Analysis of the Toll and Spaetzle Genes Involved in Toll Pathway-Dependent Antimicrobial Gene Induction in the Red Flour Beetle, Tribolium castaneum (Coleoptera; Tenebrionidae)
Abstract
1. Introduction
2. Results
2.1. Functional Assay of Toll Genes
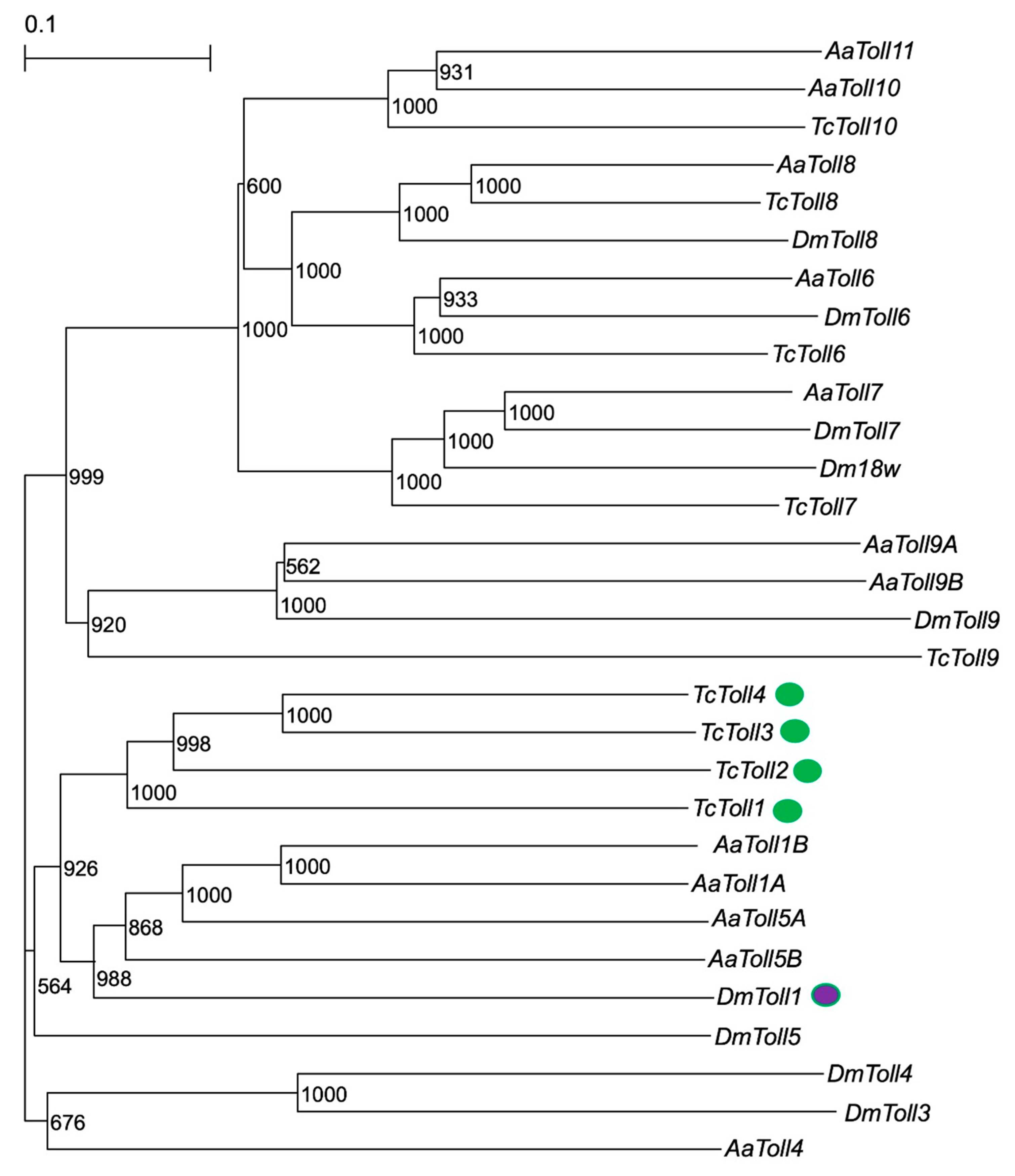
2.1.1. Phylogenetic Analysis of Toll Genes
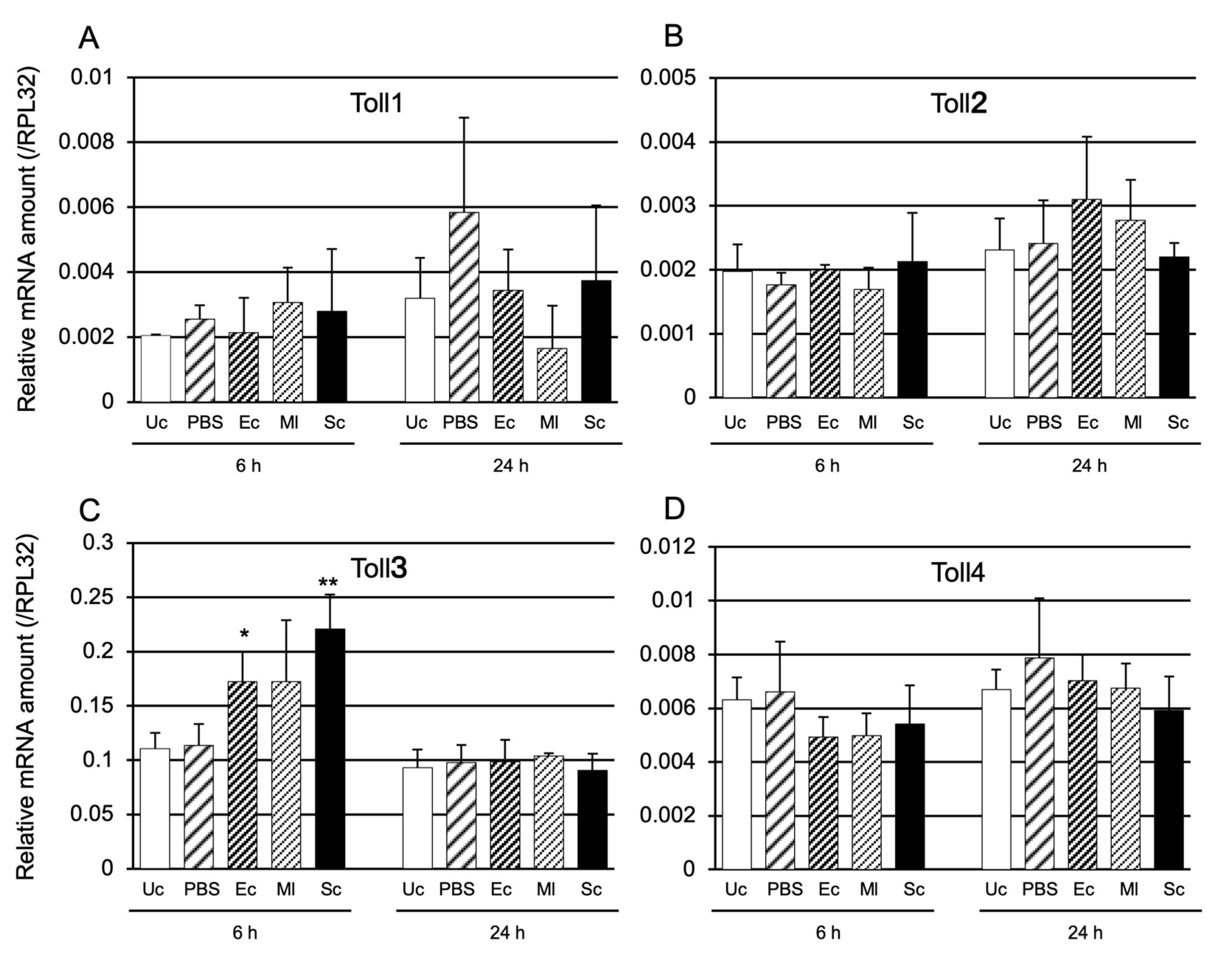
2.1.2. Toll Gene Induction by Microbial Challenge
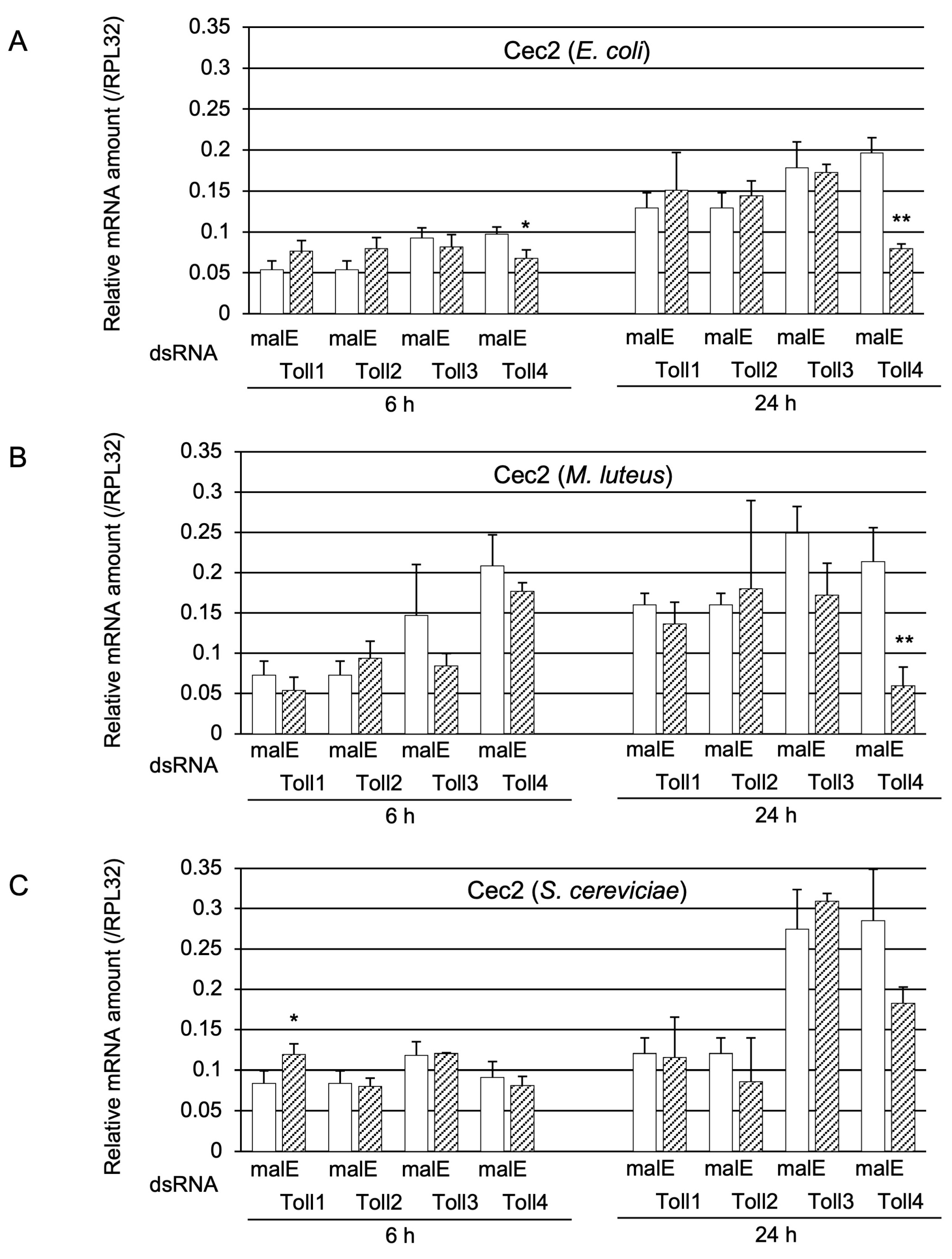
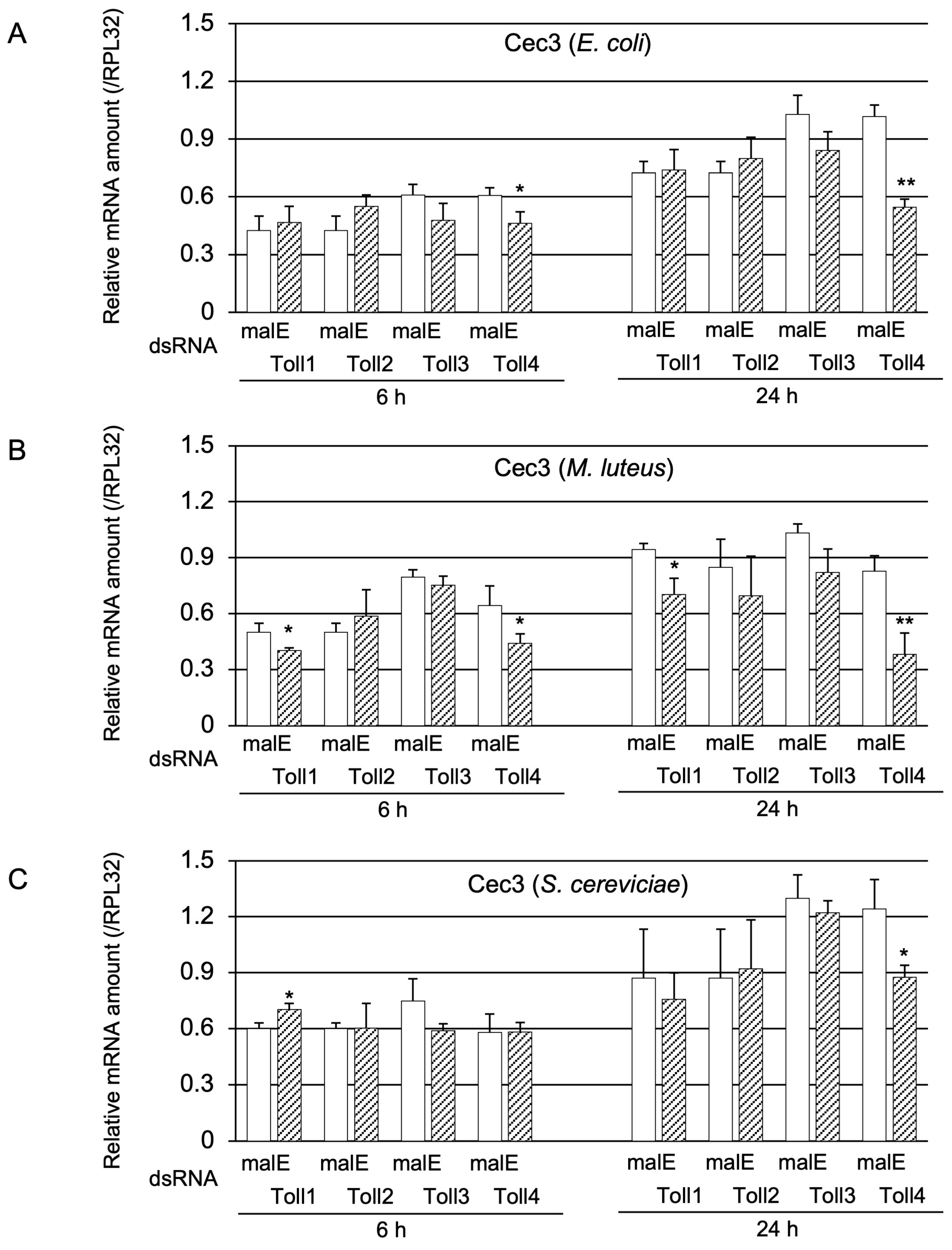
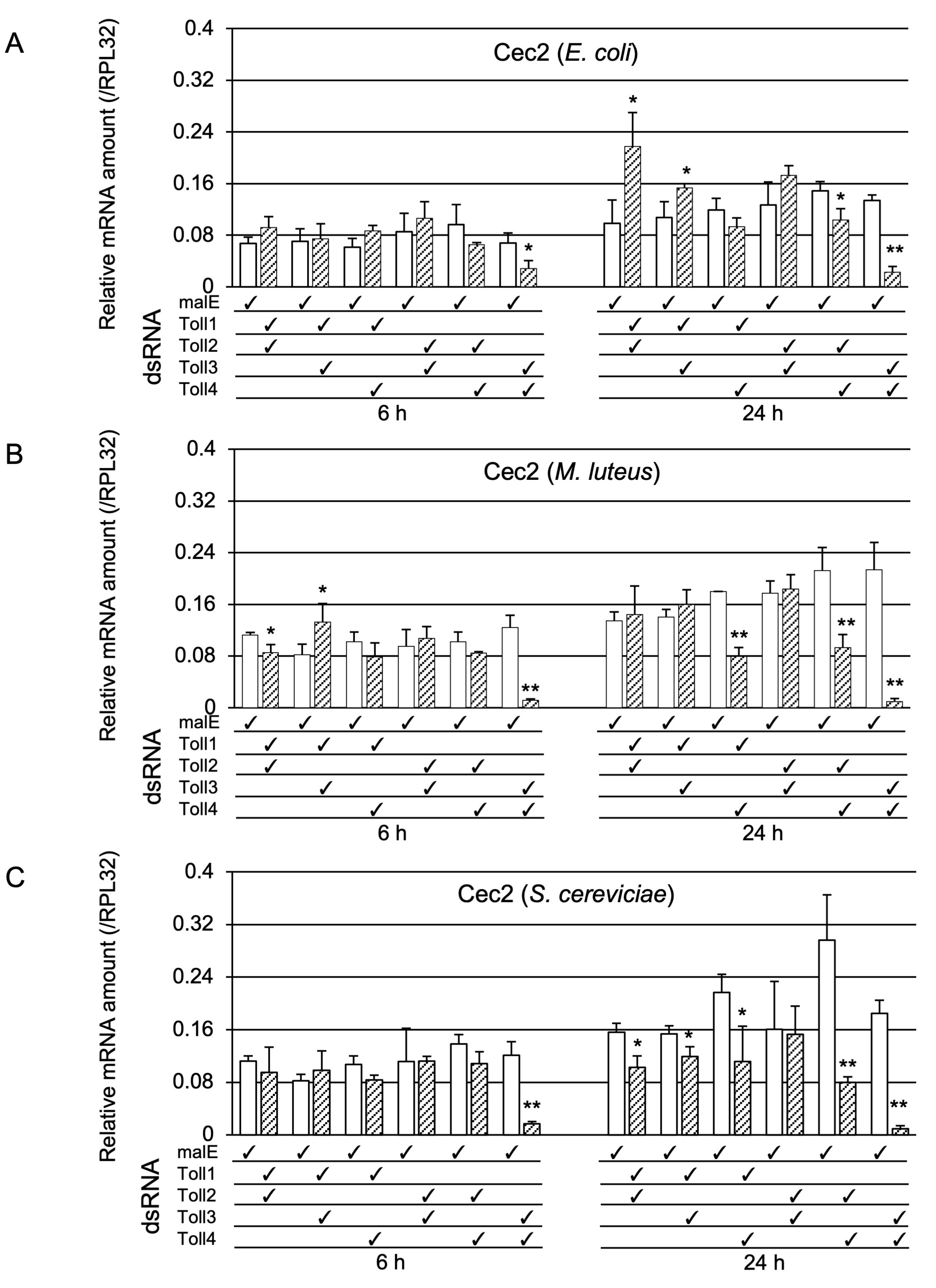
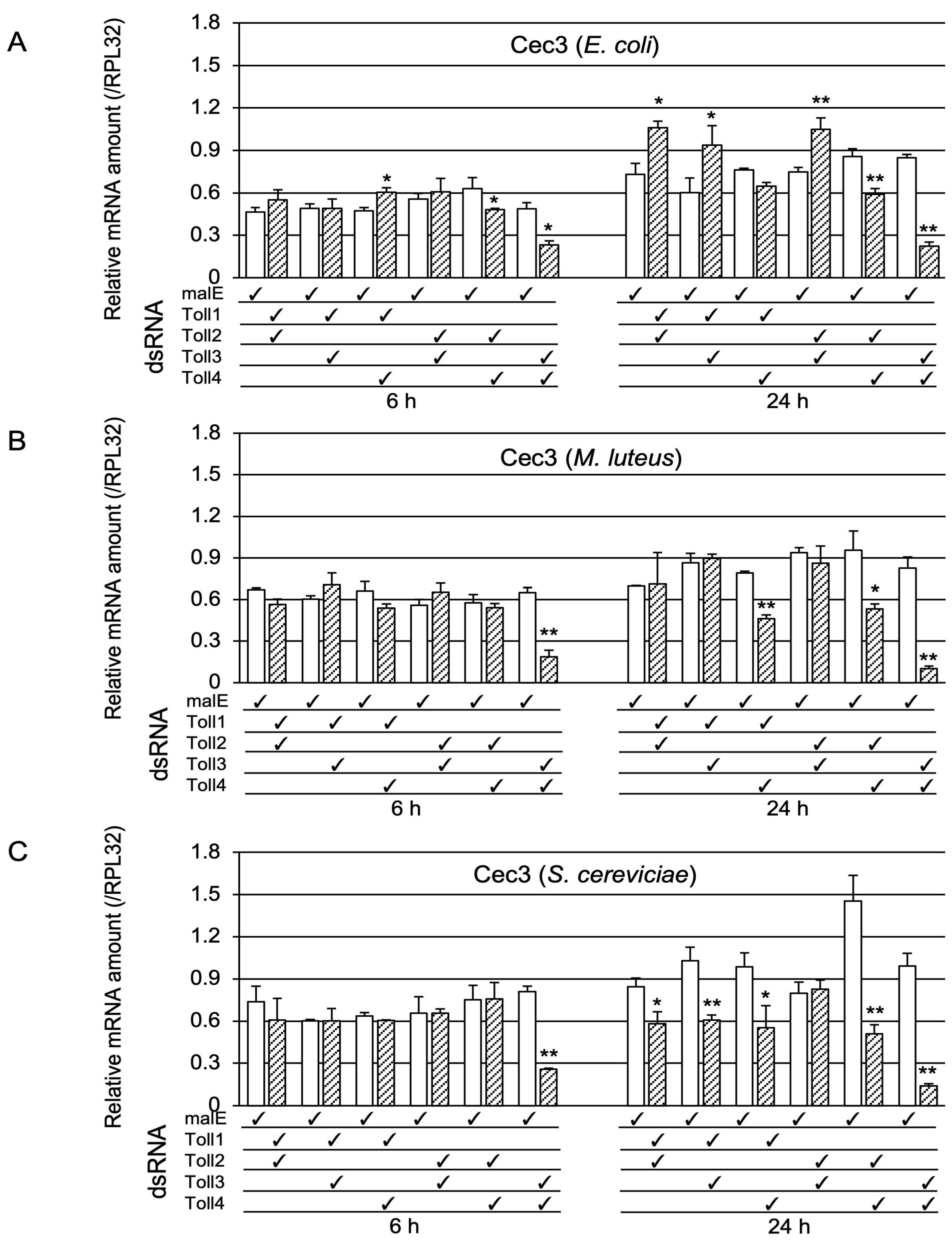
2.1.3. Determination of Whether Toll1−4 Are Involved in Toll-Pathway-Dependent AMP Gene Expression
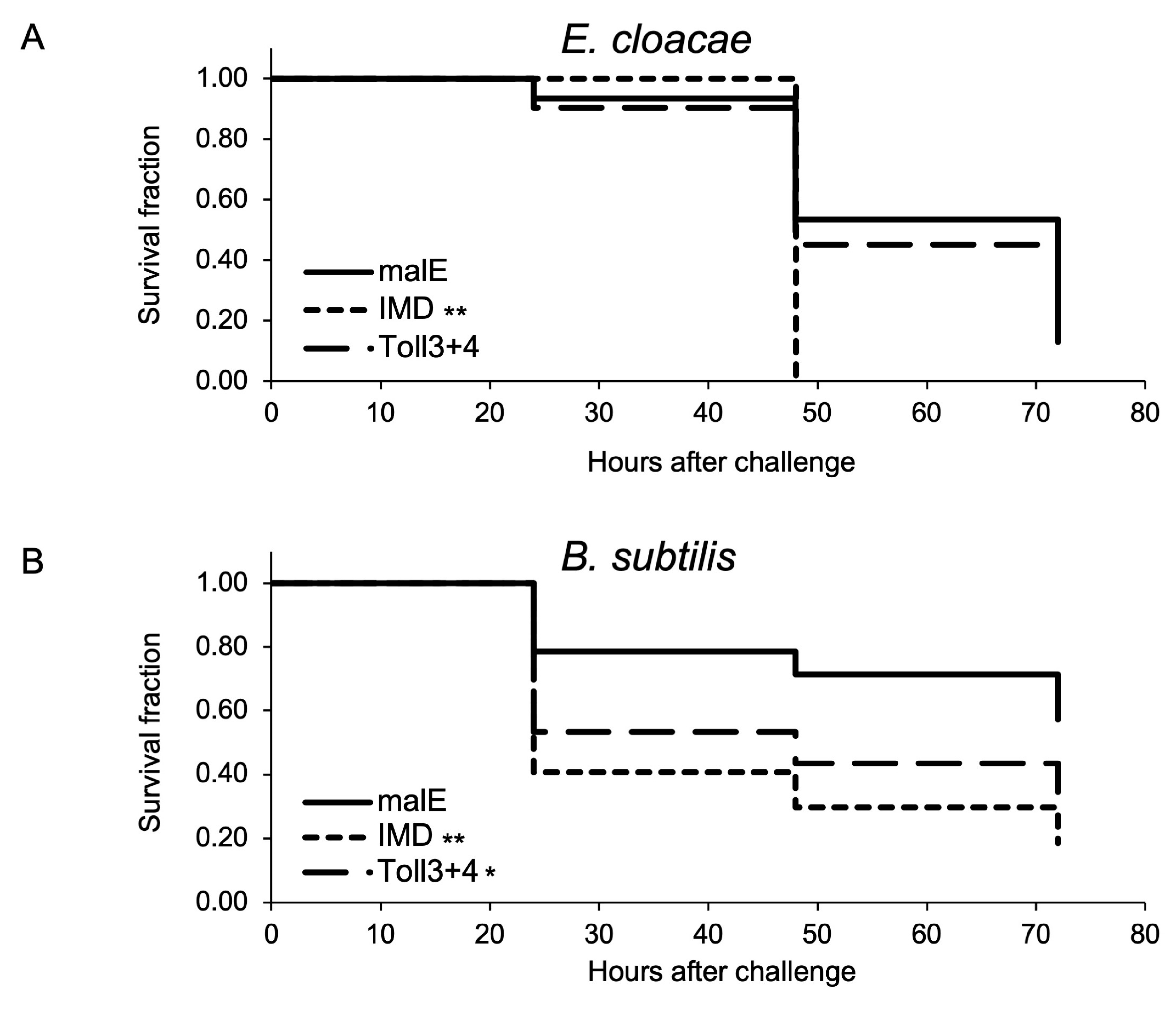
2.1.4. Survival Assay to Determine the Role of Toll3 and Toll4 in the Immune Response against the Two Entomopathogenic Bacteria
2.2. Functional Assay of spz Genes
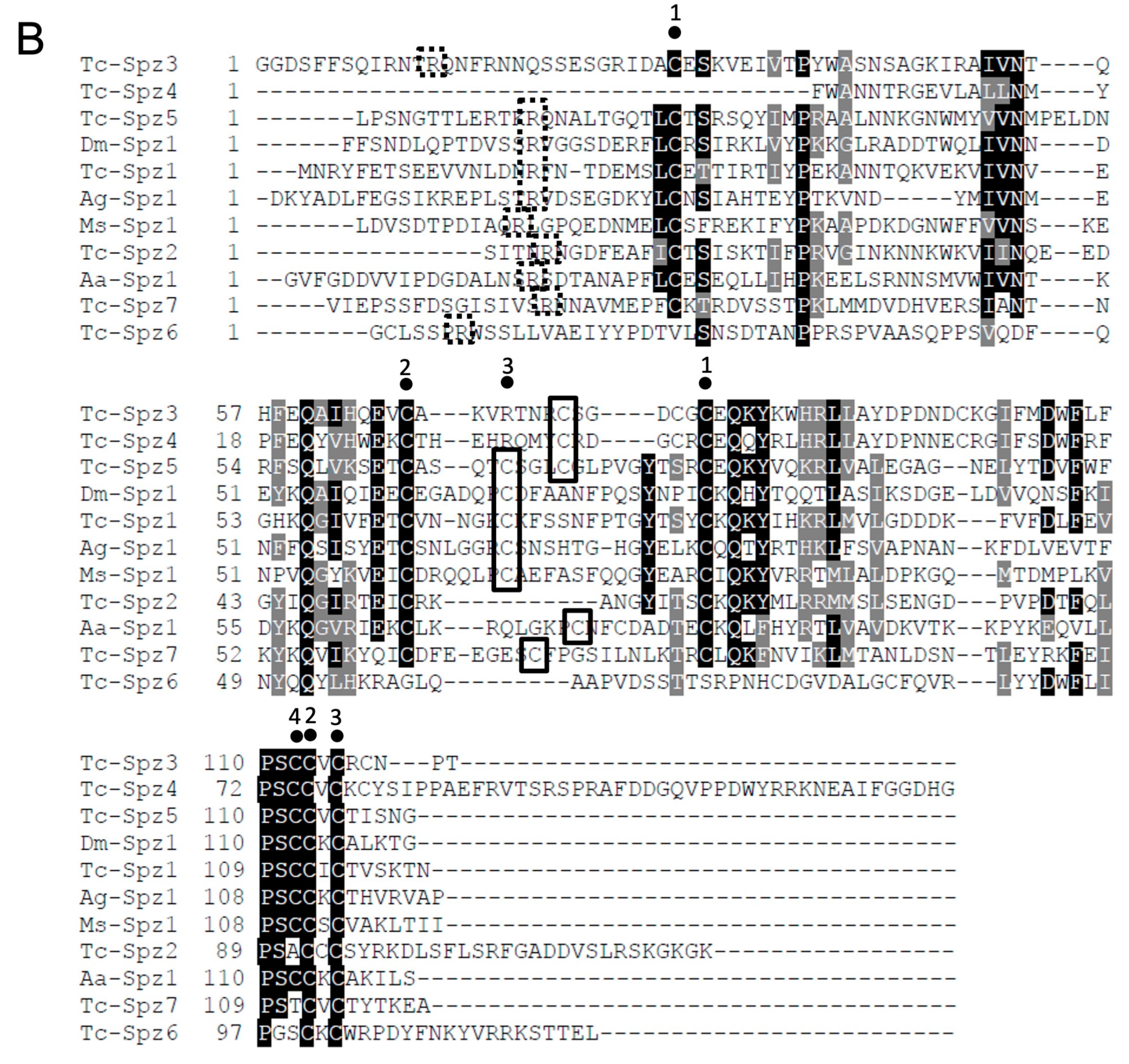
2.2.1. Phylogenetic and Sequence Analyses of the spz Gene
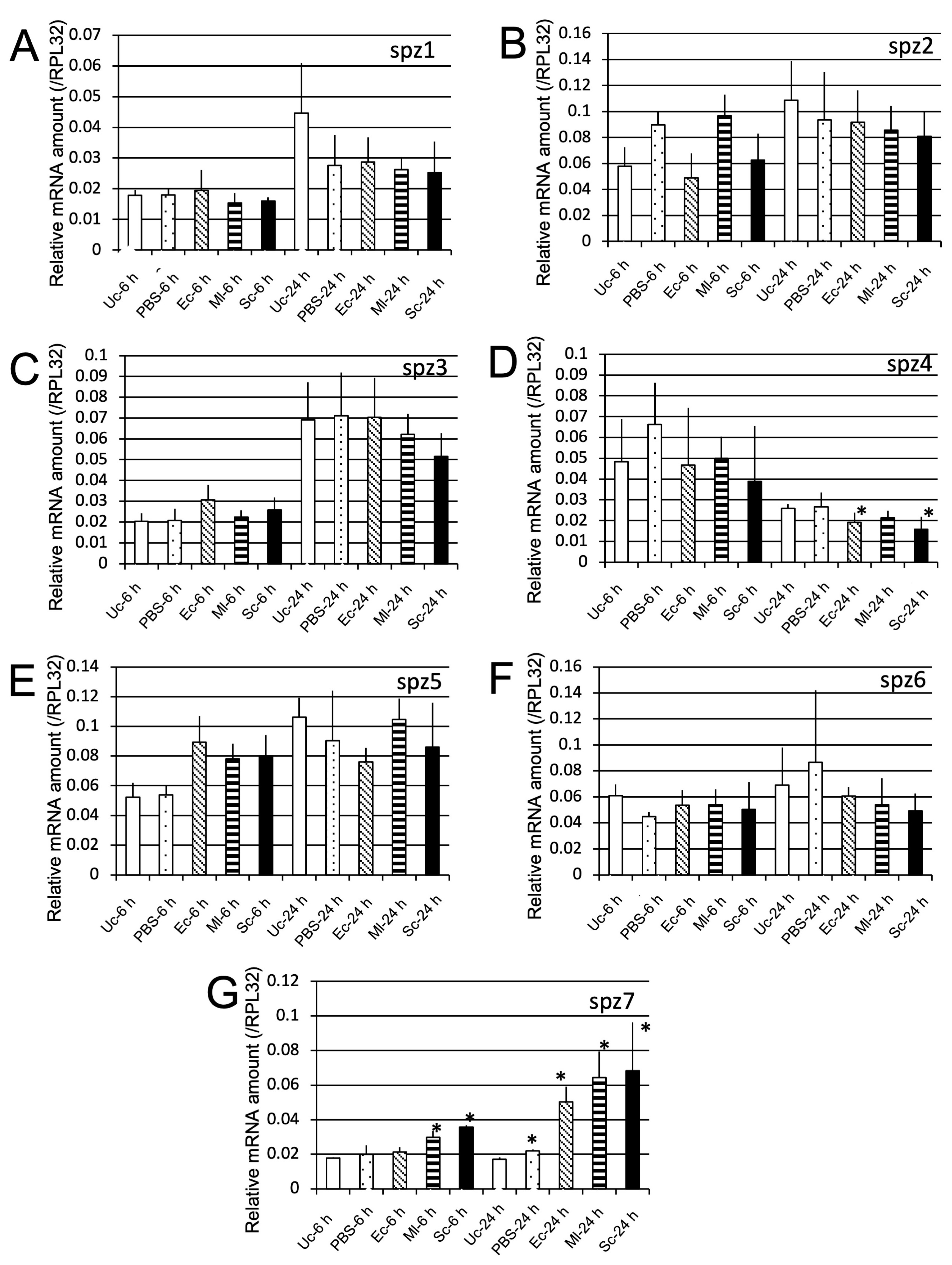
2.2.2. spz Gene Induction by Microbial Challenge
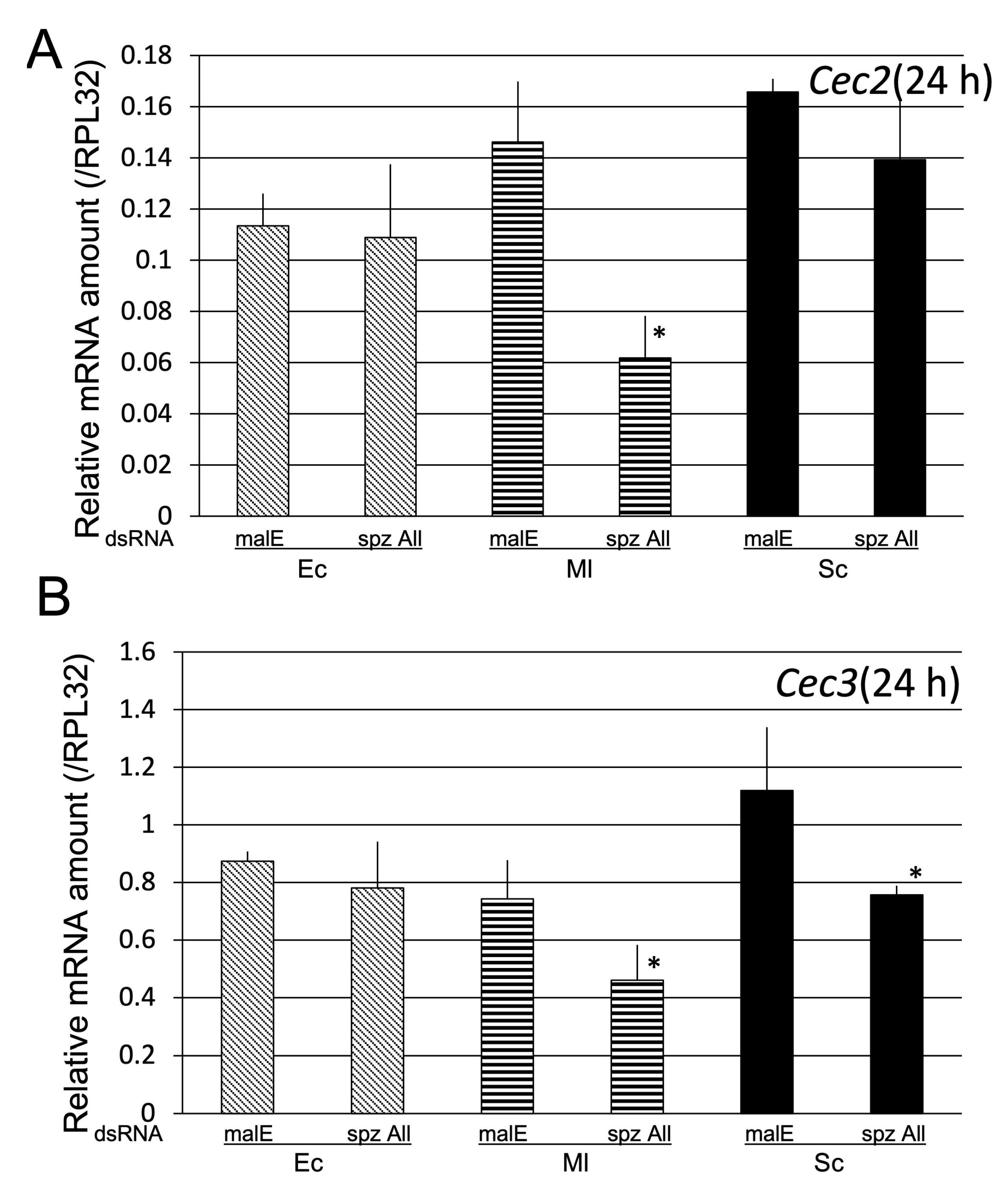
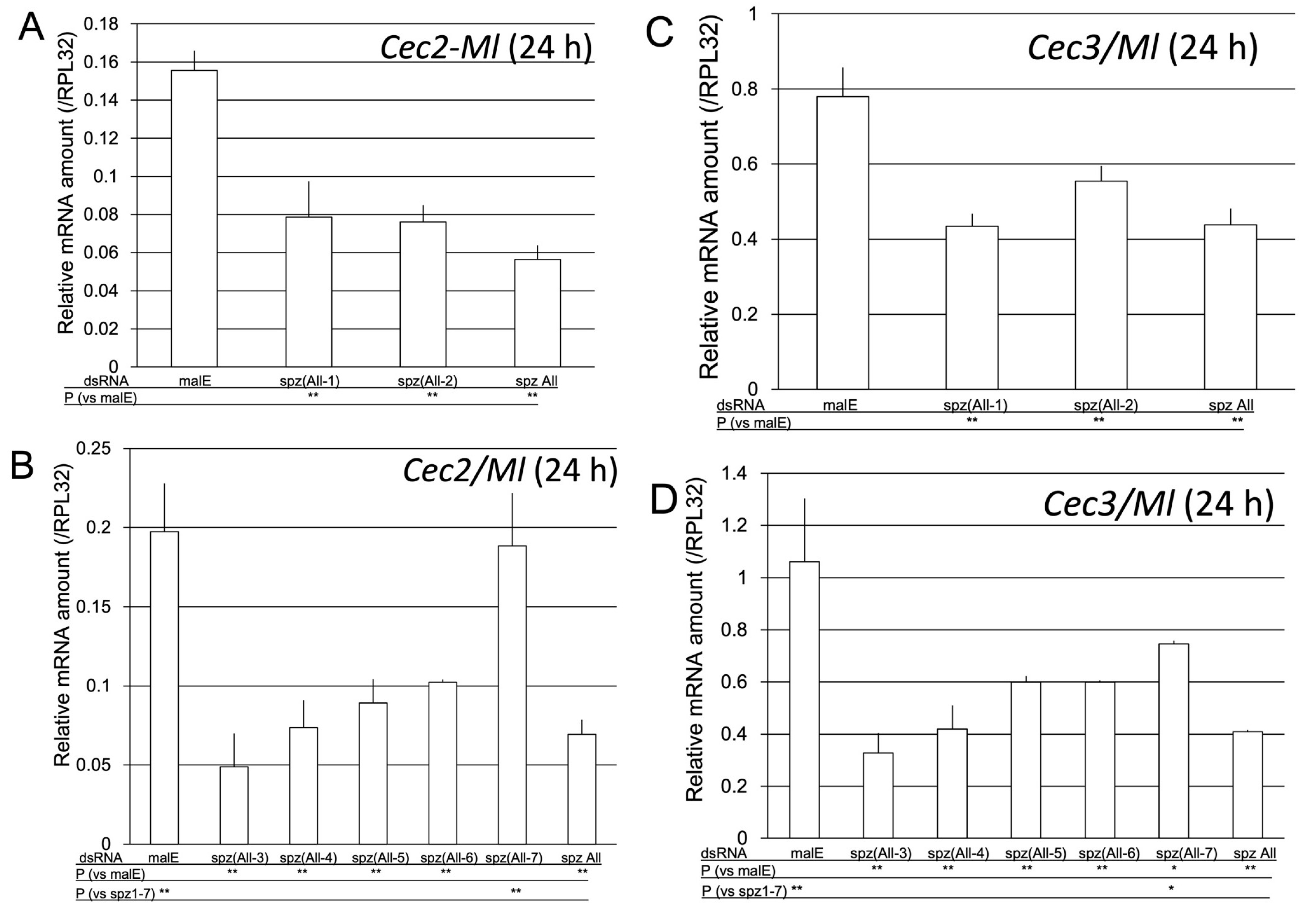
2.2.3. Determination of spz Genes Involved in Toll-Pathway-Dependent AMP Gene Expression
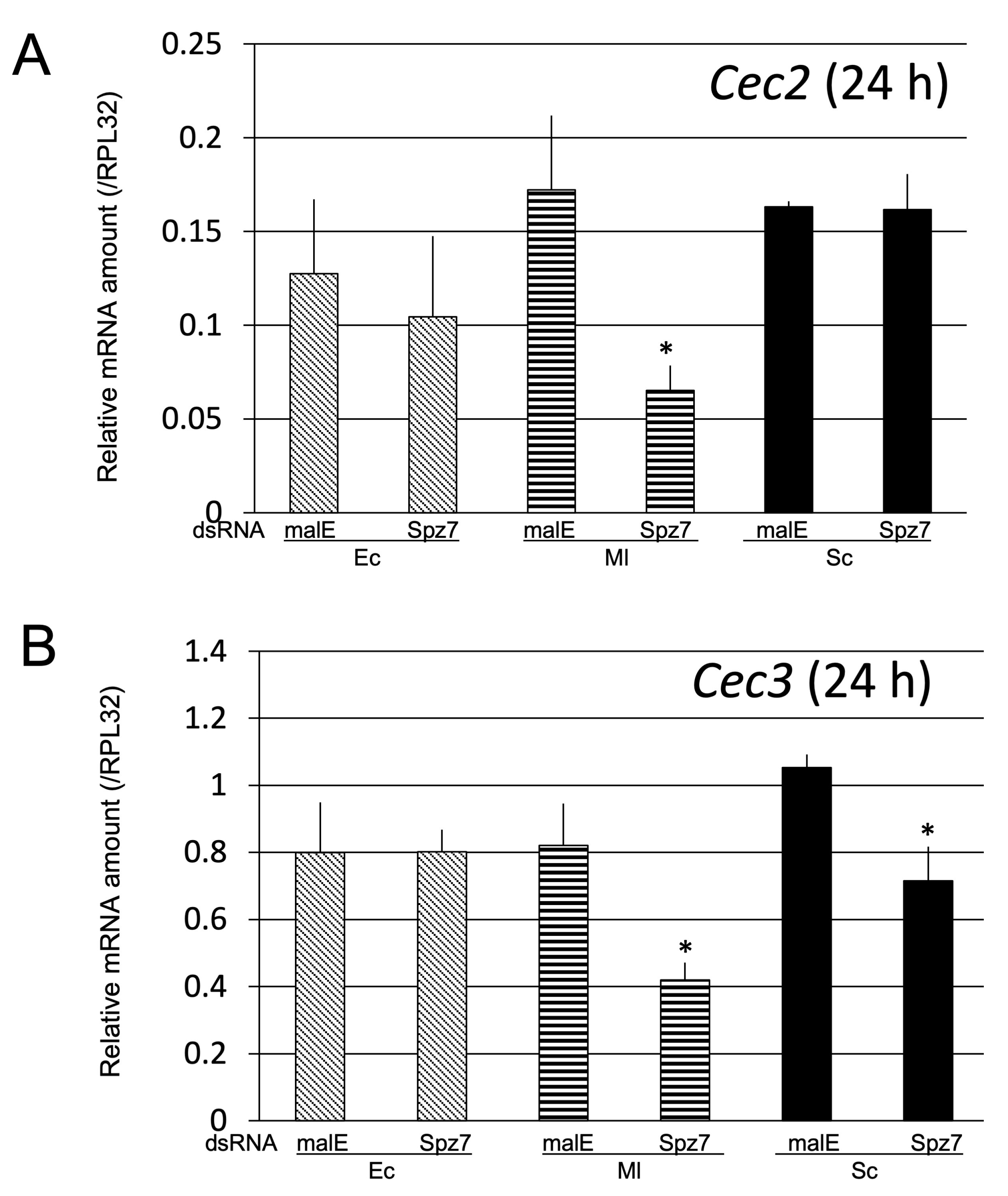
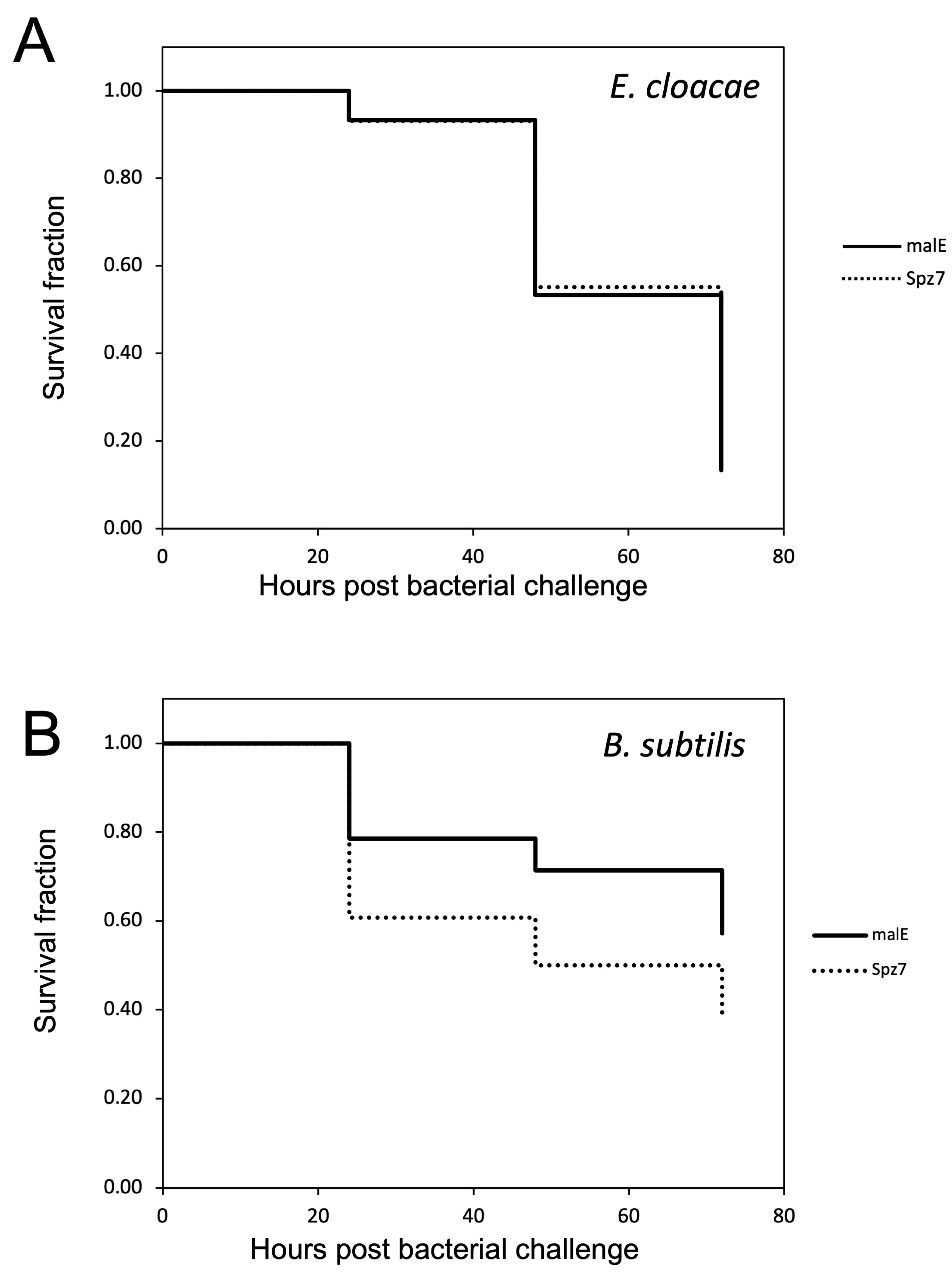
2.2.4. Survival Assay to Determine the Role of spz7 in the Immune Response against Entomopathogenic Bacteria
3. Discussion
4. Materials and Methods
4.1. Insect Rearing
4.2. Microbes and Injections
4.3. Gene Sequence Analysis
4.4. RNA Extraction and qRT-PCR
4.5. RNAi
4.6. Survival Assay
4.7. List of Abbreviations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lemaitre, B.; Hoffmann, J. The Host Defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [PubMed]
- Ferrandon, D.; Imler, J.-L.; Hetru, C.; Hoffmann, J.A. The Drosophila Systemic Immune Response: Sensing and Signalling during Bacterial and Fungal Infections. Nat. Rev. Immunol. 2007, 7, 862–874. [Google Scholar] [CrossRef]
- De Gregorio, E.; Spellman, P.T.; Rubin, G.M.; Lemaitre, B. Genome-Wide Analysis of the Drosophila Immune Response by Using Oligonucleotide Microarrays. Proc. Natl. Acad. Sci. USA 2001, 98, 12590–12595. [Google Scholar] [CrossRef]
- De Gregorio, E.; Spellman, P.T.; Tzou, P.; Rubin, G.M.; Lemaitre, B. The Toll and Imd Pathways Are the Major Regulators of the Immune Response in Drosophila. EMBO J. 2002, 21, 2568–2579. [Google Scholar] [CrossRef]
- Gobert, V.; Gottar, M.; Matskevich, A.A.; Rutschmann, S.; Royet, J.; Belvin, M.; Hoffmann, J.A.; Ferrandon, D. Dual Activation of the Drosophila Toll Pathway by Two Pattern Recognition Receptors. Science 2003, 302, 2126–2130. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Weber, A.N.R.; Atilano, M.L.; Filipe, S.R.; Gay, N.J.; Ligoxygakis, P. Sensing of Gram-Positive Bacteria in Drosophila: GNBP1 Is Needed to Process and Present Peptidoglycan to PGRP-SA. EMBO J. 2006, 25, 5005–5014. [Google Scholar] [CrossRef] [PubMed]
- Gottar, M.; Gobert, V.; Matskevich, A.A.; Reichhart, J.-M.; Wang, C.; Butt, T.M.; Belvin, M.; Hoffmann, J.A.; Ferrandon, D. Dual Detection of Fungal Infections in Drosophila via Recognition of Glucans and Sensing of Virulence Factors. Cell 2006, 127, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.N.R.; Tauszig-Delamasure, S.; Hoffmann, J.A.; Lelièvre, E.; Gascan, H.; Ray, K.P.; Morse, M.A.; Imler, J.-L.; Gay, N.J. Binding of the Drosophila Cytokine Spätzle to Toll Is Direct and Establishes Signaling. Nat. Immunol. 2003, 4, 794–800. [Google Scholar] [CrossRef]
- Jang, I.-H.; Chosa, N.; Kim, S.-H.; Nam, H.-J.; Lemaitre, B.; Ochiai, M.; Kambris, Z.; Brun, S.; Hashimoto, C.; Ashida, M.; et al. A Spätzle-Processing Enzyme Required for Toll Signaling Activation in Drosophila Innate Immunity. Dev. Cell 2006, 10, 45–55. [Google Scholar] [CrossRef]
- Gangloff, M.; Murali, A.; Xiong, J.; Arnot, C.J.; Weber, A.N.; Sandercock, A.M.; Robinson, C.V.; Sarisky, R.; Holzenburg, A.; Kao, C.; et al. Structural Insight into the Mechanism of Activation of the Toll Receptor by the Dimeric Ligand Spätzle. J. Biol. Chem. 2008, 283, 14629–14635. [Google Scholar] [CrossRef]
- Hu, X.; Yagi, Y.; Tanji, T.; Zhou, S.; Ip, Y.T. Multimerization and Interaction of Toll and Spätzle in Drosophila. Proc. Natl. Acad. Sci. USA 2004, 101, 9369–9374. [Google Scholar] [CrossRef] [PubMed]
- Valanne, S.; Wang, J.-H.; Rämet, M. The Drosophila Toll Signaling Pathway. J. Immunol. 2011, 186, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Kleino, A.; Silverman, N. The Drosophila IMD Pathway in the Activation of the Humoral Immune Response. Dev. Comp. Immunol. 2014, 42, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, K.; Koyama, H.; Minakuchi, C.; Tanaka, T.; Miura, K. Antimicrobial Peptide Gene Induction, Involvement of Toll and IMD Pathways and Defense against Bacteria in the Red Flour Beetle, Tribolium castaneum. Results Immunol. 2012, 2, 72–82. [Google Scholar] [CrossRef]
- Yokoi, K.; Koyama, H.; Ito, W.; Minakuchi, C.; Tanaka, T.; Miura, K. Involvement of NF-ΚB Transcription Factors in Antimicrobial Peptide Gene Induction in the Red Flour Beetle, Tribolium castaneum. Dev. Comp. Immunol. 2012, 38, 342–351. [Google Scholar] [CrossRef]
- Yokoi, K.; Kato, D.; Miura, K. Pelle and Tube Contribute to the Toll Pathway-Dependent Antimicrobial Peptide Production in the Red Flour Beetle, Tribolium castaneum (Coleoptera: Tenebrionidae). Eur. J. Entomol. 2022, 119, 111–121. [Google Scholar] [CrossRef]
- Koyama, H.; Kato, D.; Minakuchi, C.; Tanaka, T.; Yokoi, K.; Miura, K. Peptidoglycan Recognition Protein Genes and Their Roles in the Innate Immune Pathways of the Red Flour Beetle, Tribolium castaneum. J. Invertebr. Pathol. 2015, 132, 86–100. [Google Scholar] [CrossRef]
- Yokoi, K.; Ito, W.; Kato, D.; Miura, K. RNA Interference-Based Characterization of Caspar, DREDD and FADD Genes in Immune Signaling Pathways of the Red Flour Beetle, Tribolium castaneum (Coleoptera: Tenebrionidae). Eur. J. Entomol. 2022, 119, 23–35. [Google Scholar] [CrossRef]
- Gay, N.J.; Keith, F.J. Drosophila Toll and IL-1 Receptor. Nature 1991, 351, 355–356. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A. A Human Homologue of the Drosophila Toll Protein Signals Activation of Adaptive Immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef]
- Mizuguchi, K.; Parker, J.S.; Blundell, T.L.; Gay, N.J. Getting Knotted: A Model for the Structure and Activation of Spätzle. Trends Biocheml. Sci. 1998, 23, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.N.R.; Gangloff, M.; Moncrieffe, M.C.; Hyvert, Y.; Imler, J.-L.; Gay, N.J. Role of the Spatzle Pro-Domain in the Generation of an Active Toll Receptor Ligand. J. Biol. Chem. 2007, 282, 13522–13531. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Preston-Hurlburt, P.; Kopp, E.; Stadlen, A.; Chen, C.; Ghosh, S.; Janeway, C.A. MyD88 Is an Adaptor Protein in the HToll/IL-1 Receptor Family Signaling Pathways. Mol. Cell 1998, 2, 253–258. [Google Scholar] [CrossRef]
- Weber, A.N.R.; Moncrieffe, M.C.; Gangloff, M.; Imler, J.-L.; Gay, N.J. Ligand-Receptor and Receptor-Receptor Interactions Act in Concert to Activate Signaling in the Drosophila Toll Pathway. J. Biol. Chem. 2005, 280, 22793–22799. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Sang, Q.; Pan, G.; Li, C.; Zhou, Z. A Toll-Spätzle Pathway in the Immune Response of Bombyx mori. Insects 2020, 11, 586. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Xu, X.-X.; Yi, H.-Y.; Lin, C.; Yu, X.-Q. A Toll-Spätzle Pathway in the Tobacco Hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 2012, 42, 514–524. [Google Scholar] [CrossRef]
- An, C.; Jiang, H.; Kanost, M.R. Proteolytic Activation and Function of the Cytokine Spätzle in the Innate Immune Response of a Lepidopteran Insect, Manduca sexta. FEBS J. 2010, 277, 148–162. [Google Scholar] [CrossRef]
- Jang, H.A.; Patnaik, B.B.; Ali Mohammadie Kojour, M.; Kim, B.B.; Bae, Y.M.; Park, K.B.; Lee, Y.S.; Jo, Y.H.; Han, Y.S. TmSpz-like Plays a Fundamental Role in Response to E. coli but Not S. aureus or C. albican Infection in Tenebrio molitor via Regulation of Antimicrobial Peptide Production. Int. J. Mol. Sci. 2021, 22, 10888. [Google Scholar] [CrossRef]
- Ali Mohammadie Kojour, M.; Edosa, T.T.; Jang, H.A.; Keshavarz, M.; Jo, Y.H.; Han, Y.S. Critical Roles of Spätzle5 in Antimicrobial Peptide Production Against Escherichia coli in Tenebrio molitor Malpighian Tubules. Front. Immunol. 2021, 12, 760475. [Google Scholar] [CrossRef]
- Zou, Z.; Evans, J.D.; Lu, Z.; Zhao, P.; Williams, M.; Sumathipala, N.; Hetru, C.; Hultmark, D.; Jiang, H. Comparative Genomic Analysis of the Tribolium Immune System. Genome Biol. 2007, 8, R177. [Google Scholar] [CrossRef]
- Nishide, Y.; Kageyama, D.; Yokoi, K.; Jouraku, A.; Tanaka, H.; Futahashi, R.; Fukatsu, T. Functional Crosstalk across IMD and Toll Pathways: Insight into the Evolution of Incomplete Immune Cascades. Proc. Biol. Sci. 2019, 286, 20182207. [Google Scholar] [CrossRef]
- Horng, T.; Medzhitov, R. Drosophila MyD88 Is an Adapter in the Toll Signaling Pathway. Proc. Natl. Acad. Sci. USA 2001, 98, 12654–12658. [Google Scholar] [CrossRef] [PubMed]
- Leulier, F.; Lemaitre, B. Toll-like Receptors—Taking an Evolutionary Approach. Nat. Rev. Genet. 2008, 9, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Pervez, M.T.; ul Hasnain, M.J.; Abbas, S.H.; Moustafa, M.F.; Aslam, N.; Shah, S.S.M. A Comprehensive Review of Performance of Next-Generation Sequencing Platforms. Biomed Res. Int. 2022, 2022, 3457806. [Google Scholar] [CrossRef]
- Weinstock, G.M.; Robinson, G.E.; Gibbs, R.A.; Weinstock, G.M.; Weinstock, G.M.; Robinson, G.E.; Worley, K.C.; Evans, J.D.; Maleszka, R.; Robertson, H.M.; et al. Insights into Social Insects from the Genome of the Honeybee Apis mellifera. Nature 2006, 443, 931–949. [Google Scholar] [CrossRef]
- Wallberg, A.; Bunikis, I.; Pettersson, O.V.; Mosbech, M.-B.; Childers, A.K.; Evans, J.D.; Mikheyev, A.S.; Robertson, H.M.; Robinson, G.E.; Webster, M.T. A Hybrid de Novo Genome Assembly of the Honeybee, Apis mellifera, with Chromosome-Length Scaffolds. BMC Genom. 2019, 20, 275. [Google Scholar] [CrossRef] [PubMed]
- Elsik, C.G.; Worley, K.C.; Bennett, A.K.; Beye, M.; Camara, F.; Childers, C.P.; de Graaf, D.C.; Debyser, G.; Deng, J.; Devreese, B.; et al. Finding the Missing Honey Bee Genes: Lessons Learned from a Genome Upgrade. BMC Genom. 2014, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Tribolium Genome Sequencing Consortium; Richards, S.; Gibbs, R.A.; Herndon, N.; Weinstock, G.M.; Brown, S.J.; Denell, R.; Beeman, R.W.; Gibbs, R. The Genome of the Model Beetle and Pest Tribolium castaneum. Nature 2008, 452, 949–955. [Google Scholar] [CrossRef]
- Herndon, N.; Shelton, J.; Gerischer, L.; Ioannidis, P.; Ninova, M.; Dönitz, J.; Waterhouse, R.M.; Liang, C.; Damm, C.; Siemanowski, J.; et al. Enhanced Genome Assembly and a New Official Gene Set for Tribolium castaneum. BMC Genom. 2020, 21, 47. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Albà, M. Making Alignments Prettier. Genome Biol. 2000, 1, reports2047. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, D.; Miura, K.; Yokoi, K. Analysis of the Toll and Spaetzle Genes Involved in Toll Pathway-Dependent Antimicrobial Gene Induction in the Red Flour Beetle, Tribolium castaneum (Coleoptera; Tenebrionidae). Int. J. Mol. Sci. 2023, 24, 1523. https://doi.org/10.3390/ijms24021523
Kato D, Miura K, Yokoi K. Analysis of the Toll and Spaetzle Genes Involved in Toll Pathway-Dependent Antimicrobial Gene Induction in the Red Flour Beetle, Tribolium castaneum (Coleoptera; Tenebrionidae). International Journal of Molecular Sciences. 2023; 24(2):1523. https://doi.org/10.3390/ijms24021523
Chicago/Turabian StyleKato, Daiki, Ken Miura, and Kakeru Yokoi. 2023. "Analysis of the Toll and Spaetzle Genes Involved in Toll Pathway-Dependent Antimicrobial Gene Induction in the Red Flour Beetle, Tribolium castaneum (Coleoptera; Tenebrionidae)" International Journal of Molecular Sciences 24, no. 2: 1523. https://doi.org/10.3390/ijms24021523
APA StyleKato, D., Miura, K., & Yokoi, K. (2023). Analysis of the Toll and Spaetzle Genes Involved in Toll Pathway-Dependent Antimicrobial Gene Induction in the Red Flour Beetle, Tribolium castaneum (Coleoptera; Tenebrionidae). International Journal of Molecular Sciences, 24(2), 1523. https://doi.org/10.3390/ijms24021523




