Excess Absorbance as a Novel Approach for Studying the Self-Aggregation of Vital Dyes in Liquid Solution
Abstract
1. Introduction
2. Results and Discussion
2.1. Theoretical Aspects
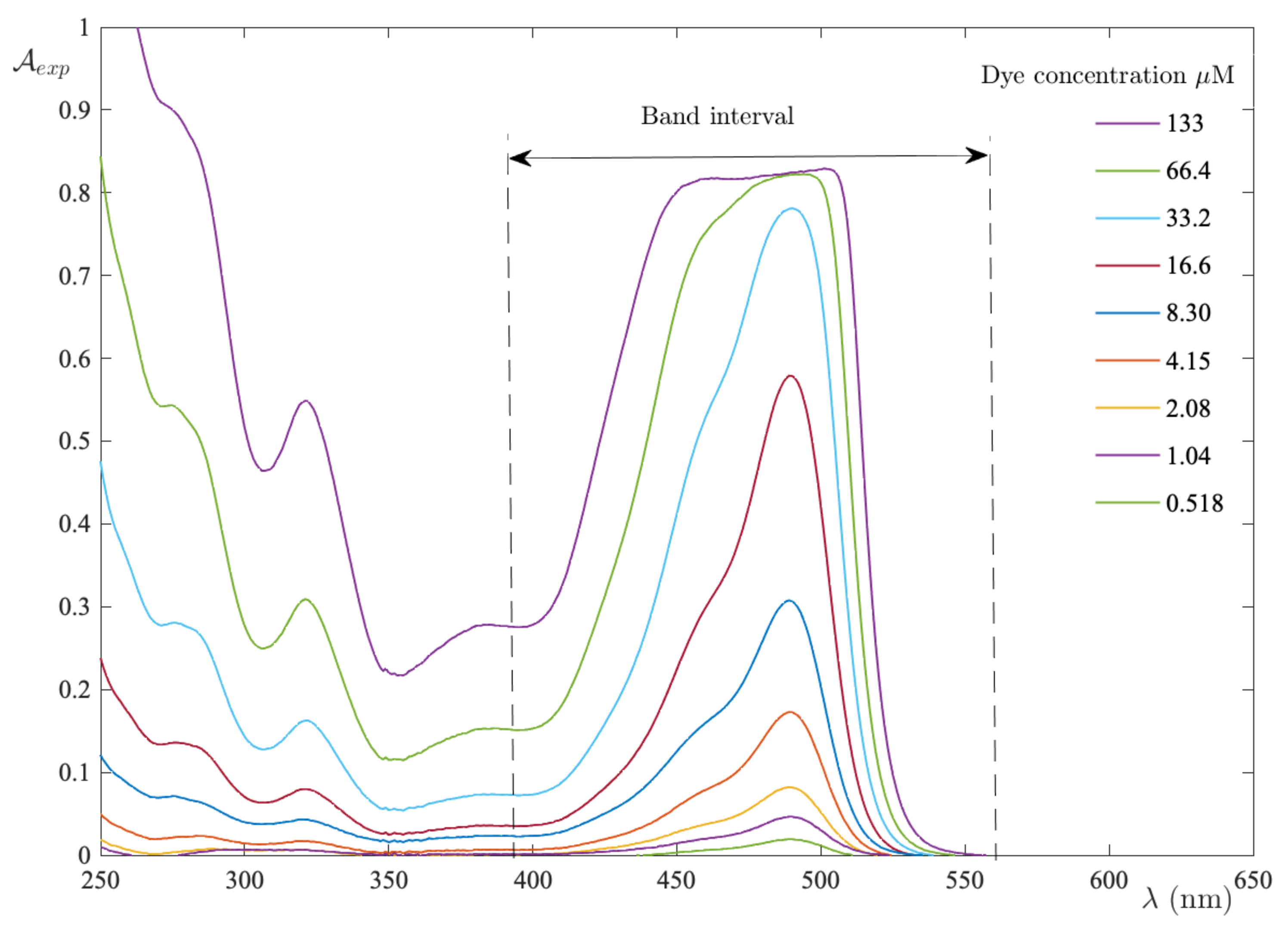
2.1.1. Integral Absorbance
2.1.2. Excess Absorbance
2.1.3. Computational Procedure
2.2. Applications
2.2.1. Anionic Dye in Water Solutions
2.2.2. Anionic Dye in Organic Solution
2.2.3. Cationic Dye in Aqueous Solution

3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of the Samples
3.2.2. Absorption Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FS | Fluorescein sodium salt; |
| ACN | Acetonitrile; |
| UPW | Ultra pure water; |
| UV-Vis | Ultraviolet-visible; |
| MB | Methylene blue |
References
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P.F. Biosensors–sense and sensitivity. Science 2000, 290, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M. Photoelectrochemical cells. In Materials For Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; World Scientific: Singapore, 2011; pp. 26–32. [Google Scholar]
- Rodrigues, E.B.; Penha, F.M.; Furlani, B.; Meyer, C.H.; Maia, M.; Farah, M.E. Historical aspects and evolution of the application of vital dyes in vitreoretinal surgery and chromovitrectomy. In Vital Dyes in Vitreoretinal Surgery; Karger Publishers: Basel, Switzerland, 2008; Volume 42, pp. 29–34. [Google Scholar]
- Zeppa, L.; Ambrosone, L.; Guerra, G.; Fortunato, M.; Costagliola, C. Using canalography to visualize the in vivo aqueous humor outflow conventional pathway in humans. JAMA Ophthalmol. 2014, 132, 1281. [Google Scholar] [CrossRef] [PubMed]
- Philip, R.; Penzkofer, A.; Bäumler, W.; Szeimies, R.; Abels, C. Absorption and fluorescence spectroscopic investigation of indocyanine green. J. Photochem. Photobiol. A Chem. 1996, 96, 137–148. [Google Scholar] [CrossRef]
- Proulx, S.T.; Luciani, P.; Derzsi, S.; Rinderknecht, M.; Mumprecht, V. Quantitative imaging of lymphatic function with liposomal indocyanine green. Cancer Res. 2010, 70, 7053–7062. [Google Scholar] [CrossRef]
- Chapman, M.; Mullen, M.; Novoa-Ortega, E.; Alhasani, M.; Elman, J.F.; Euler, W.B. Structural evolution of ultrathin films of rhodamine 6g on glass. J. Phys. Chem. C 2016, 120, 8289–8297. [Google Scholar] [CrossRef]
- Prausnitz, J.M.; Lichtenthaler, R.N.; De Azevedo, E.G. Molecular Thermodynamics of Fluid-Phase Equilibria; Prentice-Hall, Inc.: Hoboken, NJ, USA, 1998. [Google Scholar]
- Serra, F.; Terentjev, E.M. Nonlinear dynamics of absorption and photobleaching of dyes. J. Chem. Phys. 2008, 128, 224510. [Google Scholar] [CrossRef]
- Huong, A.; Tay, K.G.; Ngu, X. Towards skin tissue oxygen monitoring: An investigation of optimal visible spectral range and minimal spectral resolution. Univ. J. Electr. Electron. Eng. 2019, 6, 40–54. [Google Scholar] [CrossRef]
- Ong, P.E.; Huong, A.K.C.; Ngu, X.T.I.; Mahmud, F.; Philimon, S.P. Modified lambert beer for bilirubin concentration and blood oxygen saturation prediction. Int. J. Adv. Intell. Inform. 2019, 5, 113–122. [Google Scholar] [CrossRef]
- Contini, D.; Martelli, F.; Zaccanti, G. Photon migration through a turbid slab described by a model based on diffusion approximation. I. Theory. Appl. Opt. 1997, 36, 4587–4599. [Google Scholar] [CrossRef]
- Hiraoka, M.; Firbank, M.; Essenpreis, M.; Cope, M.; Arridge, S.R.; Van Der Zee, P.; Delpy, D.T. A Monte Carlo investigation of optical pathlength in inhomogeneous tissue and its application to near-infrared spectroscopy. Phys. Med. Biol. 1993, 38, 1859–1876. [Google Scholar] [CrossRef]
- Karamavuş, Y.; Özkan, M. Newborn jaundice determination by reflectance spectroscopy using multiple polynomial regression, neural network, and support vector regression. Biomed. Signal Process. Control 2019, 51, 253–263. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Kloo, L.; Petterson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Kim, H.; Schembri, T.; Bialas, D.; Stolte, M.; Würthner, F. Slip-stacked J-aggregate materials for organic solar cells and photodetectors. Adv. Mater. 2022, 34, 2104678. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Bian, C.; Stejskal, J.; Zheng, Y.; Jing, X. Azo dye aggregates and their roles in the morphology and conductivity of polypyrrole. Dyes Pigments 2020, 177, 108329. [Google Scholar] [CrossRef]
- Hawe, A.; Rispens, T.; Herron, J.N.; Jiskoot, W. Probing bis-ans binding sites of different affinity on aggregated igg by steady-state fluorescence, time-resolved fluorescence and isothermal titration calorimetry. J. Pharm. Sci. 2011, 100, 1294–1305. [Google Scholar] [CrossRef]
- Duff, D.; Kirkwood, D.; Stevenson, D.; Jiskoot, W. The behaviour of dyes in aqueous solutions. The influence of chemical structure on dye aggregation a polarographic study. J. Soc. Dye. Colour. 1977, 93, 303–306. [Google Scholar] [CrossRef]
- Inglesby, M.; Zeronian, S. Diffusion coefficients for direct dyes in aqueous and polar aprotic solvents by the nmr pulsed-field gradient technique. Dyes Pigments 2001, 50, 3–11. [Google Scholar] [CrossRef]
- Berlepsch, V.; Böttcher, C. H-aggregates of an indocyanine cy5 dye: Transition from strong to weak molecular coupling. J. Phys. Chem. B 2015, 119, 11900–11909. [Google Scholar] [CrossRef]
- Di Nezza, F.; Guerra, R.; Costagliola, C.; Zeppa, L.; Ambrosone, L.; Bracewell, D.G. Thermodynamic properties and photodegradation kinetics of indocyanine green in aqueous solution. Dyes Pigments 2016, 134, 342–347. [Google Scholar] [CrossRef]
- Oshinbolu, S.; Shah, G.; Finka, G.; Molloy, M.; Uden, M. Evaluation of fluorescent dyes to measure protein aggregation within mammalian cell culture supernatants. J. Chem. Technol. Biotechnol. 2018, 93, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Di Nezza, F.; Zeppa, L.; Costagliola, C.; Bufalo, G.; Ambrosone, L. A physicochemical study of ophthalmological vital dyes: From dimerization equilibrium in buffer solution to their liposomal dispersions. Dyes Pigments 2019, 162, 680–687. [Google Scholar] [CrossRef]
- Xue, H.; Thaivalappil, A.; Cao, K. The Potentials of Methylene Blue as an Anti-Aging Drug. Cells 2021, 10, 3379. [Google Scholar] [CrossRef] [PubMed]
- Courrier, E.; Renault, D.; Kaspi, M.; Marcon, A.; Lambert, V.; Garcin, T.; Chiambaretta, F.; Garhofer, G.; Thuret, G.; Gain, P. Micro-instillation of fluorescein with an inoculation loop for ocular surface staining in dry eye syndrome. Acta Ophthalmol. 2018, 96, e140–e146. [Google Scholar] [CrossRef]
- Jalli, P.; Hellsted, T.J.; Immonen, I. Early versus late staining of microaneurysms in fluorescein angiography. Retina 1997, 17, 211–215. [Google Scholar] [CrossRef]
- Romano, M.R.; Ilardi, G.; Ferrara, M.; Cennamo, G.; Parolini, B.; Mariotti, C.; Staibano, S.; Cennamo, G. Macular peeling-induced retinal damage: Clinical and histopathological evaluation after using different dyes. Graefe Arch. Clin. Exp. Ophthalmol. 2018, 256, 1573–1580. [Google Scholar] [CrossRef]
- Jaffe, H.H.; Orchin, M. Theory and Applications of Ultraviolet Spectroscopy; John Wiley & Sons, Inc.: New York, NY, USA; London, UK, 1962. [Google Scholar]
- Ambrosone, L.; Sartorio, R.; Vitagliano, V. Density measurements in the ternary system poly (vinylidene fluoride)-water-N,N-dimethyl formamide at 20 ∘C. Fluid Phase Equilibria 1993, 91, 177–185. [Google Scholar] [CrossRef]
- DeSilva, L.A.; Pitigala, P.; Gaquere-Parker, A.; Landry, R.; Hasbun, J.E.; Martin, V.; Bandara, T.M.W.J.; Perera, A.G.U. Broad absorption natural dye (Mondo-Grass berry) for dye sensitized solar cell. J. Mater. Sci. Mater. Electron. 2017, 28, 7724–7729. [Google Scholar] [CrossRef]
- Casalini, T.; Salvalaglio, M.; Perale, G.; Masi, M.; Cavallotti, C. Diffusion and aggregation of sodium fluorescein in aqueous solutions. J. Phys. Chem. B 2011, 115, 12896–12904. [Google Scholar] [CrossRef]
- Braswell, E. Evidence for trimerization in aqueous solutions of methylene blue. J. Phys. Chem. 2005, 109, 6702–6709. [Google Scholar] [CrossRef]
- Heger, D.; Jirkovsky, J.; Klan, P. Aggregation of methylene blue in frozen aqueous solutions studied by absorption spectroscopy. J. Phys. Chem. A 2005, 72, 2477–2483. [Google Scholar] [CrossRef]
- Zhao, Z.; Malinowski, E.R. Determination of the hydration of methylene blue aggregates and their dissociation constants using visible spectroscopy. Appl. Spectrosc. 1999, 53, 1567–1574. [Google Scholar] [CrossRef]
- Hemmateenejad, B.; Absalan, G.; Hasanpour, M. Application of multivariate curve resolution analysis for studying the thermodynamics of methylene blue aggregations in aqueous solutions. J. Iran. Chem. Soc. 2011, 8, 166–175. [Google Scholar] [CrossRef]
- Klika, Z.; Čapková, P.; Horáková, P.; Valášková, M.; Malỳ, P.; Macháň, R.; Pospíšil, M. Composition, structure, and luminescence of montmorillonites saturated with different aggregates of methylene blue. J. Colloid Interface Sci. 2007, 311, 14–23. [Google Scholar] [CrossRef]
- Fernandez-Perez, A.; Marban, G. Visible light spectroscopic analysis of methylene blue in water; what comes after dimer? ACS Omega 2020, 5, 29801–29815. [Google Scholar] [CrossRef]
- Sarkar, D.; Das, P.; Girigoswami, A.; Chattopadhyay, N. Spectroscopic characterization of phenazinium dye aggregates in water and acetonitrile media: Effect of methyl substitution on the aggregation phenomenon. J. Phys. Chem. A 2008, 112, 9684–9691. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minó, A.; Zeppa, L.; Ambrosone, L. Excess Absorbance as a Novel Approach for Studying the Self-Aggregation of Vital Dyes in Liquid Solution. Int. J. Mol. Sci. 2023, 24, 1645. https://doi.org/10.3390/ijms24021645
Minó A, Zeppa L, Ambrosone L. Excess Absorbance as a Novel Approach for Studying the Self-Aggregation of Vital Dyes in Liquid Solution. International Journal of Molecular Sciences. 2023; 24(2):1645. https://doi.org/10.3390/ijms24021645
Chicago/Turabian StyleMinó, Antonio, Lucio Zeppa, and Luigi Ambrosone. 2023. "Excess Absorbance as a Novel Approach for Studying the Self-Aggregation of Vital Dyes in Liquid Solution" International Journal of Molecular Sciences 24, no. 2: 1645. https://doi.org/10.3390/ijms24021645
APA StyleMinó, A., Zeppa, L., & Ambrosone, L. (2023). Excess Absorbance as a Novel Approach for Studying the Self-Aggregation of Vital Dyes in Liquid Solution. International Journal of Molecular Sciences, 24(2), 1645. https://doi.org/10.3390/ijms24021645





