The Breadth of Bacteriophages Contributing to the Development of the Phage-Based Vaccines for COVID-19: An Ideal Platform to Design the Multiplex Vaccine
Abstract
:1. Introduction
2. Phages: An Ideal Platform to Design Multiplex Vaccines
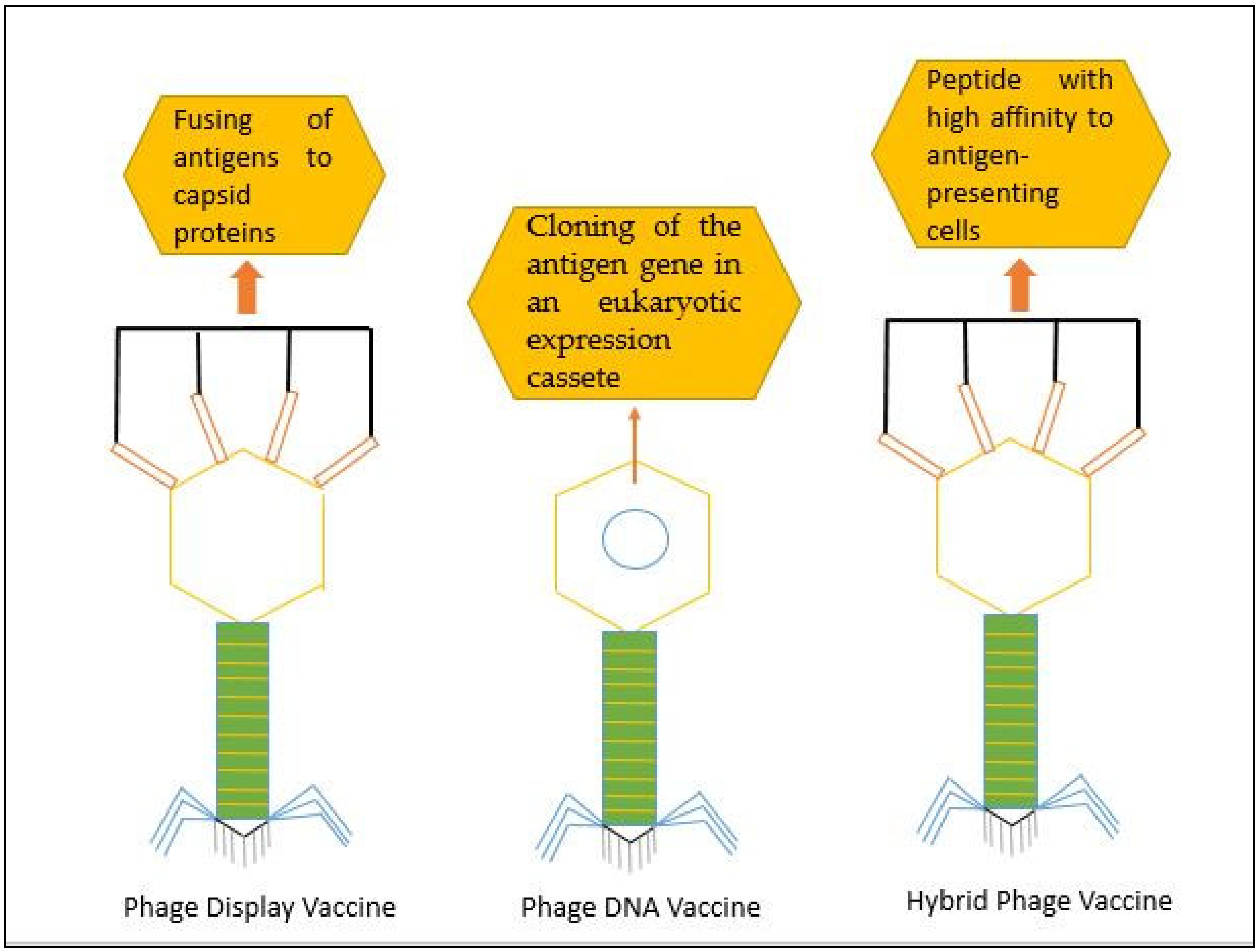
3. Phage Display Technology in Vaccine Development
4. Phage Display Technology in the Development of Antiviral Vaccines
| Phage Genera | Efficacy of Viral Phage-Based Vaccines | Reference |
|---|---|---|
| Filamentous phage particles | Phage DNA vaccine containing the herpes simplex virus 1 (HSV-1) glycoprotein D cassette triggered humoral and cellular immune responses in BALB/c mice, thus showing the efficiency of phage vaccines against HSV-1. | [20] |
| Bacteriophage PP7 | Phage-based antiviral vaccines were also found effective against human papillomavirus type 16 (HPV16) and offered comprehensive protection against HPV infection. | [21] |
| T4 phage | Phage display technology provides sufficient antiviral protection against human hepatitis virus (HCV) and human hepatitis B virus (HBV) infection. | [46] |
| Hybrid phage | The MHC class I restricted hepatitis B-specific CTL response was induced in BALB/c (H-2d) mice inoculated with a hybrid phage containing the pVIII gene with the CTL epitope [44]. | [52] |
5. The Consideration of Phage Display Technology in COVID-19
6. Bacteriophages: A Tool for the Expression and Presentation of SARS-CoV-2 Antigens
7. CRISPR Engineering in Phage Vaccines against COVID-19
8. The Pharmacological Attributes of Phage Vaccines in COVID-19
9. Safety and Biocompatibility Concerns with Phage Vaccines
10. Conclusions and Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakraborty, S.; Mallajosyula, V.; Tato, C.M.; Tan, G.S.; Wang, T.T. SARS-CoV-2 vaccines in advanced clinical trials: Where do we stand? Adv. Drug. Del. Rev. 2021, 172, 314–338. [Google Scholar] [CrossRef] [PubMed]
- Ihtisham, U.H.; Fatima, F.; Amna, S.; Abdul, B.; Firasat, H.; Israr, A.; Zarak, I.K.; Amjad, I.A.; Faisal, S.; Umair, Y.; et al. Natural Products and SARS-CoV-2. Application of Natural Products in SARS-CoV-2; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–24. ISBN 9780323950473. [Google Scholar]
- Amjad, A.; Khazeena, A.; Afshan, M.; Muhammad, A.; Muhammad, S.; Kashif, R. Chapter 2—Saponin and Its Derivatives (Glycyrrhizin) and SARS-CoV-2; Elsevier: Amsterdam, The Netherlands, 2022; pp. 25–46. [Google Scholar]
- Al-Karmalawy, A.A.; Soltane, R.; Abo Elmaaty, A.; Tantawy, M.A.; Antar, S.A.; Yahya, G.; Chrouda, A.; Pashameah, R.A.; Mustafa, M.; Abu Mraheil, M.; et al. Coronavirus Disease (COVID-19) Control between Drug Repurposing and Vaccination: A Comprehensive Overview. Vaccines 2021, 9, 1317. [Google Scholar] [CrossRef] [PubMed]
- El-Sokkary, M.M.; El-Baz, A.M.; El-Morsi, R.M.; Keuper, K.; El-Hawary, S.; Shata, A.; Yahya, G. Early forecasting of COVID-19 case progression with hematological and biochemical parameters of patients in Egypt. Pak. J. Pharm. Sci. 2022, 35, 401–408. [Google Scholar] [PubMed]
- Mostafa, I.; Mohamed, N.H.; Mohamed, B.; Almeer, R.; Abulmeaty, M.M.A.; Bungau, S.G.; El-Shazly, A.M.; Yahya, G. In-silico screening of naturally derived phytochemicals against SARS-CoV Main protease. Environ. Sci. Pollut. Res. Int. 2022, 29, 26775–26791. [Google Scholar] [CrossRef] [PubMed]
- Elmorsy, M.A.; El-Baz, A.M.; Mohamed, N.H.; Almeer, R.; Abdel-Daim, M.M.; Yahya, G. In silico screening of potent inhibitors against COVID-19 key targets from a library of FDA-approved drugs. Environ. Sci. Pollut. Res. Int. 2022, 29, 12336–12346. [Google Scholar] [CrossRef]
- Shaldam, M.A.; Yahya, G.; Mohamed, N.H.; Abdel-Daim, M.M.; Al Naggar, Y. In silico screening of potent bioactive compounds from honeybee products against COVID-19 target enzymes. Environ. Sci. Pollut. Res. Int. 2021, 28, 40507–40514. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Giesy, J.P.; Abdel-Daim, M.M.; Javed Ansari, M.; Al-Kahtani, S.N.; Yahya, G. Fighting against the second wave of COVID-19: Can honeybee products help protect against the pandemic? Saudi. J. Biol. Sci. 2021, 28, 1519–1527. [Google Scholar] [CrossRef]
- Yahya, G.; Mansour, B.; Keuper, K.; Shaldam, M.; El-Baz, M.A. Virtual Screening Attributes Male Biased COVID-19 Mortality to Predicted Antiviral Activity of Female Sex Hormones. Lett. Drug. Design. Disc. 2021, 18, 872–883. [Google Scholar] [CrossRef]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- Ga, E.; Won, Y.; Hwang, J.; Moon, S.; Yeom, M.; Lyoo, K.; Song, D.; Han, J.; Na, W. A COVID-19 Vaccine for Dogs Prevents Reverse Zoonosis. Vaccines 2022, 10, 676. [Google Scholar] [CrossRef]
- Chan, J.F.; Kok, K.H.; Zhu, Z.; Chu, H.; To, K.K.; Yuan, S.; Yuen, K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef] [Green Version]
- Rohwer, F.; Segall, A.M. In retrospect: A century of phage lessons. Nature 2015, 528, 46–48. [Google Scholar] [CrossRef] [Green Version]
- Aghebati-Maleki, L.; Bakhshinejad, B.; Baradaran, B.; Motallebnezhad, M.; Aghebati-Maleki, A.; Nickho, H.; Yousefi, M.; Majidi, J. Phage display as a promising approach for vaccine development. J. Biomed. Sci. 2016, 23, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Clementi, N.; Mancini, N.; Solforosi, L.; Castelli, M.; Clementi, M.; Burioni, R. Phage display-based strategies for cloning and optimization of monoclonal antibodies directed against human pathogens. Int. J. Mol. Sci. 2012, 13, 8273–8292. [Google Scholar] [CrossRef] [Green Version]
- Leveraging Bacteriophages in Vaccine Development. Available online: https://www.embs.org/pulse/articles/leveraging-bacteriophages-in-vaccine-development/ (accessed on 21 June 2020).
- Iborra, S.; Izquierdo, H.M.; Martínez-López, M.; Blanco-Menéndez, N.; Reis-e-Sousa, C.; Sancho, D. The DC receptor DNGR-1 mediates cross-priming of CTLs during vaccinia virus infection in mice. J Clin. Invest. 2012, 122, 1628–1643. [Google Scholar] [CrossRef] [Green Version]
- Varela-Rohena, A.; Molloy, P.E.; Dunn, S.M.; Li, Y.; Suhoski, M.M.; Carroll, R.G.; Milicic, A.; Mahon, T.; Sutton, D.H.; Laugel, B.; et al. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat. Med. 2008, 14, 1390–1395. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, H.; Bamdad, T.; Jamali, A.; Pouyanfard, S.; Mohammadi, M.G. Evaluation of humoral and cellular immune responses against HSV-1 using genetic immunization by filamentous phage particles: A comparative approach to conventional DNA vaccine. J. Virol. Methods. 2010, 163, 440–444. [Google Scholar] [CrossRef]
- Tumban, E.; Peabody, J.; Peabody, D.S.; Chackerian, B. A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PLoS ONE 2011, 6, e23310. [Google Scholar] [CrossRef]
- Tao, P.; Mahalingam, M.; Kirtley, M.L.; van Lier, C.J.; Sha, J.; Yeager, L.A.; Chopra, A.K.; Rao, V.B. Mutated and Bacteriophage T4 Nanoparticle Arrayed F1-V Immunogens From Yersinia Pestis as Next Generation Plague Vaccines. PLoS Pathog. 2013, 9, e1003495. [Google Scholar] [CrossRef] [Green Version]
- Hess, K.L.; Jewell, C.M. Phage display as a tool for vaccine and immunotherapy development. Bioeng. Transl. Med. 2020, 5, e10142. [Google Scholar] [CrossRef]
- Chauhan, G.; Madou, M.J.; Kalra, S.; Chopra, V.; Ghosh, D.; Martinez-Chapa, S.O. Nanotechnology for COVID-19: Therapeutics and vaccine research. ACS Nano 2020, 14, 7760–7782. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. npj Vaccines 2020, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Martini, F.; Maritati, M.; Mazziotta, C.; Di Mauro, G.; Lanzillotti, C.; Contini, C. SARS-CoV-2 infection: New molecular, phylogenetic, and pathogenetic insights efficacy of current vaccines and the potential risk of variants. Viruses 2021, 13, 1687. [Google Scholar] [CrossRef] [PubMed]
- Hassan, J.; Haigh, C.; Ahmed, T.; Uddin, M.J.; Das, D.B. Potential of Microneedle Systems for COVID-19 Vaccination: Current Trends and Challenges. Pharmaceutics 2022, 14, 1066. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ananthaswamy, N.; Jain, S.; Batra, H.; Tang, W.C.; Lewry, D.A.; Rao, V.B. A universal bacteriophage T4 nanoparticle platform to design multiplex SARS-CoV-2 vaccine candidates by CRISPR engineering. Sci. Adv. 2021, 7, eabh1547. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Prangishvili, D.; Forterre, P.; Garrett, R.A. Viruses of the Archaea: A unifying view. Nat. Rev. Microbiol. 2006, 4, 837–848. [Google Scholar] [CrossRef]
- Freeman, K.G.; Wetzel, K.S.; Zhang, Y.; Zack, K.M.; Jacobs-Sera, D.; Walters, S.M.; Barbeau, D.J.; McElroy, A.K.; Williams, J.V.; Hatfull, G.F. A Mycobacteriophage-Based Vaccine Platform: SARS-CoV-2 Antigen Expression and Display. Microorganisms 2021, 9, 2414. [Google Scholar] [CrossRef]
- Yadav, T.; Srivastava, N.; Mishra, G.; Dhama, K.; Kumar, S.; Puri, B.; Saxena, S.K. Recombinant vaccines for COVID-19. Hum. Vacc. Immunother. 2020, 16, 2905–2912. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Liu, Z.; Wang, Z. Phage display and its application in vaccine design. Ann. Microbiol. 2010, 60, 13–19. [Google Scholar] [CrossRef]
- Bao, Q.; Li, X.; Han, G.; Zhu, Y.; Mao, C.; Yang, M. Phage-based vaccines. Drug. Deliv. Rev. 2019, 145, 40–56. [Google Scholar] [CrossRef]
- Anand, T.; Virmani, N.; Bera, B.C.; Vaid, R.K.; Vashisth, M.; Bardajatya, P.; Kumar, A.; Tripathi, B.N. Phage Display Technique as a Tool for Diagnosis and Antibody Selection for Coronaviruses. Curr. Microbiol. 2021, 78, 1124–1134. [Google Scholar] [CrossRef]
- González-Mora, A.; Hernández-Pérez, J.; Iqbal, H.M.; Rito-Palomares, M.; Benavides, J. Bacteriophage-based vaccines: A potent approach for antigen delivery. Vaccines 2020, 8, 504. [Google Scholar] [CrossRef]
- Tao, P.; Zhu, J.; Mahalingam, M.; Batra, H.; Rao, V.B. Bacteriophage T4 nanoparticles for vaccine delivery against infectious diseases. Adv. Drug. Deliv. Rev. 2019, 145, 57–72. [Google Scholar] [CrossRef]
- Ihtisham, U.H.; Zarak, I.K.; Israr, A.; Abdul, B.; Firasat, H.; Asma, B.; Amjad, I.A.; Faisal, S.; Umair, Y.; Kashif, R. Phages and SARS-CoV-2. Application of Natural Products in SARS-CoV-2; Elsevier: Amsterdam, The Netherlands, 2022; pp. 273–292. ISBN 9780323950473. [Google Scholar]
- Sweere, J.M.; Van Belleghem, J.D.; Ishak, H.; Bach, M.S.; Popescu, M.; Sunkari, V.; Kaber, G.; Manasherob, R.; Suh, G.A.; Cao, X. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science 2019, 363, eaat9691. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Żaczek, M.; Borysowski, J. Phages in the fight against COVID-19? Future Microbiol. 2020, 15, 1095–1100. [Google Scholar] [CrossRef]
- Markosian, C.; Staquicini, D.I.; Dogra, P.; Dodero-Rojas, E.; Tang, F.H.; Smith, T.L.; Contessoto, V.G.; Libutti, S.K.; Wang, Z.; Cristini, V.; et al. Apropos of Universal Epitope Discovery for COVID-19 Vaccines: A Framework for Targeted Phage Display-Based, Delivery and Integration of New Evaluation Tools. Biorxiv 2021. [Google Scholar] [CrossRef]
- Guo, J.Y.; Liu, I.J.; Lin, H.T.; Wang, M.J.; Chang, Y.L.; Lin, S.C.; Liao, M.Y.; Hsu, W.C.; Lin, Y.L.; Liao, J.C.; et al. Identification of COVID-19 B-cell epitopes with phage-displayed peptide library. J. Biomed. Sci. 2021, 28, 1–3. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection (accessed on 8 December 2020).
- Shahin, K.; Zhang, L.; Mehraban, M.H.; Collard, J.M.; Hedayatkhah, A.; Mansoorianfar, M.; Soleimani-Delfan, A.; Wang, R. Clinical and experimental bacteriophage studies: Recommendations for possible approaches for standing against SARS-CoV-2. Microb. Pathog. 2022, 164, 105442. [Google Scholar] [CrossRef]
- Popescu, M.; Van Belleghem, J.D.; Khosravi, A.; Bollyky, P.L. Bacteriophages and the immune system. Annu. Rev. Virol. 2021, 8, 415–435. [Google Scholar] [CrossRef]
- Bazan, J.; Całkosiński, I.; Gamian, A. Phage display—A powerful technique for immunotherapy: 1. Introduction and potential of therapeutic applications. Hum. Vac. Immunother. 2012, 8, 1817–1828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samoylova, T.I.; Norris, M.D.; Samoylov, A.M.; Cochran, A.M.; Wolfe, K.G.; Petrenko, V.A.; Cox, N.R. Infective and inactivated filamentous phage as carriers for immunogenic peptides. J. Virol. Methods 2012, 183, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ledón, T.; Ferrán, B.; Pérez, C.; Suzarte, E.; Vichi, J.; Marrero, K.; Oliva, R.; Fando, R. TLP01, an mshA mutant of Vibrio cholerae O139 as vaccine candidate against cholera. Microbes. Infect. 2012, 14, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, H.; Rao, H.; Jiang, D.; Cong, X.; Feng, B.; Wang, J.; Wei, L.; Chen, H. DCs pulsed with novel HLA-A2-restricted CTL epitopes against hepatitis C virus induced a broadly reactive anti-HCV-specific T lymphocyte response. PLoS ONE 2012, 7, e38390. [Google Scholar] [CrossRef]
- Do Carmo Caldeira, J.; Medford, A.; Kines, R.C.; Lino, C.A.; Schiller, J.T.; Chackerian, B.; Peabody, D.S. Immunogenic display of diverse peptides, including a broadly cross-type neutralizing human papillomavirus L2 epitope, on virus-like particles of the RNA bacteriophage PP7. Vaccine 2010, 28, 4384–4393. [Google Scholar] [CrossRef] [Green Version]
- Slupetzky, K.; Gambhira, R.; Culp, T.D.; Shafti-Keramat, S.; Schellenbacher, C.; Christensen, N.D.; Roden, R.B.; Kirnbauer, R.A. A papillomavirus-like particle (VLP) vaccine displaying HPV16 L2 epitopes induces cross-neutralizing antibodies to HPV11. Vaccine 2007, 25, 2001–2010. [Google Scholar] [CrossRef] [Green Version]
- Castel, G.; Chtéoui, M.; Heyd, B.; Tordo, N. Phage display of combinatorial peptide libraries: Application to antiviral research. Molecules 2011, 16, 3499–3518. [Google Scholar] [CrossRef] [Green Version]
- Tao, P.; Mahalingam, M.; Zhu, J.; Moayeri, M.; Sha, J.; Lawrence, W.S.; Leppla, S.H.; Chopra, A.K.; Rao, V.B. A bacteriophage T4 nanoparticle-based dual vaccine against anthrax and plague. MBio 2018, 9, e01926-18. [Google Scholar] [CrossRef] [Green Version]
- Egbuna, C.; Chandra, S.; Awuchi, C.G.; Saklani, S.; Ulhaq, I.; Akram, M.; Khan, J. Myth surrounding the FDA disapproval of hydroxychloroquine sulfate and chloroquine phosphate as drugs for coronavirus disease 2019. In Coronavirus Drug Disc; Elsevier: Amsterdam, The Netherlands, 2022; pp. 153–168. [Google Scholar]
- Maryam, S.; Ul Haq, I.; Yahya, G.; Ul Haq, M.; Algammal, A.M.; Saber, S.; Cavalu, S. COVID-19 surveillance in wastewater: An epidemiological tool for the monitoring of SARS-CoV-2. Front. Cell. Infect. Microbiol. 2023, 12, 978643. [Google Scholar] [CrossRef]
- Basit, A.; Ulhaq, I.; Hussain, F.; Ud-Din, Z.; Rahim, K. Nucleic Acid-Based Detection of COVID-19, Coronavirus Disease-19 (COVID-19): A Perspective of New Scenario COVID-19: Epidemiology, Biochemistry, and Diagnostics. Bentham Sci 2021, 1, 405. [Google Scholar] [CrossRef]
- Ayaz, M.M.; Ulhaq, I.; Rahim, K. Histopathologic Evaluation and Scoring of SARS-CoV- 2 Infection, Coronavirus Disease-19 (COVID-19): A Perspective of New Scenario Coronavirus Disease-19 (COVID-19): Different Models and Treatment Strategies. Bentham Sci 2021, 2, 52. [Google Scholar] [CrossRef]
- Ulhaq, I.; Basit, A.; Ali., I.; Hussain, F.; Ali, Z.; Siddique, F.; Ahmed, H.; Aqib, A.I.; Rahim, K. Coronavirus Disease-2019 (COVID-19) Epidemiology, Coronavirus Disease-19 (COVID-19): A Perspective of New Scenario COVID-19: Epidemiology, Biochemistry, and Diagnostics. Bentham. Sci. 2021, 1, 1. [Google Scholar] [CrossRef]
- Petrenko, V.A.; Gillespie, J.W.; De Plano, L.M.; Shokhen, M.A. Phage-Displayed Mimotopes of SARS-CoV-2 Spike Protein Targeted to Authentic and Alternative Cellular Receptors. Viruses 2022, 14, 384. [Google Scholar] [CrossRef]
- Yang, F.; Liu, L.; Neuenschwander, P.F.; Idell, S.; Vankayalapati, R.; Jain, K.G.; Du, K.; Ji, H.; Yi, G. Phage Display-Derived Peptide for the Specific Binding of SARS-CoV-2. ACS Omega 2021, 7, 3203–3211. [Google Scholar] [CrossRef]
- Staquicini, D.I.; Tang, F.H.; Markosian, C.; Yao, V.J.; Staquicini, F.I. Design and proof-of-concept 363 for targeted phage-based COVID-19 vaccination strategies with a streamlined cold-free 364 supply chain. Proc. Natl. Acad. Sci. 2021, 118, e2105739118. [Google Scholar] [CrossRef]
- Davenport, B.J.; Catala, A.; Weston, S.M.; Johnson, R.M.; Ardanuy, J.; Hammond, H.L.; Dillen, C.; Frieman, M.B.; Catalano, C.E.; Morrison, T.E. Phage-like particle vaccines are highly immunogenic and protect against pathogenic coronavirus infection and disease. npj Vaccines 2022, 7, 1–15. [Google Scholar] [CrossRef]
- Elahi, Y.; Mazaheri Nezhad Fard, R. Phage Therapy in COVID-19 Treatment. Int. J. Infect. 2022, 9, e116630. [Google Scholar] [CrossRef]
- Chen, Y.; Batra, H.; Dong, J.; Chen, C.; Rao, V.B.; Tao, P. Genetic engineering of bacteriophages against infectious diseases. Front. Microbiol. 2019, 10, 954. [Google Scholar] [CrossRef]
- Martinecz, A.; Wojewodzic, M.W. Could bacteriophages be the answer to the COVID-19 crisis? Exp Rev. Anti-Infect. Ther 2021, 19, 557–558. [Google Scholar] [CrossRef]
- Ng, W.H.; Liu, X.; Mahalingam, S. Development of vaccines for SARS-CoV-2. F1000Research 2022, 9, 991. [Google Scholar] [CrossRef]
- Excler, J.L.; Saville, M.; Berkley, S.; Kim, J.H. Vaccine development for emerging infectious diseases. Nat. Med. 2021, 27, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Zalewska-Piątek, B.; Piątek, R. Bacteriophages as Potential Tools for Use in Antimicrobial Therapy and Vaccine Development. Pharmaceuticals 2021, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ding, Y.; Yao, J.; Zhang, M.; Zhang, Y.; Xie, Z.; Zuo, J. Nasal Nanovaccines for SARS-CoV-2 to Address COVID-19. Vaccines 2022, 10, 405. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Gaur, V.; Kumar, A. Role of Phage Therapy in COVID-19 Infection: Future Prospects. In Bacteriophages in Therapeutics; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Piekarowicz, A.; Kłyż Stein, D.C. A New Vaccination Method Based on Phage NgoΦ6 and Its Phagemid Derivatives. Front. Microbiol. 2022, 13, 793205. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Freeman, D.; Waite, F.; Rosebrock, L.; Petit, A.; Causier, C.; East, A.; Jenner, L.; Teale, A.L.; Carr, L.; Mulhall, S.; et al. Coronavirus conspiracy beliefs, mistrust, and compliance with government guidelines in England. Psychol. Med. 2022, 52, 251–263. [Google Scholar] [CrossRef]
- Charlton Hume, H.K.; Vidigal, J.; Carrondo, M.J.; Middelberg, A.P.; Roldão, A.; Lua, L.H. Synthetic biology for bioengineering virus-like particle vaccines. Biotechnol. Bioeng. 2019, 116, 919–935. [Google Scholar] [CrossRef]
- Dai, S.; Wang, H.; Deng, F. Advances and challenges in enveloped virus-like particle (VLP)-based vaccines. J. Immunol. Sci. 2018, 2, 36–41. [Google Scholar]
- Roldao, A.; Silva, A.C.; Mellado, M.C.M.; Alves, P.M.; Carrondo, M.J.T. Viruses and virus-like particles in biotechnology: Fundamentals and applications. Compr. Biotechnol. 2011, 1, 633–656. [Google Scholar]



| Phage Genera | SARS-CoV-2 Proteins | Immune Responses | References |
|---|---|---|---|
| Mycobacteriophages | SARS-CoV-2 RB motif segments | Elicited IgG-mediated antibody responses in mice against SARS-CoV-2. | [23] |
| Universal bacteriophage T4 | Envelope proteins and capsid proteins | The proteins were inserted into a phage using CRISPR engineering. Specific and broader immune responses were triggered against multiple SARS-CoV-2 targets using a single-phage backbone in which various antigens were incorporated. | [28] |
| 12-mer phage | RB domain of the S protein | A phage display peptide library was screened against RBD, which showed binding ability with the RBD. It was demonstrated that peptide no. 1 could specifically bind to the SARS-CoV-2 RBD. It was confirmed that the SARS-CoV-2-specific peptide holds great promise as a new bioreceptor and ligand for the rapid and accurate detection of SARS-CoV-2. | [60] |
| Adeno-associated virus/phage (AAVP) particles | S protein | The vaccine showed a strong targeted humoral response. A strong systemic and specific immune response was observed with phage particles against SARS-CoV-2 in immune-competent mice by aerosol pulmonary vaccination. | [61] |
| Lambda phages | CoV-2-RBD of spike proteins | Robust immune responses were observed by the activity of hCoV-RBD decorated PLPs, which induced antibodies with neutralizing activities and presented the lambda system as a promising vaccine candidate with high versatility and robustness. | [62] |
| Phage (N0315S) | ORF1ab polyprotein, spike glycoprotein (S), ORF3a, envelope protein (E), membrane glycoprotein (M), ORF7a, ORF7b, ORF8 NP phosphoprotein (N), ORF10 | A phage displayed peptide library identified B cell epitopes for SARS-CoV-2. In summary, it was concluded that this information regarding SARS-CoV-2 epitopes could help in the the rapid development of immunodiagnostic tools and showed promise for guiding epitope-based vaccine design. | [42] |
| Filamentous phage M13 | RBD of SARS-CoV-2 | A panel of phage mimotopes and spike receptor-binding protein AA clusters were identified which were able to bind to ACE2 and FGFR3. The phage probes that competed with viruses for cellular binding receptors could serve as leads in the development of vaccines. | [52] |
| T4 phage | Spike trimers on the exterior surface of the protein and the interior surface of the nucleocapsid protein | The vaccine showed valid results in providing complete protection against COVID-19. Different parts of the T4 phage genome had an enormous amount of unnecessary genetic space that could be ideally used for the development of a universal vaccine design template. | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ul Haq, I.; Krukiewicz, K.; Yahya, G.; Haq, M.U.; Maryam, S.; Mosbah, R.A.; Saber, S.; Alrouji, M. The Breadth of Bacteriophages Contributing to the Development of the Phage-Based Vaccines for COVID-19: An Ideal Platform to Design the Multiplex Vaccine. Int. J. Mol. Sci. 2023, 24, 1536. https://doi.org/10.3390/ijms24021536
Ul Haq I, Krukiewicz K, Yahya G, Haq MU, Maryam S, Mosbah RA, Saber S, Alrouji M. The Breadth of Bacteriophages Contributing to the Development of the Phage-Based Vaccines for COVID-19: An Ideal Platform to Design the Multiplex Vaccine. International Journal of Molecular Sciences. 2023; 24(2):1536. https://doi.org/10.3390/ijms24021536
Chicago/Turabian StyleUl Haq, Ihtisham, Katarzyna Krukiewicz, Galal Yahya, Mehboob Ul Haq, Sajida Maryam, Rasha A. Mosbah, Sameh Saber, and Mohammed Alrouji. 2023. "The Breadth of Bacteriophages Contributing to the Development of the Phage-Based Vaccines for COVID-19: An Ideal Platform to Design the Multiplex Vaccine" International Journal of Molecular Sciences 24, no. 2: 1536. https://doi.org/10.3390/ijms24021536
APA StyleUl Haq, I., Krukiewicz, K., Yahya, G., Haq, M. U., Maryam, S., Mosbah, R. A., Saber, S., & Alrouji, M. (2023). The Breadth of Bacteriophages Contributing to the Development of the Phage-Based Vaccines for COVID-19: An Ideal Platform to Design the Multiplex Vaccine. International Journal of Molecular Sciences, 24(2), 1536. https://doi.org/10.3390/ijms24021536







