GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives
Abstract
:1. Introduction
2. GLP-1 Receptor Agonists: Mechanisms of Action and Current Indications
3. Effects of GLP-1 Receptor Agonists on NAFLD
3.1. Effects of GLP-1 RAs on Body Weight
3.2. Effects of GLP-1 RAs on Hepatic Cytolysis
3.3. Effects of GLP-1 RAs on Liver Fibrosis
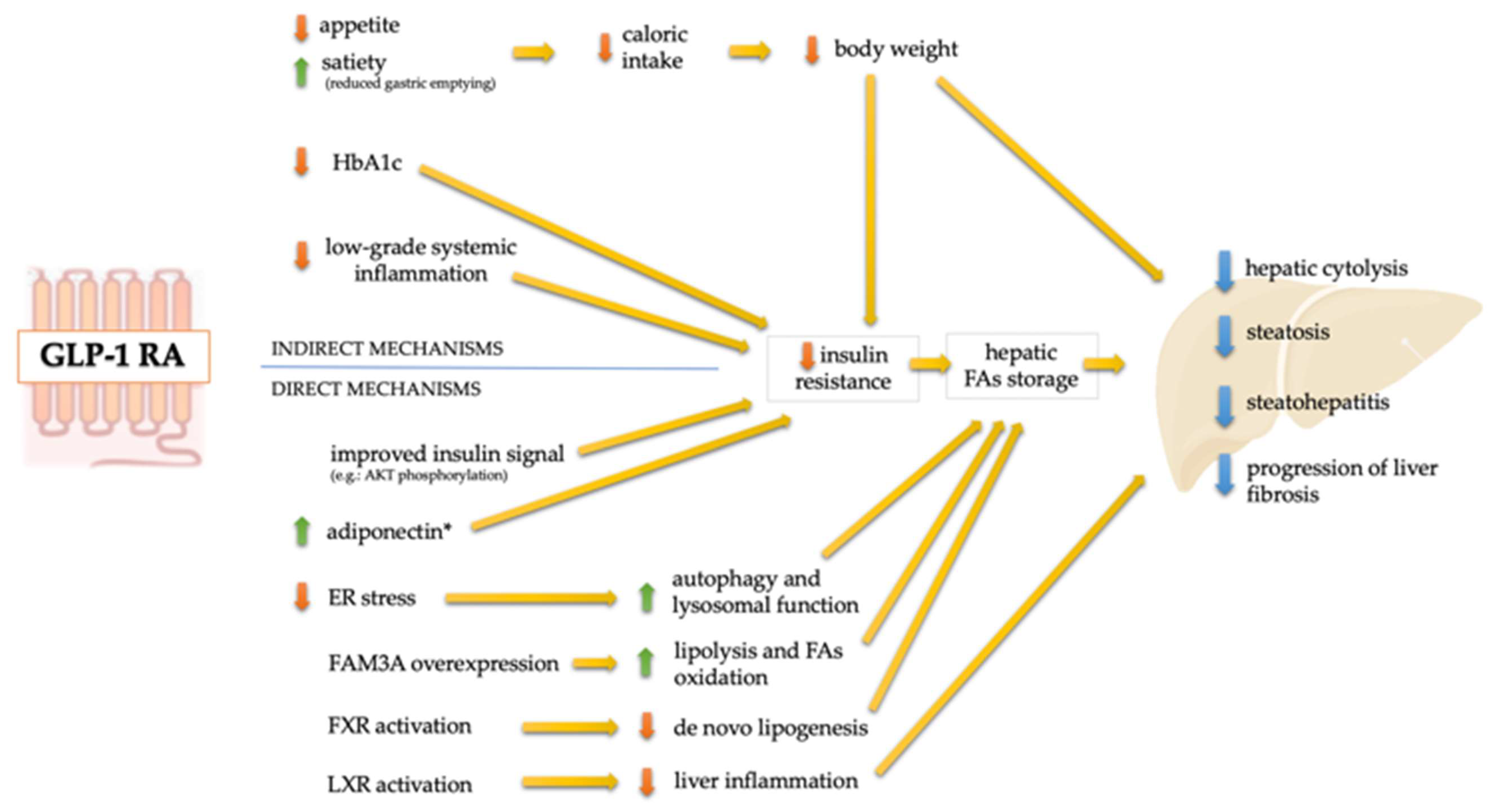
4. Potential Mechanisms of Action of GLP-1 RAs in NAFLD
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; Acierno, C.; Nevola, R.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Salvatore, T.; Adinolfi, L.E.; Sasso, F.C. Non-Alcoholic Fatty Liver Disease: From Pathogenesis to Clinical Impact. Processes 2021, 9, 135. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Acierno, C.; Caturano, A.; Pafundi, P.C.; Nevola, R.; Adinolfi, L.E.; Sasso, F.C. Nonalcoholic fatty liver disease and type 2 diabetes: Pathophysiological mechanisms shared between the two faces of the same coin. Explor. Med. 2020, 1, 287–306. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Cusi, K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep. 2019, 1, 312–328. [Google Scholar] [CrossRef] [Green Version]
- Dresner, A.; Laurent, D.; Marcucci, M.; Griffin, M.E.; Dufour, S.; Cline, G.W.; Slezak, L.A.; Andersen, D.K.; Hundal, R.S.; Rothman, D.L.; et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J. Clin. Investig. 1999, 103, 253–259. [Google Scholar] [CrossRef] [Green Version]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Associazione Italiana per lo Studio del Fegato (AISF), Società Italiana di Diabetologia (SID) and Società Italiana dell’Obesità (SIO); Members of the Guidelines Panel; Coordinator; AISF Members; SID Members; SIO Members; Metodologists. Non-alcoholic fatty liver disease in adults 2021: A clinical practice guideline of the Italian Association for the Study of the Liver (AISF), the Italian Society of Diabetology (SID) and the Italian Society of Obesity (SIO). Dig. Liver Dis. 2022, 54, 170–182. [Google Scholar] [CrossRef]
- Yen, F.S.; Yang, Y.C.; Hwu, C.M.; Wei, J.C.; Huang, Y.H.; Hou, M.C.; Hsu, C.C. Liver-related long-term outcomes of thiazolidinedione use in persons with type 2 diabetes. Liver Int. 2020, 40, 1089–1097. [Google Scholar] [CrossRef] [Green Version]
- Bril, F.; Biernacki, D.M.; Kalavalapalli, S.; Lomonaco, R.; Subbarayan, S.K.; Lai, J.; Tio, F.; Suman, A.; Orsak, B.K.; Hecht, J.; et al. Role of Vitamin E for Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2019, 42, 1481–1488. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Ratziu, V.; Loomba, R.; Rinella, M.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; Geier, A.; Beckebaum, S.; Newsome, P.N.; et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019, 394, 2184–2196, Erratum in Lancet 2020, 396, 312Erratum in Lancet 2021, 397, 2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef] [PubMed]
- Nevola, R.; Alfano, M.; Pafundi, P.C.; Brin, C.; Gragnano, F.; Calabrò, P.; Adinolfi, L.E.; Rinaldi, L.; Sasso, F.C.; Caturano, A. Cardiorenal Impact of SGLT-2 Inhibitors: A Conceptual Revolution in The Management of Type 2 Diabetes, Heart Failure and Chronic Kidney Disease. Rev. Cardiovasc. Med. 2022, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, L.; Wang, J.; Wang, T.; Chien, C.; Huang, W.; Fu, X.; Xiao, Y.; Fu, Q.; Wang, S.; et al. Network meta-analysis on the effects of finerenone versus SGLT2 inhibitors and GLP-1 receptor agonists on cardiovascular and renal outcomes in patients with type 2 diabetes mellitus and chronic kidney disease. Cardiovasc. Diabetol. 2022, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Jensterle, M.; Rizzo, M.; Haluzík, M.; Janež, A. Efficacy of GLP-1 RA Approved for Weight Management in Patients With or Without Diabetes: A Narrative Review. Adv. Ther. 2022, 39, 2452–2467. [Google Scholar] [CrossRef]
- Del Prato, S.; Gallwitz, B.; Holst, J.J.; Meier, J.J. The incretin/glucagon system as a target for pharmacotherapy of obesity. Obes. Rev. 2022, 23, e13372. [Google Scholar] [CrossRef]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef] [Green Version]
- Rajeev, S.P.; Wilding, J. GLP-1 as a target for therapeutic intervention. Curr. Opin. Pharmacol. 2016, 31, 44–49. [Google Scholar] [CrossRef]
- Salvatore, T.; Nevola, R.; Pafundi, P.C.; Monaco, L.; Ricozzi, C.; Imbriani, S.; Rinaldi, L.; Sasso, F.C. Incretin Hormones: The Link between Glycemic Index and Cardiometabolic Diseases. Nutrients 2019, 11, 1878. [Google Scholar] [CrossRef]
- Lovshin, J.A.; Drucker, D.J. Incretin-based therapies for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Agersø, H.; Jensen, L.B.; Elbrønd, B.; Rolan, P.; Zdravkovic, M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia 2002, 45, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, J.M.; Nuffer, W.; Smith, B.A. GLP-1 receptor agonists: An updated review of head-to-head clinical studies. Ther. Adv. Endocrinol. Metab. 2021, 12, 2042018821997320. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [Green Version]
- Marfella, R.; Sardu, C.; Balestrieri, M.L.; Siniscalchi, M.; Minicucci, F.; Signoriello, G.; Calabrò, P.; Mauro, C.; Pieretti, G.; Coppola, A.; et al. Effects of incretin treatment on cardiovascular outcomes in diabetic STEMI-patients with culprit obstructive and multivessel non obstructive-coronary-stenosis. Diabetol. Metab. Syndr. 2018, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Mann, J.F.E.; Ørsted, D.D.; Brown-Frandsen, K.; Marso, S.P.; Poulter, N.R.; Rasmussen, S.; Tornøe, K.; Zinman, B.; Buse, J.B.; LEADER Steering Committee and Investigators. Liraglutide and Renal Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 839–848. [Google Scholar] [CrossRef] [Green Version]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Botros, F.T.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and renal outcomes in type 2 diabetes: An exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 2019, 394, 131–138. [Google Scholar] [CrossRef]
- Lingvay, I.; Sumithran, P.; Cohen, R.V.; le Roux, C.W. Obesity management as a primary treatment goal for type 2 diabetes: Time to reframe the conversation. Lancet 2022, 399, 394–405, Erratum in Lancet 2022, 399, 358. [Google Scholar] [CrossRef]
- O’Neil, P.M.; Birkenfeld, A.L.; McGowan, B.; Mosenzon, O.; Pedersen, S.D.; Wharton, S.; Carson, C.G.; Jepsen, C.H.; Kabisch, M.; Wilding, J.P.H. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: A randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet 2018, 392, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Pratley, R.E.; Aroda, V.R.; Lingvay, I.; Lüdemann, J.; Andreassen, C.; Navarria, A.; Viljoen, A.; SUSTAIN 7 Investigators. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): A randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018, 6, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.; le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Davies, M.; Færch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; et al. STEP 2 Study Group. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021, 397, 971–984. [Google Scholar] [CrossRef]
- Wadden, T.A.; Bailey, T.S.; Billings, L.K.; Davies, M.; Frias, J.P.; Koroleva, A.; Lingvay, I.; O’Neil, P.M.; Rubino, D.M.; Skovgaard, D.; et al. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA 2021, 325, 1403–1413. [Google Scholar] [CrossRef]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 2021, 325, 1414–1425. [Google Scholar] [CrossRef]
- Astrup, A.; Rössner, S.; Van Gaal, L.; Rissanen, A.; Niskanen, L.; Al Hakim, M.; Madsen, J.; Rasmussen, M.F.; Lean, M.E.; NN8022-1807 Study Group. Effects of liraglutide in the treatment of obesity: A randomised, double-blind, placebo-controlled study. Lancet 2009, 374, 1606–1616, Erratum in Lancet 2010, 375, 984. [Google Scholar] [CrossRef]
- Blundell, J.; Finlayson, G.; Axelsen, M.; Flint, A.; Gibbons, C.; Kvist, T.; Hjerpsted, J.B. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes. Metab. 2017, 19, 1242–1251. [Google Scholar] [CrossRef] [Green Version]
- Kelly, A.S.; Auerbach, P.; Barrientos-Perez, M.; Gies, I.; Hale, P.M.; Marcus, C.; Mastrandrea, L.D.; Prabhu, N.; Arslanian, S.; NN8022-4180 Trial Investigators. A Randomized, Controlled Trial of Liraglutide for Adolescents with Obesity. N. Engl. J. Med. 2020, 382, 2117–2128. [Google Scholar] [CrossRef]
- de Boer, S.A.; Lefrandt, J.D.; Petersen, J.F.; Boersma, H.H.; Mulder, D.J.; Hoogenberg, K. The effects of GLP-1 analogues in obese, insulin-using type 2 diabetes in relation to eating behaviour. Int. J. Clin. Pharm. 2016, 38, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadden, T.A.; Tronieri, J.S.; Sugimoto, D.; Lund, M.T.; Auerbach, P.; Jensen, C.; Rubino, D. Liraglutide 3.0 mg and Intensive Behavioral Therapy (IBT) for Obesity in Primary Care: The SCALE IBT Randomized Controlled Trial. Obesity 2020, 28, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Hollander, P.; Klein, S.; Niswender, K.; Woo, V.; Hale, P.M.; Aronne, L.; NN8022-1923 Investigators. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: The SCALE Maintenance randomized study. Int. J. Obes. 2013, 37, 1443–1451, Erratum in Int. J. Obes. 2013, 37, 1514Erratum in Int. J. Obes. 2015, 39, 187. [Google Scholar] [CrossRef] [Green Version]
- Blackman, A.; Foster, G.D.; Zammit, G.; Rosenberg, R.; Aronne, L.; Wadden, T.; Claudius, B.; Jensen, C.B.; Mignot, E. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: The SCALE Sleep Apnea randomized clinical trial. Int. J. Obes. 2016, 40, 1310–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halawi, H.; Khemani, D.; Eckert, D.; O’Neill, J.; Kadouh, H.; Grothe, K.; Clark, M.M.; Burton, D.D.; Vella, A.; Acosta, A.; et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: A randomised, placebo-controlled pilot trial. Lancet Gastroenterol. Hepatol. 2017, 2, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Rubino, D.M.; Greenway, F.L.; Khalid, U.; O’Neil, P.M.; Rosenstock, J.; Sørrig, R.; Wadden, T.A.; Wizert, A.; Garvey, W.T.; STEP 8 Investigators. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults With Overweight or Obesity Without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA 2022, 327, 138–150. [Google Scholar] [CrossRef]
- Davies, M.J.; Bergenstal, R.; Bode, B.; Kushner, R.F.; Lewin, A.; Skjøth, T.V.; Andreasen, A.H.; Jensen, C.B.; DeFronzo, R.A.; NN8022-1922 Study Group. Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. JAMA 2015, 314, 687–699, Erratum in JAMA 2016, 315, 90. [Google Scholar] [CrossRef] [Green Version]
- Garvey, W.T.; Birkenfeld, A.L.; Dicker, D.; Mingrone, G.; Pedersen, S.D.; Satylganova, A.; Skovgaard, D.; Sugimoto, D.; Jensen, C.; Mosenzon, O. Efficacy and Safety of Liraglutide 3.0 mg in Individuals With Overweight or Obesity and Type 2 Diabetes Treated With Basal Insulin: The SCALE Insulin Randomized Controlled Trial. Diabetes Care 2020, 43, 1085–1093. [Google Scholar] [CrossRef] [Green Version]
- Weghuber, D.; Barrett, T.; Barrientos-Pérez, M.; Gies, I.; Hesse, D.; Jeppesen, O.K.; Kelly, A.S.; Mastrandrea, L.D.; Sørrig, R.; Arslanian, S.; et al. Once-Weekly Semaglutide in Adolescents with Obesity. N. Engl. J. Med. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Yan, J.; Yao, B.; Kuang, H.; Yang, X.; Huang, Q.; Hong, T.; Li, Y.; Dou, J.; Yang, W.; Qin, G.; et al. Liraglutide, Sitagliptin, and Insulin Glargine Added to Metformin: The Effect on Body Weight and Intrahepatic Lipid in Patients With Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 2414–2426. [Google Scholar] [CrossRef]
- van Bloemendaal, L.; Veltman, D.J.; Ten Kulve, J.S.; Groot, P.F.; Ruhé, H.G.; Barkhof, F.; Sloan, J.H.; Diamant, M.; Ijzerman, R.G. Brain reward-system activation in response to anticipation and consumption of palatable food is altered by glucagon-like peptide-1 receptor activation in humans. Diabetes Obes. Metab. 2015, 17, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, K.; Kaji, T. Liraglutide, a GLP-1 Receptor Agonist, Which Decreases Hypothalamic 5-HT2A Receptor Expression, Reduces Appetite and Body Weight Independently of Serotonin Synthesis in Mice. J. Diabetes Res. 2018, 2018, 6482958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonogaki, K.; Kaji, T. The acute anorexic effect of liraglutide, a GLP-1 receptor agonist, does not require functional leptin receptor, serotonin, and hypothalamic POMC and CART activities in mice. Diabetes Res. Clin. Pract. 2016, 120, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Gabery, S.; Salinas, C.G.; Paulsen, S.J.; Ahnfelt-Rønne, J.; Alanentalo, T.; Baquero, A.F.; Buckley, S.T.; Farkas, E.; Fekete, C.; Frederiksen, K.S.; et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020, 5, e133429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umapathysivam, M.M.; Lee, M.Y.; Jones, K.L.; Annink, C.E.; Cousins, C.E.; Trahair, L.G.; Rayner, C.K.; Chapman, M.J.; Nauck, M.A.; Horowitz, M.; et al. Comparative effects of prolonged and intermittent stimulation of the glucagon-like peptide 1 receptor on gastric emptying and glycemia. Diabetes 2014, 63, 785–790. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Abouda, G.; Aldersley, M.A.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Petit, J.M.; Cercueil, J.P.; Loffroy, R.; Denimal, D.; Bouillet, B.; Fourmont, C.; Chevallier, O.; Duvillard, L.; Vergès, B. Effect of Liraglutide Therapy on Liver Fat Content in Patients With Inadequately Controlled Type 2 Diabetes: The Lira-NAFLD Study. J. Clin. Endocrinol. Metab. 2017, 102, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Frøssing, S.; Nylander, M.; Chabanova, E.; Frystyk, J.; Holst, J.J.; Kistorp, C.; Skouby, S.O.; Faber, J. Effect of liraglutide on ectopic fat in polycystic ovary syndrome: A randomized clinical trial. Diabetes Obes. Metab. 2018, 20, 215–218. [Google Scholar] [CrossRef]
- Feng, W.H.; Bi, Y.; Li, P.; Yin, T.T.; Gao, C.X.; Shen, S.M.; Gao, L.J.; Yang, D.H.; Zhu, D.L. Effects of liraglutide, metformin and gliclazide on body composition in patients with both type 2 diabetes and non-alcoholic fatty liver disease: A randomized trial. J. Diabetes Investig. 2019, 10, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Matikainen, N.; Söderlund, S.; Björnson, E.; Pietiläinen, K.; Hakkarainen, A.; Lundbom, N.; Taskinen, M.R.; Borén, J. Liraglutide treatment improves postprandial lipid metabolism and cardiometabolic risk factors in humans with adequately controlled type 2 diabetes: A single-centre randomized controlled study. Diabetes Obes. Metab. 2019, 21, 84–94. [Google Scholar] [CrossRef]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.S.; Harrison, S.A.; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Sanchez, P.; Bril, F.; Maximos, M.; Lomonaco, R.; Biernacki, D.; Orsak, B.; Subbarayan, S.; Webb, A.; Hecht, J.; Cusi, K. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J. Clin. Endocrinol. Metab. 2015, 100, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Franzén, L.E.; Mathiesen, U.L.; Thorelius, L.; Holmqvist, M.; Bodemar, G.; Kechagias, S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006, 44, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Loomba, R.; Sanyal, A.J.; Lavine, J.E.; Van Natta, M.L.; Abdelmalek, M.F.; Chalasani, N.; Dasarathy, S.; Diehl, A.M.; Hameed, B.; et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet 2015, 385, 956–965, Erratum in Lancet 2015, 385, 946Erratum in Lancet 2016, 387, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Friedman, S.L.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Lazo-Del Vallin, S.; Diago, M.; Adams, L.A. Serum biomarkers can predict a change in liver fibrosis 1 year after lifestyle intervention for biopsy-proven NASH. Liver Int. 2017, 37, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Promrat, K.; Kleiner, D.E.; Niemeier, H.M.; Jackvony, E.; Kearns, M.; Wands, J.R.; Fava, J.L.; Wing, R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010, 51, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5, quiz e14–e15. [Google Scholar] [CrossRef]
- Friedman, S.L.; Ratziu, V.; Harrison, S.A.; Abdelmalek, M.F.; Aithal, G.P.; Caballeria, J.; Francque, S.; Farrell, G.; Kowdley, K.V.; Craxi, A.; et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018, 67, 1754–1767. [Google Scholar] [CrossRef] [Green Version]
- Buse, J.B.; Klonoff, D.C.; Nielsen, L.L.; Guan, X.; Bowlus, C.L.; Holcombe, J.H.; Maggs, D.G.; Wintle, M.E. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: An interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin. Ther. 2007, 29, 139–153. [Google Scholar] [CrossRef]
- Klonoff, D.C.; Buse, J.B.; Nielsen, L.L.; Guan, X.; Bowlus, C.L.; Holcombe, J.H.; Wintle, M.E.; Maggs, D.G. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr. Med. Res. Opin. 2008, 24, 275–286. [Google Scholar] [CrossRef]
- Sathyanarayana, P.; Jogi, M.; Muthupillai, R.; Krishnamurthy, R.; Samson, S.L.; Bajaj, M. Effects of combined exenatide and pioglitazone therapy on hepatic fat content in type 2 diabetes. Obesity 2011, 19, 2310–2315. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Pan, Q.; Xu, Y.; Yang, X. Exenatide improves type 2 diabetes concomitant with non-alcoholic fatty liver disease. Arq. Bras. Endocrinol. Metabol. 2013, 57, 702–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, M.J.; Houlihan, D.D.; Rowe, I.A.; Clausen, W.H.; Elbrønd, B.; Gough, S.C.; Tomlinson, J.W.; Newsome, P.N. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: Individual patient data meta-analysis of the LEAD program. Aliment. Pharmacol. Ther. 2013, 37, 234–242. [Google Scholar] [CrossRef]
- Blaslov, K.; Zibar, K.; Bulum, T.; Duvnjak, L. Effect of exenatide therapy on hepatic fat quantity and hepatic biomarkers in type 2 diabetic patients. Clin. Res. Hepatol. Gastroenterol. 2014, 38, e61–e63. [Google Scholar] [CrossRef] [Green Version]
- Gluud, L.L.; Knop, F.K.; Vilsbøll, T. Effects of lixisenatide on elevated liver transaminases: Systematic review with individual patient data meta-analysis of randomised controlled trials on patients with type 2 diabetes. BMJ Open 2014, 4, e005325. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Kitajima, Y.; Hyogo, H.; Takahashi, H.; Kojima, M.; Ono, M.; Araki, N.; Tanaka, K.; Yamaguchi, M.; Matsuda, Y.; et al. Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J). Hepatol. Res. 2015, 45, 269–278. [Google Scholar] [CrossRef]
- Feng, W.; Gao, C.; Bi, Y.; Wu, M.; Li, P.; Shen, S.; Chen, W.; Yin, T.; Zhu, D. Randomized trial comparing the effects of gliclazide, liraglutide, and metformin on diabetes with non-alcoholic fatty liver disease. J. Diabetes 2017, 9, 800–809. [Google Scholar] [CrossRef] [Green Version]
- Seko, Y.; Sumida, Y.; Tanaka, S.; Mori, K.; Taketani, H.; Ishiba, H.; Hara, T.; Okajima, A.; Umemura, A.; Nishikawa, T.; et al. Effect of 12-week dulaglutide therapy in Japanese patients with biopsy-proven non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol. Res. 2017, 47, 1206–1211. [Google Scholar] [CrossRef]
- Cusi, K.; Sattar, N.; García-Pérez, L.E.; Pavo, I.; Yu, M.; Robertson, K.E.; Karanikas, C.A.; Haupt, A. Dulaglutide decreases plasma aminotransferases in people with Type 2 diabetes in a pattern consistent with liver fat reduction: A post hoc analysis of the AWARD programme. Diabet. Med. 2018, 35, 1434–1439. [Google Scholar] [CrossRef]
- Newsome, P.; Francque, S.; Harrison, S.; Ratziu, V.; Van Gaal, L.; Calanna, S.; Hansen, M.; Linder, M.; Sanyal, A. Effect of semaglutide on liver enzymes and markers of inflammation in subjects with type 2 diabetes and/or obesity. Aliment. Pharmacol. Ther. 2019, 50, 193–203. [Google Scholar] [CrossRef]
- Colosimo, S.; Ravaioli, F.; Petroni, M.L.; Brodosi, L.; Marchignoli, F.; Barbanti, F.A.; Sasdelli, A.S.; Marchesini, G.; Pironi, L. Effects of antidiabetic agents on steatosis and fibrosis biomarkers in type 2 diabetes: A real-world data analysis. Liver Int. 2021, 41, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Saxena, N.K.; Lin, S.; Gupta, N.A.; Anania, F.A. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 2006, 43, 173–181, Erratum in Hepatology 2006, 44, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.A.; Mells, J.; Dunham, R.M.; Grakoui, A.; Handy, J.; Saxena, N.K.; Anania, F.A. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 2010, 51, 1584–1592. [Google Scholar] [CrossRef] [Green Version]
- Smits, M.M.; Tonneijck, L.; Muskiet, M.H.; Kramer, M.H.; Pouwels, P.J.; den Pieters-van Bos, I.C.; Hoekstra, T.; Diamant, M.; van Raalte, D.H.; Cahen, D.L. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: A randomised placebo-controlled trial. Diabetologia 2016, 59, 2588–2593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, A.; Rabasa-Lhoret, R.; Castel, H.; Wartelle-Bladou, C.; Gilbert, G.; Massicotte-Tisluck, K.; Chartrand, G.; Olivié, D.; Julien, A.S.; de Guise, J.; et al. Effects of Insulin Glargine and Liraglutide Therapy on Liver Fat as Measured by Magnetic Resonance in Patients With Type 2 Diabetes: A Randomized Trial. Diabetes Care 2015, 38, 1339–1346. [Google Scholar] [CrossRef] [Green Version]
- Jendle, J.; Nauck, M.A.; Matthews, D.R.; Frid, A.; Hermansen, K.; Düring, M.; Zdravkovic, M.; Strauss, B.J.; Garber, A.J.; LEAD-2 and LEAD-3 Study Groups. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes. Metab. 2009, 11, 1163–1172. [Google Scholar] [CrossRef]
- Cuthbertson, D.J.; Irwin, A.; Gardner, C.J.; Daousi, C.; Purewal, T.; Furlong, N.; Goenka, N.; Thomas, E.L.; Adams, V.L.; Pushpakom, S.P.; et al. Improved glycaemia correlates with liver fat reduction in obese, type 2 diabetes, patients given glucagon-like peptide-1 (GLP-1) receptor agonists. PLoS ONE 2012, 7, e50117. [Google Scholar] [CrossRef] [Green Version]
- Dutour, A.; Abdesselam, I.; Ancel, P.; Kober, F.; Mrad, G.; Darmon, P.; Ronsin, O.; Pradel, V.; Lesavre, N.; Martin, J.C.; et al. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: A prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes. Metab. 2016, 18, 882–891. [Google Scholar] [CrossRef]
- Vanderheiden, A.; Harrison, L.B.; Warshauer, J.T.; Adams-Huet, B.; Li, X.; Yuan, Q.; Hulsey, K.; Dimitrov, I.; Yokoo, T.; Jaster, A.W.; et al. Mechanisms of Action of Liraglutide in Patients With Type 2 Diabetes Treated With High-Dose Insulin. J. Clin. Endocrinol. Metab. 2016, 101, 1798–1806. [Google Scholar] [CrossRef]
- Bouchi, R.; Nakano, Y.; Fukuda, T.; Takeuchi, T.; Murakami, M.; Minami, I.; Izumiyama, H.; Hashimoto, K.; Yoshimoto, T.; Ogawa, Y. Reduction of visceral fat by liraglutide is associated with ameliorations of hepatic steatosis, albuminuria, and micro-inflammation in type 2 diabetic patients with insulin treatment: A randomized control trial. Endocr. J. 2017, 64, 269–281. [Google Scholar] [CrossRef]
- Sharma, S.; Mells, J.E.; Fu, P.P.; Saxena, N.K.; Anania, F.A. GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS ONE 2011, 6, e25269. [Google Scholar] [CrossRef] [PubMed]
- Mells, J.E.; Fu, P.P.; Sharma, S.; Olson, D.; Cheng, L.; Handy, J.A.; Saxena, N.K.; Sorescu, D.; Anania, F.A. Glp-1 analog, liraglutide, ameliorates hepatic steatosis and cardiac hypertrophy in C57BL/6J mice fed a Western diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G225–G235.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015, 149, 389–397.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, R.S.; Taylor, R.J.; Bayliss, S.; Hagström, H.; Nasr, P.; Schattenberg, J.M.; Ishigami, M.; Toyoda, H.; Wai-Sun Wong, V.; Peleg, N.; et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1611–1625.e12. [Google Scholar] [CrossRef] [Green Version]
- Lomonaco, R.; Godinez Leiva, E.; Bril, F.; Shrestha, S.; Mansour, L.; Budd, J.; Portillo Romero, J.; Schmidt, S.; Chang, K.L.; Samraj, G.; et al. Advanced Liver Fibrosis Is Common in Patients With Type 2 Diabetes Followed in the Outpatient Setting: The Need for Systematic Screening. Diabetes Care 2021, 44, 399–406. [Google Scholar] [CrossRef]
- Kenny, P.R.; Brady, D.E.; Torres, D.M.; Ragozzino, L.; Chalasani, N.; Harrison, S.A. Exenatide in the treatment of diabetic patients with non-alcoholic steatohepatitis: A case series. Am. J. Gastroenterol. 2010, 105, 2707–2709. [Google Scholar] [CrossRef]
- Ohki, T.; Isogawa, A.; Iwamoto, M.; Ohsugi, M.; Yoshida, H.; Toda, N.; Tagawa, K.; Omata, M.; Koike, K. The effectiveness of liraglutide in nonalcoholic fatty liver disease patients with type 2 diabetes mellitus compared to sitagliptin and pioglitazone. Sci. World J. 2012, 2012, 496453. [Google Scholar] [CrossRef]
- Tan, Y.; Zhen, Q.; Ding, X.; Shen, T.; Liu, F.; Wang, Y.; Zhang, Q.; Lin, R.; Chen, L.; Peng, Y.; et al. Association between use of liraglutide and liver fibrosis in patients with type 2 diabetes. Front. Endocrinol. 2022, 13, 935180. [Google Scholar] [CrossRef]
- Rakipovski, G.; Rolin, B.; Nøhr, J.; Klewe, I.; Frederiksen, K.S.; Augustin, R.; Hecksher-Sørensen, J.; Ingvorsen, C.; Polex-Wolf, J.; Knudsen, L.B. The GLP-1 Analogs Liraglutide and Semaglutide Reduce Atherosclerosis in ApoE-/- and LDLr-/- Mice by a Mechanism That Includes Inflammatory Pathways. JACC Basic Transl. Sci. 2018, 3, 844–857. [Google Scholar] [CrossRef]
- Kojima, M.; Takahashi, H.; Kuwashiro, T.; Tanaka, K.; Mori, H.; Ozaki, I.; Kitajima, Y.; Matsuda, Y.; Ashida, K.; Eguchi, Y.; et al. Glucagon-Like Peptide-1 Receptor Agonist Prevented the Progression of Hepatocellular Carcinoma in a Mouse Model of Nonalcoholic Steatohepatitis. Int. J. Mol. Sci. 2020, 21, 5722. [Google Scholar] [CrossRef]
- Di Francia, R.; Rinaldi, L.; Troisi, A.; Di Benedetto, F.; Berretta, M. Effect of anti-oxidant agents in patients with hepatocellular di-seases. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3993–3995. [Google Scholar] [PubMed]
- Di Francia, R.; Rinaldi, L.; Cillo, M.; Varriale, E.; Facchini, G.; D’Aniello, C.; Marotta, G.; Berretta, M. Antioxidant diet and geno-typing as tools for the prevention of liver disease. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 5155–5163. [Google Scholar]
- Savvidou, S.; Karatzidou, K.; Tsakiri, K.; Gagalis, A.; Hytiroglou, P.; Goulis, J. Circulating adiponectin levels in type 2 diabetes mellitus patients with or without non-alcoholic fatty liver disease: Results of a small, open-label, randomized controlled intervention trial in a subgroup receiving short-term exenatide. Diabetes Res. Clin. Pract. 2016, 113, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Wang, J.; Fu, P.P.; Sharma, S.; Nagalingam, A.; Mells, J.; Handy, J.; Page, A.J.; Cohen, C.; Anania, F.A.; et al. Adiponectin antagonizes the oncogenic actions of leptin in hepatocellular carcinogenesis. Hepatology 2010, 52, 1713–1722. [Google Scholar] [CrossRef] [Green Version]
- Samson, S.L.; Gonzalez, E.V.; Yechoor, V.; Bajaj, M.; Oka, K.; Chan, L. Gene therapy for diabetes: Metabolic effects of helper-dependent adenoviral exendin 4 expression in a diet-induced obesity mouse model. Mol. Ther. 2008, 16, 1805–1812, Erratum in Mol. Ther. 2009, 17, 1831. [Google Scholar] [CrossRef]
- Mantovani, A.; Byrne, C.D.; Targher, G. Efficacy of peroxisome proliferator-activated receptor agonists, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors for treatment of non-alcoholic fatty liver disease: A systematic review. Lancet Gastroenterol. Hepatol. 2022, 7, 367–378. [Google Scholar] [CrossRef]
- Gupta, N.A.; Kolachala, V.L.; Jiang, R.; Abramowsky, C.; Romero, R.; Fifadara, N.; Anania, F.; Knechtle, S.; Kirk, A. The glucagon-like peptide-1 receptor agonist Exendin 4 has a protective role in ischemic injury of lean and steatotic liver by inhibiting cell death and stimulating lipolysis. Am. J. Pathol. 2012, 181, 1693–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Ji, L.; Zhu, C.; Xiao, Y.; Zhang, J.; Lu, J.; Yin, J.; Wei, L. Liraglutide Alleviates Hepatic Steatosis by Activating the TFEB-Regulated Autophagy-Lysosomal Pathway. Front. Cell Dev. Biol. 2020, 8, 602574. [Google Scholar] [CrossRef]
- Yokomori, H.; Ando, W. Spatial expression of glucagon-like peptide 1 receptor and caveolin-1 in hepatocytes with macrovesicular steatosis in non-alcoholic steatohepatitis. BMJ Open Gastroenterol. 2020, 7, e000370. [Google Scholar] [CrossRef]
- Svegliati-Baroni, G.; Saccomanno, S.; Rychlicki, C.; Agostinelli, L.; De Minicis, S.; Candelaresi, C.; Faraci, G.; Pacetti, D.; Vivarelli, M.; Nicolini, D.; et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011, 31, 1285–1297. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Hong, S.W.; Rhee, E.J.; Lee, W.Y. GLP-1 Receptor Agonist and Non-Alcoholic Fatty Liver Disease. Diabetes Metab. J. 2012, 36, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Piro, S.; Spadaro, L.; Russello, M.; Spampinato, D.; Oliveri, C.E.; Vasquez, E.; Benigno, R.; Brancato, F.; Purrello, F.; Rabuazzo, A.M. Molecular determinants of insulin resistance, cell apoptosis and lipid accumulation in non-alcoholic steatohepatitis. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kataria, M.A.; Saini, V.; Yadav, A. Role of leptin and adiponectin in insulin resistance. Clin. Chim. Acta 2013, 417, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Handy, J.A.; Fu, P.P.; Kumar, P.; Mells, J.E.; Sharma, S.; Saxena, N.K.; Anania, F.A. Adiponectin inhibits leptin signalling via multiple mechanisms to exert protective effects against hepatic fibrosis. Biochem. J. 2011, 440, 385–395. [Google Scholar] [CrossRef] [Green Version]
- Ben-Shlomo, S.; Zvibel, I.; Shnell, M.; Shlomai, A.; Chepurko, E.; Halpern, Z.; Barzilai, N.; Oren, R.; Fishman, S. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J. Hepatol. 2011, 54, 1214–1223. [Google Scholar] [CrossRef]
- Panzitt, K.; Wagner, M. FXR in liver physiology: Multiple faces to regulate liver metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166133. [Google Scholar] [CrossRef] [PubMed]
- Errafii, K.; Khalifa, O.; Al-Akl, N.S.; Arredouani, A. Comparative Transcriptome Analysis Reveals That Exendin-4 Improves Steatosis in HepG2 Cells by Modulating Signaling Pathways Related to Lipid Metabolism. Biomedicines 2022, 10, 1020. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, Y.; Yan, L.; Gao, M.; Liu, D. Synthetic FXR agonist GW4064 prevents diet-induced hepatic steatosis and insulin resistance. Pharm. Res. 2013, 30, 1447–1457. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Jia, Y.; Yang, G.; Zhang, X.; Boddu, P.C.; Petersen, B.; Narsingam, S.; Zhu, Y.J.; Thimmapaya, B.; Kanwar, Y.S.; et al. PPARα-Deficient ob/ob Obese Mice Become More Obese and Manifest Severe Hepatic Steatosis Due to Decreased Fatty Acid Oxidation. Am. J. Pathol. 2015, 185, 1396–1408. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, K.; Xu, Y.; Xu, Y.; Li, Y.; Xu, J.; Zhu, Y.; Adorini, L.; Lee, Y.K.; Kasumov, T.; Yin, L.; et al. Reversal of metabolic disorders by pharmacological activation of bile acid receptors TGR5 and FXR. Mol. Metab. 2018, 9, 131–140. [Google Scholar] [CrossRef]
- Pettinelli, P.; Videla, L.A. Up-regulation of PPAR-gamma mRNA expression in the liver of obese patients: An additional reinforcing lipogenic mechanism to SREBP-1c induction. J. Clin. Endocrinol. Metab. 2011, 96, 1424–1430. [Google Scholar] [CrossRef]
- Decara, J.; Arrabal, S.; Beiroa, D.; Rivera, P.; Vargas, A.; Serrano, A.; Pavón, F.J.; Ballesteros, J.; Dieguez, C.; Nogueiras, R.; et al. Antiobesity efficacy of GLP-1 receptor agonist liraglutide is associated with peripheral tissue-specific modulation of lipid metabolic regulators. Biofactors 2016, 42, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.H.; Lee, J.; Hong, S.W.; Rhee, E.J.; Park, S.E.; Park, C.Y.; Oh, K.W.; Park, S.W.; Lee, W.Y. Exendin-4 Inhibits Hepatic Lipogenesis by Increasing β-Catenin Signaling. PLoS ONE 2016, 11, e0166913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Hong, S.W.; Kim, M.J.; Moon, S.J.; Kwon, H.; Park, S.E.; Rhee, E.J.; Lee, W.Y. Dulaglutide Ameliorates Palmitic Acid-Induced Hepatic Steatosis by Activating FAM3A Signaling Pathway. Endocrinol. Metab. 2022, 37, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Lima-Cabello, E.; García-Mediavilla, M.V.; Miquilena-Colina, M.E.; Vargas-Castrillón, J.; Lozano-Rodríguez, T.; Fernández-Bermejo, M.; Olcoz, J.L.; González-Gallego, J.; García-Monzón, C.; Sánchez-Campos, S. Enhanced expression of pro-inflammatory mediators and liver X-receptor-regulated lipogenic genes in non-alcoholic fatty liver disease and hepatitis C. Clin. Sci. 2011, 120, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Wouters, K.; van Bilsen, M.; van Gorp, P.J.; Bieghs, V.; Lütjohann, D.; Kerksiek, A.; Staels, B.; Hofker, M.H.; Shiri-Sverdlov, R. Intrahepatic cholesterol influences progression, inhibition and reversal of non-alcoholic steatohepatitis in hyperlipidemic mice. FEBS Lett. 2010, 584, 1001–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Han, X.; Bian, Z.; Peng, Y.; You, Z.; Wang, Q.; Chen, X.; Qiu, D.; Ma, X. Activation of liver X receptors attenuates endotoxin-induced liver injury in mice with nonalcoholic fatty liver disease. Dig. Dis. Sci. 2012, 57, 390–398. [Google Scholar] [CrossRef]
- Ito, A.; Hong, C.; Rong, X.; Zhu, X.; Tarling, E.J.; Hedde, P.N.; Gratton, E.; Parks, J.; Tontonoz, P. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. Elife 2015, 4, e08009. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef] [Green Version]
- Cuervo, A.M.; Bergamini, E.; Brunk, U.T.; Dröge, W.; Ffrench, M.; Terman, A. Autophagy and aging: The importance of maintaining "clean" cells. Autophagy 2005, 1, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Rahman, K.; Liu, Y.; Kumar, P.; Smith, T.; Thorn, N.E.; Farris, A.B.; Anania, F.A. C/EBP homologous protein modulates liraglutide-mediated attenuation of non-alcoholic steatohepatitis. Lab. Investig. 2016, 96, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Albanese, G.; Di Martino, A.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Sasso, F.C. The Diabetic Cardiomyopathy: The Contributing Pathophysiological Mechanisms. Front. Med. 2021, 30, 695792. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Mawatari, H.; Fujita, K.; Iida, H.; Yonemitsu, K.; Kato, S.; Takahashi, H.; Kirikoshi, H.; Inamori, M.; Nozaki, Y.; et al. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J. Gastroenterol. 2007, 42, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, A.; Patel, P.; Makdissi, A.; Dhindsa, S.; Chaudhuri, A.; Dandona, P. Clinical use of liraglutide in type 2 diabetes and its effects on cardiovascular risk factors. Endocr. Pract. 2012, 18, 140–145. [Google Scholar] [CrossRef]
- Chiquette, E.; Toth, P.P.; Ramirez, G.; Cobble, M.; Chilton, R. Treatment with exenatide once weekly or twice daily for 30 weeks is associated with changes in several cardiovascular risk markers. Vasc. Health Risk Manag. 2012, 8, 621–629. [Google Scholar] [CrossRef] [Green Version]
- Derosa, G.; Franzetti, I.G.; Querci, F.; Carbone, A.; Ciccarelli, L.; Piccinni, M.N.; Fogari, E.; Maffioli, P. Exenatide plus metformin compared with metformin alone on β-cell function in patients with Type 2 diabetes. Diabet. Med. 2012, 29, 1515–1523. [Google Scholar] [CrossRef]
- Wei, H.; Bu, R.; Yang, Q.; Jia, J.; Li, T.; Wang, Q.; Chen, Y. Exendin-4 Protects against Hyperglycemia-Induced Cardiomyocyte Pyroptosis via the AMPK-TXNIP Pathway. J. Diabetes Res. 2019, 2019, 8905917. [Google Scholar] [CrossRef]
- Lee, Y.S.; Park, M.S.; Choung, J.S.; Kim, S.S.; Oh, H.H.; Choi, C.S.; Ha, S.Y.; Kang, Y.; Kim, Y.; Jun, H.S. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia 2012, 55, 2456–2468. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Jun, H.S. Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control. Mediat. Inflamm. 2016, 2016, 3094642. [Google Scholar] [CrossRef] [Green Version]
- Ren, Q.; Chen, S.; Chen, X.; Niu, S.; Yue, L.; Pan, X.; Li, Z.; Chen, X. An Effective Glucagon-Like Peptide-1 Receptor Agonists, Semaglutide, Improves Sarcopenic Obesity in Obese Mice by Modulating Skeletal Muscle Metabolism. Drug Des. Dev. Ther. 2022, 16, 3723–3735. [Google Scholar] [CrossRef]
- Hong, Y.; Lee, J.H.; Jeong, K.W.; Choi, C.S.; Jun, H.S. Amelioration of muscle wasting by glucagon-like peptide-1 receptor agonist in muscle atrophy. J. Cachexia Sarcopenia Muscle 2019, 10, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, M.; Mizoguchi, F.; Yasuda, S. Amelioration of inflammatory myopathies by glucagon-like peptide-1 receptor agonist via suppressing muscle fibre necroptosis. J. Cachexia Sarcopenia Muscle 2022, 13, 2118–2131. [Google Scholar] [CrossRef]
- Joo, S.K.; Kim, W. Interaction between Sarcopenia and NAFLD. Clin. Mol. Hepatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, P.; Gane, E.; Buti, M.; Afdhal, N.; Sievert, W.; Jacobson, I.M.; Washington, M.K.; Germanidis, G.; Flaherty, J.F.; Aguilar Schall, R.; et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet 2013, 381, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G. Regression of HCV cirrhosis: Time will tell. Hepatology 2018, 67, 1651–1653. [Google Scholar] [CrossRef] [Green Version]
- Rosato, V.; Ascione, A.; Nevola, R.; Fracanzani, A.L.; Piai, G.; Messina, V.; Claar, E.; Coppola, C.; Fontanella, L.; Lombardi, R.; et al. Factors affecting long-term changes of liver stiffness in direct-acting anti-hepatitis C virus therapy: A multicentre prospective study. J. Viral Hepat. 2022, 29, 26–34. [Google Scholar] [CrossRef]
- Gallwitz, B. Clinical perspectives on the use of the GIP/GLP-1 receptor agonist tirzepatide for the treatment of type-2 diabetes and obesity. Front. Endocrinol. 2022, 13, 1004044. [Google Scholar] [CrossRef]
- Capozzi, M.E.; DiMarchi, R.D.; Tschöp, M.H.; Finan, B.; Campbell, J.E. Targeting the Incretin/Glucagon System With Triagonists to Treat Diabetes. Endocr. Rev. 2018, 39, 719–738. [Google Scholar] [CrossRef] [Green Version]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Pfeiffer, A.F.H. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes Obes. Metab. 2021, 23 (Suppl. 3), 5–29. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.E.; Drucker, D.J. Islet α cells and glucagon--critical regulators of energy homeostasis. Nat. Rev. Endocrinol. 2015, 11, 329–338. [Google Scholar] [CrossRef]
- Galsgaard, K.D.; Pedersen, J.; Knop, F.K.; Holst, J.J.; Wewer Albrechtsen, N.J. Glucagon Receptor Signaling and Lipid Metabolism. Front. Physiol. 2019, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.M.; Field, B.C.; McCullough, K.A.; Troke, R.C.; Chambers, E.S.; Salem, V.; Gonzalez Maffe, J.; Baynes, K.C.; De Silva, A.; Viardot, A.; et al. Coadministration of glucagon-like peptide-1 during glucagon infusion in humans results in increased energy expenditure and amelioration of hyperglycemia. Diabetes 2013, 62, 1131–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, J.A.; McCullough, K.A.; Field, B.C.; Minnion, J.S.; Martin, N.M.; Ghatei, M.A.; Bloom, S.R. Glucagon and GLP-1 inhibit food intake and increase c-fos expression in similar appetite regulating centres in the brainstem and amygdala. Int. J. Obes. 2013, 37, 1391–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Garrido, M.A.; Brandt, S.J.; Clemmensen, C.; Müller, T.D.; DiMarchi, R.D.; Tschöp, M.H. GLP-1/glucagon receptor co-agonism for treatment of obesity. Diabetologia 2017, 60, 1851–1861. [Google Scholar] [CrossRef] [Green Version]
- Pocai, A.; Carrington, P.E.; Adams, J.R.; Wright, M.; Eiermann, G.; Zhu, L.; Du, X.; Petrov, A.; Lassman, M.E.; Jiang, G.; et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes 2009, 58, 2258–2266. [Google Scholar] [CrossRef] [Green Version]
- Day, J.W.; Ottaway, N.; Patterson, J.T.; Gelfanov, V.; Smiley, D.; Gidda, J.; Findeisen, H.; Bruemmer, D.; Drucker, D.J.; Chaudhary, N.; et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol. 2009, 5, 749–757. [Google Scholar] [CrossRef]
- Day, J.W.; Gelfanov, V.; Smiley, D.; Carrington, P.E.; Eiermann, G.; Chicchi, G.; Erion, M.D.; Gidda, J.; Thornberry, N.A.; Tschöp, M.H.; et al. Optimization of co-agonism at GLP-1 and glucagon receptors to safely maximize weight reduction in DIO-rodents. Biopolymers 2012, 98, 443–450. [Google Scholar] [CrossRef]
- Elvert, R.; Herling, A.W.; Bossart, M.; Weiss, T.; Zhang, B.; Wenski, P.; Wandschneider, J.; Kleutsch, S.; Butty, U.; Kannt, A.; et al. Running on mixed fuel-dual agonistic approach of GLP-1 and GCG receptors leads to beneficial impact on body weight and blood glucose control: A comparative study between mice and non-human primates. Diabetes Obes. Metab. 2018, 20, 1836–1851. [Google Scholar] [CrossRef]
- Samms, R.J.; Coghlan, M.P.; Sloop, K.W. How May GIP Enhance the Therapeutic Efficacy of GLP-1? Trends Endocrinol. Metab. 2020, 31, 410–421. [Google Scholar] [CrossRef] [Green Version]
- Cegla, J.; Troke, R.C.; Jones, B.; Tharakan, G.; Kenkre, J.; McCullough, K.A.; Lim, C.T.; Parvizi, N.; Hussein, M.; Chambers, E.S.; et al. Coinfusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake. Diabetes 2014, 63, 3711–3720. [Google Scholar] [CrossRef] [Green Version]
- Ambery, P.; Parker, V.E.; Stumvoll, M.; Posch, M.G.; Heise, T.; Plum-Moerschel, L.; Tsai, L.F.; Robertson, D.; Jain, M.; Petrone, M.; et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: A randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet 2018, 391, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, Q.; Han, J.; Liu, X.; Fu, J.; Yin, J. Xenopus GLP-1-based glycopeptides as dual glucagon-like peptide 1 receptor/glucagon receptor agonists with improved in vivo stability for treating diabetes and obesity. Chin. J. Nat. Med. 2022, 20, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Finan, B.; Ma, T.; Ottaway, N.; Müller, T.D.; Habegger, K.M.; Heppner, K.M.; Kirchner, H.; Holland, J.; Hembree., J.; Raver, C.; et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci. Transl. Med. 2013, 5, 209ra151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nørregaard, P.K.; Deryabina, M.A.; Tofteng Shelton, P.; Fog, J.U.; Daugaard, J.R.; Eriksson, P.O.; Larsen, L.F.; Jessen, L. A novel GIP analogue, ZP4165, enhances glucagon-like peptide-1-induced body weight loss and improves glycaemic control in rodents. Diabetes Obes. Metab. 2018, 20, 60–68. [Google Scholar] [CrossRef]
- Killion, E.A.; Wang, J.; Yie, J.; Shi, S.D.; Bates, D.; Min, X.; Komorowski, R.; Hager, T.; Deng, L.; Atangan, L.; et al. Anti-obesity effects of GIPR antagonists alone and in combination with GLP-1R agonists in preclinical models. Sci. Transl. Med. 2018, 10, eaat3392. [Google Scholar] [CrossRef]
- Frias, J.P.; Nauck, M.A.; Van, J.; Kutner, M.E.; Cui, X.; Benson, C.; Urva, S.; Gimeno, R.E.; Milicevic, Z.; Robins, D.; et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: A randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018, 392, 2180–2193. [Google Scholar] [CrossRef]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K.; SURPASS-2 Investigators. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef]
- Ludvik, B.; Giorgino, F.; Jódar, E.; Frias, J.P.; Fernández Landó, L.; Brown, K.; Bray, R.; Rodríguez, Á. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): A randomised, open-label, parallel-group, phase 3 trial. Lancet 2021, 398, 583–598. [Google Scholar] [CrossRef]
- Samms, R.J.; Christe, M.E.; Collins, K.A.; Pirro, V.; Droz, B.A.; Holland, A.K.; Friedrich, J.L.; Wojnicki, S.; Konkol, D.L.; Cosgrove, R.; et al. GIPR agonism mediates weight-independent insulin sensitization by tirzepatide in obese mice. J. Clin. Investig. 2021, 131, e146353. [Google Scholar] [CrossRef]
- Jall, S.; Sachs, S.; Clemmensen, C.; Finan, B.; Neff, F.; DiMarchi, R.D.; Tschöp, M.H.; Müller, T.D.; Hofmann, S.M. Monomeric GLP-1/GIP/glucagon triagonism corrects obesity, hepatosteatosis, and dyslipidemia in female mice. Mol. Metab. 2017, 6, 440–446. [Google Scholar] [CrossRef]
- Finan, B.; Yang, B.; Ottaway, N.; Smiley, D.L.; Ma, T.; Clemmensen, C.; Chabenne, J.; Zhang, L.; Habegger, K.M.; Fischer, K.; et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat. Med. 2015, 21, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kannt, A.; Madsen, A.N.; Kammermeier, C.; Elvert, R.; Klöckener, T.; Bossart, M.; Haack, T.; Evers, A.; Lorenz, K.; Hennerici, W.; et al. Incretin combination therapy for the treatment of non-alcoholic steatohepatitis. Diabetes Obes. Metab. 2020, 22, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Nahra, R.; Wang, T.; Gadde, K.M.; Oscarsson, J.; Stumvoll, M.; Jermutus, L.; Hirshberg, B.; Ambery, P. Effects of Cotadutide on Metabolic and Hepatic Parameters in Adults With Overweight or Obesity and Type 2 Diabetes: A 54-Week Randomized Phase 2b Study. Diabetes Care 2021, 44, 1433–1442, Erratum in Diabetes Care 2022, 45, 3112. [Google Scholar] [CrossRef] [PubMed]
- Jouihan, H.; Will, S.; Guionaud, S.; Boland, M.L.; Oldham, S.; Ravn, P.; Celeste, A.; Trevaskis, J.L. Superior reductions in hepatic steatosis and fibrosis with co-administration of a glucagon-like peptide-1 receptor agonist and obeticholic acid in mice. Mol. Metab. 2017, 6, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
| Year | First Author | Ref. | Sample Size | Study Typology | Evaluated Drugs | Benefit | Results |
|---|---|---|---|---|---|---|---|
| 2009 | Astrup | [38] | 564 non-diabetic obese | Prospective double-blind | Liraglutide vs orlistat vs placebo | Yes | Liraglutide resulted in greater weight loss than placebo or orlistat |
| 2013 | Wadden | [43] | 422 obese/overweight | Prospective randomised | Liraglutide vs placebo | Yes | Liraglutide plus diet and exercise maintained weight loss achieved by caloric restriction and induced further weight loss |
| 2015 | Pi-Sunyer | [33] | 3731 non-diabetic obese | Prospective double-blind | Liraglutide vs placebo | Yes | Liraglutide induced greater weight loss (8.4 Kg) than placebo (2.8 Kg) |
| 2015 | Davies | [47] | 846 obese/overweight T2DM | Prospective double-blind | Liraglutide vs placebo | Yes | Liraglutide (3.0 mg/day), compared with placebo, resulted in greater weight loss |
| 2016 | de Boer | [41] | 151 obese insulin-using T2DM | Prospective | Liraglutide or Exenatide | Yes | Liraglutide or Exenatide led to sustained weight reduction and daily insulin dose |
| 2016 | Blackman | [44] | 359 non-diabetic obese | Prospective double-blind | Liraglutide vs placebo | Yes | Liraglutide (3.0 mg/day) plus lifestyle therapy led to a greater weight loss than placebo plus lifestyle therapy |
| 2016 | Armstrong | [56] | 52 NASH patients | Multicentre, double-blinded | Liraglutide vs placebo | Yes | Liraglutide 1.8 mg/day led to a greater weight loss than placebo |
| 2017 | Halawi | [45] | 40 obese patients | Prospective double-blind | Liraglutide vs placebo | Yes | Liraglutide delays gastric emptying and reduces body weight more than placebo |
| 2017 | Petit | [57] | 68 uncontrolled T2DM patients | Prospective single-center | Liraglutide | Yes | Liraglutide 1.2 mg/day significantly reduced body weight |
| 2018 | Frøssing | [58] | 72 obese/overweight with PCOS | Prospective double-blind | Liraglutide vs placebo | Yes | Liraglutide results in greater weight loss than placebo |
| 2019 | Feng | [59] | 85 T2DM and NAFLD patients | Prospective, randomized | Liraglutide vs gliclazide | Yes | Liraglutide results in greater weight loss than gliclazide |
| 2019 | Yan | [50] | 75 NAFLD and metformin-uncontrolled T2DM patients | RCT | Liraglutide vs sitagliptin vs insulin glargine | Yes | Combined with metformin, both liraglutide and sitagliptin, but not insulin glargine, reduced body weight |
| 2020 | Kelly | [40] | 251 obese adolescents | Prospective double-blind | Liraglutide vs placebo | Yes | Liraglutide (3.0 mg/day) plus lifestyle therapy led to a greater weight loss than placebo plus lifestyle therapy in adolescent with obesity |
| 2020 | Wadden | [42] | 282 obese | Prospective double-blind | Liraglutide vs placebo | Yes | Liraglutide (3.0 mg/day) amplifies weight loss due to intensive behavioral therapy |
| 2020 | Garvey | [48] | 396 obese/overweight and insulin-treated T2DM patients | Prospective double-blind | Liraglutide vs placebo | Yes | Liraglutide (3.0 mg/day) led to greater weight loss than placebo |
| 2017 | Blundell | [39] | 28 obese | Prospective double-blind | Semaglutide vs placebo | Yes | Semaglutide resulted in lower ad libitum energy intake and greater weight loss than placebo |
| 2018 | O’Neil | [31] | 957 non-diabetic obese | Prospective double-blind | Semaglutide vs Liraglutide | Yes | Semaglutide (≥0.2 mg/day) resulted in greater weight loss than liraglutide (any dose) |
| 2018 | Pratley | [32] | 1201 T2DM patients | Prospective randomised | Semaglutide vs Dulaglutide | Yes | Semaglutide 0.5 mg/week resulted in greater weight loss than dulaglutide 0.75 mg/week. Semaglutide 1 mg/week resulted in greater weight loss than dulaglutide 1.5 mg/week |
| 2019 | Matikainen | [60] | 22 controlled T2DM patients | Prospective single-blind | Liraglutide vs placebo | No | Similar weight loss between 16-week liraglutide 1.8 mg/day and placebo group |
| 2021 | Wilding | [34] | 1961 non-diabetic obese | Prospective double-blind | Semaglutide vs placebo | Yes | Semaglutide (2.4 mg/week) plus lifestyle intervention resulted in a greater reduction in BMI than lifestyle intervention alone |
| 2021 | Davies | [35] | 1210 insulin-untreated diabetic obese/overweight patients | Prospective double-blind | Semaglutide 2.4 vs 1 mg/week vs placebo | Yes | Semaglutide 2.4 mg/week resulted in a greater weight loss than Semaglutide 1.0 mg/week or placebo |
| 2021 | Wadden | [36] | 611 non-diabetic obese/overweight patients | Prospective double-blind | Semaglutide vs placebo | Yes | Semaglutide (2.4 mg/week) plus lifestyle intervention resulted in a greater reduction in BMI than lifestyle intervention alone |
| 2021 | Rubino | [37] | 902 non-diabetic obese/overweight patients | Prospective double-blind | Semaglutide vs placebo | Yes | Semaglutide (2.4 mg/week) plus lifestyle intervention resulted in a greater reduction in BMI than lifestyle intervention alone |
| 2021 | Newsome | [61] | 320 obese/overweight NASH patients | Prospective double-blind | Semaglutide vs placebo | Yes | Semaglutide treatment resulted in a greater reduction in BMI than placebo |
| 2022 | Rubino | [46] | 338 non-diabetic obese/overweight patients | Prospective randomised | Semaglutide vs Liraglutide | Yes | Semaglutide (2.4 mg/week) resulted in greater weight loss than liraglutide (3 mg/day) |
| 2022 | Weghuber | [49] | 201 obese adolescents | Prospective double-blind | Semaglutide vs placebo | Yes | Semaglutide (2.4 mg/week) plus lifestyle intervention resulted in a greater reduction in BMI than lifestyle intervention alone |
| Year | First Author | Ref. | Sample Size | Study Typology | Evaluated Drugs | Benefit | Results |
|---|---|---|---|---|---|---|---|
| 2007 | Buse | [69] | 283 T2DM patients | Multicenter, double-blind trials | Exenatide vs placebo | Yes | Exenatide resulted in progressive reduction in weight and improvements in hepatic injury biomarkers |
| 2008 | Klonoff | [70] | 217 T2DM patients | Open-label clinical trial | Exenatide vs placebo | Yes | Exenatide significantly reduced liver enzymes vs placebo |
| 2011 | Sathyanarayana | [71] | 21 T2DM patients | NS | Exenatide vs pioglitazone | Yes | Hepatic injury biomarkers were significantly decreased by Exenatide and Pioglitazone. The reduction in ALT was significantly greater following combined therapy. |
| 2013 | Fan | [72] | 117 T2DM and NAFLD patients | Prospective randomized trial | Exenatide vs metformin | Yes | Exenatide treatment results in significant improvement in liver enzymes compared with metformin. |
| 2013 | Armstrong | [73] | 4442 T2DM patients | Meta-analysis | Liraglutide vs placebo | Yes | Liraglutide 1.8 mg/day reduced ALT vs placebo with a dose-dependent effect: no significant differences vs placebo with liraglutide 0.6 or 1.2 mg/day |
| 2014 | Blaslov | [74] | 125 T2DM patients | Open label parallel-group uncontrolled | Exenatide vs other oral hypoglycemic agents | Yes | Exenatide results in reduction of NAFLD marker levels and intrahepatic fat quantity calculated by FLI |
| 2014 | Gluud | [75] | 1070 T2DM patients with increased ALT | Systematic review | Lixisenatide | Yes | Lixisenatide increases the proportion of obese or overweight T2DM patients who achieve normalisation of ALT |
| 2015 | Eguchi | [76] | 19 NASH patients | Prospective uncontrolled | Liraglutide | Yes | Liraglutide significantly improved liver enzymes in NASH patients |
| 2016 | Armstrong | [56] | 52 NASH patients | Multicentre, double-blinded, phase 2 trial | Liraglutide vs placebo | Yes | Liraglutide 1.8 mg/day led to significative ALT reduction vs placebo |
| 2017 | Feng | [77] | 87 T2DM and NAFLD patients | RCT | Liraglutide vs gliclazide vs metformin | Yes | Liraglutide and metformin reduce ALT and liver fat content |
| 2017 | Seko | [78] | 15 biopsy-proven NAFLD and T2DM patients | Retrospective | Dulaglutide | Yes | Dulaglutide significantly reduced liver enzymes |
| 2018 | Cusi | [79] | 1499 T2DM and NAFLD patients | Placebo-controlled clinical trial: post hoc analysis | Dulaglutide vs placebo | Yes | Dulaglutide significantly reduced liver enzymes vs placebo |
| 2019 | Newsome | [80] | 4254 T2DM and/or obesity patients | Post hoc analysis | Semaglutide | Yes | Semaglutide significantly reduced ALT |
| 2021 | Colosimo | [81] | 637 T2DM patients | Retrospective | DPP-4i vs GLP-1 RA vs SGLT-2i vs others | Yes | GLP-1 RA and SGLT-2i improve biomarkers of liver injury |
| 2021 | Newsome | [61] | 320 obese/overweight NASH patients | Prospective double-blind | Semaglutide vs placebo | Yes | Semaglutide treatment resulted in a greater dose-dipendent reduction in liver enzymes than placebo |
| Year | First Author | Ref. | Sample Size | Study Typology | Evaluated Drugs | Benefit | Results |
|---|---|---|---|---|---|---|---|
| 2009 | Jendle | [86] | 314 uncontrolled T2DM patients | Randomized, double-blind, parallel-group | Metformin + Liraglutide (0.6, 1.2 or 1.8 mg/day) or glimepiride or placebo | Yes | Liraglutide (with or without metformin) significantly reduced fat mass and fat percentage vs glimepiride |
| 2011 | Sathyanarayana | [71] | 21 T2DM patients | NS | Exenatide vs pioglitazone | Yes | The addition of exenatide to pioglitazone is associated with a greater reduction in hepatic fat content as compared to pioglitazone monotherapy |
| 2012 | Cuthbertson | [87] | 25 obese, T2DM and NAFLD patients | Prospective single arm | Exenatide | Yes | Exenatide significantly decreased liver fat (evaluated by MR) |
| 2014 | Braslov | [74] | 125 T2DM patients | Open label parallel-group uncontrolled | Exenatide vs other oral hypoglycaemic agents | Yes | Exenatide induces greater improvement in FLI than other oral hypoglycaemic agents |
| 2015 | Tang | [85] | 35 uncontrolled T2DM patients | RCT | Liraglutide vs insulin glargine | No | Twelve-week liraglutide treatment liraglutide does not modify liver proton density fat fraction |
| 2015 | Eguchi | [76] | 19 NASH patients | Prospective uncontrolled | Liraglutide | Yes | Liraglutide significantly improved histological features in NASH |
| 2016 | Dutour | [88] | 44 obese T2DM patients | RCT | Exenatide vs other hypoglycaemics | Yes | Exenatide induced a significant reduction in hepatic triglyceride content compared with the reference treatment |
| 2016 | Smits | [84] | 52 overweight T2DM patients | Randomised, placebo-controlled | Liraglutide 1.8 mg vs sitagliptin vs placebo | No | Twelve-week liraglutide treatment does not reduce hepatic steatosis |
| 2016 | Vanderheiden | [89] | 71 T2DM patients requiring high-dose insulin treatment | Single-center, randomized, double-blind | Liraglutide vs placebo | Yes | Liraglutide significantly decreased liver fat (evaluated by MR) vs placebo |
| 2016 | Armstrong | [56] | 52 NASH patients | Multicentre, double-blinded, phase 2 trial | Liraglutide vs placebo | Yes | Liraglutide 1.8 mg/day led to histological resolution of NASH in 39% of patients |
| 2017 | Bouchi | [90] | 17 insulin-treated T2DM patients | Randomized, open-label | Liraglutide + insulin vs insulin | Yes | Liraglutide reduces visceral adiposity and hepatic fat accumulation |
| 2017 | Feng | [77] | 87 T2DM and NAFLD patients | RCT | Liraglutide vs gliclazide vs metformin | Yes | Liraglutide causes a greater reduction of intrahepatic fat than gliclazide |
| 2017 | Petit | [57] | 68 uncontrolled T2DM patients | Prospective single-center | Liraglutide | Yes | Liraglutide 1.2 mg/day significantly reduced liver fat content by body weight reduction |
| 2018 | Frøssing | [58] | 72 obese/overweight patients with PCOS | Prospective double-blind | Liraglutide vs placebo | Yes | Liraglutide treatment reduced liver fat content by 44% (evaluated by MR spectroscopy) compared with placebo |
| 2019 | Yan | [50] | 75 NAFLD and metformin-uncontrolled T2DM patients | RCT | Liraglutide vs sitagliptin vs insulin glargine | Yes | Combined with metformin, both liraglutide and sitagliptin, but not insulin glargine, reduced intrahepatic lipid and visceral adipose tissue |
| 2021 | Colosimo | [81] | 637 T2DM patients | Retrospective | DPP-4i vs GLP-1 RA vs SGLT-2i vs others | Yes | GLP-1 RA and SGLT-2Is improve biomarkers of steatosis (FLI) |
| 2021 | Newsome | [61] | 320 obese/overweight NASH patients | Prospective double-blind | Semaglutide vs placebo | Yes | Semaglutide treatment resulted in a significantly higher percentage of patients with NASH resolution than placebo |
| Year | First Author | Ref. | Sample Size | Study Typology | Evaluated Drugs | Benefit | Results |
|---|---|---|---|---|---|---|---|
| 2010 | Kenny | [96] | 8 T2DM and biopsy-proven NAFLD | Prospective, open-labeled case series | Exenatide | NA | Three of eight patients show improvement in liver histology |
| 2012 | Ohki | [97] | 82 NAFLD and T2DM patients | Retrospective | Liraglutide vs pioglitazone vs sitagliptin | Yes | Liraglutide or pioglitazone significantly reduce APRI |
| 2016 | Armstrong | [56] | 52 NASH patients | Multicentre, double-blinded, randomized, phase 2 trial | Liraglutide vs placebo | Yes | Liraglutide led to lower biopsy proven fibrosis progression compared with placebo |
| 2021 | Colosimo | [81] | 637 T2DM patients | Retrospective | DPP-4i vs GLP-1 RA vs SGLT-2i vs others | Yes | GLP-1 RA and SGLT-2Is improve biomarkers of fibrosis (FIB-4) |
| 2021 | Newsome | [61] | 320 obese/overweight NASH patients | Prospective double-blind | Semaglutide vs placebo | No | No significant improvement in fibrosis stage |
| Yes | Semaglutide 2.1 mg/week led to lower biopsy proven fibrosis progression compared with placebo | ||||||
| 2022 | Tan | [98] | 1765 T2DM patients | Prospective cohort | Liraglutide | Yes | Liraglutide treatment is associated with decreased liver fibrosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nevola, R.; Epifani, R.; Imbriani, S.; Tortorella, G.; Aprea, C.; Galiero, R.; Rinaldi, L.; Marfella, R.; Sasso, F.C. GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 1703. https://doi.org/10.3390/ijms24021703
Nevola R, Epifani R, Imbriani S, Tortorella G, Aprea C, Galiero R, Rinaldi L, Marfella R, Sasso FC. GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives. International Journal of Molecular Sciences. 2023; 24(2):1703. https://doi.org/10.3390/ijms24021703
Chicago/Turabian StyleNevola, Riccardo, Raffaella Epifani, Simona Imbriani, Giovanni Tortorella, Concetta Aprea, Raffaele Galiero, Luca Rinaldi, Raffaele Marfella, and Ferdinando Carlo Sasso. 2023. "GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives" International Journal of Molecular Sciences 24, no. 2: 1703. https://doi.org/10.3390/ijms24021703
APA StyleNevola, R., Epifani, R., Imbriani, S., Tortorella, G., Aprea, C., Galiero, R., Rinaldi, L., Marfella, R., & Sasso, F. C. (2023). GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives. International Journal of Molecular Sciences, 24(2), 1703. https://doi.org/10.3390/ijms24021703







