Pathogenesis and Treatment of Pruritus Associated with Chronic Kidney Disease and Cholestasis
Abstract
:1. Introduction
2. Clinical Feature of CKD-Associated Pruritus
2.1. CKD-Associated Pruritus and Patients Characteristics
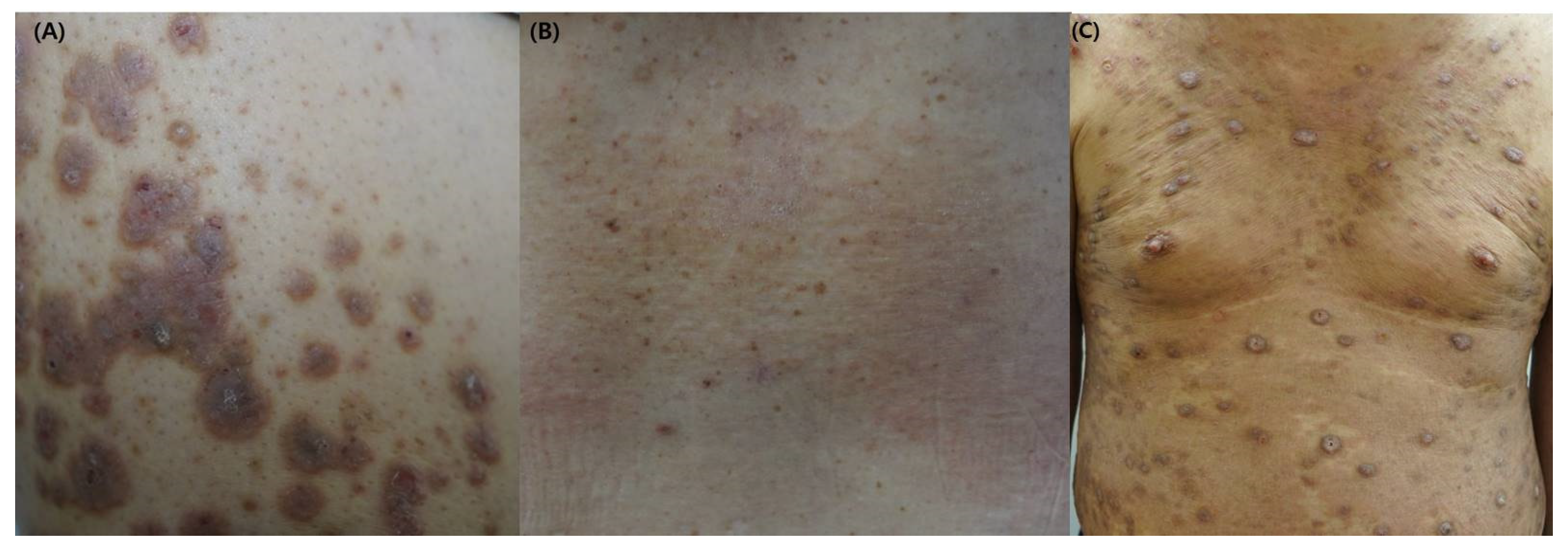
2.2. Clinical Manifestations of CKD-Associated Pruritus
2.3. The Quality of Life Affected by CKD-Associated Pruritus
3. Pathogenesis of CKD-Associated Pruritus
3.1. Dysregulation of the Endogenous Opioid System
3.2. Uremic Neuropathy
3.3. Alteration of Metabolism
3.4. Immune System Dysregulation
3.5. Xerosis
3.6. Neuropeptide Natriuretic Polypeptide b (NPPB)
4. Treatment of CKD-Associated Pruritus
4.1. Gabapentin or Pregabalin
4.2. TRPV1 and TRPM8 Agonist
4.3. Specific Opioid Agonists and Antagonists
4.4. Cannabinoids
4.5. Dialysis Modification
4.6. Others
5. Clinical Feature of Cholestasis-Associated Pruritus
6. Pathogenesis of Cholestasis-Associated Pruritus
6.1. Bile Acid
6.2. Bilirubin and MRGPRX4 Receptor
6.3. Substance P (SP)
6.4. Lysophosphatidic Acid (LPA) and Autotaxin
6.5. Lysophosphatidylcholine (LPC) and TRPV4
6.6. Histamine
6.7. Bovine Adrenal Medulla 8-22 (BAM8-22)
6.8. Others
7. Treatment of Cholestasis-Associated Pruritus
7.1. Treatment for Underlying Disease
7.2. Ursodeoxycholic Acid (UDCA) and Obeticholic Acid (OCA)
7.3. Benzofibrate/Elafibranor
7.4. Cholestyramine
7.5. Rifampicin
7.6. Naltrexone
7.7. Selective serotonin Reuptake Inhibitor (SSRI)
7.8. Maralixibat
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CA | Cholic acid |
| CaP | Calcium phosphate |
| CDCA | Chenodeoxycholic acid |
| CKD | Chronic kidney disease |
| DCA | Deoxycholic acid |
| DOR | δ-opioid receptor |
| GABA | Gamma-aminobutyric acid |
| KOR | κ-opioid receptor |
| LPA | Lysophosphatidic acid |
| LPC | Lysophosphatidylcholilne |
| MOR | μ-opioid receptor |
| MRGPRX4 | Mas-Related G Protein-coupled Receptor member X4 |
| SP | Substance P |
| TGR5 | Takeda G protein-coupled receptor 5 |
| TRP | Transient receptor potential |
| UV | Ultraviolet |
References
- Kimata, N.; Karaboyas, A.; Bieber, B.A.; Pisoni, R.L.; Morgenstern, H.; Gillespie, B.W.; Saito, A.; Akizawa, T.; Fukuhara, S.; Robinson, B.M.; et al. Gender, low Kt/V, and mortality in Japanese hemodialysis patients: Opportunities for improvement through modifiable practices. Hemodial. Int. 2014, 18, 596–606. [Google Scholar] [CrossRef] [Green Version]
- Mettang, T.; Kremer, A.E. Uremic pruritus. Kidney Int. 2015, 87, 685–691. [Google Scholar] [CrossRef] [Green Version]
- Rayner, H.C.; Larkina, M.; Wang, M.; Graham-Brown, M.; van der Veer, S.N.; Ecder, T.; Hasegawa, T.; Kleophas, W.; Bieber, B.A.; Tentori, F.; et al. International Comparisons of Prevalence, Awareness, and Treatment of Pruritus in People on Hemodialysis. Clin. J. Am. Soc. Nephrol. 2017, 12, 2000–2007. [Google Scholar] [CrossRef] [Green Version]
- Patel, T.S.; Freedman, B.I.; Yosipovitch, G. An update on pruritus associated with CKD. Am. J. Kidney Dis. 2007, 50, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisoni, R.L.; Wikström, B.; Elder, S.J.; Akizawa, T.; Asano, Y.; Keen, M.L.; Saran, R.; Mendelssohn, D.C.; Young, E.W.; Port, F.K. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transplant. 2006, 21, 3495–3505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, J.W.; Kim, S.H.; Kim, Y.O.; Jin, D.C.; Song, H.C.; Choi, E.J.; Kim, Y.L.; Kim, Y.S.; Kang, S.W.; Kim, N.H.; et al. Comparison of uremic pruritus between patients undergoing hemodialysis and peritoneal dialysis. Kidney Res. Clin. Pr. 2016, 35, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwab, M.; Mikus, G.; Mettang, T.; Pauli-Magnus, C.; Kuhlmann, U. Urämischer pruritus im Kindes-und Jugendalter. Mon. Kinderheilkd. 1999, 147, 232. [Google Scholar]
- Tessari, G.; Dalle Vedove, C.; Loschiavo, C.; Tessitore, N.; Rugiu, C.; Lupo, A.; Girolomoni, G. The impact of pruritus on the quality of life of patients undergoing dialysis: A single centre cohort study. J. Nephrol. 2009, 22, 241–248. [Google Scholar]
- Narita, I.; Alchi, B.; Omori, K.; Sato, F.; Ajiro, J.; Saga, D.; Kondo, D.; Skatsume, M.; Maruyama, S.; Kazama, J.J.; et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006, 69, 1626–1632. [Google Scholar] [CrossRef] [Green Version]
- Hiroshige, K.; Kabashima, N.; Takasugi, M.; Kuroiwa, A. Optimal dialysis improves uremic pruritus. Am. J. Kidney Dis. 1995, 25, 413–419. [Google Scholar] [CrossRef]
- Masi, C.M.; Cohen, E.P. Dialysis efficacy and itching in renal failure. Nephron 1992, 62, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Massry, S.G.; Popovtzer, M.M.; Coburn, J.W.; Makoff, D.L.; Maxwell, M.H.; Kleeman, C.R. Intractable pruritus as a manifestation of secondary hyperparathyroidism in uremia. Disappearance of itching after subtotal parathyroidectomy. N. Engl. J. Med. 1968, 279, 697–700. [Google Scholar] [CrossRef]
- Chou, F.F.; Ho, J.C.; Huang, S.C.; Sheen-Chen, S.M. A study on pruritus after parathyroidectomy for secondary hyperparathyroidism. J. Am. Coll. Surg. 2000, 190, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Blachley, J.D.; Blankenship, D.M.; Menter, A.; Parker, T.F., 3rd; Knochel, J.P. Uremic pruritus: Skin divalent ion content and response to ultraviolet phototherapy. Am. J. Kidney Dis. 1985, 5, 237–241. [Google Scholar] [CrossRef]
- Duque, M.I.; Thevarajah, S.; Chan, Y.H.; Tuttle, A.B.; Freedman, B.I.; Yosipovitch, G. Uremic pruritus is associated with higher kt/V and serum calcium concentration. Clin. Nephrol. 2006, 66, 184–191. [Google Scholar] [CrossRef]
- Cawley, E.P.; Hoch-ligeti, C.; Bond, G.M. The eccrine sweat glands of patients in uremia. Arch. Dermatol. 1961, 84, 889–897. [Google Scholar] [CrossRef]
- Goicoechea, M.; de Sequera, P.; Ochando, A.; Andrea, C.; Caramelo, C. Uremic pruritus: An unresolved problem in hemodialysis patients. Nephron 1999, 82, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Friga, V.; Linos, A.; Linos, D.A. Is aluminum toxicity responsible for uremic pruritus in chronic hemodialysis patients? Nephron 1997, 75, 48–53. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Mora-Fernández, C.; García-Pérez, J. Reviews: Clinical implications of disordered magnesium homeostasis in chronic renal failure and dialysis. In Seminars in Dialysis; Blackwell Publishing Ltd: Oxford, UK, 2009; pp. 37–44. [Google Scholar]
- Carmichael, A.J.; McHugh, M.M.; Martin, A.M.; Farrow, M. Serological markers of renal itch in patients receiving long term haemodialysis. Br. Med. J. (Clin. Res. Ed.) 1988, 296, 1575. [Google Scholar] [CrossRef] [Green Version]
- Mistik, S.; Utas, S.; Ferahbas, A.; Tokgoz, B.; Unsal, G.; Sahan, H.; Ozturk, A.; Utas, C. An epidemiology study of patients with uremic pruritus. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 672–678. [Google Scholar] [CrossRef]
- Wikström, B. Itchy skin—A clinical problem for haemodialysis patients. Nephrol. Dial. Transplant. 2007, 22, v3–v7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, V.S.; Lindberg, J.; Germain, M.; Block, G.; Tumlin, J.; Smith, M.; Grewal, M.; McGuire, D. A longitudinal study of uremic pruritus in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2010, 5, 1410–1419. [Google Scholar] [CrossRef]
- Mettang, T.; Pauli-Magnus, C.; Alscher, D.M. Uraemic pruritus--new perspectives and insights from recent trials. Nephrol. Dial. Transplant. 2002, 17, 1558–1563. [Google Scholar] [CrossRef]
- Bencini, P.L.; Montagnino, G.; Citterio, A.; Graziani, G.; Crosti, C.; Ponticelli, C. Cutaneous abnormalities in uremic patients. Nephron 1985, 40, 316–321. [Google Scholar] [CrossRef]
- Hayani, K.; Weiss, M.; Weisshaar, E. Clinical Findings and Provision of Care in Haemodialysis Patients with Chronic Itch: New Results from the German Epidemiological Haemodialysis Itch Study. Acta Derm.-Venereol. 2016, 96, 361–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieczorek, A.; Krajewski, P.; Kozioł-Gałczyńska, M.; Szepietowski, J.C. Opioid receptors expression in the skin of haemodialysis patients suffering from uraemic pruritus. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2368–2372. [Google Scholar] [CrossRef]
- Akiyama, T.; Carstens, E. Frontiers in Neuroscience Spinal Coding of Itch and Pain. In Itch: Mechanisms and Treatment; Carstens, E., Akiyama, T., Eds.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Kardon, A.P.; Polgár, E.; Hachisuka, J.; Snyder, L.M.; Cameron, D.; Savage, S.; Cai, X.; Karnup, S.; Fan, C.R.; Hemenway, G.M.; et al. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 2014, 82, 573–586. [Google Scholar] [CrossRef] [Green Version]
- Phan, N.Q.; Bernhard, J.D.; Luger, T.A.; Ständer, S. Antipruritic treatment with systemic μ-opioid receptor antagonists: A review. J. Am. Acad. Dermatol. 2010, 63, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, H.; Ebata, T.; Takamori, K.; Muramatsu, T.; Nakamoto, H.; Suzuki, H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: A Phase III, randomized, double-blind, placebo-controlled study. Nephrol. Dial. Transpl. 2010, 25, 1251–1257. [Google Scholar] [CrossRef] [Green Version]
- Schricker, S.; Kimmel, M. Unravelling the pathophysiology of chronic kidney disease-associated pruritus. Clin. Kidney J. 2021, 14, i23–i31. [Google Scholar] [CrossRef]
- Fantini, F.; Baraldi, A.; Sevignani, C.; Spattini, A.; Pincelli, C.; Giannetti, A. Cutaneous innervation in chronic renal failure patients. An immunohistochemical study. Acta Derm.-Venereol. 1992, 72, 102–105. [Google Scholar] [PubMed]
- Johansson, O.; Hilliges, M.; Ståhle-Bäckdahl, M. Intraepidermal neuron-specific enolase (NSE)-immunoreactive nerve fibres: Evidence for sprouting in uremic patients on maintenance hemodialysis. Neurosci. Lett. 1989, 99, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Weisshaar, E.; Dunker, N.; Röhl, F.W.; Gollnick, H. Antipruritic effects of two different 5-HT3 receptor antagonists and an antihistamine in haemodialysis patients. Exp. Dermatol. 2004, 13, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Keshari, S.; Sipayung, A.D.; Hsieh, C.C.; Su, L.J.; Chiang, Y.R.; Chang, H.C.; Yang, W.C.; Chuang, T.H.; Chen, C.L.; Huang, C.M. IL-6/p-BTK/p-ERK signaling mediates calcium phosphate-induced pruritus. FASEB J. 2019, 33, 12036–12046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhag, S.; Rivas, N.; Tejovath, S.; Mustaffa, N.; Deonarine, N.; Abdullah Hashmi, M.; Yerneni, S.; Hamid, P. Chronic Kidney Disease-Associated Pruritus: A Glance at Novel and Lesser-Known Treatments. Cureus 2022, 14, e21127. [Google Scholar] [CrossRef]
- Lu, P.H.; Wang, J.Y.; Chuo, H.E.; Lu, P.H. Effects of Uremic Clearance Granules in Uremic Pruritus: A Meta-Analysis. Toxins 2021, 13, 702. [Google Scholar] [CrossRef]
- Verduzco, H.A.; Shirazian, S. CKD-Associated Pruritus: New Insights Into Diagnosis, Pathogenesis, and Management. Kidney Int. Rep. 2020, 5, 1387–1402. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, H.; Ding, J.R.; Hong, Z.Y.; Wu, H.; Zhu, Z.Y.; Guo, Z.Y.; Chai, Y.F. UPLC-QTOF MS-Based Serum Metabolomic Profiling Analysis Reveals the Molecular Perturbations Underlying Uremic Pruritus. Biomed Res. Int. 2018, 2018, 4351674. [Google Scholar] [CrossRef] [Green Version]
- Mettang, T.; Fischer, F.P.; Dollenbacher, U.; Kuhlman, U. Uraemic pruritus is not related to beta-endorphin serum levels in haemodialysis patients. Nephrol. Dial. Transpl. 1998, 13, 231–232. [Google Scholar] [CrossRef] [Green Version]
- Seckin, D.; Demircay, Z.; Akin, O. Generalized pruritus treated with narrowband UVB. Int. J. Dermatol. 2007, 46, 367–370. [Google Scholar] [CrossRef]
- Agarwal, P.; Garg, V.; Karagaiah, P.; Szepietowski, J.C.; Grabbe, S.; Goldust, M. Chronic Kidney Disease-Associated Pruritus. Toxins 2021, 13, 527. [Google Scholar] [CrossRef]
- Malekmakan, L.; Malekmakan, A.; Sayadi, M.; Pakfetrat, M.; Sepaskhah, M.; Roozbeh, J. Association of high-sensitive C-reactive protein and dialysis adequacy with uremic pruritus. Saudi J. Kidney Dis. Transpl. 2015, 26, 890–895. [Google Scholar] [CrossRef]
- Kimmel, M.; Alscher, D.M.; Dunst, R.; Braun, N.; Machleidt, C.; Kiefer, T.; Stülten, C.; van der Kuip, H.; Pauli-Magnus, C.; Raub, U.; et al. The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol. Dial. Transpl. 2006, 21, 749–755. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.K.; Hoon, M.A. The cells and circuitry for itch responses in mice. Science 2013, 340, 968–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solinski, H.J.; Dranchak, P.; Oliphant, E.; Gu, X.; Earnest, T.W.; Braisted, J.; Inglese, J.; Hoon, M.A. Inhibition of natriuretic peptide receptor 1 reduces itch in mice. Sci. Transl. Med. 2019, 11, eaav5464. [Google Scholar] [CrossRef] [PubMed]
- Francos, G.C.; Kauh, Y.C.; Gittlen, S.D.; Schulman, E.S.; Besarab, A.; Goyal, S.; Burke, J.F., Jr. Elevated plasma histamine in chronic uremia. Effects of ketotifen on pruritus. Int. J. Dermatol. 1991, 30, 884–889. [Google Scholar] [CrossRef]
- Mettang, T.; Fritz, P.; Weber, J.; Machleidt, C.; Hübel, E.; Kuhlmann, U. Uremic pruritus in patients on hemodialysis or continuous ambulatory peritoneal dialysis (CAPD). The role of plasma histamine and skin mast cells. Clin. Nephrol. 1990, 34, 136–141. [Google Scholar] [PubMed]
- Amirkhanlou, S.; Rashedi, A.; Taherian, J.; Hafezi, A.A.; Parsaei, S. Comparison of Gabapentin and Ketotifen in Treatment of Uremic Pruritus in Hemodialysis Patients. Pak. J. Med. Sci. 2016, 32, 22–26. [Google Scholar] [CrossRef]
- Foroutan, N.; Etminan, A.; Nikvarz, N.; Shojai Shahrokh Abadi, M. Comparison of pregabalin with doxepin in the management of uremic pruritus: A randomized single blind clinical trial. Hemodial. Int. 2017, 21, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Gunal, A.I.; Ozalp, G.; Yoldas, T.K.; Gunal, S.Y.; Kirciman, E.; Celiker, H. Gabapentin therapy for pruritus in haemodialysis patients: A randomized, placebo-controlled, double-blind trial. Nephrol. Dial. Transpl. 2004, 19, 3137–3139. [Google Scholar] [CrossRef] [Green Version]
- Tol, H.; Atalay, H.; Güney, İ.; Gökbel, H.; Altıntepe, L.; Büyükbaş, S.; Bodur, S.; Selçuk, N.Y.; Tonbul, H.Z.; Yeksan, M.; et al. The effects of gabapentin therapy on pruritus, quality of life, depression and sleep quality in pruritic hemodialysis patients. Balk. Med. J. 2010, 27, 1–5. [Google Scholar]
- Naini, A.E.; Harandi, A.A.; Khanbabapour, S.; Shahidi, S.; Seirafiyan, S.; Mohseni, M. Gabapentin: A promising drug for the treatment of uremic pruritus. Saudi J. Kidney Dis. Transpl. 2007, 18, 378–381. [Google Scholar]
- Nofal, E.; Farag, F.; Nofal, A.; Eldesouky, F.; Alkot, R.; Abdelkhalik, Z. Gabapentin: A promising therapy for uremic pruritus in hemodialysis patients: A randomized-controlled trial and review of literature. J. Dermatol. Treat. 2016, 27, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Solak, Y.; Biyik, Z.; Atalay, H.; Gaipov, A.; Guney, F.; Turk, S.; Covic, A.; Goldsmith, D.; Kanbay, M. Pregabalin versus gabapentin in the treatment of neuropathic pruritus in maintenance haemodialysis patients: A prospective, crossover study. Nephrology 2012, 17, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Jiao, S.; Xiao, Y.; Ren, W.; Zhao, T.; Meng, J. Comparison of pregabalin with ondansetron in treatment of uraemic pruritus in dialysis patients: A prospective, randomized, double-blind study. Int. Urol. Nephrol. 2015, 47, 161–167. [Google Scholar] [CrossRef]
- Matsuda, K.M.; Sharma, D.; Schonfeld, A.R.; Kwatra, S.G. Gabapentin and pregabalin for the treatment of chronic pruritus. J. Am. Acad. Dermatol. 2016, 75, 619–625.e6. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Bíró, T. A TR(I)P to pruritus research: Role of TRPV3 in inflammation and itch. J. Investig. Dermatol. 2009, 129, 531–535. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, Z.L.; Yeo, M.; Zhang, Q.J.; López-Romero, A.E.; Ding, H.P.; Zhang, X.; Zeng, Q.; Morales-Lázaro, S.L.; Moore, C.; et al. Epithelia-Sensory Neuron Cross Talk Underlies Cholestatic Itch Induced by Lysophosphatidylcholine. Gastroenterology 2021, 161, 301–317.e16. [Google Scholar] [CrossRef]
- Liu, B.; Jordt, S.E. Cooling the Itch via TRPM8. J. Investig. Dermatol. 2018, 138, 1254–1256. [Google Scholar] [CrossRef] [Green Version]
- Tarng, D.C.; Cho, Y.L.; Liu, H.N.; Huang, T.P. Hemodialysis-related pruritus: A double-blind, placebo-controlled, crossover study of capsaicin 0.025% cream. Nephron 1996, 72, 617–622. [Google Scholar] [CrossRef]
- Cho, Y.L.; Liu, H.N.; Huang, T.P.; Tarng, D.C. Uremic pruritus: Roles of parathyroid hormone and substance P. J. Am. Acad. Dermatol. 1997, 36, 538–543. [Google Scholar] [CrossRef]
- Denda, M.; Tsutsumi, M.; Denda, S. Topical application of TRPM8 agonists accelerates skin permeability barrier recovery and reduces epidermal proliferation induced by barrier insult: Role of cold-sensitive TRP receptors in epidermal permeability barrier homoeostasis. Exp. Dermatol. 2010, 19, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Choi, M.G.; Wei, E.T.; Selescu, T.; Lee, S.Y.; Kim, J.C.; Chung, B.Y.; Park, C.W.; Kim, H.O. TRPM8 agonist (cryosim-1) gel for scalp itch: A randomised, vehicle-controlled clinical trial. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e588–e589. [Google Scholar] [CrossRef]
- Jung, M.J.; Kim, J.C.; Wei, E.T.; Selescu, T.; Chung, B.Y.; Park, C.W.; Kim, H.O. A randomized, vehicle-controlled clinical trial of a synthetic TRPM8 agonist (Cryosim-1) gel for itch. J. Am. Acad. Dermatol. 2021, 84, 869–871. [Google Scholar] [CrossRef]
- Fishbane, S.; Jamal, A.; Munera, C.; Wen, W.; Menzaghi, F. A Phase 3 Trial of Difelikefalin in Hemodialysis Patients with Pruritus. N. Engl. J. Med. 2020, 382, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Hawi, A.; Alcorn, H.; Berg, J.; Hines, C.; Hait, H.; Sciascia, T. Pharmacokinetics of nalbuphine hydrochloride extended release tablets in hemodialysis patients with exploratory effect on pruritus. BMC Nephrol. 2015, 16, 47. [Google Scholar] [CrossRef] [Green Version]
- Peer, G.; Kivity, S.; Agami, O.; Fireman, E.; Silverberg, D.; Blum, M.; Iaina, A. Randomised crossover trial of naltrexone in uraemic pruritus. Lancet 1996, 348, 1552–1554. [Google Scholar] [CrossRef]
- Pauli-Magnus, C.; Mikus, G.; Alscher, D.M.; Kirschner, T.; Nagel, W.; Gugeler, N.; Risler, T.; Berger, E.D.; Kuhlmann, U.; Mettang, T. Naltrexone does not relieve uremic pruritus: Results of a randomized, double-blind, placebo-controlled crossover study. J. Am. Soc. Nephrol. 2000, 11, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, H.; Ebata, T.; Takamori, K.; Miyasato, K.; Muramatsu, T.; Nakamoto, H.; Kurihara, M.; Yanagita, T.; Suzuki, H. Efficacy and safety of a novel ĸ-agonist for managing intractable pruritus in dialysis patients. Am. J. Nephrol. 2012, 36, 175–183. [Google Scholar] [CrossRef]
- Avila, C.; Massick, S.; Kaffenberger, B.H.; Kwatra, S.G.; Bechtel, M. Cannabinoids for the treatment of chronic pruritus: A review. J. Am. Acad. Dermatol. 2020, 82, 1205–1212. [Google Scholar] [CrossRef]
- Rein, J.L.; Wyatt, C.M. Marijuana and Cannabinoids in ESRD and Earlier Stages of CKD. Am. J. Kidney Dis. 2018, 71, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, H.; Zhang, M.; Yin, C.-l.; Long, G. Effect of high-flux dialysis membrane on uremic pruritus and solute clearance of maintenance hemodialysis patients. Chin. J. Tissue Eng. Res. 2011, 15, 5493. [Google Scholar]
- Jiang, X.; Ji, F.; Chen, Z.W.; Huang, Q.L. Comparison of high-flux hemodialysis with hemodialysis filtration in treatment of uraemic pruritus: A randomized controlled trial. Int. Urol. Nephrol. 2016, 48, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Li, M.X.; Yu, H.; Zhao, J.; Xiao, F.L.; Xuan, F.; Zhao, Y.X. Combination of Multiple Hemodialysis Modes: Better Treatment Options for Patients Under Maintenance Hemodialysis. Ther. Clin. Risk Manag. 2021, 17, 127–133. [Google Scholar] [CrossRef]
- Chen, Z.J.; Cao, G.; Tang, W.X.; Lv, X.Y.; Huang, S.M.; Qin, W.; Ping, F.; Ye, T. A randomized controlled trial of high-permeability haemodialysis against conventional haemodialysis in the treatment of uraemic pruritus. Clin. Exp. Dermatol. 2009, 34, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A.; Rowe, J.W.; Brown, R.S.; Steinman, T.I.; Arndt, K.A. Relief of uremic pruritus with ultraviolet phototherapy. N. Engl. J. Med. 1977, 297, 136–138. [Google Scholar] [CrossRef]
- Gilchrest, B.A. Ultraviolet phototherapy of uremic pruritus. Int. J. Dermatol. 1979, 18, 741–748. [Google Scholar] [CrossRef]
- Ko, M.J.; Yang, J.Y.; Wu, H.Y.; Hu, F.C.; Chen, S.I.; Tsai, P.J.; Jee, S.H.; Chiu, H.C. Narrowband ultraviolet B phototherapy for patients with refractory uraemic pruritus: A randomized controlled trial. Br. J. Dermatol. 2011, 165, 633–639. [Google Scholar] [CrossRef]
- Tan, J.K.; Haberman, H.F.; Coldman, A.J. Identifying effective treatments for uremic pruritus. J. Am. Acad. Dermatol. 1991, 25, 811–818. [Google Scholar] [CrossRef]
- Mahmudpour, M.; Roozbeh, J.; Raiss Jalali, G.A.; Pakfetrat, M.; Ezzat Zadegan, S.; Sagheb, M.M. Therapeutic Effect of Montelukast for Treatment of Uremic Pruritus in Hemodialysis Patients. Iran. J. Kidney Dis. 2017, 11, 50–55. [Google Scholar]
- Kinugasa, E.; Igawa, K.; Shimada, H.; Kondo, M.; Funakoshi, S.; Imada, N.; Itami, N.; Fukazawa, N.; Takubo, R.; Kawata, Y.; et al. Anti-pruritic effect of nemolizumab in hemodialysis patients with uremic pruritus: A phase II, randomized, double-blind, placebo-controlled clinical study. Clin. Exp. Nephrol. 2021, 25, 875–884. [Google Scholar] [CrossRef]
- Lu, P.H.; Chuo, H.E.; Kuo, K.L.; Liao, J.F.; Lu, P.H. Clinical Efficacy and Safety of Sodium Thiosulfate in the Treatment of Uremic Pruritus: A Meta-Analysis of Randomized Controlled Trials. Toxins 2021, 13, 769. [Google Scholar] [CrossRef]
- Bunchorntavakul, C.; Reddy, K.R. Pruritus in chronic cholestatic liver disease. Clin. Liver Dis. 2012, 16, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Mela, M.; Mancuso, A.; Burroughs, A.K. Review article: Pruritus in cholestatic and other liver diseases. Aliment. Pharmacol. Ther. 2003, 17, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Rishe, E.; Azarm, A.; Bergasa, N.V. Itch in primary biliary cirrhosis: A patients’ perspective. Acta Derm.-Venereol. 2008, 88, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Broomé, U.; Olsson, R.; Lööf, L.; Bodemar, G.; Hultcrantz, R.; Danielsson, A.; Prytz, H.; Sandberg-Gertzén, H.; Wallerstedt, S.; Lindberg, G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut 1996, 38, 610–615. [Google Scholar] [CrossRef]
- Tischendorf, J.J.; Hecker, H.; Krüger, M.; Manns, M.P.; Meier, P.N. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A single center study. Am. J. Gastroenterol. 2007, 102, 107–114. [Google Scholar] [CrossRef]
- McPhedran, N.T.; Henderson, R.D. PRURITUS AND JAUNDICE. Can. Med. Assoc. J. 1965, 92, 1258–1260. [Google Scholar]
- Cipriani, S.; Renga, B.; D’Amore, C.; Simonetti, M.; De Tursi, A.A.; Carino, A.; Monti, M.C.; Sepe, V.; Zampella, A.; Fiorucci, S. Impaired Itching Perception in Murine Models of Cholestasis Is Supported by Dysregulation of GPBAR1 Signaling. PLoS ONE 2015, 10, e0129866. [Google Scholar] [CrossRef] [Green Version]
- Meixiong, J.; Vasavda, C.; Green, D.; Zheng, Q.; Qi, L.; Kwatra, S.G.; Hamilton, J.P.; Snyder, S.H.; Dong, X. Identification of a bilirubin receptor that may mediate a component of cholestatic itch. Elife 2019, 8, e44116. [Google Scholar] [CrossRef]
- Patel, S.P.; Vasavda, C.; Ho, B.; Meixiong, J.; Dong, X.; Kwatra, S.G. Cholestatic pruritus: Emerging mechanisms and therapeutics. J. Am. Acad. Dermatol. 2019, 81, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; John, S. Cholestatic Jaundice. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Talaga, Z.J.; Vaidya, P.N. Dubin Johnson Syndrome. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Muñoz, M.; Coveñas, R. Involvement of substance P and the NK-1 receptor in human pathology. Amino Acids 2014, 46, 1727–1750. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.Y.; Lin, H.C.; Tsai, Y.T.; Chang, F.Y.; Lu, R.H.; Hou, M.C.; Li, C.P.; Chu, C.J.; Wang, S.S.; Lee, S.D. Plasma substance P levels in patients with liver cirrhosis: Relationship to systemic and portal hemodynamics. Am. J. Gastroenterol. 1997, 92, 2080–2084. [Google Scholar]
- Trivedi, M.; Bergasa, N.V. Serum concentrations of substance P in cholestasis. Ann. Hepatol. 2010, 9, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Andoh, T.; Nagasawa, T.; Satoh, M.; Kuraishi, Y. Substance P induction of itch-associated response mediated by cutaneous NK1 tachykinin receptors in mice. J. Pharmacol. Exp. Ther. 1998, 286, 1140–1145. [Google Scholar]
- Azimi, E.; Reddy, V.B.; Pereira, P.J.S.; Talbot, S.; Woolf, C.J.; Lerner, E.A. Substance P activates Mas-related G protein-coupled receptors to induce itch. J. Allergy Clin. Immunol. 2017, 140, 447–453.e3. [Google Scholar] [CrossRef] [Green Version]
- Ständer, S.; Yosipovitch, G. Substance P and neurokinin 1 receptor are new targets for the treatment of chronic pruritus. Br. J. Dermatol. 2019, 181, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Tigyi, G. Aiming drug discovery at lysophosphatidic acid targets. Br. J. Pharmacol. 2010, 161, 241–270. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.W.; Herr, D.R.; Noguchi, K.; Yung, Y.C.; Lee, C.W.; Mutoh, T.; Lin, M.E.; Teo, S.T.; Park, K.E.; Mosley, A.N.; et al. LPA receptors: Subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 157–186. [Google Scholar] [CrossRef]
- Kihara, Y.; Maceyka, M.; Spiegel, S.; Chun, J. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br. J. Pharmacol. 2014, 171, 3575–3594. [Google Scholar] [CrossRef] [Green Version]
- Kremer, A.E.; Martens, J.J.; Kulik, W.; Ruëff, F.; Kuiper, E.M.; van Buuren, H.R.; van Erpecum, K.J.; Kondrackiene, J.; Prieto, J.; Rust, C.; et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology 2010, 139, 1008–1018.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanjel, B.; Shim, W.S. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: A review. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165958. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, C.; Baird, A.W.; Nolan, N.; McCormick, P.A. Cholestatic pruritus—the role of cutaneous mast cells and nerves. Aliment. Pharmacol. Ther. 2004, 19, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Rioux, K.P.; Sharkey, K.A.; Wallace, J.L.; Swain, M.G. Hepatic mucosal mast cell hyperplasia in rats with secondary biliary cirrhosis. Hepatology 1996, 23, 888–895. [Google Scholar] [CrossRef]
- Sikand, P.; Dong, X.; LaMotte, R.H. BAM8-22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J. Neurosci. 2011, 31, 7563–7567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swain, M.G.; MacArthur, L.; Vergalla, J.; Jones, E.A. Adrenal secretion of BAM-22P, a potent opioid peptide, is enhanced in rats with acute cholestasis. Am. J. Physiol. 1994, 266, G201–G205. [Google Scholar] [CrossRef]
- Moser, H.R.; Giesler, G.J., Jr. Itch elicited by intradermal injection of serotonin, intracisternal injection of morphine, and their synergistic interactions in rats. Neuroscience 2014, 274, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Bergasa, N.V. Pruritus in primary biliary cirrhosis: Pathogenesis and therapy. Clin. Liver Dis. 2008, 12, 385–406. [Google Scholar] [CrossRef]
- Ostadhadi, S.; Haddadi, N.S.; Foroutan, A.; Azimi, E.; Elmariah, S.; Dehpour, A.R. Development of resistance to serotonin-induced itch in bile duct ligated mice. Clin. Exp. Pharmacol. Physiol. 2017, 44, 680–685. [Google Scholar] [CrossRef]
- Ostadhadi, S.; Foroutan, A.; Momeny, M.; Norouzi-Javidan, A.; Azimi, E.; Kordjazy, N.; Dehpour, A.R. Evidence for the involvement of nitric oxide in cholestasis-induced itch associated response in mice. Biomed. Pharmacother. 2016, 84, 1367–1374. [Google Scholar] [CrossRef]
- Kremer, A.E.; Beuers, U.; Oude-Elferink, R.P.; Pusl, T. Pathogenesis and treatment of pruritus in cholestasis. Drugs 2008, 68, 2163–2182. [Google Scholar] [CrossRef] [PubMed]
- Lindor, K.D.; Gershwin, M.E.; Poupon, R.; Kaplan, M.; Bergasa, N.V.; Heathcote, E.J. Primary biliary cirrhosis. Hepatology 2009, 50, 291–308. [Google Scholar] [CrossRef]
- Bolier, R.; de Vries, E.S.; Parés, A.; Helder, J.; Kemper, E.M.; Zwinderman, K.; Elferink, R.P.O.; Beuers, U. Fibrates for the treatment of cholestatic itch (FITCH): Study protocol for a randomized controlled trial. Trials 2017, 18, 230. [Google Scholar] [CrossRef] [Green Version]
- Nevens, F.; Andreone, P.; Mazzella, G.; Strasser, S.I.; Bowlus, C.; Invernizzi, P.; Drenth, J.P.; Pockros, P.J.; Regula, J.; Beuers, U.; et al. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N. Engl. J. Med. 2016, 375, 631–643. [Google Scholar] [CrossRef]
- de Vries, E.; Bolier, R.; Goet, J.; Parés, A.; Verbeek, J.; de Vree, M.; Drenth, J.; van Erpecum, K.; van Nieuwkerk, K.; van der Heide, F.; et al. Fibrates for Itch (FITCH) in Fibrosing Cholangiopathies: A Double-Blind, Randomized, Placebo-Controlled Trial. Gastroenterology 2021, 160, 734–743.e6. [Google Scholar] [CrossRef] [PubMed]
- Corpechot, C.; Chazouillères, O.; Rousseau, A.; Le Gruyer, A.; Habersetzer, F.; Mathurin, P.; Goria, O.; Potier, P.; Minello, A.; Silvain, C.; et al. A Placebo-Controlled Trial of Bezafibrate in Primary Biliary Cholangitis. N. Engl. J. Med. 2018, 378, 2171–2181. [Google Scholar] [CrossRef]
- Kremer, A.E.; Maillette de Buy Wenniger, L.; Oude Elferink, R.P.; Beuers, U. Pruritus in liver disease. Pathogenesis and treatment. Ned. Tijdschr. Voor Geneeskd. 2011, 155, A4045. [Google Scholar]
- Khurana, S.; Singh, P. Rifampin is safe for treatment of pruritus due to chronic cholestasis: A meta-analysis of prospective randomized-controlled trials. Liver Int. 2006, 26, 943–948. [Google Scholar] [CrossRef]
- EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. J. Hepatol. 2009, 51, 237–267. [CrossRef]
- Bachs, L.; Parés, A.; Elena, M.; Piera, C.; Rodés, J. Effects of long-term rifampicin administration in primary biliary cirrhosis. Gastroenterology 1992, 102, 2077–2080. [Google Scholar] [CrossRef]
- Prince, M.I.; Burt, A.D.; Jones, D.E. Hepatitis and liver dysfunction with rifampicin therapy for pruritus in primary biliary cirrhosis. Gut 2002, 50, 436–439. [Google Scholar] [CrossRef]
- Hirschfield, G.M.; Beuers, U.; Corpechot, C.; Invernizzi, P.; Jones, D.; Marzioni, M.; Schramm, C. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef]
- Gonzales, E.; Hardikar, W.; Stormon, M.; Baker, A.; Hierro, L.; Gliwicz, D.; Lacaille, F.; Lachaux, A.; Sturm, E.; Setchell, K.D.R.; et al. Efficacy and safety of maralixibat treatment in patients with Alagille syndrome and cholestatic pruritus (ICONIC): A randomised phase 2 study. Lancet 2021, 398, 1581–1592. [Google Scholar] [CrossRef]


| Risk Factors | Characteristics |
|---|---|
| Inadequate dialysis | High level of BUN was a significant risk factor for severe uremic pruritus [9,10,11]. |
| Hyperparathyroidism product | Hyperparathyroidism, the product due to the alteration of metabolism, can be involved in the pathogenesis and risk factor [12,13]. |
| Elevated calcium x phosphorus product | Calcium and phosphate, the product due to the alteration of metabolism, can be involved in the pathogenesis and risk factor [9,14,15]. |
| Xerosis | Xerosis is a common complication in HD patients and has an impact on the severity of itch [2,16,17]. |
| Elevated serum magnesium and aluminum concentrations | Magnesium and aluminum, the product due to the alteration of metabolism, can be involved in the pathogenesis and risk factor [18,19,20]. |
| Drug | Class | Effectiveness |
|---|---|---|
| Difelikefalin | KOR agonist |
|
| Nalbuphine | KOR agonist MOR antagonist |
|
| Naltrexone | Nonselective opioid antagonist |
|
| Nalfurafine hydrochloride | KOR agonist |
|
| Specific Liver Disease | Characteristics | Incidence |
|---|---|---|
| Intrahepatic cholestasis of pregnancy | Characterized by pruritus and an elevation in serum bile acid levels, developing in the second or third trimester and resolving after delivery. | 100% [85] |
| Primary biliary cholangitis | It is common in females between the ages of 30 and 65 years. Pruritus often precedes the development of jaundice and can be accompanied by skin findings. | 70–80% by 10 years [86,87] |
| Primary sclerosing cholangitis | Chronic progressive disorder characterized by inflammation and fibrosis. The incidence increases as the disease progresses. | 20–40% (Initial) [88,89] |
| Malignant biliary tract obstruction | It can be due to the presence of tumor in the gallbladder, bile duct, ampulla, duodenum, or pancreas. | 45% [90] |
| Chronic viral hepatitis | The hepatitis B and C viruses can cause chronic hepatitis and can lead to cirrhosis, liver failure, and liver cancer with pruritus. | 20% [90] |
| Nonmalignant biliary tract obstruction | Choledocholithiasis, cholecystitis, and stricture of commone bile duct can cause pruritus. | 17% [90] |
| Cirrhosis | Patients with decompensated cirrhosis can present with jaundice, pruritus and gastrointestinal bleeding. | 7% [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-C.; Shim, W.-S.; Kwak, I.-S.; Lee, D.-H.; Park, J.-S.; Lee, S.-Y.; Kang, S.-Y.; Chung, B.-Y.; Park, C.-W.; Kim, H.-O. Pathogenesis and Treatment of Pruritus Associated with Chronic Kidney Disease and Cholestasis. Int. J. Mol. Sci. 2023, 24, 1559. https://doi.org/10.3390/ijms24021559
Kim J-C, Shim W-S, Kwak I-S, Lee D-H, Park J-S, Lee S-Y, Kang S-Y, Chung B-Y, Park C-W, Kim H-O. Pathogenesis and Treatment of Pruritus Associated with Chronic Kidney Disease and Cholestasis. International Journal of Molecular Sciences. 2023; 24(2):1559. https://doi.org/10.3390/ijms24021559
Chicago/Turabian StyleKim, Jin-Cheol, Won-Sik Shim, In-Suk Kwak, Dong-Hun Lee, Jin-Seo Park, So-Yeon Lee, Seok-Young Kang, Bo-Young Chung, Chun-Wook Park, and Hye-One Kim. 2023. "Pathogenesis and Treatment of Pruritus Associated with Chronic Kidney Disease and Cholestasis" International Journal of Molecular Sciences 24, no. 2: 1559. https://doi.org/10.3390/ijms24021559
APA StyleKim, J.-C., Shim, W.-S., Kwak, I.-S., Lee, D.-H., Park, J.-S., Lee, S.-Y., Kang, S.-Y., Chung, B.-Y., Park, C.-W., & Kim, H.-O. (2023). Pathogenesis and Treatment of Pruritus Associated with Chronic Kidney Disease and Cholestasis. International Journal of Molecular Sciences, 24(2), 1559. https://doi.org/10.3390/ijms24021559






