Role of Sirtuins in the Pathogenesis of Rheumatoid Arthritis
Abstract
1. Introduction
2. Rheumatoid Arthritis
3. Sirtuins Family
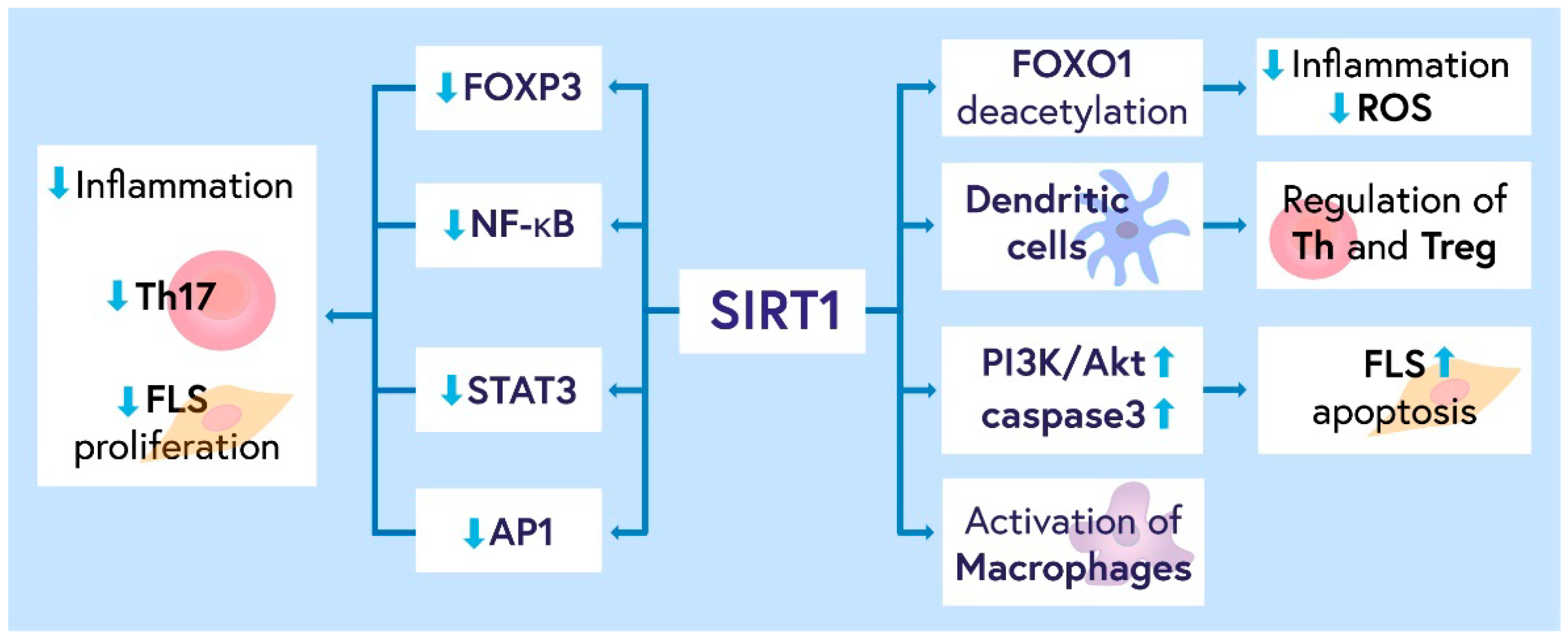
3.1. Role of SIRT1 in RA Pathogenesis
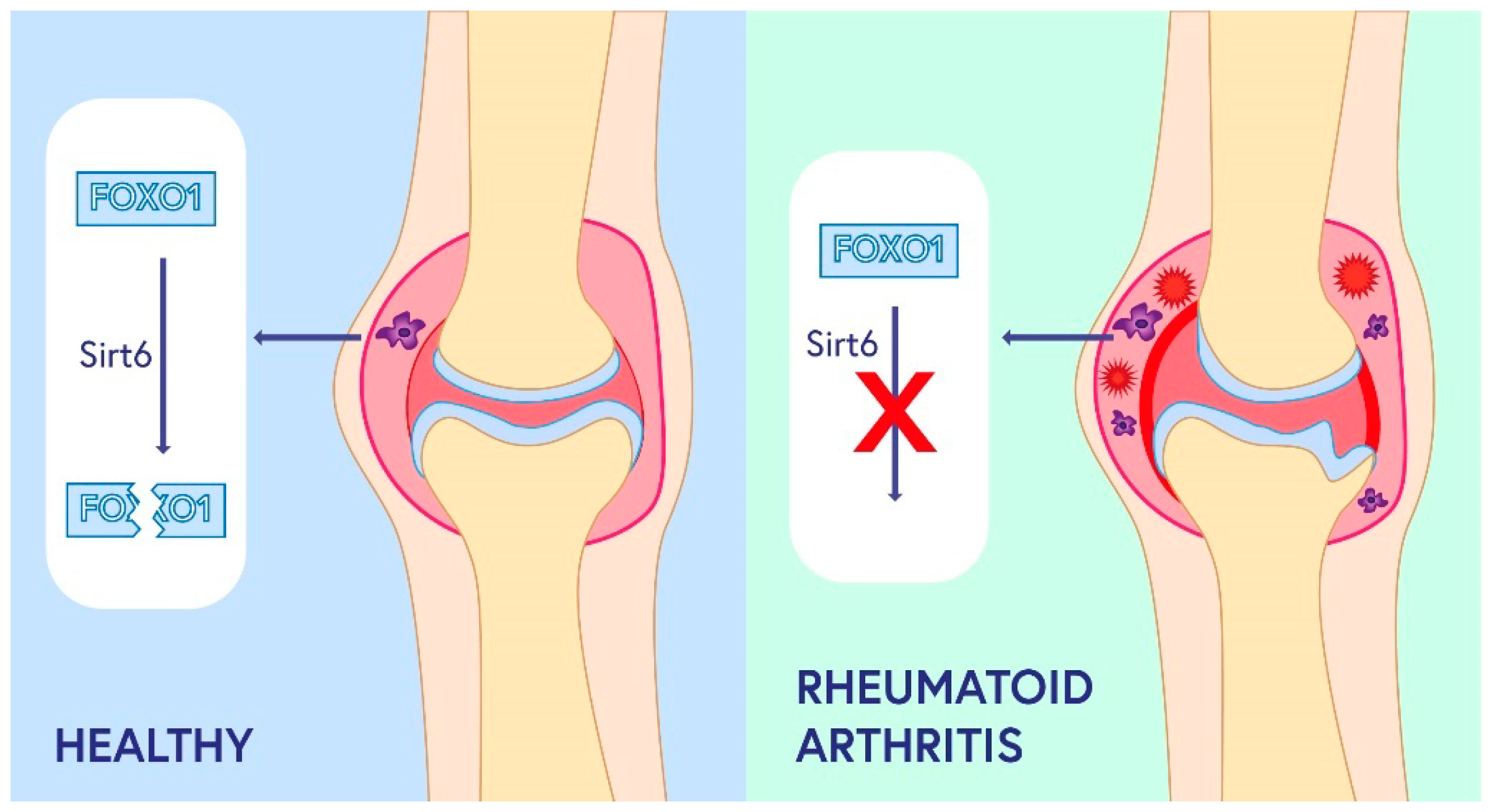
3.2. Role of SIRT2-SIRT 7 in RA Pathogenesis
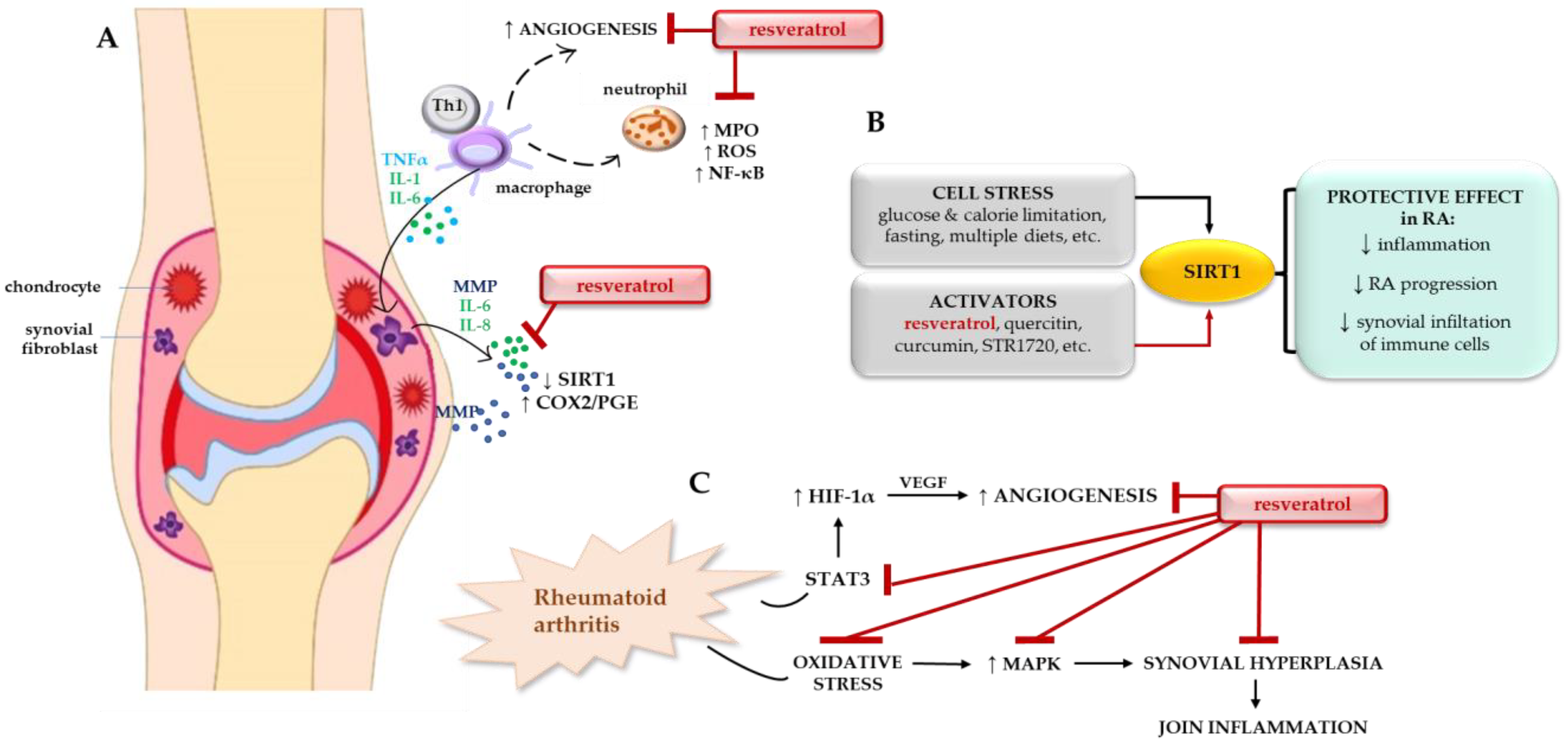
4. Regulation of SIRT1 as a Potential Target in RA Therapy
4.1. Resveratrol in RA Therapy
4.2. Healthy Diet
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, D.M.; Weinblatt, M.E. Rheumatoid arthritis. Lancet 2001, 358, 903–911. [Google Scholar] [CrossRef]
- Klein, K.; Gay, S. Epigenetics in rheumatoid arthritis. Curr. Opin. Rheumatol. 2015, 27, 76–82. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 27, 15. [Google Scholar] [CrossRef]
- van der Woude, D.; van der Helm-van Mil, A.H.M. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2018, 32, 174–187. [Google Scholar] [CrossRef]
- Szumilas, K.; Szumilas, P.; Słuczanowska-Głąbowska, S.; Zgutka, K.; Pawlik, A. Role of Adiponectin in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 8265. [Google Scholar] [CrossRef]
- Morand, S.; Staats, H.; Creeden, J.F.; Iqbal, A.; Kahaleh, B.; Stanbery, L.; Dworkin, L.; Nemunaitis, J. Molecular mechanisms underlying rheumatoid arthritis and cancer development and treatment. Future Oncol. 2020, 16, 483–495. [Google Scholar] [CrossRef]
- De Cock, D.; Hyrich, K. Malignancy and rheumatoid arthritis: Epidemiology, risk factors and management. Best Pract. Res. Clin. Rheumatol. 2018, 32, 869–886. [Google Scholar] [CrossRef]
- Pundole, X.; Suarez-Almazor, M.E. Cancer and Rheumatoid Arthritis. Rheum. Dis. Clin. N. Am. 2020, 46, 445–462. [Google Scholar] [CrossRef]
- Khurana, R.; Wolf, R.; Berney, S.; Caldito, G.; Hayat, S.; Berney, S.M. Risk of development of lung cancer is increased in patients with rheumatoid arthritis: A large case control study in US veterans. J. Rheumatol. 2008, 35, 1704–1708. [Google Scholar]
- Klein, A.; Polliack, A.; Gafter-Gvili, A. Rheumatoid arthritis and lymphoma: Incidence, pathogenesis, biology, and outcome. Hematol. Oncol. 2018, 36, 733–739. [Google Scholar] [CrossRef]
- Nakayama, H.; Yaguchi, T.; Yoshiya, S.; Nishizaki, T. Resveratrol induces apoptosis MH7A human rheumatoid arthritis synovial cells in a sirtuin 1-dependent manner. Rheumatol. Int. 2012, 32, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.Z.; Haris, M.S.; Khan, M.S.; Mahjabeen, I. Role of mitochondrial sirtuins in rheumatoid arthritis. Biochem Biophys Res Commun. 2021, 584, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Schoonjans, K.; Auwerx, J. Sirtuin Functions in Health and Disease. Mol. Endocrinol. 2007, 21, 1745–1755. [Google Scholar] [CrossRef]
- Wu, Y.J.; Fang, W.J.; Pan, S.; Zhang, S.S.; Li, D.F.; Wang, Z.F.; Chen, W.G.; Yin, Q.; Zuo, J. Regulation of Sirt1 on energy metabolism and immune response in rheumatoid arthritis. Int. Immunopharmacol. 2021, 101, 108175. [Google Scholar] [CrossRef] [PubMed]
- Morris, B.J. Seven sirtuins for seven deadly diseases of aging. Free Radic. Biol. Med. 2013, 56, 133–171. [Google Scholar] [CrossRef]
- Lavu, S.; Boss, O.; Elliott, P.J.; Lambert, P.D. Sirtuins--novel therapeutic targets to treat age-associated diseases. Nat. Rev. Drug Discov. 2008, 7, 841–853. [Google Scholar] [CrossRef]
- Shen, P.; Deng, X.; Chen, Z.; Ba, X.; Qin, K.; Huang, Y.; Huang, Y.; Li, T.; Yan, J.; Tu, S. SIRT1: A Potential Therapeutic Target in Autoimmune Diseases. Front. Immunol. 2021, 12, 779177. [Google Scholar] [CrossRef]
- Li, G.; Xia, Z.; Liu, Y.; Meng, F.; Wu, X.; Fang, Y.; Zhang, C.; Liu, D. SIRT1 inhibits rheumatoid arthritis fibroblast-like synoviocyte aggressiveness and inflammatory response via suppressing NF-κB pathway. Biosci. Rep. 2018, 38, BSR20180541. [Google Scholar] [CrossRef]
- Niederer, F.; Ospelt, C.; Brentano, F.; Hottiger, M.O.; Gay, R.E.; Gay, S.; Detmar, M.; Kyburz, D. SIRT1 overexpression in the rheumatoid arthritis synovium contributes to proinflammatory cytokine production and apoptosis resistance. Ann. Rheum. Dis. 2011, 70, 1866–1873. [Google Scholar] [CrossRef]
- Qu ZA, Ma XJ, Huang SB, Hao XR, Li DM, Feng KY, Wang WM SIRT2 inhibits oxidative stress and inflammatory response in diabetic osteoarthritis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2855–2864.
- Taneja, A.; Ravi, V.; Hong, J.Y.; Lin, H.; Sundaresan, N.R. Emerging roles of Sirtuin 2 in cardiovascular diseases. FASEB J. 2021, 35, e21841. [Google Scholar] [CrossRef] [PubMed]
- Rodier, G.; Kirsh, O.; Baraibar, M.; Houleès, T.; Lacroix, M.; Delpech, H.; Hatchi, E.; Arnould, S.; Severac, D.; Dubois, E.; et al. The transcription factor E4F1 coordinates CHK1-dependent checkpoint and mitochondrial functions. Cell Rep. 2015, 11, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Mathias, R.A.; Greco, T.M.; Oberstein, A.; Budayeva, H.G.; Chakrabarti, R.; Rowland, E.A.; Kang, Y.; Shenk, T.; Cristea, I.M. Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell 2014, 159, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, H.; Yuen Kwan Law, B.; De Seabra Rodrigues Dias, I.R.; Qiu, C.L.; Zeng, W.; Pan, H.D.; Chen, J.Y.; Bai, Y.F.; Lv, J.; et al. Sirtuin 5 deficiency increases disease severity in rats with adjuvant-induced arthritis. Cell. Mol. Immunol. 2020, 17, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, K.; Xu, W.; Zhao, S.; Ye, D.; Wang, Y.; Xu, Y.; Zhou, L.; Chu, Y.; Zhang, C.; et al. SIRT5 desuccinylates and activates pyruvate kinase M2 to block macrophage IL-1β production and to prevent DSS-induced colitis in mice. Cell Rep. 2017, 19, 2331–2344. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Ka, S.O.; Lee, S.M.; Lee, S.I.; Park, J.W.; Park, B.H. Overexpression of sirtuin 6 suppresses inflammatory responses and bone destruction in mice with collagen-induced arthritis. Arthritis Rheumatol. 2013, 65, 1776–1785. [Google Scholar] [CrossRef]
- Woo, S.J.; Noh, H.S.; Lee, N.Y.; Cheon, Y.H.; Yi, S.M.; Jeon, H.M.; Bae, E.J.; Lee, S.I.; Park, B.H. Myeloid sirtuin 6 deficiency accelerates experimental rheumatoid arthritis by enhancing macrophage activation and infiltration into synovium. EBioMedicine 2018, 38, 228–237. [Google Scholar] [CrossRef]
- Barber, M.F.; Michishita-Kioi, E.; Xi, Y.; Tasselli, L.; Kioi, M.; Moqtaderi, Z.; Tennen, R.I.; Paredes, S.; Young, N.L.; Chen, K.; et al. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature 2012, 487, 114–118. [Google Scholar] [CrossRef]
- Ford, E.; Voit, R.; Liszt, G.; Magin, C.; Grummt, I.; Guarente, L. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 2006, 20, 1075–1080. [Google Scholar] [CrossRef]
- Tanno, M.; Sakamoto, J.; Miura, T.; Shimamoto, K.; Horio, Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J. Biol. Chem. 2007, 282, 6823–6832. [Google Scholar] [CrossRef]
- Kong, S.; Yeung, P.; Fang, D. The class III histone deacetylase sirtuin 1 in immune suppression and its therapeutic potential in rheumatoid arthritis. J. Genet. Genom. 2013, 40, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Polzer, K.; Schett, G.; Zwerina, J. The lonely death: Chondrocyte apoptosis in TNF-induced arthritis. Autoimmunity 2007, 40, 333–336. [Google Scholar] [CrossRef]
- Yatsugi, N.; Tsukazaki, T.; Osaki, M.; Koji, T.; Yamashita, S.; Shindo, H. Apoptosis of articular chondrocytes in rheumatoid arthritis and osteoarthritis: Correlation of apoptosis with degree of cartilage destruction and expression of apoptosis-related proteins of p53 and c-myc. J. Orthop. Sci. 2000, 5, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Peck, Y.; Leom, L.T.; Low, P.F.P.; Wang, D.A. Establishment of an in vitro three-dimensional model for cartilage damage in rheumatoid arthritis. J. Tissue Eng. Regen. Med. 2018, 12, e237–e249. [Google Scholar] [CrossRef]
- Wang, A.Z.; Wang, J.C.; Fisher, G.W.; Diamond, H.S. Interleukin-1β-stimulated invasion of articular cartilage by rheumatoid synovial fibroblasts is inhibited by antibodies to specific integrin receptors and by collagenase inhibitors. Arthritis Rheum. 1997, 40, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wang, Z.; Jin, J.; Wang, Y.; Bao, N.; Gao, Q.; Zhao, J. SIRT1 protects osteoblasts against particle-induced inflammatory responses and apoptosis in aseptic prosthesis loosening. Acta Biomater. 2017, 49, 541–554. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, S.W.; Lee, S.Y.; Hong, K.W.; Bae, S.S.; Kim, K.; Kim, C.D. SIRT1/Adenosine Monophosphate-Activated Protein Kinase α Signaling Enhances Macrophage Polarization to an Anti-inflammatory Phenotype in Rheumatoid Arthritis. Front. Immunol. 2017, 8, 1135. [Google Scholar] [CrossRef]
- Cohen-Kfir, E.; Artsi, H.; Levin, A.; Abramowitz, E.; Bajayo, A.; Gurt, I.; Zhong, L.; D’Urso, A.; Toiber, D.; Mostoslavsky, R.; et al. Sirt1 Is a Regulator of Bone Mass and a Repressor of Sost Encoding for Sclerostin, a Bone Formation Inhibitor. Endocrinology 2011, 152, 4514–4524. [Google Scholar] [CrossRef]
- Huang, W.; Shang, W.L.; Wang, H.D.; Wu, W.W.; Hou, S.X. Sirt1 overexpression protects murine osteoblasts against TNF-alpha-induced injury in vitro by suppressing the NF-κB signaling pathway. Acta Pharmacol. Sin. 2012, 33, 668–674. [Google Scholar] [CrossRef]
- Moon, M.H.; Jeong, J.K.; Lee, Y.J.; Seol, J.W.; Jackson, C.J.; Park, S.Y. SIRT1, a class III histone deacetylase, regulates TNF-α-induced inflammation in human chondrocytes. Osteoarthr. Cartil. 2013, 21, 470–480. [Google Scholar] [CrossRef]
- Müller-Ladner, U.; Ospelt, C.; Gay, S.; Distler, O.; Pap, T. Cells of the synovium in rheumatoid arthritis. Synovial fibroblasts. Arthritis Res. Ther. 2007, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.; Schafer, N.; Breitenstein, A.; Besler, C.; Winnik, S.; Lohmann, C.; Heinrich, K.; Brokopp, C.E.; Handschin, C.; Landmesser, U.; et al. SIRT1 reduces endothelial activation without affecting vascular function in ApoE−/− mice. Aging 2010, 2, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Meednu, N.; Zhang, H.; Owen, T.; Sun, W.; Wang, V.; Cistrone, C.; Rangel-Moreno, J.; Xing, L.; Anolik, J.H. Production of RANKL by memory B cells: A link between B cells and bone erosion in rheumatoid arthritis. Arthritis Rheumatol. 2016, 68, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lee, S.M.; Shannon, S.; Gao, B.; Chen, W.; Chen, A.; Divekar, R.; McBurney, M.W.; Braley-Mullen, H.; Zaghouani, H.; et al. The Type III Histone Deacetylase Sirt1 is Essential for Maintenance of T Cell Tolerance in Mice. J. Clin. Invest. 2009, 119, 3048–3058. [Google Scholar] [CrossRef]
- Ofosu-Appiah, W.A.; Warrington, R.J.; Wilkins, J.A. Interleukin 2 responsive T cell clones from rheumatoid and normal subjects: Proliferative responses to connective tissue elements. Clin. Immunol. Immunopathol. 1989, 50, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.J.; Lee, S.M.; Lim, H.S.; Hah, Y.S.; Jung, I.D.; Park, Y.M.; Kim, H.O.; Cheon, Y.H.; Jeon, M.G.; Jang, K.Y.; et al. Myeloid Deletion of SIRT1 Suppresses Collagen-Induced Arthritis in Mice by Modulating Dendritic Cell Maturation. Exp. Mol. Med. 2016, 48, e221. [Google Scholar] [CrossRef]
- Yang, G.; Chang, C.C.; Yang, Y.; Yuan, L.; Xu, L.; Ho, C.T.; Li, S. Resveratrol Alleviates Rheumatoid Arthritis via Reducing ROS and Inflammation, Inhibiting MAPK Signaling Pathways, and Suppressing Angiogenesis. J. Agric. Food Chem. 2018, 66, 12953–12960. [Google Scholar] [CrossRef]
- Michishita, E.; Park, J.Y.; Burneskis, J.M.; Barrett, J.C.; Horikawa, I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell 2005, 16, 4623–4635. [Google Scholar] [CrossRef]
- Kara, M.; Yolbaş, S.; Şahin, C.; Koca, S.S. Changes in sirtuin 2 and sirtuin 3 mRNA expressions in rheumatoid arthritis. Eur. J. Rheumatol. 2017, 4, 83–86. [Google Scholar] [CrossRef]
- Lin, J.; Sun, B.; Jiang, C.; Hong, H.; Zheng, Y. Sirt2 suppresses inflammatory responses in collagen-induced arthritis. Biochem. Biophys. Res. Commun. 2013, 441, 897–903. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, S.; Li, J.; Li, J.; Lan, Y.; Nie, H.; Zuo, Y. SIRT4 suppresses the inflammatory response and oxidative stress in osteoarthritis. Am. J. Transl. Res. 2020, 12, 1965. [Google Scholar] [PubMed]
- Ahuja, N.; Schwer, B.; Carobbio, S.; Waltregny, D.; North, B.J.; Castronovo, V.; Maechler, P.; Verdin, E. Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J. Biol. Chem. 2007, 282, 33583–33592. [Google Scholar] [CrossRef] [PubMed]
- Haigis, M.C.; Mostoslavsky, R.; Haigis, K.M.; Fahie, K.; Christodoulou, D.C.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Karow, M.; Blander, G.; et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 2006, 126, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Li, M.; Hou, T.; Gao, T.; Zhu, W.G.; Yang, Y. Sirtuins in glucose and lipid metabolism. Oncotarget 2017, 8, 1845–1859. [Google Scholar] [CrossRef] [PubMed]
- Rardin, M.J.; He, W.; Nishida, Y.; Newman, J.C.; Carrico, C.; Danielson, S.R.; Guo, A.; Gut, P.; Sahu, A.K.; Li, B.; et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 2013, 18, 920–933. [Google Scholar] [CrossRef]
- Schlicker, C.; Gertz, M.; Papatheodorou, P.; Kachholz, B.; Becker, C.F.; Steegborn, C. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J. Mol. Biol. 2008, 382, 790–801. [Google Scholar] [CrossRef]
- Zhu, S.; Batushansky, A.; Jopkiewicz, A.; Makosa, D.; Humphries, K.M.; Van Remmen, H.; Griffin, T.M. Sirt5 Deficiency Causes Posttranslational Protein Malonylation and Dysregulated Cellular Metabolism in Chondrocytes Under Obesity Conditions. Cartilage 2021, 13, 1185S–1199S. [Google Scholar] [CrossRef]
- Mostoslavsky, R.; Chua, K.F.; Lombard, D.B.; Pang, W.W.; Fischer, M.R.; Gellon, L.; Liu, P.; Mostoslavsky, G.; Franco, S.; Murphy, M.M.; et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 2006, 124, 315–329. [Google Scholar] [CrossRef]
- Knobloch, J.; Wahl, C.; Feldmann, M.; Jungck, D.; Strauch, J.; Stoelben, E.; Koch, A. Resveratrol attenuates the release of inflammatory cytokines from human bronchial smooth muscle cells exposed to lipoteichoic acid in chronic obstructive pulmonary disease. Basic Clin. Pharmacol. Toxicol. 2014, 114, 202–209. [Google Scholar] [CrossRef]
- Sebastián, C.; Zwaans, B.M.; Silberman, D.M.; Gymrek, M.; Goren, A.; Zhong, L.; Ram, O.; Truelove, J.; Guimaraes, A.R.; Toiber, D.; et al. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell 2012, 151, 1185–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.W.; Wu, X.; Liu, D.; Zhou, W.; Tan, W.; Fang, Y.X.; Zhang, Y.; Liu, Y.Q.; Li, G.Q. Long non-coding RNA PVT1 knockdown suppresses fibroblast-like synoviocyte inflammation and induces apoptosis in rheumatoid arthritis through demethylation of sirt6. J. Biol. Eng. 2019, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K. Sirtuin-7 as a Novel Therapeutic Target in Vascular Smooth Muscle Cell Proliferation and Remodeling. Circ. J. 2021, 85, 2241–2242. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Jung, W.; Lee, E.; Chang, H.; Choi, J.H.; Kim, H.G.; Kim, A.; Kim, B.H. SIRT7, H3K18ac, and ELK4 Immunohistochemical Expression in Hepatocellular Carcinoma. J. Pathol. Transl. Med. 2016, 50, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Jing, Z.X.; Ke, Z.; Yi, P. Sirtuin 7 plays an oncogenic role in human osteosarcoma via downregulating CDC4 expression. Am. J. Cancer Res. 2017, 7, 1788–1803. [Google Scholar] [PubMed]
- Liu, H.; Hu, L.; Yu, G.; Yang, H.; Cao, Y.; Wang, S.; Fan, Z. LncRNA, PLXDC2-OT promoted the osteogenesis potentials of MSCs by inhibiting the deacetylation function of RBM6/SIRT7 complex and OSX specific isoform. Stem Cells. 2021, 39, 1049–1066. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Luo, W.; Wang, B.; Wang, X.; Gong, P.; Xiong, Y. Resveratrol promotes osteogenesis via activating SIRT1/FoxO1 pathway in osteoporosis mice. Life Sci. 2020, 246, 117422. [Google Scholar] [CrossRef]
- Wang, G.; Xie, X.; Yuan, L.; Qiu, J.; Duan, W.; Xu, B.; Chen, X. Resveratrol ameliorates rheumatoid arthritis via activation of SIRT1-Nrf2 signaling pathway. BioFactors 2020, 46, 441–453. [Google Scholar] [CrossRef]
- Švajger, U.; Jeras, M. Anti-inflammatory effects of resveratrol and its potential use in therapy of immune-mediated diseases. Int. Rev. Immunol. 2012, 31, 202–222. [Google Scholar] [CrossRef]
- Imperatore, F.; Maurizio, J.; Vargas Aguilar, S.; Busch, C.J.; Favret, J.; Kowenz-Leutz, E.; Cathou, W.; Gentek, R.; Perrin, P.; Leutz, A.; et al. SIRT1 regulates macrophage self-renewal. EMBO J. 2017, 36, 2353–2372. [Google Scholar] [CrossRef]
- Alesci, A.; Nicosia, N.; Fumia, A.; Giorgianni, F.; Santini, A.; Cicero, N. Resveratrol and Immune Cells: A Link to Improve Human Health. Molecules 2022, 27, 424. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, L. Influence of resveratrol on the immune response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef]
- Bai, X.; Yao, L.; Ma, X.; Xu, X. Small Molecules as SIRT Modulators. Mini. Rev. Med. Chem. 2018, 18, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Deniz, F.S.Ş.; Eren, G.; Orhan, I.E. Flavonoids as Sirtuin Modulators. Curr. Top. Med. Chem. 2022, 22, 790–805. [Google Scholar] [PubMed]
- Villalba, J.M.; Alcaín, F.J. Sirtuin activators and inhibitors. BioFactors 2012, 38, 349–359. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Ceribelli, A.; Marotto, D.; Batticciotto, A.; Atzeni, F. Systemic rheumatic diseases: From biological agents to small molecules. Autoimmun. Rev. 2019, 18, 583–592. [Google Scholar] [CrossRef]
- Senolt, L.; Vencovský, J.; Pavelka, K.; Ospelt, C.; Gay, S. Prospective new biological therapies for rheumatoid arthritis. Autoimmun. Rev. 2009, 9, 102–107. [Google Scholar] [CrossRef]
- Massalska, M.; Maslinski, W.; Ciechomska, M. Small Molecule Inhibitors in the Treatment of Rheumatoid Arthritis and Beyond: Latest Updates and Potential Strategy for Fighting COVID-19. Cells 2020, 9, 1876. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Cornelius, C.; Leso, V.; Trovato-Salinaro, A.; Ventimiglia, B.; Cavallaro, M.; Scuto, M.; Rizza, S.; Zanoli, L.; Neri, S.; et al. Oxidative stress, glutathione status, sirtuin and cellular stress response in type 2 diabetes. Biochim. Biophys. Acta 2012, 1822, 729–736. [Google Scholar] [CrossRef]
- Iwashige, K.; Kouda, K.; Kouda, M.; Horiuchi, K.; Takahashi, M.; Nagano, A.; Tanaka, T.; Takeuchi, H. Calorie restricted diet and urinary pentosidine in patients with rheumatoid arthritis. J. Physiol. Anthropol. Appl. Human Sci. 2004, 23, 19–24. [Google Scholar] [CrossRef]
- Abendroth, A.; Michalsen, A.; Lüdtke, R.; Rüffer, A.; Musial, F.; Dobos, G.J.; Langhorst, J. Changes of Intestinal Microflora in Patients with Rheumatoid Arthritis during Fasting or a Mediterranean Diet. Forsch. Komplement. Res. Complement. Med. 2010, 17, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Hafström, I.; Ringerz, B.; Gyllenhammar, H.; Palmblad, J.; Harms-Ringdahl, M. Effects of fasting on disease activity, neutrophil function, fatty acid composition, and leukotriene biosynthesis in patients with rheumatoid arthritis. Arthritis Rheum. 1998, 31, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Michalsen, A.; Riegert, M.; Lüdtke, R.; Bäcker, M.; Langhorst, J.; Schwickert, M.; Dobos, G.J. Mediterranean diet or extended fasting’s influence on changing the intestinal microflora, immunoglobulin A secretion and clinical outcome in patients with rheumatoid arthritis and fibromyalgia: An observational study. BMC Complement. Altern. Med. 2005, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.Y.; Lee, C.; Longo, V.D. Nutrition and fasting mimicking diets in the prevention and treatment of autoimmune diseases and immunosenescence. Mol. Cell Endocrinol. 2017, 455, 4–12. [Google Scholar] [CrossRef]
- Muller, H.; de Toledo, F.W.; Resch, K.L. Fasting followed by vegetarian diet in patients with rheumatoid arthritis: A systematic review. Scand. J. Rheumatol. 2001, 30, 1–10. [Google Scholar]
- Kjeldsen-Kragh, J.; Haugen, M.; Borchgrevink, C.F.; Laerum, E.; Eek, M.; Mowinkel, P.; Hovi, K.; Førre, O. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 1991, 338, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen-Kragh, J.; Haugen, M.; Borchgrevink, C.F.; Førre, Ø. Vegetarian diet for patients with rheumatoid arthritis—Status: Two years after introduction of the diet. Clin. Rheumatol. 1994, 13, 475–482. [Google Scholar] [CrossRef]
- McDougall, J.; Bruce, B.; Spiller, G.; Westerdahl, J.; McDougall, M. Effects of a Very Low-Fat, Vegan Diet in Subjects with Rheumatoid Arthritis. J. Altern. Complement. Med. 2002, 8, 71–75. [Google Scholar] [CrossRef]
- Hafström, I.; Ringertz, B.; Spångberg, A.; von Zweigbergk, L.; Brannemark, S.; Nylander, I.; Rönnelid, J.; Laasonen, L.; Klareskog, L. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: The effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology 2001, 40, 1175–1179. [Google Scholar] [CrossRef]
- McKellar, G.; Morrison, E.; McEntegart, A.; Hampson, R.; Tierney, A.; Mackle, G.; Scoular, J.; Scott, J.A.; Capell, H.A. A pilot study of a Mediterranean-type diet intervention in female patients with rheumatoid arthritis living in areas of social deprivation in Glasgow. Ann. Rheum. Dis. 2007, 66, 1239–1243. [Google Scholar] [CrossRef]
- Johansson, K.; Askling, J.; Alfredsson, L.; Di Giuseppe, D.; EIRA Study Group. Mediterranean diet and risk of rheumatoid arthritis: A population-based case-control study. Arthritis Res. Ther. 2018, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- Ciaffi, J.; Mitselman, D.; Mancarella, L.; Brusi, V.; Lisi, L.; Ruscitti, P.; Cipriani, P.; Meliconi, R.; Giacomelli, R.; Borghi, C.; et al. The Effect of Ketogenic Diet on Inflammatory Arthritis and Cardiovascular Health in Rheumatic Conditions: A Mini Review. Front. Med. 2021, 8, 792846. [Google Scholar] [CrossRef] [PubMed]
| Name | Function | References |
|---|---|---|
| SIRT1 |
| [18,19] |
| SIRT2 |
| [20] |
| SIRT3 |
| [21] |
| SIRT4 |
| [22,23] |
| SIRT5 |
| [24,25] |
| SIRT6 |
| [26,27] |
| SIRT7 |
| [28,29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poniewierska-Baran, A.; Bochniak, O.; Warias, P.; Pawlik, A. Role of Sirtuins in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2023, 24, 1532. https://doi.org/10.3390/ijms24021532
Poniewierska-Baran A, Bochniak O, Warias P, Pawlik A. Role of Sirtuins in the Pathogenesis of Rheumatoid Arthritis. International Journal of Molecular Sciences. 2023; 24(2):1532. https://doi.org/10.3390/ijms24021532
Chicago/Turabian StylePoniewierska-Baran, Agata, Oliwia Bochniak, Paulina Warias, and Andrzej Pawlik. 2023. "Role of Sirtuins in the Pathogenesis of Rheumatoid Arthritis" International Journal of Molecular Sciences 24, no. 2: 1532. https://doi.org/10.3390/ijms24021532
APA StylePoniewierska-Baran, A., Bochniak, O., Warias, P., & Pawlik, A. (2023). Role of Sirtuins in the Pathogenesis of Rheumatoid Arthritis. International Journal of Molecular Sciences, 24(2), 1532. https://doi.org/10.3390/ijms24021532








