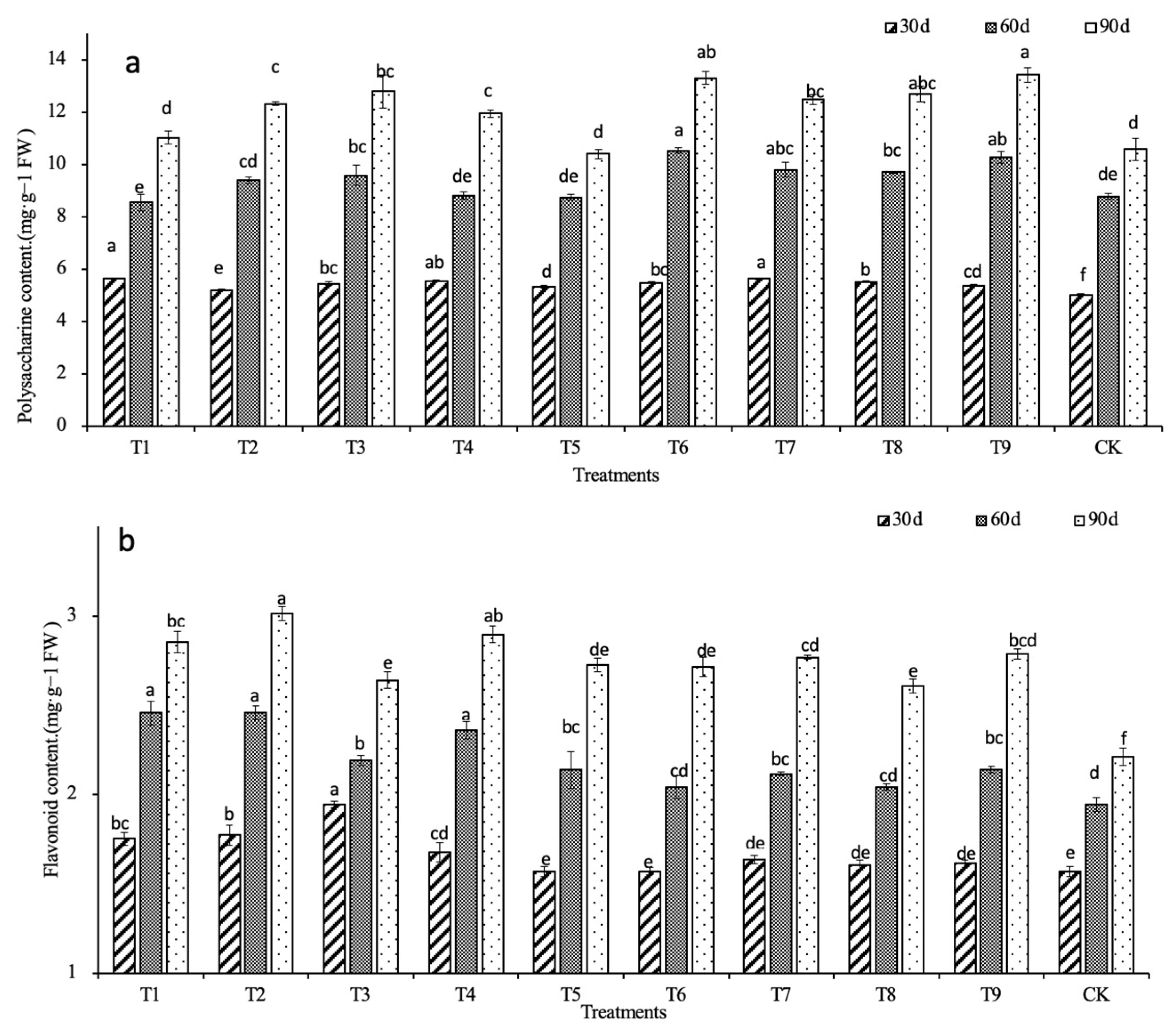

| Chlorophyll | Treatment | 30 d | 60 d | 90 d |
|---|---|---|---|---|
| (mg·g−1 FW) | (mg·g−1 FW) | (mg·g−1 FW) | ||
| Chl. A | T1 | 0.46 ± 0.01 de | 0.75 ± 0.01 d | 0.82 ± 0.03 c |
| T2 | 0.51 ± 0.02 c | 0.83 ± 0.02 b | 0.89 ± 0.01 a | |
| T3 | 0.43 ± 0.02 f | 0.75 ± 0.01 d | 0.78 ± 0.03 d | |
| T4 | 0.49 ± 0.02 cd | 0.77 ± 0.01 c | 0.84 ± 0.01 c | |
| T5 | 0.52 ± 0.01 bc | 0.81 ± 0.02 b | 0.86 ± 0.02 b | |
| T6 | 0.49 ± 0.04 bc | 0.75 ± 0.03 d | 0.79 ± 0.02 d | |
| T7 | 0.57 ± 0.02 a | 0.86 ± 0.01 a | 0.90 ± 0.03 a | |
| T8 | 0.55 ± 0.02 ab | 0.85 ± 0.02 a | 0.91 ± 0.02 a | |
| T9 | 0.56 ± 0.03 a | 0.87 ± 0.02 a | 0.90 ± 0.04 a | |
| CK | 0.45 ± 0.02 ef | 0.72 ± 0.03 e | 0.74 ± 0.05 e | |
| Chl. B | T1 | 0.24 ± 0.02 de | 0.38 ± 0.01 f | 0.40 ± 0.02 e |
| T2 | 0.26 ± 0.03 b | 0.45 ± 0.02 ab | 0.46 ± 0.01 ab | |
| T3 | 0.22 ± 0.02 f | 0.39 ± 0.01 f | 0.42 ± 0.04 cde | |
| T4 | 0.25 ± 0.01 bc | 0.43 ± 0.02 ab | 0.44 ± 0.02 bc | |
| T5 | 0.30 ± 0.02 a | 0.41 ± 0.01 cd | 0.43 ± 0.03 cd | |
| T6 | 0.25 ± 0.01 cd | 0.41 ± 0.02 de | 0.42 ± 0.01 cde | |
| T7 | 0.29 ± 0.03 a | 0.45 ± 0.01 a | 0.44 ± 0.05 bc | |
| T8 | 0.29 ± 0.02 a | 0.43 ± 0.01 bc | 0.47 ± 0.03 a | |
| T9 | 0.30 ± 0.02 a | 0.44 ± 0.02 ab | 0.46 ± 0.02 ab | |
| CK | 0.23 ± 0.02 ef | 0.39 ± 0.02 ef | 0.40 ± 0.02 e |
| Number of Blades | Maximum Leaf Area | Plant Height | Stem Diameter | Chl. A | Chl. B | Soluble Protein | Soluble Sugar | SOD | POD | CAT | Polysaccharides | Flavonoids | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of blades | 1 | ||||||||||||
| Maximum leaf area | 0.320 | 1 | |||||||||||
| Plant height | 0.720 * | 0.847 ** | 1 | ||||||||||
| Stem diameter | 0.472 | 0.67 1* | 0.772 ** | 1 | |||||||||
| Chl. A | 0.398 | 0.898 ** | 0.878 ** | 0.805** | 1 | ||||||||
| Chl. B | 0.331 | 0.894 ** | 0.776 ** | 0.578 | 0.865 ** | 1 | |||||||
| Soluble protein | 0.290 | 0.708 * | 0.656 * | 0.537 | 0.737 * | 0.734 * | 1 | ||||||
| Soluble sugar | 0.046 | 0.832 ** | 0.569 | 0.440 | 0.755 * | 0.646 * | 0.676 * | 1 | |||||
| SOD | 0.179 | 0.504 | 0.360 | 0.458 | 0.640 * | 0.531 | 0.505 | 0.670 * | 1 | ||||
| POD | 0.331 | 0.638* | 0.599 | 0.745 * | 0.722 * | 0.724 * | 0.749 * | 0.468 | 0.624 | 1 | |||
| CAT | −0.066 | 0.556 | 0.266 | 0.179 | 0.430 | 0.473 | 0.353 | 0.719 * | 0.537 | 0.471 | 1 | ||
| Polysaccharides | 0.168 | 0.511 | 0.342 | 0.476 | 0.447 | 0.582 | 0.693* | 0.476 | 0.559 | 0.835 ** | 0.577 | 1 | |
| Flavonoids | 0.776 ** | 0.579 | 0.748 * | 0.692 * | 0.594 | 0.472 | 0.403 | 0.390 | 0.463 | 0.406 | 0.021 | 0.335 | 1 |
| Treatments | Components | Comprehensive Score | Ranking | |
|---|---|---|---|---|
| 1 | 2 | |||
| T1 | −1.004 | −1.736 | −0.834 | 9 |
| T2 | 0.527 | −1.367 | 0.151 | 4 |
| T3 | −0.634 | 0.460 | −0.331 | 7 |
| T4 | 0.233 | −0.281 | 0.108 | 5 |
| T5 | −0.485 | −0.320 | −0.338 | 8 |
| T6 | −0.073 | 0.688 | 0.0421 | 6 |
| T7 | 1.070 | 0.254 | 0.688 | 2 |
| T8 | 0.899 | −0.211 | 0.524 | 3 |
| T9 | 1.301 | 1.197 | 0.949 | 1 |
| CK | −1.834 | 1.312 | −0.959 | 10 |
| Treatment | N(CH4N2O) mg·L−1 | P(Ca(H2PO4)2·H2O) mg·L−1 | K(K2SO4) mg·L−1 |
|---|---|---|---|
| T1 (N1P1K1) | 500 | 1000 | 300 |
| T2 (N1P2K2) | 500 | 2000 | 500 |
| T3 (N1P3K3) | 500 | 3000 | 700 |
| T4 (N2P1K2) | 1000 | 1000 | 500 |
| T5 (N2P2K3) | 1000 | 2000 | 700 |
| T6 (N2P3K1) | 1000 | 3000 | 300 |
| T7 (N3P1K3) | 1500 | 1000 | 700 |
| T8 (N3P2K1) | 1500 | 2000 | 300 |
| T9 (N3P3K2) | 1500 | 3000 | 500 |
| CK | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Xu, E.; Wang, G.; He, D.; Ma, J.; Liu, Y.; Li, X.; Luo, A. Transcriptional and Physiological Analysis Reveal New Insights into the Regulation of Fertilization (N, P, K) on the Growth and Synthesis of Medicinal Components of Dendrobium denneanum. Int. J. Mol. Sci. 2023, 24, 1522. https://doi.org/10.3390/ijms24021522
Fan Y, Xu E, Wang G, He D, Ma J, Liu Y, Li X, Luo A. Transcriptional and Physiological Analysis Reveal New Insights into the Regulation of Fertilization (N, P, K) on the Growth and Synthesis of Medicinal Components of Dendrobium denneanum. International Journal of Molecular Sciences. 2023; 24(2):1522. https://doi.org/10.3390/ijms24021522
Chicago/Turabian StyleFan, Yijun, Erya Xu, Gang Wang, Dingxin He, Jie Ma, Yuanyuan Liu, Xuebing Li, and Aoxue Luo. 2023. "Transcriptional and Physiological Analysis Reveal New Insights into the Regulation of Fertilization (N, P, K) on the Growth and Synthesis of Medicinal Components of Dendrobium denneanum" International Journal of Molecular Sciences 24, no. 2: 1522. https://doi.org/10.3390/ijms24021522





