GC-MS Techniques Investigating Potential Biomarkers of Dying in the Last Weeks with Lung Cancer
Abstract
:1. Introduction
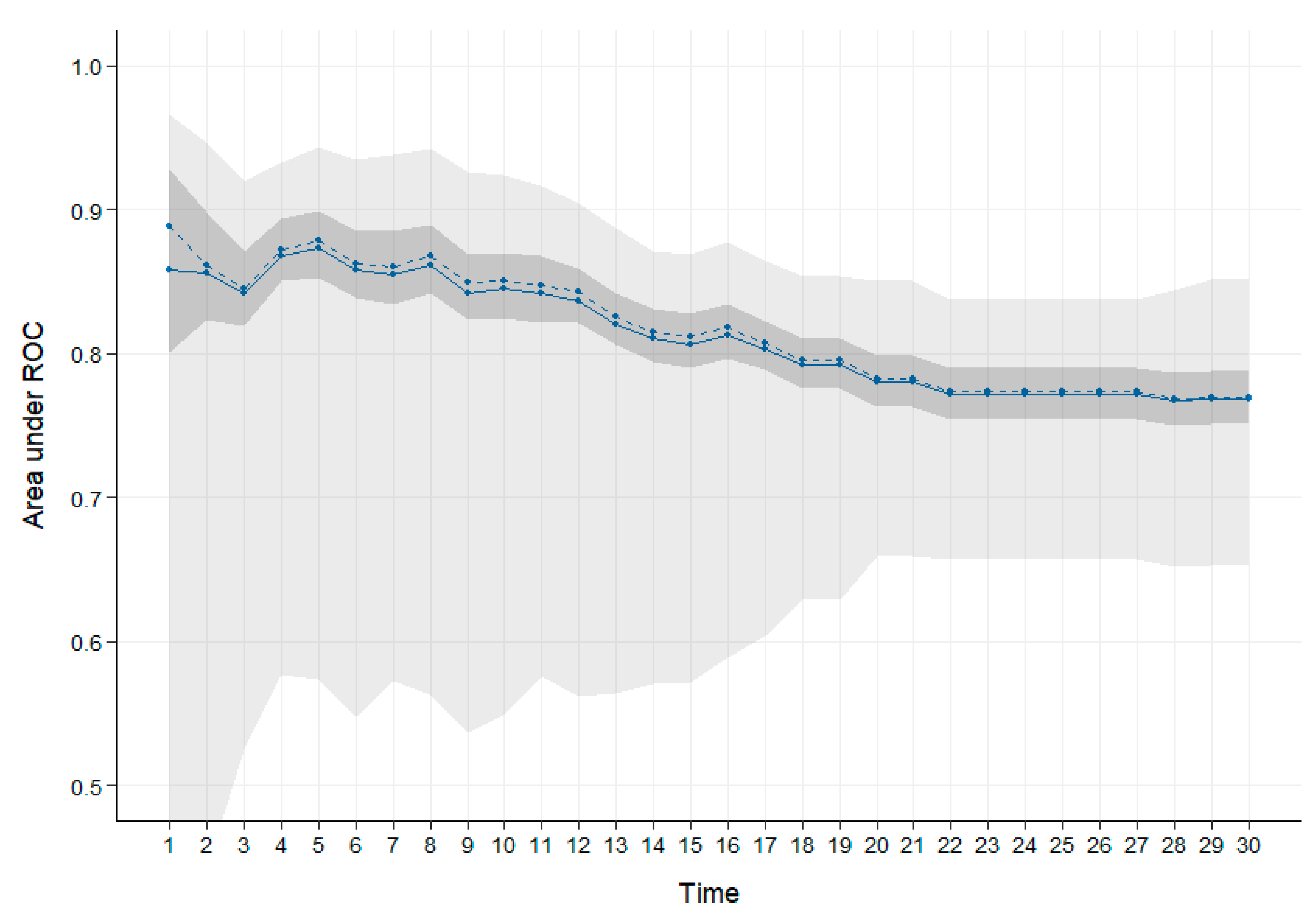
2. Results
2.1. VOCs Changed toward Death in Acid-Treated Urine Dataset
2.2. VOCs Changed toward Death in Alkali-Treated Urine Dataset
2.3. Comparing Acid and Alkali Datasets
3. Discussion
4. Materials and Methods
4.1. Setting, Patient Recruitment and Ethical Consent
4.2. GCMS Methods
4.2.1. Urine Samples for GCMS VOC Analysis
4.2.2. Urine Sample Preparation
4.2.3. Headspace-SPME-GC-MS Analysis
4.2.4. System Suitability and Quality Control
4.2.5. GC-MS VOC Library Building and Data Analysis
4.2.6. Statistical Analysis
4.2.7. Cox Lasso Prediction Modelling
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Neuberger, J. More Care, Less Pathway: A Review of the Liverpool Care Pathway; Department of Health: London, UK, 2013. [Google Scholar]
- Reid, V.L.; McDonald, R.; Nwosu, A.C.; Mason, S.R.; Probert, C.; Ellershaw, J.E.; Coyle, S. A systematically structured review of biomarkers of dying in cancer patients in the last months of life; An exploration of the biology of dying. PLoS ONE 2017, 12, e0175123. [Google Scholar] [CrossRef] [Green Version]
- Reuben, D.B.; Mor, V.; Hiris, J. Clinical symptoms and length of survival in patients with terminal cancer. Arch. Intern Med. 1988, 148, 1586–1591. [Google Scholar] [CrossRef]
- Simmons, C.P.L.; McMillan, D.C.; McWilliams, K.; Sande, T.A.; Fearon, K.C.; Tuck, S.; Fallon, M.T.; Laird, B.J. Prognostic Tools in Patients with Advanced Cancer: A Systematic Review. J. Pain Symptoms Manag. 2017, 53, 962–970.e910. [Google Scholar] [CrossRef] [Green Version]
- Stone, P.; Vickerstaff, V.; Kalpakidou, A.; Todd, C.; Griffiths, J.; Keeley, V.; Spencer, K.; Buckle, P.; Finlay, D.; Omar, R.Z. Prognostic tools or clinical predictions: Which are better in palliative care? PLoS ONE 2021, 16, e0249763. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.C.; Kalpakidou, A.; Todd, C.; Griffiths, J.; Keeley, V.; Spencer, K.; Buckle, P.; Finlay, D.; Vickerstaff, V.; Omar, R.Z.; et al. The Prognosis in Palliative care Study II (PiPS2): A prospective observational validation study of a prognostic tool with an embedded qualitative evaluation. PLoS ONE 2021, 16, e0249297. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Lee, W.Y. Urinary metabolites for urological cancer detection: A review on the application of volatile organic compounds for cancers. Am. J. Clin. Exp. Urol. 2019, 7, 232–248. [Google Scholar]
- Wen, Q.; Boshier, P.; Myridakis, A.; Belluomo, I.; Hanna, G.B. Urinary Volatile Organic Compound Analysis for the Diagnosis of Cancer: A Systematic Literature Review and Quality Assessment. Metabolites 2020, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Aggio, R.B.; de Lacy Costello, B.; White, P.; Khalid, T.; Ratcliffe, N.M.; Persad, R.; Probert, C.S. The use of a gas chromatography-sensor system combined with advanced statistical methods, towards the diagnosis of urological malignancies. J. Breath Res. 2016, 10, 017106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raftery, D. Mass Spectrometry in Metabolomics: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2014; Volume 1198. [Google Scholar]
- Cozzolino, R.; De Magistris, L.; Saggese, P.; Stocchero, M.; Martignetti, A.; Di Stasio, M.; Malorni, A.; Marotta, R.; Boscaino, F.; Malorni, L. Use of solid-phase microextraction coupled to gas chromatography-mass spectrometry for determination of urinary volatile organic compounds in autistic children compared with healthy controls. Anal. Bioanal. Chem. 2014, 406, 4649–4662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Raftery, D. Headspace SPME-GC-MS metabolomics analysis of urinary volatile organic compounds (VOCs). Methods Mol. Biol. 2014, 1198, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Drabinska, N.; Flynn, C.; Ratcliffe, N.; Belluomo, I.; Myridakis, A.; Gould, O.; Fois, M.; Smart, A.; Devine, T.; Costello, B.L. A literature survey of all volatiles from healthy human breath and bodily fluids: The human volatilome. J. Breath Res. 2021, 15, 034001. [Google Scholar] [CrossRef] [PubMed]
- De Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef] [PubMed]
- Janfaza, S.; Khorsand, B.; Nikkhah, M.; Zahiri, J. Digging deeper into volatile organic compounds associated with cancer. Biol. Methods Protoc. 2019, 4, bpz014. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Forleo, A.; Ferramosca, A.; Notari, T.; Pappalardo, S.; Siciliano, P.; Capone, S.; Montano, L. Blood, urine and semen Volatile Organic Compound (VOC) pattern analysis for assessing health environmental impact in highly polluted areas in Italy. Environ. Pollut. 2021, 286, 117410. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, N.; Wieczorek, T.; Drabinska, N.; Gould, O.; Osborne, A.; De Lacy Costello, B. A mechanistic study and review of volatile products from peroxidation of unsaturated fatty acids: An aid to understanding the origins of volatile organic compounds from the human body. J. Breath Res. 2020, 14, 034001. [Google Scholar] [CrossRef]
- Vas, G.; Vekey, K. Solid-phase microextraction: A powerful sample preparation tool prior to mass spectrometric analysis. J. Mass Spectrom. 2004, 39, 233–254. [Google Scholar] [CrossRef]
- Hough, R.; Archer, D.; Probert, C. A comparison of sample preparation methods for extracting volatile organic compounds (VOCs) from equine faeces using HS-SPME. Metabolomics 2018, 14, 19. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, B.R.B.; De Martinis, B.S. Analysis of urinary VOCs using mass spectrometric methods to diagnose cancer: A review. Clin. Mass Spectrom. 2020, 18, 27–37. [Google Scholar] [CrossRef]
- Khalid, T.; Aggio, R.; White, P.; De Lacy Costello, B.; Persad, R.; Al-Kateb, H.; Jones, P.; Probert, C.S.; Ratcliffe, N. Urinary Volatile Organic Compounds for the Detection of Prostate Cancer. PLoS ONE 2015, 10, e0143283. [Google Scholar] [CrossRef] [Green Version]
- Lett, L.; George, M.; Slater, R.; De Lacy Costello, B.; Ratcliffe, N.; Garcia-Finana, M.; Lazarowicz, H.; Probert, C. Investigation of urinary volatile organic compounds as novel diagnostic and surveillance biomarkers of bladder cancer. Br. J. Cancer 2022, 127, 329–336. [Google Scholar] [CrossRef]
- Aggarwal, P.; Baker, J.; Boyd, M.T.; Coyle, S.; Probert, C.; Chapman, E.A. Optimisation of Urine Sample Preparation for Headspace-Solid Phase Microextraction Gas Chromatography-Mass Spectrometry: Altering Sample pH, Sulphuric Acid Concentration and Phase Ratio. Metabolites 2020, 10, 482. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; May, S. Applied Survival Analysis: Regression Modeling of Time-to-Event Data, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2008; pp. 13–392. [Google Scholar]
- Santos, P.M.; Del Nogal Sanchez, M.; Pozas, A.P.C.; Pavon, J.L.P.; Cordero, B.M. Determination of ketones and ethyl acetate-a preliminary study for the discrimination of patients with lung cancer. Anal. Bioanal. Chem. 2017, 409, 5689–5696. [Google Scholar] [CrossRef]
- Perez Anton, A.; Ramos, A.G.; Del Nogal Sanchez, M.; Pavon, J.L.; Cordero, B.M.; Pozas, A.P. Headspace-programmed temperature vaporization-mass spectrometry for the rapid determination of possible volatile biomarkers of lung cancer in urine. Anal. Bioanal. Chem. 2016, 408, 5239–5246. [Google Scholar] [CrossRef]
- Porto-Figueira, P.; Pereira, J.; Miekisch, W.; Camara, J.S. Exploring the potential of NTME/GC-MS, in the establishment of urinary volatomic profiles. Lung cancer patients as case study. Sci. Rep. 2018, 8, 13113. [Google Scholar] [CrossRef] [Green Version]
- Hanai, Y.; Shimono, K.; Matsumura, K.; Vachani, A.; Albelda, S.; Yamazaki, K.; Beauchamp, G.K.; Oka, H. Urinary volatile compounds as biomarkers for lung cancer. Biosci. Biotechnol. Biochem. 2012, 76, 679–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasparri, R.; Capuano, R.; Guaglio, A.; Caminiti, V.; Canini, F.; Catini, A.; Sedda, G.; Paolesse, R.; Di Natale, C.; Spaggiari, L. Volatolomic urinary profile analysis for diagnosis of the early stage of lung cancer. J. Breath Res. 2022, 16, 046008. [Google Scholar] [CrossRef]
- Janfaza, S.; Banan Nojavani, M.; Khorsand, B.; Nikkhah, M.; Zahiri, J. Cancer Odor Database (COD): A critical databank for cancer diagnosis research. Database 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- COD. Cancer Odor Database. Available online: http://bioinf.modares.ac.ir/software/COD/index.html (accessed on 10 December 2022).
- HMDB. Human Metabolome Database: Showing Metabocard for Acetone (HMDB0001659). Available online: https://hmdb.ca/metabolites/HMDB0001659 (accessed on 10 December 2022).
- Zimmermann, D.; Hartmann, M.; Moyer, M.P.; Nolte, J.; Baumbach, J.I. Determination of volatile products of human colon cell line metabolism by GC/MS analysis. Metabolomics 2007, 3, 13–17. [Google Scholar] [CrossRef]
- Huang, J.; Kumar, S.; Abbassi-Ghadi, N.; Spanel, P.; Smith, D.; Hanna, G.B. Selected ion flow tube mass spectrometry analysis of volatile metabolites in urine headspace for the profiling of gastro-esophageal cancer. Anal. Chem 2013, 85, 3409–3416. [Google Scholar] [CrossRef]
- Wang, C.; Sun, B.; Guo, L.; Wang, X.; Ke, C.; Liu, S.; Zhao, W.; Luo, S.; Guo, Z.; Zhang, Y.; et al. Volatile organic metabolites identify patients with breast cancer, cyclomastopathy, and mammary gland fibroma. Sci. Rep. 2014, 4, 5383. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, C.; Chi, C.; Wang, X.; Liu, S.; Zhao, W.; Ke, C.; Xu, G.; Li, E. Exhaled breath volatile biomarker analysis for thyroid cancer. Transl. Res. 2015, 166, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Amal, H.; Leja, M.; Funka, K.; Lasina, I.; Skapars, R.; Sivins, A.; Ancans, G.; Kikuste, I.; Vanags, A.; Tolmanis, I.; et al. Breath testing as potential colorectal cancer screening tool. Int. J. Cancer 2016, 138, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Huang, J.; Abbassi-Ghadi, N.; Spanel, P.; Smith, D.; Hanna, G.B. Selected ion flow tube mass spectrometry analysis of exhaled breath for volatile organic compound profiling of esophago-gastric cancer. Anal. Chem. 2013, 85, 6121–6128. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Huang, J.; Abbassi-Ghadi, N.; Mackenzie, H.A.; Veselkov, K.A.; Hoare, J.M.; Lovat, L.B.; Spanel, P.; Smith, D.; Hanna, G.B. Mass Spectrometric Analysis of Exhaled Breath for the Identification of Volatile Organic Compound Biomarkers in Esophageal and Gastric Adenocarcinoma. Ann. Surg. 2015, 262, 981–990. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Pan, F.; Liu, J.; Wang, K.; Zhang, C.; Cheng, S.; Lu, L.; Zhang, W.; Zhang, Z.; et al. Breath Analysis Based on Surface-Enhanced Raman Scattering Sensors Distinguishes Early and Advanced Gastric Cancer Patients from Healthy Persons. ACS Nano 2016, 10, 8169–8179. [Google Scholar] [CrossRef]
- Filipiak, W.; Filipiak, A.; Sponring, A.; Schmid, T.; Zelger, B.; Ager, C.; Klodzinska, E.; Denz, H.; Pizzini, A.; Lucciarini, P.; et al. Comparative analyses of volatile organic compounds (VOCs) from patients, tumors and transformed cell lines for the validation of lung cancer-derived breath markers. J. Breath Res. 2014, 8, 027111. [Google Scholar] [CrossRef]
- Rudnicka, J.; Kowalkowski, T.; Ligor, T.; Buszewski, B. Determination of volatile organic compounds as biomarkers of lung cancer by SPME-GC-TOF/MS and chemometrics. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 3360–3366. [Google Scholar] [CrossRef]
- Machado, R.F.; Laskowski, D.; Deffenderfer, O.; Burch, T.; Zheng, S.; Mazzone, P.J.; Mekhail, T.; Jennings, C.; Stoller, J.K.; Pyle, J.; et al. Detection of lung cancer by sensor array analyses of exhaled breath. Am. J. Respir. Crit. Care Med. 2005, 171, 1286–1291. [Google Scholar] [CrossRef] [Green Version]
- Buszewski, B.; Ulanowska, A.; Kowalkowski, T.; Cieslinski, K. Investigation of lung cancer biomarkers by hyphenated separation techniques and chemometrics. Clin. Chem. Lab. Med. 2011, 50, 573–581. [Google Scholar] [CrossRef]
- Ligor, M.; Ligor, T.; Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Denz, H.; Fiegl, M.; Hilbe, W.; Weiss, W.; et al. Determination of volatile organic compounds in exhaled breath of patients with lung cancer using solid phase microextraction and gas chromatography mass spectrometry. Clin. Chem. Lab. Med. 2009, 47, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Xie, G.; Jia, W. Metabonomics of human colorectal cancer: New approaches for early diagnosis and biomarker discovery. J. Proteome Res. 2014, 13, 3857–3870. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Song, R.; Ma, C.; Zhou, L.; Liu, X.; Yin, P.; Zhang, Z.; Sun, Y.; Xu, C.; Lu, X.; et al. Discovery and validation of potential urinary biomarkers for bladder cancer diagnosis using a pseudotargeted GC-MS metabolomics method. Oncotarget 2017, 8, 20719–20728. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.L.; Passos, M.; Camara, J.S. Solid phase microextraction, mass spectrometry and metabolomic approaches for detection of potential urinary cancer biomarkers—A powerful strategy for breast cancer diagnosis. Talanta 2012, 89, 360–368. [Google Scholar] [CrossRef]
- Calzada, E.; Onguka, O.; Claypool, S.M. Phosphatidylethanolamine Metabolism in Health and Disease. Int. Rev. Cell Mol. Biol. 2016, 321, 29–88. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.; Hatch, G.M.; Wang, Y.; Yu, F.; Wang, M. The relationship between phospholipids and insulin resistance: From clinical to experimental studies. J. Cell Mol. Med. 2019, 23, 702–710. [Google Scholar] [CrossRef] [Green Version]
- CRUK. Lung Cancer Incidence Statistics|Cancer Research UK. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/incidence#heading-One (accessed on 10 December 2022).
- Young, R.P.; Hopkins, R.J.; Christmas, T.; Black, P.N.; Metcalf, P.; Gamble, G.D. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur. Respir. J. 2009, 34, 380–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDougall, F.A.; Kvaal, K.; Matthews, F.E.; Paykel, E.; Jones, P.B.; Dewey, M.E.; Brayne, C.; Ageing, S.; Medical Research Council Cognitive Function. Prevalence of depression in older people in England and Wales: The MRC CFA Study. Psychol. Med. 2007, 37, 1787–1795. [Google Scholar] [CrossRef]
- Aitken, G.R.; Roderick, P.J.; Fraser, S.; Mindell, J.S.; O’Donoghue, D.; Day, J.; Moon, G. Change in prevalence of chronic kidney disease in England over time: Comparison of nationally representative cross-sectional surveys from 2003 to 2010. BMJ Open 2014, 4, e005480. [Google Scholar] [CrossRef]
- Roger, V.L. Epidemiology of heart failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef]
- Roger, V.L. Epidemiology of Heart Failure: A Contemporary Perspective. Circ. Res. 2021, 128, 1421–1434. [Google Scholar] [CrossRef]
- Whicher, C.A.; O’Neill, S.; Holt, R.I.G. Diabetes in the UK: 2019. Diabet. Med. 2020, 37, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldrich, S. Analytical Vials. Available online: https://www.sigmaaldrich.com/GB/en/products/analytical-chemistry/analytical-chromatography/analytical-vials (accessed on 10 December 2022).
- McFarlan, E.M.; Mozdia, K.E.; Daulton, E.; Arasaradnam, R.; Covington, J.; Nwokolo, C. Pre-analytical and analytical variables that influence urinary volatile organic compound measurements. PLoS ONE 2020, 15, e0236591. [Google Scholar] [CrossRef]
- Smith, S.; Burden, H.; Persad, R.; Whittington, K.; de Lacy Costello, B.; Ratcliffe, N.M.; Probert, C.S. A comparative study of the analysis of human urine headspace using gas chromatography-mass spectrometry. J. Breath Res. 2008, 2, 037022. [Google Scholar] [CrossRef]
- Alwis, K.U.; Blount, B.C.; Britt, A.S.; Patel, D.; Ashley, D.L. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS). Anal. Chim. Acta 2012, 750, 152–160. [Google Scholar] [CrossRef]
- Dixon, E.; Clubb, C.; Pittman, S.; Ammann, L.; Rasheed, Z.; Kazmi, N.; Keshavarzian, A.; Gillevet, P.; Rangwala, H.; Couch, R.D. Solid-phase microextraction and the human fecal VOC metabolome. PLoS ONE 2011, 6, e18471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzone, P.J.; Wang, X.F.; Lim, S.; Choi, H.; Jett, J.; Vachani, A.; Zhang, Q.; Beukemann, M.; Seeley, M.; Martino, R.; et al. Accuracy of volatile urine biomarkers for the detection and characterization of lung cancer. BMC Cancer 2015, 15, 1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalid, T.; White, P.; De Lacy Costello, B.; Persad, R.; Ewen, R.; Johnson, E.; Probert, C.S.; Ratcliffe, N. A pilot study combining a GC-sensor device with a statistical model for the identification of bladder cancer from urine headspace. PLoS ONE 2013, 8, e69602. [Google Scholar] [CrossRef] [Green Version]
- Stone, P.; Kalpakidou, A.; Todd, C.; Griffiths, J.; Keeley, V.; Spencer, K.; Buckle, P.; Finlay, D.A.; Vickerstaff, V.; Omar, R.Z. Prognostic models of survival in patients with advanced incurable cancer: The PiPS2 observational study. Health Technol. Assess. 2021, 25, 1–118. [Google Scholar] [CrossRef]
- Coyle, S.; Scott, A.; Nwosu, A.C.; Latten, R.; Wilson, J.; Mayland, C.R.; Mason, S.; Probert, C.; Ellershaw, J. Collecting biological material from palliative care patients in the last weeks of life: A feasibility study. BMJ Open 2016, 6, e011763. [Google Scholar] [CrossRef] [Green Version]
- Aggio, R.B.; Mayor, A.; Coyle, S.; Reade, S.; Khalid, T.; Ratcliffe, N.M.; Probert, C.S. Freeze-drying: An alternative method for the analysis of volatile organic compounds in the headspace of urine samples using solid phase micro-extraction coupled to gas chromatography—Mass spectrometry. Chem. Cent. J. 2016, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 2018, 14, 72. [Google Scholar] [CrossRef] [Green Version]
- Aggio, R.; Villas-Boas, S.G.; Ruggiero, K. Metab: An R package for high-throughput analysis of metabolomics data generated by GC-MS. Bioinformatics 2011, 27, 2316–2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCBI. NCBI Medical Subject Headings (MeSH). Available online: https://pubchem.ncbi.nlm.nih.gov/source/11939 (accessed on 10 December 2022).
- Team, R.S. RStudio: Integrated Development for R, Publisher = RStudio, PBC, Boston, MA. Available online: http://www.rstudio.com/ (accessed on 10 December 2022).
- Stacklies, W.; Redestig, H.; Scholz, M.; Walther, D.; Selbig, J. pcaMethods--a bioconductor package providing PCA methods for incomplete data. Bioinformatics 2007, 23, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Wang, J.; Su, M.; Jia, E.; Chen, S.; Chen, T.; Ni, Y. Missing Value Imputation Approach for Mass Spectrometry-based Metabolomics Data. Sci. Rep. 2018, 8, 663. [Google Scholar] [CrossRef] [Green Version]
- Armitage, E.G.; Godzien, J.; Alonso-Herranz, V.; Lopez-Gonzalvez, A.; Barbas, C. Missing value imputation strategies for metabolomics data. Electrophoresis 2015, 36, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Parsons, H.M.; Ludwig, C.; Gunther, U.L.; Viant, M.R. Improved classification accuracy in 1- and 2-dimensional NMR metabolomics data using the variance stabilising generalised logarithm transformation. BMC Bioinform. 2007, 8, 234. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Simon, N.; Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Cox’s Proportional Hazards Model via Coordinate Descent. J. Stat. Softw. 2011, 39, 1–13. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J.H. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer: New York, NY, USA, 2009; pp. 12–745. [Google Scholar]
- Uno, H.; Cai, T.; Tian, L.; Wei, L.J. Evaluating Prediction Rules for t-Year Survivors With Censored Regression Models. J. Am. Stat. Assoc. 2007, 102, 527–537. [Google Scholar] [CrossRef]
- Ulanowska, A.; Trawiańska, E.; Sawrycki, P.; Buszewski, B. Chemotherapy control by breath profile with application of SPME-GC/MS method. J. Sep. Sci. 2012, 35, 2908–2913. [Google Scholar] [CrossRef]
- Wang, C.; Ke, C.; Wang, X.; Chi, C.; Guo, L.; Luo, S.; Guo, Z.; Xu, G.; Zhang, F.; Li, E. Noninvasive detection of colorectal cancer by analysis of exhaled breath. Anal. Bioanal. Chem. 2014, 406, 4757–4763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zou, L.; Xu, H. Anodic alumina coating for extraction of volatile organic compounds in human exhaled breath vapor. Talanta 2015, 132, 528–534. [Google Scholar] [CrossRef]
- Ahmed, I.; Fayyaz, F.; Nasir, M.; Niaz, Z.; Furnari, M.; Perry, L. Extending landscape of volatile metabolites as novel diagnostic biomarkers of inflammatory bowel disease—A review. Scand. J. Gastroenterol. 2016, 51, 385–392. [Google Scholar] [CrossRef]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The Human Urine Metabolome. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Povedano, M.M.; Calderón-Santiago, M.; Priego-Capote, F.; Luque de Castro, M.D. Development of a method for enhancing metabolomics coverage of human sweat by gas chromatography-mass spectrometry in high resolution mode. Anal. Chim. Acta 2016, 905, 115–125. [Google Scholar] [CrossRef]
- Garner, C.E.; Smith, S.; Lacy Costello, B.; White, P.; Spencer, R.; Probert, C.S.; Ratcliffe, N.M. Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. FASEB J. 2007, 21, 1675–1688. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, E.M.; Blendis, L.M.; Sandler, S. A gas chromatographic--mass spectrometric study of profiles of volatile metabolites in hepatic encephalopathy. J. Chromatogr. 1981, 226, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Guneral, F.; Bachmann, C. Age-related reference values for urinary organic acids in a healthy Turkish pediatric population. Clin. Chem. 1994, 40. [Google Scholar] [CrossRef]
- Libert, R.; Draye, J.P.; van Hoof, F.; Schanck, A.; Soumillion, J.P.; de Hoffmann, E. Study of reactions induced by hydroxylamine treatment of esters of organic acids and of 3-ketoacids: Application to the study of urines from patients under valproate therapy. Biol. Mass Spectrom. 1991, 20, 75–86. [Google Scholar] [CrossRef]
- Raman, M.; Ahmed, I.; Gillevet, P.M.; Probert, C.S.; Ratcliffe, N.M.; Smith, S.; Greenwood, R.; Sikaroodi, M.; Lam, V.; Crotty, P.; et al. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2013, 11, 868–875.e3. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Hayashi, T.; Sato, M.; Tatsumi, M.; Takagi, N. Simultaneous separation and sensitive determination of free fatty acids in blood plasma by high-performance liquid chromatography. J. Chromatogr. 1984, 309, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Matsunaga, T.; Narimatsu, S.; Yamamoto, I.; Yoshimura, H. Mouse hepatic microsomal oxidation of aliphatic aldehydes (C8 to C011) to carboxylic acids. Biochem. Biophys. Res. Commun. 1992, 188. [Google Scholar] [CrossRef]
- Yanagisawa, I.; Yamane, M.; Urayama, T. Simultaneous separation and sensitive determination of free fatty acids in blood plasma by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1985, 345, 229–240. [Google Scholar] [CrossRef] [PubMed]




| Acid Dataset | Alkali Dataset | |
| Total samples run on GC-MS | n = 205 | n = 150 |
| Samples closest to death, 1 sample/patient | n = 144 | n = 116 |
| Patients | Absolute number /144 (%) | Absolute number /116 (%) |
| Sex | ||
| Female:male | 70:74 (49:51) | 53:63 (46:54) |
| Diagnosis | ||
| NSCLC † (Adenocarcinoma) | 54 (38) | 43 (37) |
| NSCLC † (Squamous) | 29 (20) | 23 (20) |
| NSCLC † (Large Cell) | 1 (1) | 1 (1) |
| SCLC ‡ | 28 (19) | 24 (21) |
| Radiological or Clinical Diagnosis § | 31 (22) | 24 (21) |
| Mesothelioma | 1 (1) | 1 (1) |
| Age (years) | ||
| Median (range) | 70.50 (47–90) | 70.00 (47–90) |
| 40–49 | 4 (3) | 3 (3) |
| 50–59 | 22 (15) | 16 (14) |
| 60–69 | 46 (32) | 41 (35) |
| 70–79 | 43 (30) | 31 (27) |
| 80–89 | 29 (20) | 24 (21) |
| 90–100 | 0 (0) | 1 (1) |
| Ethnicity | ||
| Mixed—White and Black African | 1 (1) | 0 (0) |
| White—British | 141 (98) | 115 (99) |
| White—Irish | 2 (1) | 1 (1) |
| Smoking status | ||
| Ex-smoker | 25 (17) | 19 (16) |
| Current | 87 (60) | 71 (61) |
| Never | 32 (22) | 26 (22) |
| Co-morbidities | ||
| Chronic Obstructive Pulmonary Disease | 54 (38) | 47 (41) |
| Chronic Kidney Disease | 8 (6) | 6 (5) |
| Heart failure | 9 (6) | 7 (6) |
| Depression | 13 (9) | 8 (7) |
| Diabetes Mellitus | 21 (15) | 18 (16) |
| Time of urine sample in relationship to death (time before death) | ||
| Day 0–21 | 55 (38) | 43 (37) |
| Day 22+ | 89 (62) | 73 (63) |
| Time of urine sample in relationship to death (time before death) | ||
| Week 01 | 25 (17) | 17 (15) |
| Week 02 | 16 (11) | 13 (11) |
| Week 03 | 14 (10) | 13 (11) |
| Week 04+ | 33 (23) | 25 (22) |
| Week 12+ | 56 (39) | 48 (41) |
| Univariate | Linear Regression | |||||
|---|---|---|---|---|---|---|
| RT | IUPAC Compound Name | BH pvals | Fold Change | Trend towards Death | Trend towards Death | padj Hetero |
| 28.87 | nonan-2-one or 5-methylhexan-2-one | 0.005 | 1.757 | ↑ | ↑ | 0.000 |
| 21.71 | heptan-2-one | 0.012 | 1.913 | ↑ | ↑ | 0.008 |
| 25.35 | Benzaldehyde * | 0.018 | 2.152 | ↑ | ||
| 32.67 | (Z)-oct-2-enoic acid | 0.038 | 1.370 | ↑ | ↑ | 0.015 |
| 31.31 | 5-ethyl-5-methyloxolan-2-one * | 0.040 | 2.015 | ↑ | ↑ | 0.000 |
| 7.31 | propan-2-one | 0.042 | 1.382 | ↑ | ↑ | 0.015 |
| 24.67 | 2-methyl-5-methylsulfanylfuran * | 0.044 | 1.863 | ↑ | ↑ | 0.017 |
| 23.69 | 3,4-dimethylhexan-2-one | 0.044 | 1.523 | ↑ | ↑ | 0.015 |
| 19.65 | hexane-3,4-dione ** | 0.044 | 1.255 | ↑ | ↑ | 0.046 |
| 22.81 | Cyclohexanone ** | 0.051 | 1.848 | ↑ | ↑ | 0.006 |
| 27.76 | Phenol * | 0.056 | 2.261 | ↑ | ||
| 27.57 | 4-methylpent-3-enoic acid | ↑ | 0.000 | |||
| 37.09 | 1,2,4-triazole-3,4-diamine | ↑ | 0.010 | |||
| 29.16 | (E)-non-3-en-2-one ** | ↑ | 0.030 | |||
| 38.58 | (E)-1-(2,6,6-trimethylcyclohexa-1,3-dien-1-yl)but-2-en-1-one | 0.012 | 1.685 | ↓ | ↓ | 0.000 |
| 34.94 | 1,1,4a-trimethyl-4,5,6,7-tetrahydro-3H-naphthalen-2-one * | 0.012 | 2.715 | ↓ | ↓ | 0.000 |
| 26.01 | 1-methyl-4-propan-2-ylbenzene (p-Cimene) * | 0.018 | 2.044 | ↓ | ↓ | 0.001 |
| 28.37 | 5-(3,3-dimethyloxiran-2-yl)-3-methylpent-1-en-3-ol | 0.030 | 1.935 | ↓ | ↓ | 0.000 |
| 37.32 | 2-buta-1,3-dienyl-1,3,5-trimethylbenzene * | 0.038 | 2.139 | ↓ | ↓ | 0.000 |
| 34.49 | (5R)-5-methyl-2-propan-2-ylidenecyclohexan-1-one-(Pulegone) | 0.044 | 1.351 | ↓ | ||
| 26.44 | 1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane (Eucalyptol) * | 0.044 | 2.009 | ↓ | ↓ | 0.031 |
| 35.9 | 2,6,6,10-tetramethyl-1-oxaspiro[4.5]dec-9-ene | ↓ | 0.000 | |||
| 28.85 | 3,7-dimethyloctan-3-ol or 3-methylpentan-3-ol | ↓ | 0.003 | |||
| 25.75 | 1-hydroperoxyhexane | ↓ | 0.004 | |||
| 23.44 | 1-(furan-2-yl)ethenone * | ↓ | 0.012 | |||
| 29.92 | 2-methoxyphenol * | ↓ | 0.015 | |||
| 32.21 | (1,4-dimethylpent-2-enyl)benzene | ↓ | 0.015 | |||
| 26.89 | 1-methyl-4-propan-2-ylcyclohexa-1,4-diene | ↓ | 0.015 | |||
| 30.94 | 1-methyl-4-prop-1-en-2-ylcyclohexa-1,3-diene or 1,2,4,5-tetramethylbenzene | ↓ | 0.015 | |||
| 28.55 | 1-methyl-4-prop-1-en-2-ylbenzene or 1-methyl-2-prop-1-en-2-ylbenzene * | ↓ | 0.016 | |||
| 34.77 | 2-methyl-5-prop-1-en-2-ylcyclohex-2-en-1-one (Carvone) * | ↓ | 0.018 | |||
| 25.53 | 4,7,7-trimethylbicyclo[4.1.0]hept-2-ene((+)-4-Carene) | ↓ | 0.022 | |||
| 25.59 | 1-methyl-4-propan-2-yl-7-oxabicyclo[2.2.1]heptane | ↓ | 0.022 | |||
| 22.14 | 3,4-dimethylthiophene | ↓ | 0.028 | |||
| 28.92 | 2-(5-ethenyl-5-methyloxolan-2-yl)propan-2-ol | ↓ | 0.033 | |||
| 28.11 | 2,6-dimethyloct-7-en-2-ol | ↓ | 0.045 | |||
| 32.14 | 5-methyl-2-propan-2-ylcyclohexan-1-ol (Menthol) * | ↓ | 0.045 | |||
| RT | IUPAC Compound Name | Coefficient in Cox Lasso Model | |
|---|---|---|---|
| 1 | 7.31 | propan-2-one | 0.205 |
| 2 | 19.65 | hexane-3,4-dione ** | 0.009 |
| 3 | 26.44 | 1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane(Eucalyptol) * | −0.038 |
| 4 | 28.85 | 3,7-dimethyloctan-3-ol or 3-methylpentan-3-ol | −0.029 |
| 5 | 28.87 | nonan-2-one or 5-methylhexan-2-one | 0.208 |
| 6 | 31.31 | 5-ethyl-5-methyloxolan-2-one * | 0.007 |
| 7 | 34.94 | 1,1,4a-trimethyl-4,5,6,7-tetrahydro-3H-naphthalen-2-one * | −0.078 |
| 8 | 38.58 | (E)-1-(2,6,6-trimethylcyclohexa-1,3-dien-1-yl)but-2-en-1-one | −0.015 |
| Univariate | Linear Regression | |||||
|---|---|---|---|---|---|---|
| RT | IUPAC Compound Name | BH pvals | Fold Change | Trend towards Death | Trend towards Death | padj Hetero |
| 26.93 | 2-ethylhexan-1-ol * | 0.010 | 4.962 | ↑ | ↑ | 0.007 |
| 7.31 | propan-2-one | ↑ | 0.065 | |||
| 34.77 | 2-methyl-5-prop-1-en-2-ylcyclohex-2-en-1-one (Carvone) | 0.016 | 0.630 | ↓ | ↓ | 0.007 |
| 33.25 | 1-[2-(furan-2-yl)cyclopropyl]ethanone | ↓ | 0.016 | |||
| 17.5 | hexan-3-one ** | ↓ | 0.052 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapman, E.A.; Baker, J.; Aggarwal, P.; Hughes, D.M.; Nwosu, A.C.; Boyd, M.T.; Mayland, C.R.; Mason, S.; Ellershaw, J.; Probert, C.S.; et al. GC-MS Techniques Investigating Potential Biomarkers of Dying in the Last Weeks with Lung Cancer. Int. J. Mol. Sci. 2023, 24, 1591. https://doi.org/10.3390/ijms24021591
Chapman EA, Baker J, Aggarwal P, Hughes DM, Nwosu AC, Boyd MT, Mayland CR, Mason S, Ellershaw J, Probert CS, et al. GC-MS Techniques Investigating Potential Biomarkers of Dying in the Last Weeks with Lung Cancer. International Journal of Molecular Sciences. 2023; 24(2):1591. https://doi.org/10.3390/ijms24021591
Chicago/Turabian StyleChapman, Elinor A., James Baker, Prashant Aggarwal, David M. Hughes, Amara C. Nwosu, Mark T. Boyd, Catriona R. Mayland, Stephen Mason, John Ellershaw, Chris S. Probert, and et al. 2023. "GC-MS Techniques Investigating Potential Biomarkers of Dying in the Last Weeks with Lung Cancer" International Journal of Molecular Sciences 24, no. 2: 1591. https://doi.org/10.3390/ijms24021591





