Clinical Significance of Combined Epithelial–Mesenchymal Transition Markers Expression and Role of Rac1 in Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Results
2.1. Clinicopathologic Characteristics and EMT Markers of the Patients
2.2. Comparison of Clinicopathological Data between HCC Patients with Combined EMT Markers Expression and HCC Patients without Combined EMT Markers Expression
2.3. Sensitivity and Specificity of EMT Markers for Prediction of MVI in HCC
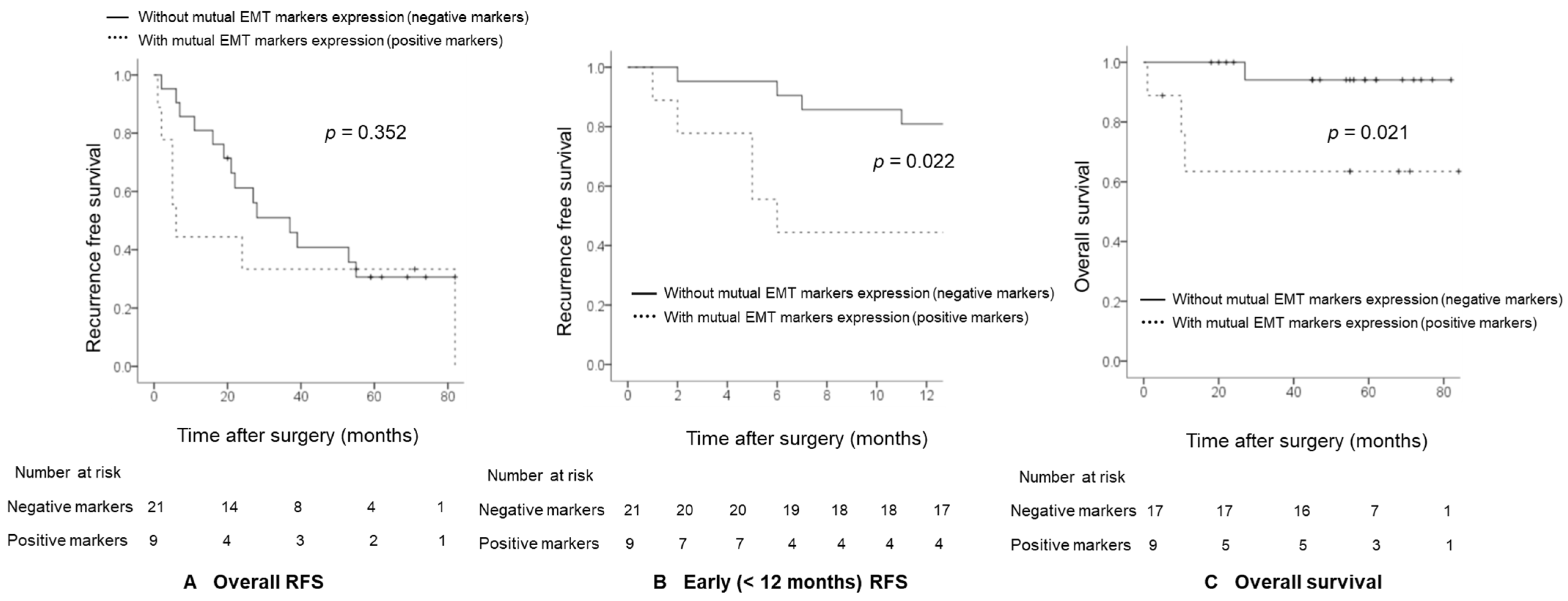
2.4. Comparison of Recurrence-Free Survival (RFS) and Overall Survival between HCC Patients with Combined EMT Markers Expression and HCC Patients without Combined EMT Markers Expression
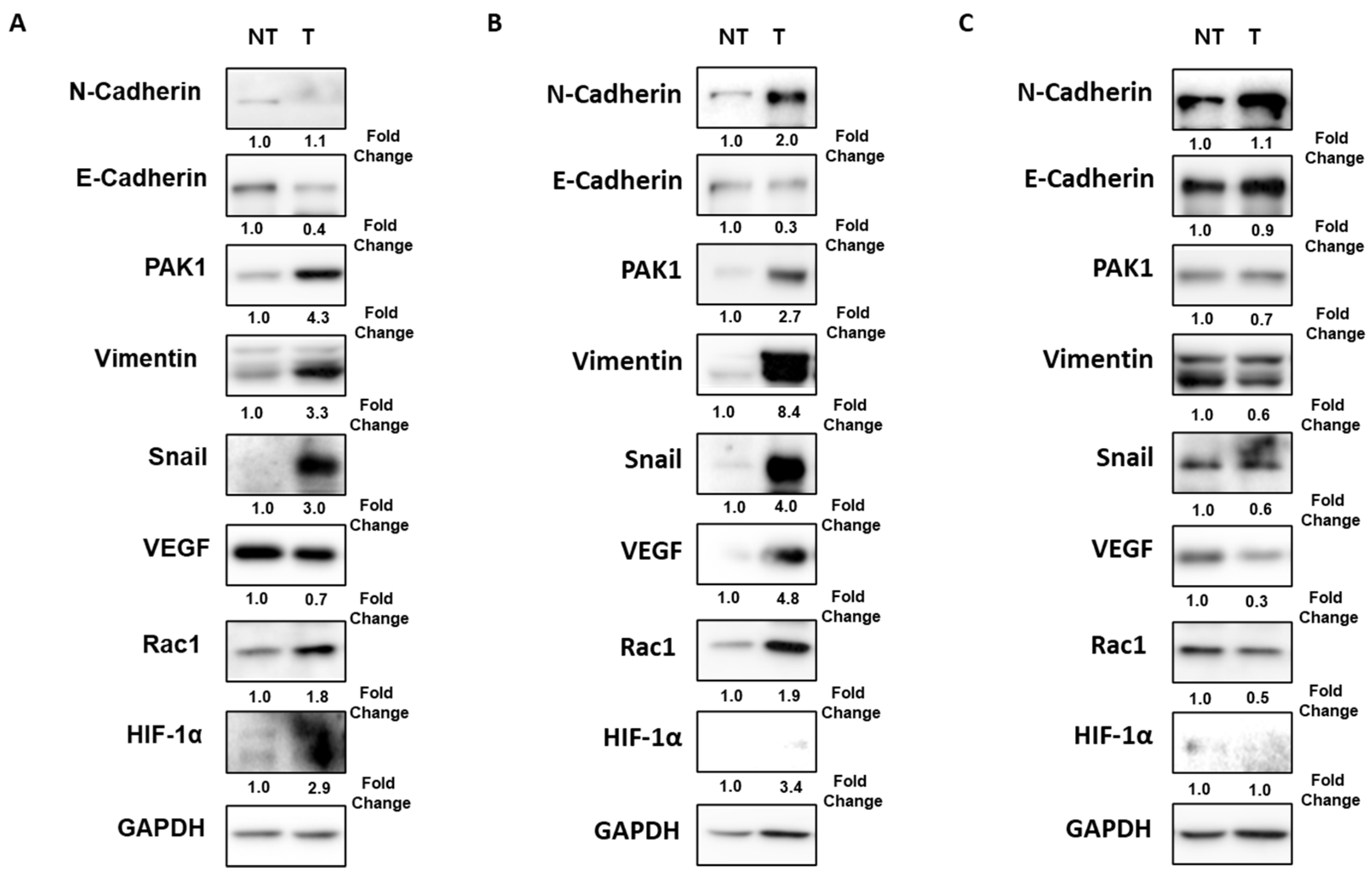
2.5. Rac1 Knockdown Decreases the Expressions of PAK1/Snail in Hypoxia-Exposed Hep3B Cells
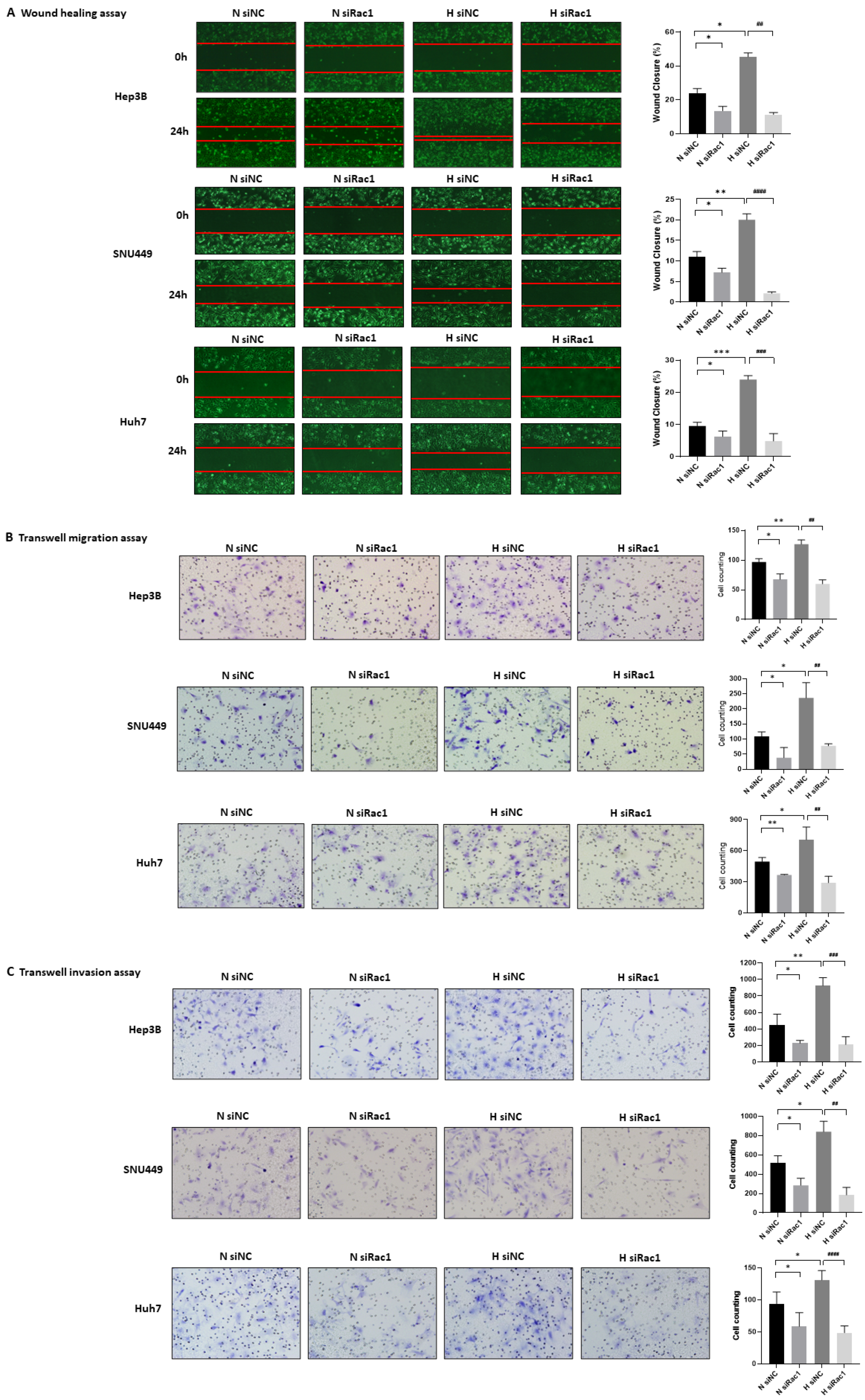
2.6. Rac1 Knockdown Suppresses the Migration and Invasion of Hypoxia-Exposed HCC Cells
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Follow-Up and Tumor Recurrence
4.3. Cell Lines and Hypoxic Condition
4.4. RNA Interference and Transfection
4.5. Western Blot Analysis
4.6. Definition of Combined EMT Markers Expression
4.7. Wound HEALING assay
4.8. Transwell Migration Assay
4.9. Matrigel Invasion Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Ding, S.M.; Zhou, L.; Xie, H.Y.; Chen, K.J.; Zhang, W.; Xing, C.Y.; Guo, H.J.; Zheng, S.S. FOXC1 contributes to microvascular invasion in primary hepatocellular carcinoma via regulating epithelial-mesenchymal transition. Int. J. Biol. Sci. 2012, 8, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, Y.; Zhong, J.; Wang, Q.; Wang, X.; Wei, H.; Li, J.; Xiu, P. The clinical significance of microvascular invasion in the surgical planning and postoperative sequential treatment in hepatocellular carcinoma. Sci. Rep. 2021, 11, 2415. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nistico, P.; Bissell, M.J.; Radisky, D.C. Epithelial-mesenchymal transition: General principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harb. Perspect. Biol. 2012, 4, a011908. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [Green Version]
- Song, J. Targeting epithelial-mesenchymal transition pathway in hepatocellular carcinoma. Clin. Mol. Hepatol. 2020, 26, 484–486. [Google Scholar] [CrossRef]
- Chae, Y.C.; Kim, J.H.; Kim, K.L.; Kim, H.W.; Lee, H.Y.; Heo, W.D.; Meyer, T.; Suh, P.G.; Ryu, S.H. Phospholipase D activity regulates integrin-mediated cell spreading and migration by inducing GTP-Rac translocation to the plasma membrane. Mol. Biol. Cell 2008, 19, 3111–3123. [Google Scholar] [CrossRef] [Green Version]
- Collins, C.; Tzima, E. Rac[e] to the pole: Setting up polarity in endothelial cells. Small GTPases 2014, 5, e28650. [Google Scholar] [CrossRef] [Green Version]
- Nobes, C.D.; Hall, A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 1995, 81, 53–62. [Google Scholar] [CrossRef]
- Zhou, K.; Rao, J.; Zhou, Z.H.; Yao, X.H.; Wu, F.; Yang, J.; Yang, L.; Zhang, X.; Cui, Y.H.; Bian, X.W.; et al. RAC1-GTP promotes epithelial-mesenchymal transition and invasion of colorectal cancer by activation of STAT3. Lab. Investig. J. Tech. Methods Pathol. 2018, 98, 989–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, C.; Cho, S.J.; Chang, K.K.; Park, D.J.; Ryeom, S.W.; Yoon, S.S. Role of Rac1 Pathway in Epithelial-to-Mesenchymal Transition and Cancer Stem-like Cell Phenotypes in Gastric Adenocarcinoma. Mol. Cancer Res. MCR 2017, 15, 1106–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radu, M.; Semenova, G.; Kosoff, R.; Chernoff, J. PAK signalling during the development and progression of cancer. Nat. Rev. Cancer 2014, 14, 13–25. [Google Scholar] [CrossRef]
- Rane, C.K.; Minden, A. P21 activated kinases: Structure, regulation, and functions. Small GTPases 2014, 5, e28003. [Google Scholar] [CrossRef] [Green Version]
- Somanath, P.R.; Byzova, T.V. 14-3-3beta-Rac1-p21 activated kinase signaling regulates Akt1-mediated cytoskeletal organization, lamellipodia formation and fibronectin matrix assembly. J. Cell. Physiol. 2009, 218, 394–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goc, A.; Abdalla, M.; Al-Azayzih, A.; Somanath, P.R. Rac1 activation driven by 14-3-3zeta dimerization promotes prostate cancer cell-matrix interactions, motility and transendothelial migration. PloS ONE 2012, 7, e40594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Z.; Hu, M.; Zhen, J.; Lin, J.; Wang, Q.; Wang, R. Rac1/PAK1 signaling promotes epithelial-mesenchymal transition of podocytes in vitro via triggering beta-catenin transcriptional activity under high glucose conditions. Int. J. Biochem. Cell Biol. 2013, 45, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Son, H.; Moon, A. Epithelial-mesenchymal Transition and Cell Invasion. Toxicol. Res. 2010, 26, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Nawshad, A.; Hay, E.D. TGFbeta3 signaling activates transcription of the LEF1 gene to induce epithelial mesenchymal transformation during mouse palate development. J. Cell Biol. 2003, 163, 1291–1301. [Google Scholar] [CrossRef]
- Peinado, H.; Portillo, F.; Cano, A. Transcriptional regulation of cadherins during development and carcinogenesis. Int. J. Dev. Biol. 2004, 48, 365–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.; Massague, J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell 2004, 118, 277–279. [Google Scholar] [CrossRef] [Green Version]
- Lundgren, K.; Holm, C.; Landberg, G. Hypoxia and breast cancer: Prognostic and therapeutic implications. Cell. Mol. Life Sci. CMLS 2007, 64, 3233–3247. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Rayala, S.; Nguyen, D.; Vadlamudi, R.K.; Chen, S.; Kumar, R. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail’s subcellular localization and functions. Cancer Res. 2005, 65, 3179–3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.H.; Chen, C.L.; Chau, G.Y.; Chiou, S.H.; Su, C.W.; Chou, T.Y.; Peng, W.L.; Wu, J.C. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology 2009, 50, 1464–1474. [Google Scholar] [CrossRef]
- Zhai, X.; Zhu, H.; Wang, W.; Zhang, S.; Zhang, Y.; Mao, G. Abnormal expression of EMT-related proteins, S100A4, vimentin and E-cadherin, is correlated with clinicopathological features and prognosis in HCC. Med. Oncol. 2014, 31, 970. [Google Scholar] [CrossRef] [PubMed]
- Mima, K.; Hayashi, H.; Kuroki, H.; Nakagawa, S.; Okabe, H.; Chikamoto, A.; Watanabe, M.; Beppu, T.; Baba, H. Epithelial-mesenchymal transition expression profiles as a prognostic factor for disease-free survival in hepatocellular carcinoma: Clinical significance of transforming growth factor-beta signaling. Oncol. Lett. 2013, 5, 149–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyoshi, A.; Kitajima, Y.; Kido, S.; Shimonishi, T.; Matsuyama, S.; Kitahara, K.; Miyazaki, K. Snail accelerates cancer invasion by upregulating MMP expression and is associated with poor prognosis of hepatocellular carcinoma. Br. J. Cancer 2005, 92, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Ching, Y.P.; Leong, V.Y.; Lee, M.F.; Xu, H.T.; Jin, D.Y.; Ng, I.O. P21-activated protein kinase is overexpressed in hepatocellular carcinoma and enhances cancer metastasis involving c-Jun NH2-terminal kinase activation and paxillin phosphorylation. Cancer Res. 2007, 67, 3601–3608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Peralvarez, M.; Luong, T.V.; Andreana, L.; Meyer, T.; Dhillon, A.P.; Burroughs, A.K. A systematic review of microvascular invasion in hepatocellular carcinoma: Diagnostic and prognostic variability. Ann. Surg. Oncol. 2013, 20, 325–339. [Google Scholar] [CrossRef]
- Yao, D.F.; Wu, X.H.; Zhu, Y.; Shi, G.S.; Dong, Z.Z.; Yao, D.B.; Wu, W.; Qiu, L.W.; Meng, X.Y. Quantitative analysis of vascular endothelial growth factor, microvascular density and their clinicopathologic features in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis. Int. 2005, 4, 220–226. [Google Scholar] [PubMed]
- Huang, G.W.; Yang, L.Y.; Lu, W.Q. Expression of hypoxia-inducible factor 1alpha and vascular endothelial growth factor in hepatocellular carcinoma: Impact on neovascularization and survival. World J. Gastroenterol. 2005, 11, 1705–1708. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Nomoto, S.; Nishikawa, Y.; Sugimoto, H.; Kanazumi, N.; Takeda, S.; Nakao, A. Correlations of the expression of vascular endothelial growth factor B and its isoforms in hepatocellular carcinoma with clinico-pathological parameters. J. Surg. Oncol. 2008, 98, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Liu, J.; Tu, X.; Zang, Y.; Zhu, J.; Chen, J.; Dong, L.; Zhang, J. miR-30 inhibits TGF-beta1-induced epithelial-to-mesenchymal transition in hepatocyte by targeting Snail1. Biochem. Biophys. Res. Commun. 2012, 417, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Ma, X.; Li, X.; Dong, H.; Yi, J.; Zeng, W.; Yang, Z. miR-153 inhibits epithelial-to-mesenchymal transition in hepatocellular carcinoma by targeting Snail. Oncol. Rep. 2015, 34, 655–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichl, P.; Dengler, M.; van Zijl, F.; Huber, H.; Fuhrlinger, G.; Reichel, C.; Sieghart, W.; Peck-Radosavljevic, M.; Grubinger, M.; Mikulits, W. Axl activates autocrine transforming growth factor-beta signaling in hepatocellular carcinoma. Hepatology 2015, 61, 930–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, F.; Yin, L.X. PAK1 promotes proliferation, migration and invasion of hepatocellular carcinoma by facilitating EMT via directly up-regulating Snail. Genomics 2020, 112, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Rane, C.K.; Minden, A. P21 activated kinase signaling in cancer. Semin. Cancer Biol. 2019, 54, 40–49. [Google Scholar] [CrossRef]
- Jagadeeshan, S.; Subramanian, A.; Tentu, S.; Beesetti, S.; Singhal, M.; Raghavan, S.; Surabhi, R.P.; Mavuluri, J.; Bhoopalan, H.; Biswal, J.; et al. P21-activated kinase 1 (Pak1) signaling influences therapeutic outcome in pancreatic cancer. Ann. Oncol. 2016, 27, 1546–1556. [Google Scholar] [CrossRef]
- Chen, C.; Lou, T. Hypoxia inducible factors in hepatocellular carcinoma. Oncotarget 2017, 8, 46691–46703. [Google Scholar] [CrossRef]
- Hirota, K.; Semenza, G.L. Rac1 activity is required for the activation of hypoxia-inducible factor 1. J. Biol. Chem. 2001, 276, 21166–21172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Hernandez, M.A.; Chapresto-Garzon, R.; Cadenas, M.; Navarro-Villaran, E.; Negrete, M.; Gomez-Bravo, M.A.; Victor, V.M.; Padillo, F.J.; Muntane, J. Differential effectiveness of tyrosine kinase inhibitors in 2D/3D culture according to cell differentiation, p53 status and mitochondrial respiration in liver cancer cells. Cell Death Dis. 2020, 11, 339. [Google Scholar] [CrossRef]
- Bao, M.H.; Wong, C.C. Hypoxia, Metabolic Reprogramming, and Drug Resistance in Liver Cancer. Cells 2021, 10, 1715–1732. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, Y.J.; Han, S.H.; Lee, Y.E.; Kim, S.; Kim, Y.J.; Cho, J.H.; Kwon, K.A.; Kim, J.H.; Kim, S.H. Dexamethasone inhibits hypoxia-induced epithelial-mesenchymal transition in colon cancer. World J. Gastroenterol. 2015, 21, 9887–9899. [Google Scholar] [CrossRef]

| Variable | HCC Patients Who Underwent Surgical Resection (n = 30) |
|---|---|
| Age (years) | 58.0 ± 9.7 |
| Male gender | 22 (77.3) |
| LC | 25 (83.3) |
| Etiology | |
| HBV | 24 (80.0) |
| HCV | 1 (3.3) |
| HBV + HCV | 1 (3.3) |
| Alcohol | 1 (3.3) |
| NASH | 2 (6.7) |
| Unknown | 1 (3.3) |
| Tumor size (cm) | 4.0 ± 2.5 |
| Tumor number | |
| Single | 25 (83.3) |
| Multiple | 5 (16.7) |
| Tumor grade | |
| 1 | 2 (6.7) |
| 2 | 5 (16.7) |
| 3 | 19 (63.3) |
| 4 | 4 (13.3) |
| Macrovascular invasion | 3 (10.0) |
| MVI | 9 (30.0) |
| Glisson capsule invasion with perforation | 4 (13.3) |
| Adjacent organ invasion | 2 (6.7) |
| TNM stage | |
| IA | 6 (20.0) |
| IB | 12 (40.0) |
| II | 7 (23.3) |
| IIIA | 2 (6.7) |
| IIIB | 3 (10.0) |
| IV | 0 (0) |
| A-FP (ng/dL) | 1505.4 ± 3963.5 |
| PIVKA-II (mAU/mL) | 3691.2 ± 14,921.4 |
| Down-expression of E-cadherin | 22 (73.3) |
| Overexpression of PAK1 | 18 (60.0) |
| Overexpression of Snail | 17 (56.7) |
| Combined EMT markers expression | 9 (30.0) |
| Variable | With Combined EMT Markers Expression (n = 9) | Without Combined EMT Markers Expression (n = 21) | p-Value |
|---|---|---|---|
| Age (years) | 55 (50.0, 66.0) | 59.5 (49.5, 67.5) | 0.717 |
| Male gender | 7 (77.8) | 15 (71.4) | 0.999 |
| LC | 5 (55.6) | 20 (95.2) | 0.019 |
| Etiology | 0.897 | ||
| HBV | 9 (100) | 15 (71.4) | |
| HCV | 0 (0) | 1 (4.8) | |
| HBV+HCV | 0 (0) | 1 (4.8) | |
| Alcohol | 0 (0) | 1 (4.8) | |
| NASH | 0 (0) | 2 (9.5) | |
| Unknown | 0 (0) | 1 (4.8) | |
| Tumor size (cm) | 4.0 (3.2, 11.9) | 2.7 (1.9, 4.0) | 0.021 |
| Tumor number | 0.999 | ||
| Single | 8 (88.9) | 17 (81.0) | |
| Multiple | 1 (11.1) | 4 (19.0) | |
| Tumor grade | 0.713 | ||
| 1 | 0 (0) | 2 (9.5) | |
| 2 | 1 (11.1) | 4 (19.0) | |
| 3 | 6 (66.7) | 13 (61.9) | |
| 4 | 2 (22.2) | 2 (9.5) | |
| Macrovascular invasion | 3 (33.3) | 0 (0) | 0.021 |
| MVI | 7 (77.8) | 2 (9.5) | 0.001 |
| Glisson capsule invasion with perforation | 3 (33.3) | 1 (4.8) | 0.069 |
| Adjacent organ invasion | 2 (22.2) | 0 (0) | 0.083 |
| TNM stage | 0.015 | ||
| IA | 1 (11.1) | 5 (23.8) | |
| IB | 1 (11.1) | 11 (52.4) | |
| II | 3 (33.3) | 4 (19.0) | |
| IIIA | 1 (11.1) | 1 (4.8) | |
| IIIB | 3 (33.3) | 0 (0) | |
| IV | 0 (0) | 0 (0) | |
| A-FP (ng/dL) | 17.1 (5.0, 8134.0) | 18.2 (6.8, 469.1) | 0.665 |
| PIVKA-II (mAU/mL) | 1437 (16.3, 5169.5) | 54.5 (22.0, 180.5) | 0.600 |
| Overexpression of Rac1 | 8 (88.9) | 9 (42.9) | 0.042 |
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUROC (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| Down-expression of E-cadherin | 22.2 | 71.4 | 25.0 | 68.2 | 0.468 (0.242–0.694) | 0.786 |
| Overexpression of Snail | 88.9 | 57.1 | 47.1 | 92.3 | 0.730 (0.542–0.918) | 0.049 |
| Overexpression of PAK1 | 100 | 57.1 | 50.0 | 100 | 0.786 (0.626–0.945) | 0.015 |
| Overexpression of VEGF | 66.7 | 57.1 | 40.0 | 80.0 | 0.619 (0.399–0.840) | 0.309 |
| Down-expression of E-cadherin and overexpression of Vimentin | 66.7 | 76.2 | 54.5 | 84.2 | 0.714 (0.503–0.925) | 0.067 |
| Combined EMT markers expression | 77.8 | 90.5 | 77.8 | 90.5 | 0.841 (0.630–0.999) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.K.; Ryu, S.; Nam, S.; Ha, S.Y.; Kwon, O.S.; Kim, Y.S.; Kim, S.-H.; Kim, J.H. Clinical Significance of Combined Epithelial–Mesenchymal Transition Markers Expression and Role of Rac1 in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 1765. https://doi.org/10.3390/ijms24021765
Shin SK, Ryu S, Nam S, Ha SY, Kwon OS, Kim YS, Kim S-H, Kim JH. Clinical Significance of Combined Epithelial–Mesenchymal Transition Markers Expression and Role of Rac1 in Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2023; 24(2):1765. https://doi.org/10.3390/ijms24021765
Chicago/Turabian StyleShin, Seung Kak, Sujin Ryu, Seungyoon Nam, Seung Yeon Ha, Oh Sang Kwon, Yun Soo Kim, Se-Hee Kim, and Ju Hyun Kim. 2023. "Clinical Significance of Combined Epithelial–Mesenchymal Transition Markers Expression and Role of Rac1 in Hepatocellular Carcinoma" International Journal of Molecular Sciences 24, no. 2: 1765. https://doi.org/10.3390/ijms24021765
APA StyleShin, S. K., Ryu, S., Nam, S., Ha, S. Y., Kwon, O. S., Kim, Y. S., Kim, S.-H., & Kim, J. H. (2023). Clinical Significance of Combined Epithelial–Mesenchymal Transition Markers Expression and Role of Rac1 in Hepatocellular Carcinoma. International Journal of Molecular Sciences, 24(2), 1765. https://doi.org/10.3390/ijms24021765






