Nebulized Menthol Impairs Mucociliary Clearance via TRPM8 and MUC5AC/MUC5B in Primary Airway Epithelial Cells
Abstract
1. Introduction
2. Results
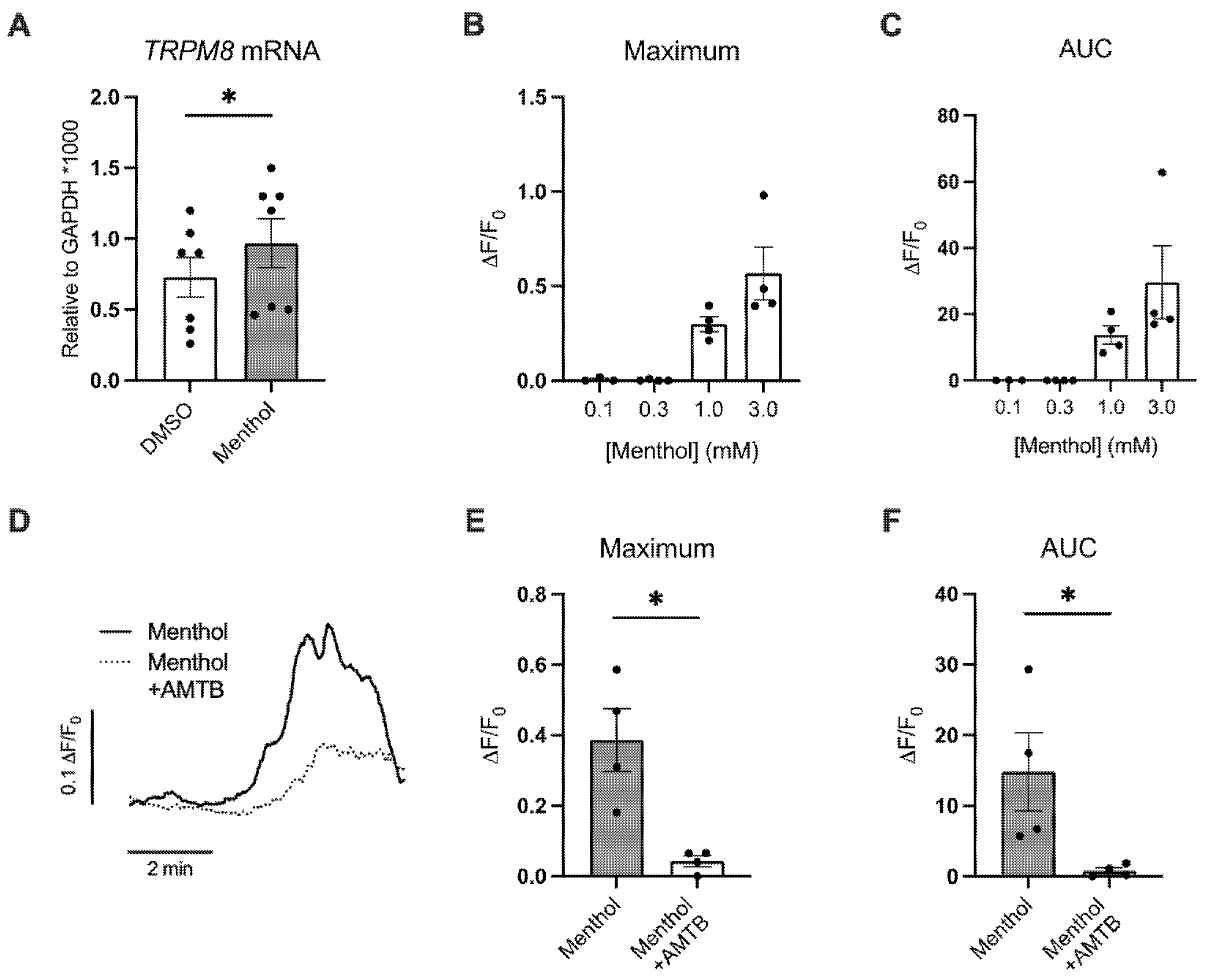
2.1. Menthol Increases TRPM8 mRNA Expression and Induces TRPM8-Mediated Ca2+ Responses
2.2. Menthol Reduces Ciliary Beating and ASL Volumes
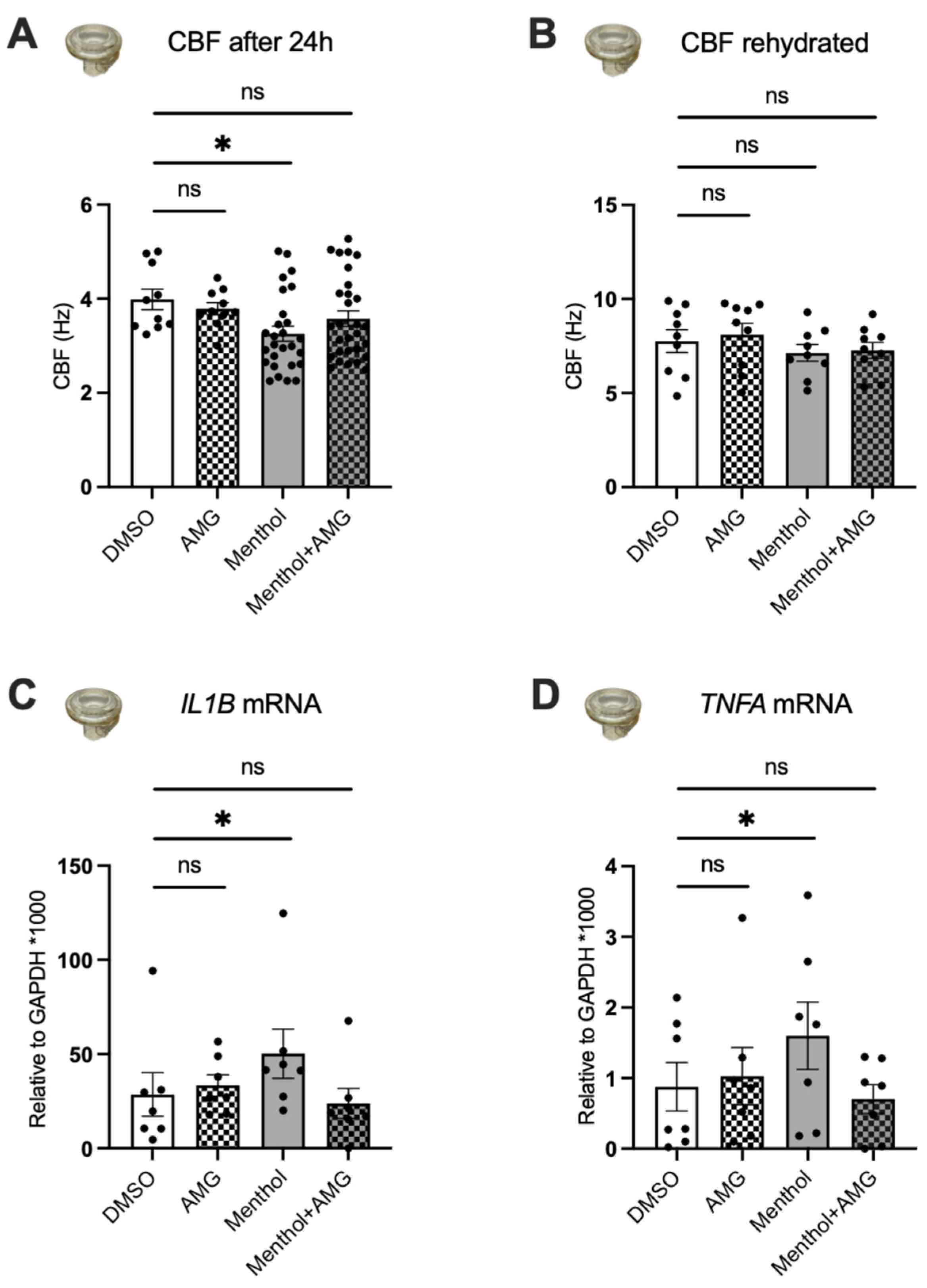
2.3. Nebulized Menthol Decreases CBF and Increases Inflammatory Markers
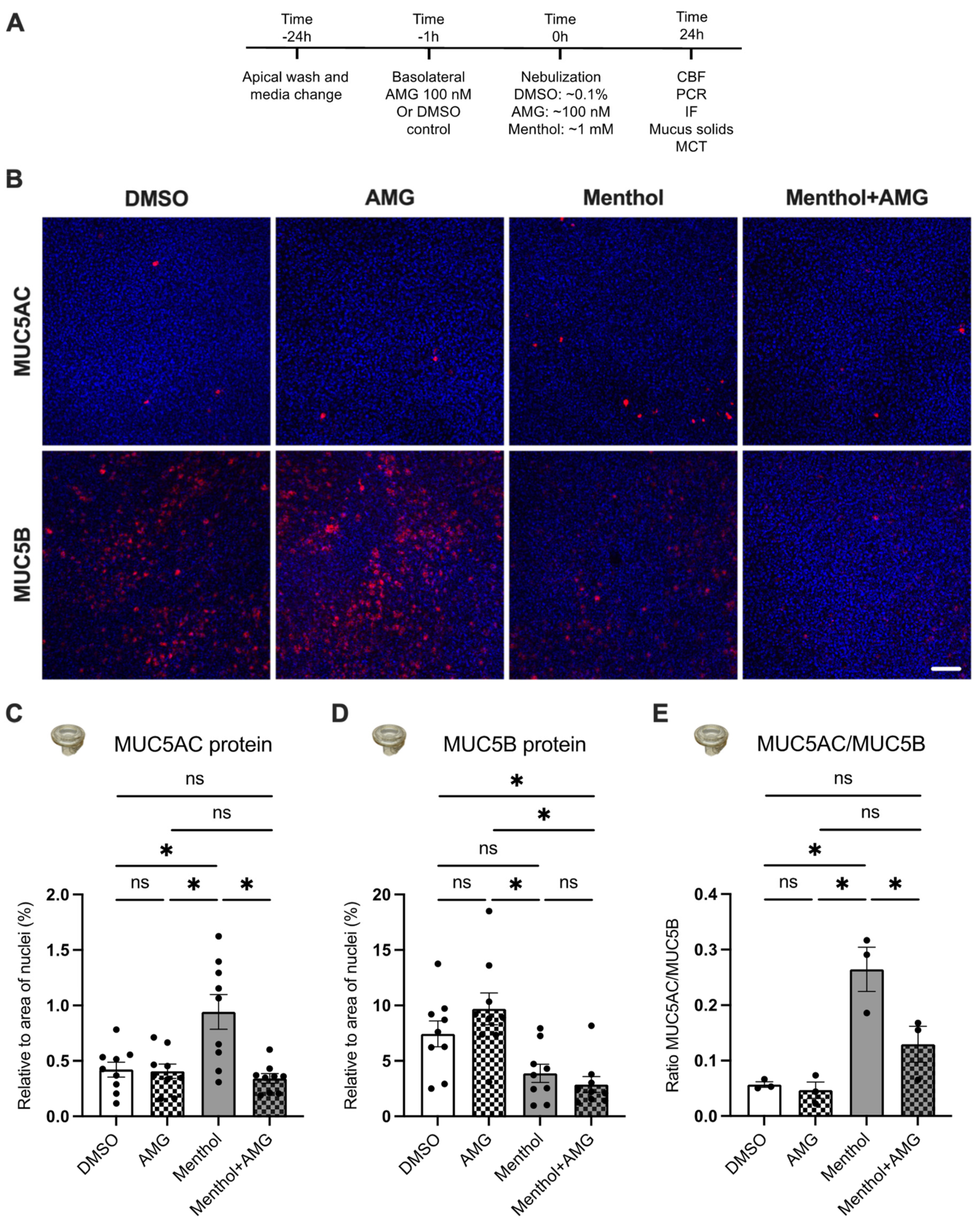
2.4. Nebulized Menthol Alters Mucin Secretions
2.5. Blocking TRPM8 Prevents Menthol-Induced Increases in MUC5AC Expression, Reductions in CBF, and Increases in IL1B and TNFA mRNA Expressions
2.6. Blocking TRPM8 Reverses Menthol-Induced Reductions in Additional Parameters of MCC
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Air–Liquid Interface (ALI) Cultures
4.3. Calcium Imaging
4.4. Airway Surface Liquid (ASL) Volumes
4.5. Ciliary Beat Frequency (CBF)
4.6. Nebulization of Menthol and AMG333 via the Cloud System and Media Treatment
4.7. Immunofluorescence
4.8. Mucus Solids Measurements
4.9. Mucociliary Transport (MCT) Measurements
4.10. Quantitative PCR (qPCR) and Droplet Digital PCR (ddPCR)
4.11. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sassano, M.F.; Davis, E.S.; Keating, J.E.; Zorn, B.T.; Kochar, T.K.; Wolfgang, M.C.; Glish, G.L.; Tarran, R. Evaluation of e-liquid toxicity using an open-source high-throughput screening assay. PLoS Biol. 2018, 16, e2003904. [Google Scholar] [CrossRef]
- Clapp, P.W.; Lavrich, K.S.; van Heusden, C.A.; Lazarowski, E.R.; Carson, J.L.; Jaspers, I. Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2019, 316, L470–L486. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, C.L.; Boitano, S. Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine. Respir. Res. 2016, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Muthumalage, T.; Lamb, T.; Friedman, M.R.; Rahman, I. E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci. Rep. 2019, 9, 19035. [Google Scholar] [CrossRef]
- Park, H.R.; O’Sullivan, M.; Vallarino, J.; Shumyatcher, M.; Himes, B.E.; Park, J.A.; Christiani, D.C.; Allen, J.; Lu, Q. Transcriptomic response of primary human airway epithelial cells to flavoring chemicals in electronic cigarettes. Sci. Rep. 2019, 9, 1400. [Google Scholar] [CrossRef]
- Wang, T.W.; Gentzke, A.S.; Neff, L.J.; Glidden, E.V.; Jamal, A.; Park-Lee, E.; Ren, C.; Cullen, K.A.; King, B.A.; Hacker, K.A. Characteristics of e-Cigarette Use Behaviors Among US Youth, 2020. JAMA Netw. Open. 2021, 4, e2111336. [Google Scholar] [CrossRef]
- Omaiye, E.E.; Luo, W.; McWhirter, K.J.; Pankow, J.F.; Talbot, P. Flavour chemicals, synthetic coolants and pulegone in popular mint-flavoured and menthol-flavoured e-cigarettes. Tob. Control. 2022, 31, e3–e9. [Google Scholar] [CrossRef]
- Eccles, R. Menthol and related cooling compounds. J. Pharm. Pharm. 1994, 46, 618–630. [Google Scholar] [CrossRef]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP channel that senses cold stimuli and menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, A.; Murray, A.N.; Mathur, J.; Earley, T.J.; Petrus, M.J.; Patapoutian, A. TRPM8 is required for cold sensation in mice. Neuron 2007, 54, 371–378. [Google Scholar] [CrossRef]
- Liu, Y.; Mikrani, R.; He, Y.; Faran Ashraf Baig, M.M.; Abbas, M.; Naveed, M.; Tang, M.; Zhang, Q.; Li, C.; Zhou, X. TRPM8 channels: A review of distribution and clinical role. Eur. J. Pharm. 2020, 882, 173312. [Google Scholar] [CrossRef]
- Sabnis, A.S.; Shadid, M.; Yost, G.S.; Reilly, C.A. Human lung epithelial cells express a functional cold-sensing TRPM8 variant. Am. J. Respir. Cel.l Mo.l Biol. 2008, 39, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Demouveaux, B.; Gouyer, V.; Gottrand, F.; Narita, T.; Desseyn, J.L. Gel-forming mucin interactome drives mucus viscoelasticity. Adv. Colloid. Interface. Sci. 2018, 252, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Rubin, B.K.; Voynow, J.A. Mucins, Mucus, and Goblet Cells. Chest 2018, 154, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Q.; Yang, G.; Kolosov, V.P.; Perelman, J.M.; Zhou, X.D. Cold temperature induces mucin hypersecretion from normal human bronchial epithelial cells in vitro through a transient receptor potential melastatin 8 (TRPM8)-mediated mechanism. J. Allergy. Clin. Immunol. 2011, 128, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Naumov, D.; Gassan, D.; Kotova, O.; Prikhodko, A.; Pinegina, E.; Perelman, J.; Kolosov, V.; Zhou, X.; Li, Q. Cold air alters MUC5AC and MUC5B expression in the airways of asthma patients. Eur. Respir. J. 2018, 52, PA1272. [Google Scholar]
- Kesimer, M.; Ford, A.A.; Ceppe, A.; Radicioni, G.; Cao, R.; Davis, C.W.; Doerschuk, C.M.; Alexis, N.E.; Anderson, W.H.; Henderson, A.G.; et al. Airway Mucin Concentration as a Marker of Chronic Bronchitis. N Engl. J. Med. 2017, 377, 911–922. [Google Scholar] [CrossRef]
- Roy, M.G.; Livraghi-Butrico, A.; Fletcher, A.A.; McElwee, M.M.; Evans, S.E.; Boerner, R.M.; Alexander, S.N.; Bellinghausen, L.K.; Song, A.S.; Petrova, Y.M.; et al. Muc5b is required for airway defence. Nature 2014, 505, 412–416. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, Y.S.; Kim, H.I.; Park, J.Y.; Park, S.H.; Hwang, Y.I.; Jang, S.H.; Jung, K.S.; Park, H.S.; Park, C.S. Activation of Transient Receptor Potential Melastatin Family Member 8 (TRPM8) Receptors Induces Proinflammatory Cytokine Expressions in Bronchial Epithelial Cells. Allergy. Asthma. Immunol. Res. 2020, 12, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Baumlin, N.; Dennis, J.S.; Moore, R.; Salathe, S.F.; Whitney, P.L.; Sabater, J.; Abraham, W.M.; Kim, M.D.; Salathe, M. Electronic Cigarette Vapor with Nicotine Causes Airway Mucociliary Dysfunction Preferentially via TRPA1 Receptors. Am. J. Respi.r Crit. Care. Med. 2019, 200, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Li, M.C.; Perelman, J.M.; Kolosov, V.P.; Zhou, X.D. Effects of transient receptor potential melastatin 8 cation channels on inflammatory reaction induced by cold temperatures in human airway epithelial cells. Zhonghua Jie He He Hu Xi Za Zhi 2011, 34, 757–761. [Google Scholar] [PubMed]
- Chen, Y.; Val, S.; Krueger, A.; Zhong, L.; Panigrahi, A.; Nino, G.; Wolf, S.; Preciado, D. Human primary middle ear epithelial cell culture: A novel in vitro model to study otitis media. Laryngoscope Investig Otolaryngol 2019, 4, 663–672. [Google Scholar] [CrossRef]
- Song, K.S.; Seong, J.K.; Chung, K.C.; Lee, W.J.; Kim, C.H.; Cho, K.N.; Kang, C.D.; Koo, J.S.; Yoon, J.H. Induction of MUC8 gene expression by interleukin-1 beta is mediated by a sequential ERK MAPK/RSK1/CREB cascade pathway in human airway epithelial cells. J. Biol. Chem. 2003, 278, 34890–34896. [Google Scholar] [CrossRef] [PubMed]
- Liegeois, M.A.; Fahy, J.V. The Mucin Gene MUC5B Is Required for Normal Lung Function. Am. J. Respir. Crit. Care. Med. 2022, 205, 737–739. [Google Scholar] [CrossRef]
- Kim, M.D.; Baumlin, N.; Dennis, J.S.; Yoshida, M.; Kis, A.; Aguiar, C.; Schmid, A.; Mendes, E.; Salathe, M. Losartan reduces cigarette smoke-induced airway inflammation and mucus hypersecretion. ERJ Open. Res. 2021, 7. [Google Scholar] [CrossRef]
- Horne, D.B.; Biswas, K.; Brown, J.; Bartberger, M.D.; Clarine, J.; Davis, C.D.; Gore, V.K.; Harried, S.; Horner, M.; Kaller, M.R.; et al. Discovery of TRPM8 Antagonist (S)-6-(((3-Fluoro-4-(trifluoromethoxy)phenyl)(3-fluoropyridin-2-yl)methyl)carbamoyl)nicotinic Acid (AMG 333), a Clinical Candidate for the Treatment of Migraine. J. Med. Chem. 2018, 61, 8186–8201. [Google Scholar] [CrossRef]
- Liu, S.C.; Lu, H.H.; Fan, H.C.; Wang, H.W.; Chen, H.K.; Lee, F.P.; Yu, C.J.; Chu, Y.H. The identification of the TRPM8 channel on primary culture of human nasal epithelial cells and its response to cooling. Medicine 2017, 96, e7640. [Google Scholar] [CrossRef]
- Neher, A.; Gstottner, M.; Thaurer, M.; Augustijns, P.; Reinelt, M.; Schobersberger, W. Influence of essential and fatty oils on ciliary beat frequency of human nasal epithelial cells. Am. J. Rhinol. 2008, 22, 130–134. [Google Scholar] [CrossRef]
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and Mucociliary Clearance. Cold Spring Harb. Perspect. Biol. 2017, 9, a028241. [Google Scholar] [CrossRef]
- Fischer, A.J.; Pino-Argumedo, M.I.; Hilkin, B.M.; Shanrock, C.R.; Gansemer, N.D.; Chaly, A.L.; Zarei, K.; Allen, P.D.; Ostedgaard, L.S.; Hoffman, E.A.; et al. Mucus strands from submucosal glands initiate mucociliary transport of large particles. JCI Insight 2019, 4, e124863. [Google Scholar] [CrossRef]
- Bonser, L.R.; Zlock, L.; Finkbeiner, W.; Erle, D.J. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J. Clin. Invest. 2016, 126, 2367–2371. [Google Scholar] [CrossRef]
- Evans, C.M.; Raclawska, D.S.; Ttofali, F.; Liptzin, D.R.; Fletcher, A.A.; Harper, D.N.; McGing, M.A.; McElwee, M.M.; Williams, O.W.; Sanchez, E.; et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat Commun 2015, 6, 6281. [Google Scholar] [CrossRef] [PubMed]
- Costain, G.; Liu, Z.; Mennella, V.; Radicioni, G.; Goczi, A.N.; Albulescu, A.; Walker, S.; Ngan, B.; Manson, D.; Vali, R.; et al. Hereditary Mucin Deficiency Caused by Biallelic Loss of Function of MUC5B. Am. J. Respir. Crit. Care. Med. 2022, 205, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.D.; Chung, S.; Dennis, J.S.; Yoshida, M.; Aguiar, C.; Aller, S.P.; Mendes, E.S.; Schmid, A.; Sabater, J.; Baumlin, N.; et al. Vegetable glycerin e-cigarette aerosols cause airway inflammation and ion channel dysfunction. Front. Pharm. 2022, 13, 1012723. [Google Scholar] [CrossRef]
- Gellatly, S.; Pavelka, N.; Crue, T.; Schweitzer, K.S.; Day, B.J.; Min, E.; Numata, M.; Voelker, D.R.; Scruggs, A.; Petrache, I.; et al. Nicotine-Free e-Cigarette Vapor Exposure Stimulates IL6 and Mucin Production in Human Primary Small Airway Epithelial Cells. J. Inflamm. Res. 2020, 13, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Reidel, B.; Radicioni, G.; Clapp, P.W.; Ford, A.A.; Abdelwahab, S.; Rebuli, M.E.; Haridass, P.; Alexis, N.E.; Jaspers, I.; Kesimer, M. E-Cigarette Use Causes a Unique Innate Immune Response in the Lung, Involving Increased Neutrophilic Activation and Altered Mucin Secretion. Am. J. Respir. Crit. Care. Med. 2018, 197, 492–501. [Google Scholar] [CrossRef]
- Ghosh, A.; Coakley, R.C.; Mascenik, T.; Rowell, T.R.; Davis, E.S.; Rogers, K.; Webster, M.J.; Dang, H.; Herring, L.E.; Sassano, M.F.; et al. Chronic E-Cigarette Exposure Alters the Human Bronchial Epithelial Proteome. Am. J. Respir. Crit. Care. Med. 2018, 198, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.S.; Baxter, M.; Dubuis, E.; Birrell, M.A.; Belvisi, M.G. Transient receptor potential (TRP) channels in the airway: Role in airway disease. Br. J. Pharm. 2014, 171, 2593–2607. [Google Scholar] [CrossRef]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Baumlin-Schmid, N.; Salathe, M.; Fregien, N.L. Optimal Lentivirus Production and Cell Culture Conditions Necessary to Successfully Transduce Primary Human Bronchial Epithelial Cells. J. Vis. Exp. 2016, 113, e54176. [Google Scholar] [CrossRef] [PubMed]
- Bengtson, C.D.; Kim, M.D.; Anabtawi, A.; He, J.; Dennis, J.S.; Miller, S.; Yoshida, M.; Baumlin, N.; Salathe, M. Hyperglycaemia in cystic fibrosis adversely affects BK channel function critical for mucus clearance. Eur. Respir. J. 2021, 57, 2000509. [Google Scholar] [PubMed]
- Kim, M.D.; Bengtson, C.D.; Yoshida, M.; Niloy, A.J.; Dennis, J.S.; Baumlin, N.; Salathe, M. Losartan ameliorates TGF-beta1-induced CFTR dysfunction and improves correction by cystic fibrosis modulator therapies. J. Clin. Invest. 2022, 132, e155241. [Google Scholar] [CrossRef]
- Bengtson, C.; Silswal, N.; Baumlin, N.; Yoshida, M.; Dennis, J.; Yerrathota, S.; Kim, M.; Salathe, M. The CFTR Amplifier Nesolicaftor Rescues TGF-beta1 Inhibition of Modulator-Corrected F508del CFTR Function. Int. J. Mol. Sci. 2022, 23, 10956. [Google Scholar] [CrossRef]
- Garcia-Arcos, I.; Geraghty, P.; Baumlin, N.; Campos, M.; Dabo, A.J.; Jundi, B.; Cummins, N.; Eden, E.; Grosche, A.; Salathe, M.; et al. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax 2016, 71, 1119–1129. [Google Scholar] [CrossRef]
- Kim, M.D.; Baumlin, N.; Guerrero-Cignarella, A.; Schmid, A.; Aguiar, C.; Mohiuddin, M.; Dennis, J.S.; Ahluwalia, J.S.; Leavens, E.L.; Nollen, N.; et al. Persistence of airway inflammation in smokers who switch to electronic cigarettes. ERJ Open. Res. 2022, 8. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumlin, N.; Silswal, N.; Dennis, J.S.; Niloy, A.J.; Kim, M.D.; Salathe, M. Nebulized Menthol Impairs Mucociliary Clearance via TRPM8 and MUC5AC/MUC5B in Primary Airway Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 1694. https://doi.org/10.3390/ijms24021694
Baumlin N, Silswal N, Dennis JS, Niloy AJ, Kim MD, Salathe M. Nebulized Menthol Impairs Mucociliary Clearance via TRPM8 and MUC5AC/MUC5B in Primary Airway Epithelial Cells. International Journal of Molecular Sciences. 2023; 24(2):1694. https://doi.org/10.3390/ijms24021694
Chicago/Turabian StyleBaumlin, Nathalie, Neerupma Silswal, John S. Dennis, Asef J. Niloy, Michael D. Kim, and Matthias Salathe. 2023. "Nebulized Menthol Impairs Mucociliary Clearance via TRPM8 and MUC5AC/MUC5B in Primary Airway Epithelial Cells" International Journal of Molecular Sciences 24, no. 2: 1694. https://doi.org/10.3390/ijms24021694
APA StyleBaumlin, N., Silswal, N., Dennis, J. S., Niloy, A. J., Kim, M. D., & Salathe, M. (2023). Nebulized Menthol Impairs Mucociliary Clearance via TRPM8 and MUC5AC/MUC5B in Primary Airway Epithelial Cells. International Journal of Molecular Sciences, 24(2), 1694. https://doi.org/10.3390/ijms24021694





