Different Contacted Cell Types Contribute to Acquiring Different Properties in Brain Microglial Cells upon Intercellular Interaction
Abstract
:1. Introduction
2. Results
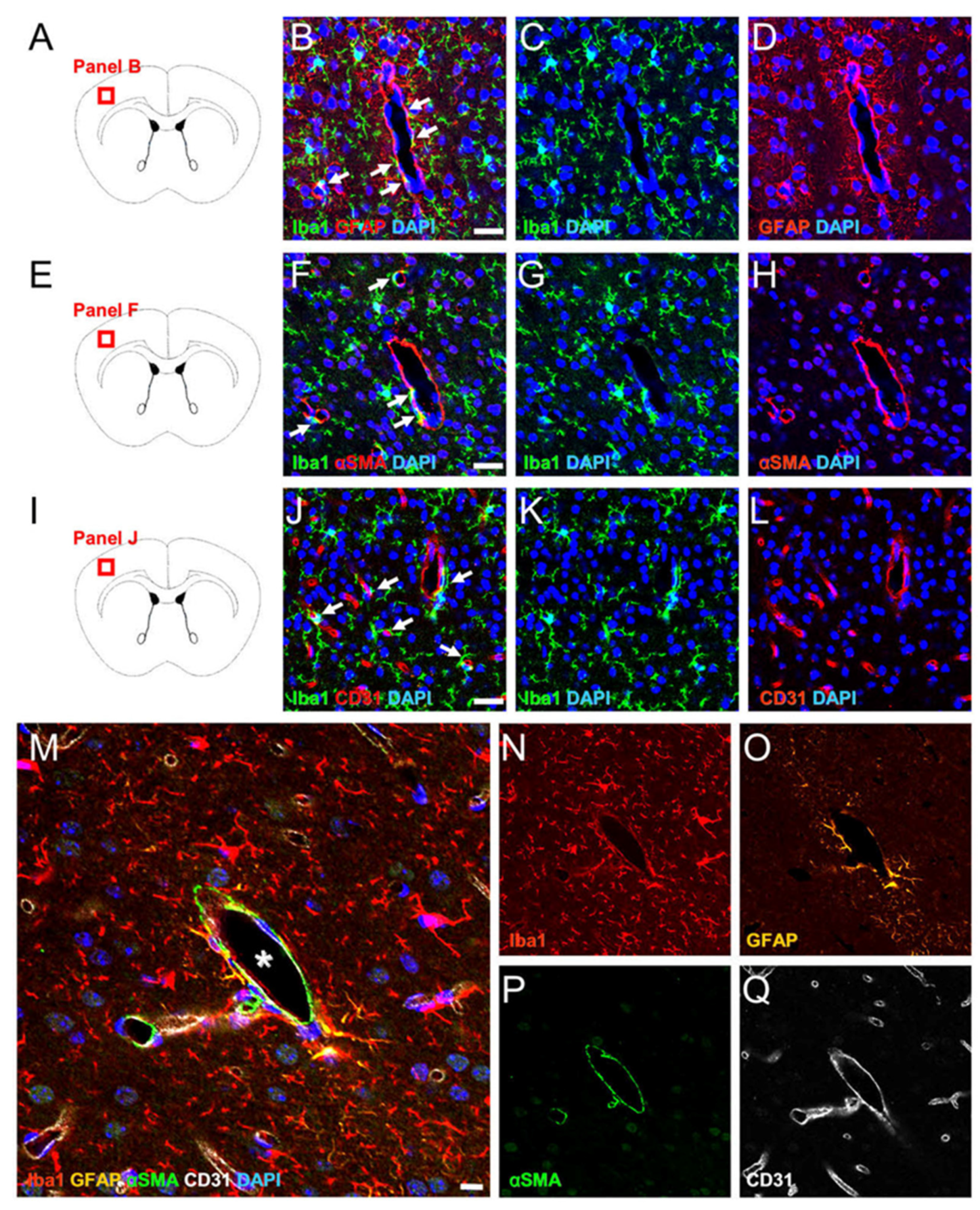
2.1. MG, AC, PC, and EC Expression Patterns in Healthy Brain Samples
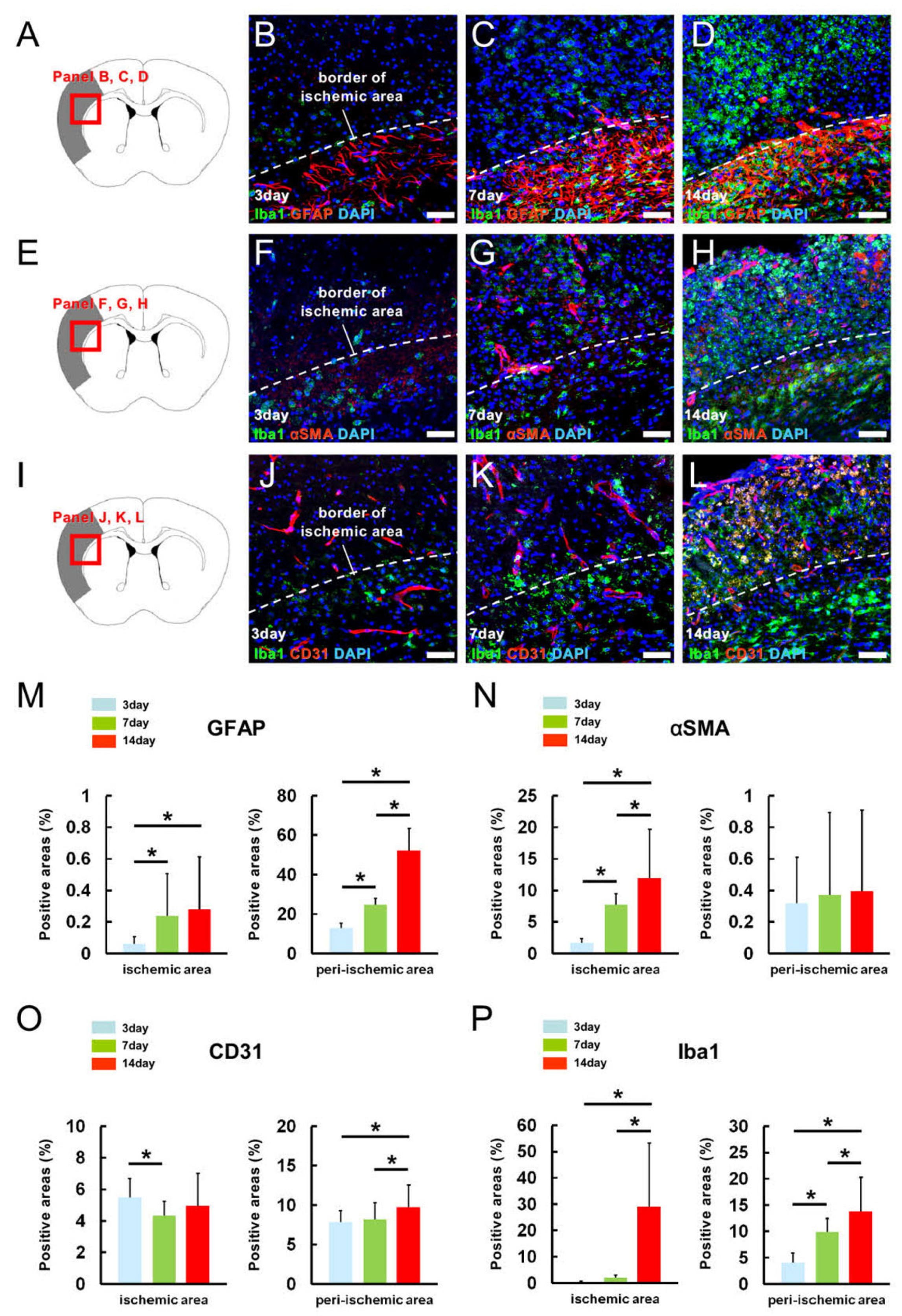
2.2. MG, AC, PC, and EC Expression Patterns in Pathological Brain Samples after Ischemic Stroke
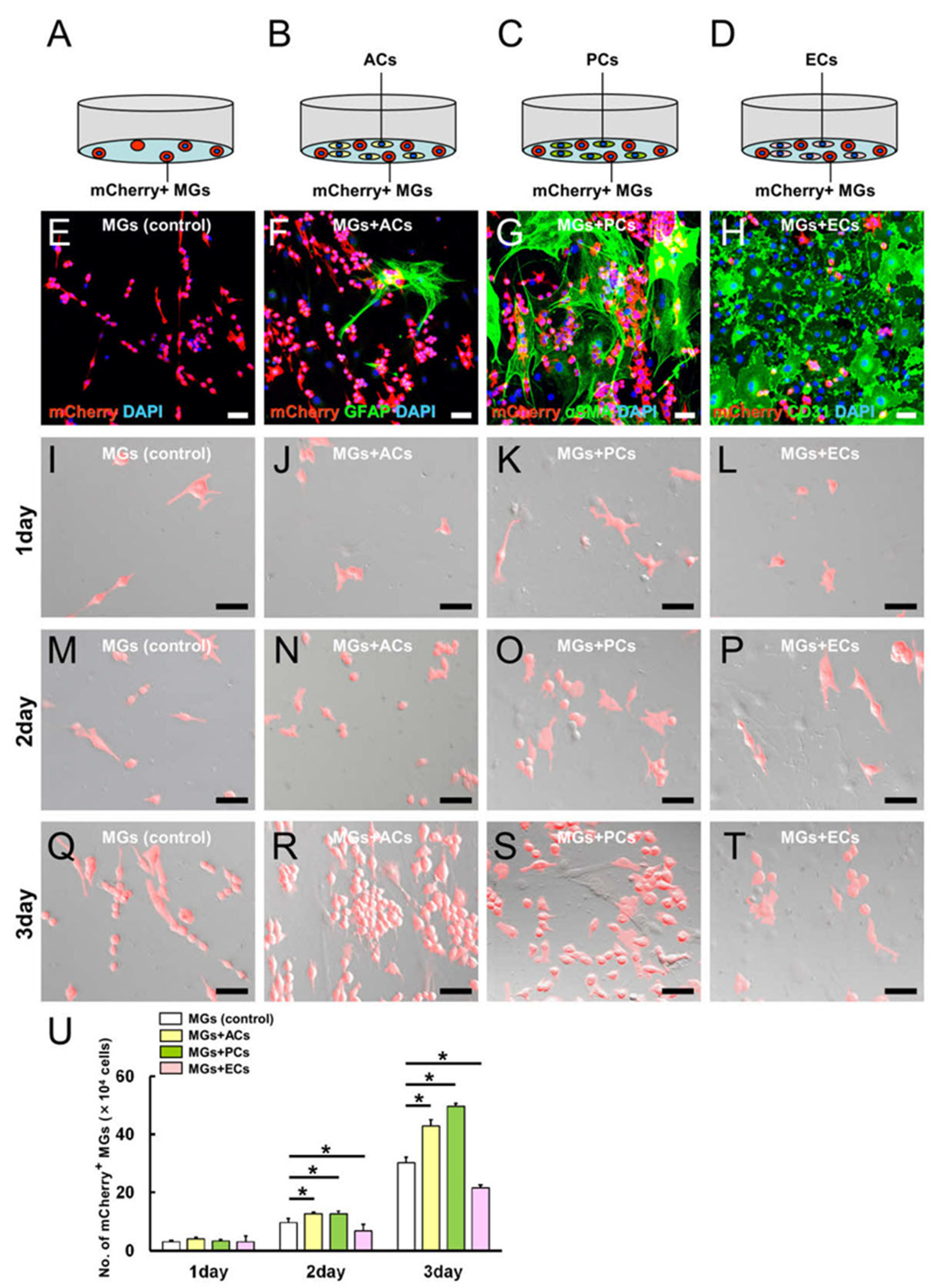
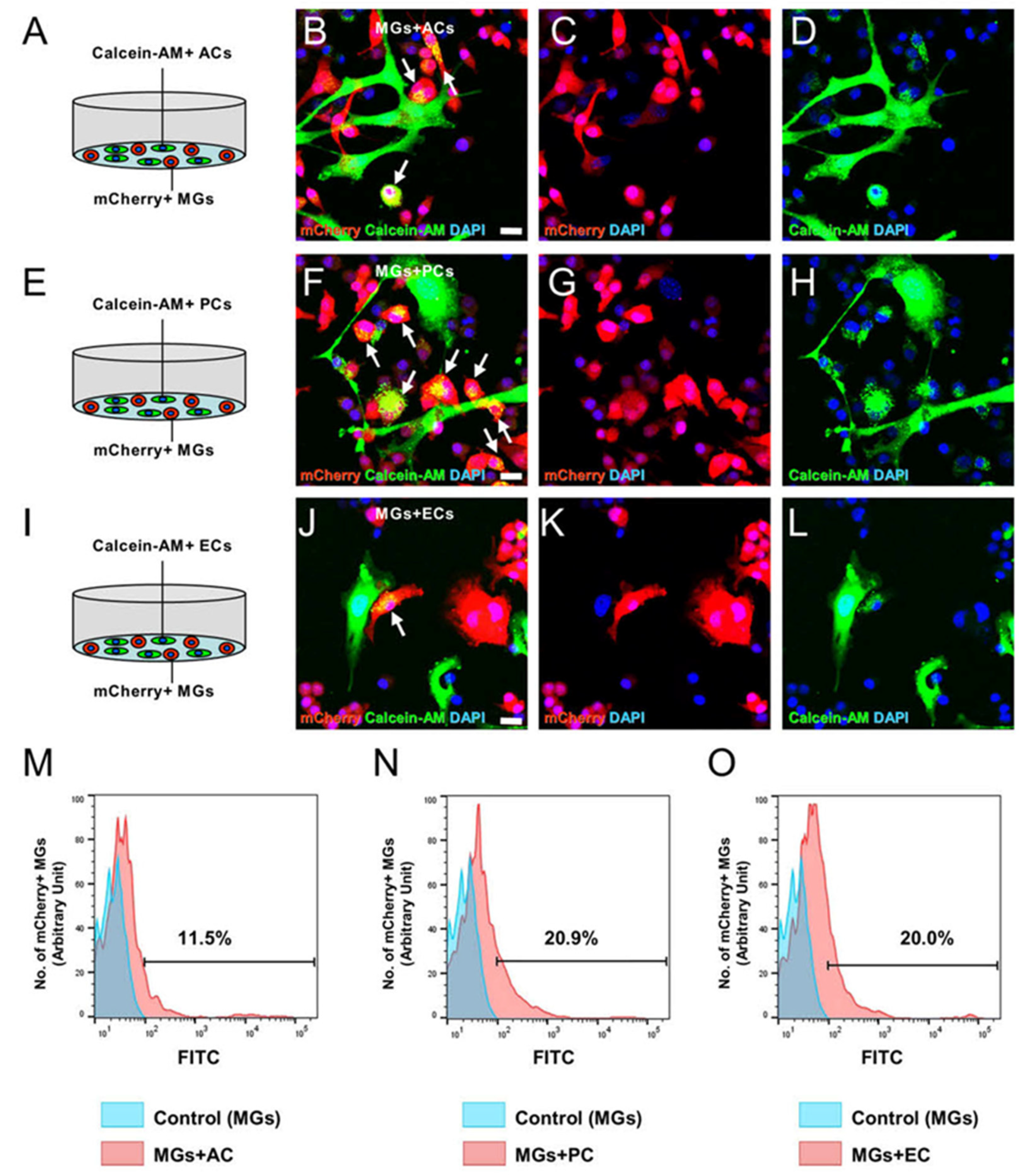
2.3. AC and PC Contact Increased the Number of MGs, while EC Contact Reduced Them
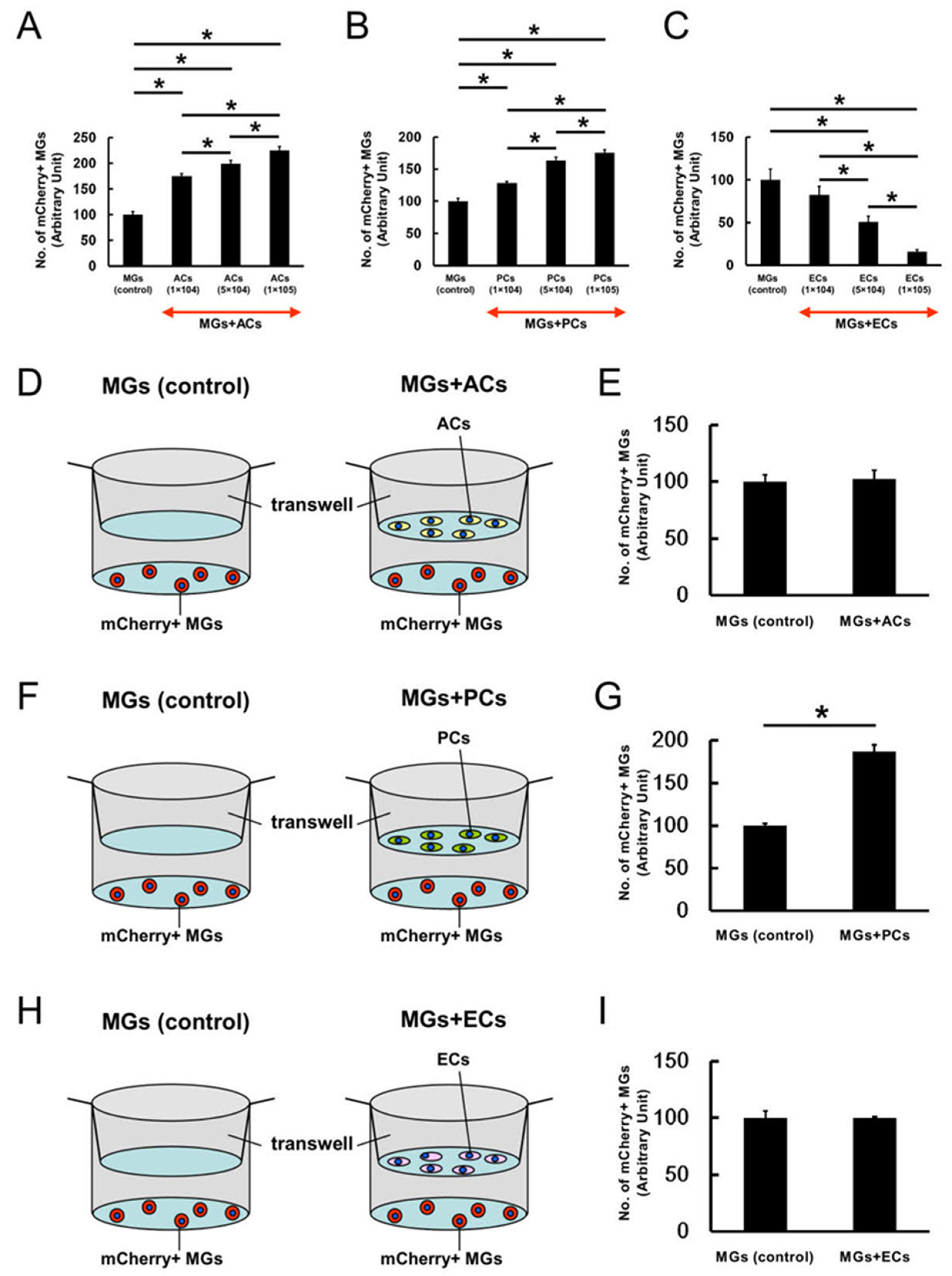
2.4. ACs, PCs, and ECs Affect Microglial Numbers Dose-Dependently
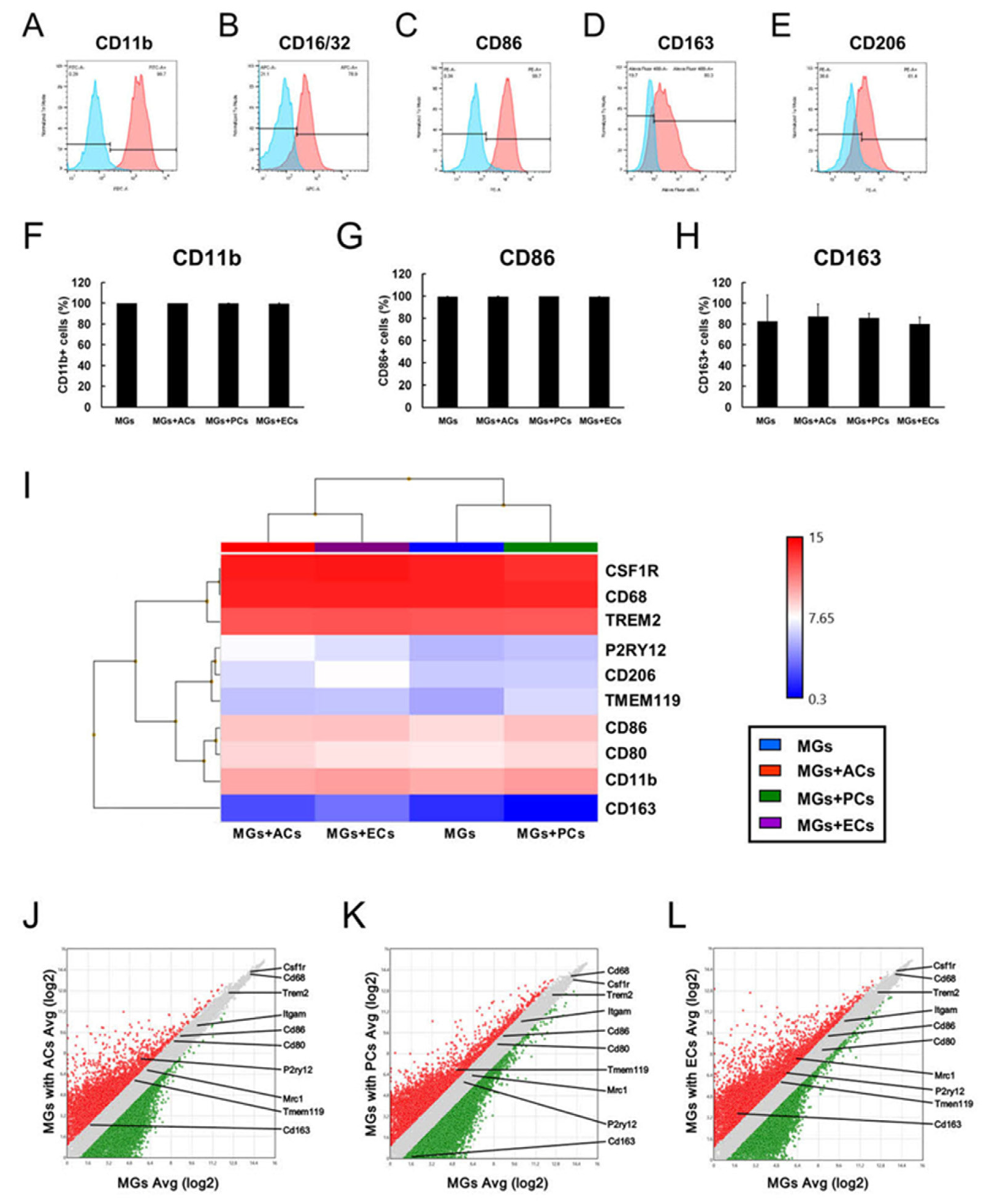
2.5. Contact with ACs, PCs, or ECs Altered Microglial Phenotypes
2.6. Cell Cycle-Related Gene Expression Patterns in MGs upon AC, PC, or EC Contact
2.7. Apoptosis-Related Gene Expression Patterns in MGs upon AC, PC, or EC Contact
2.8. M1/M2 Marker Expression Patterns in MGs upon AC, PC, or EC Contact
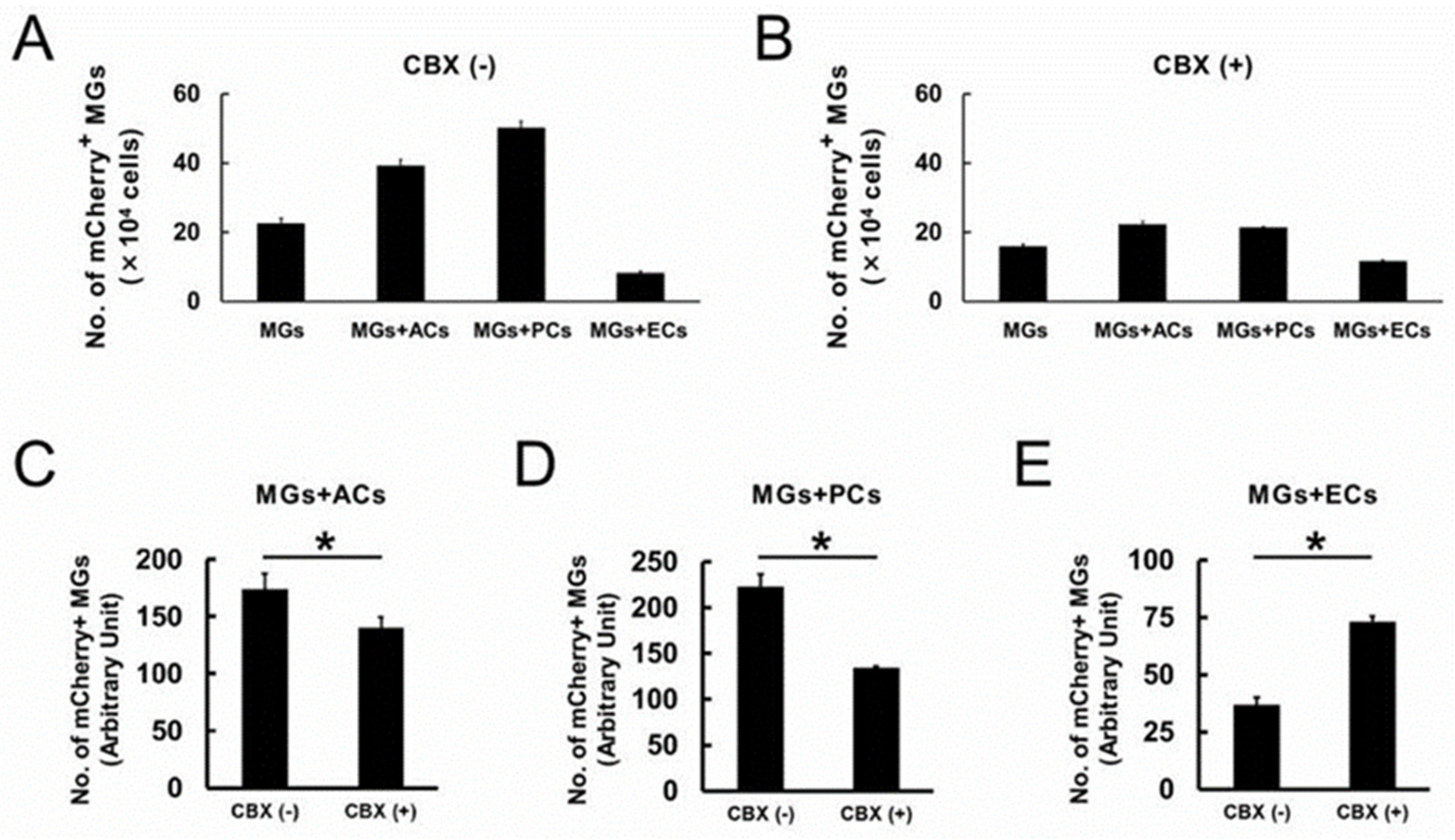
2.9. Gja1 Involvement in Microglial Regulation upon AC, PC, or EC Contact
3. Discussion
4. Materials and Methods
4.1. Animals and Brain Sample Preparation
4.2. Immunohistochemistry
4.3. Electron Microscope
4.4. Cell Cultures
4.5. Flow Cytometric Analysis
4.6. Microarray Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, C.; Zhou, L.Q.; Ma, X.T.; Hu, Z.W.; Yang, S.; Chen, M.; Bosco, D.B.; Wu, L.J.; Tian, D.S. Dual Functions of Microglia in Ischemic Stroke. Neurosci. Bull. 2019, 35, 921–933. [Google Scholar] [CrossRef]
- Su, I.J.; Hsu, C.Y.; Shen, S.; Chao, P.K.; Hsu, J.T.; Hsueh, J.T.; Liang, J.J.; Hsu, Y.T.; Shie, F.S. The Beneficial Effects of Combining Anti-Abeta Antibody NP106 and Curcumin Analog TML-6 on the Treatment of Alzheimer’s Disease in APP/PS1 Mice. Int. J. Mol. Sci. 2022, 23, 556. [Google Scholar] [CrossRef] [PubMed]
- Donat, C.K.; Scott, G.; Gentleman, S.M.; Sastre, M. Microglial Activation in Traumatic Brain Injury. Front. Aging Neurosci. 2017, 9, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geribaldi-Doldan, N.; Fernandez-Ponce, C.; Quiroz, R.N.; Sanchez-Gomar, I.; Escorcia, L.G.; Velasquez, E.P.; Quiroz, E.N. The Role of Microglia in Glioblastoma. Front. Oncol. 2021, 10, 603495. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Stanimirovic, D.B.; Friedman, A. Pathophysiology of the neurovascular unit: Disease cause or consequence? J. Cereb. Blood Flow Metab. 2012, 32, 1207–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.R.; Liu, J.C.; Bao, J.S.; Bai, Q.Q.; Wang, G.Q. Interaction of Microglia and Astrocytes in the Neurovascular Unit. Front. Immunol. 2020, 11, 1024. [Google Scholar] [CrossRef]
- Thurgur, H.; Pinteaux, E. Microglia in the Neurovascular Unit: Blood-Brain Barrier-microglia Interactions after Central Nervous System Disorders. Neuroscience 2019, 405, 55–67. [Google Scholar] [CrossRef]
- Armulik, A.; Genove, G.; Mae, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [Green Version]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef]
- Bell, R.D.; Winkler, E.A.; Sagare, A.P.; Singh, I.; LaRue, B.; Deane, R.; Zlokovic, B.V. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010, 68, 409–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naranjo, O.; Osborne, O.; Torices, S.; Toborek, M. In Vivo Targeting of the Neurovascular Unit: Challenges and Advancements. Cell Mol. Neurobiol. 2021, 42, 2131–2146. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Ronnback, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Csaszar, E.; Lenart, N.; Cserep, C.; Kornyei, Z.; Fekete, R.; Posfai, B.; Balazsfi, D.; Hangya, B.; Schwarcz, A.D.; Szabadits, E.; et al. Microglia modulate blood flow, neurovascular coupling, and hypoperfusion via purinergic actions. J. Exp. Med. 2022, 219, e20211071. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson, P.T.; Davis, T.P. Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity. J. Cereb. Blood Flow. Metab. 2020, 40, S6–S24. [Google Scholar] [CrossRef]
- Zhou, W.; Liesz, A.; Bauer, H.; Sommer, C.; Lahrmann, B.; Valous, N.; Grabe, N.; Veltkamp, R. Postischemic brain infiltration of leukocyte subpopulations differs among murine permanent and transient focal cerebral ischemia models. Brain. Pathol. 2012, 23, 34–44. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakagomi, N.; Doe, N.; Nakano-Doi, A.; Sawano, T.; Takagi, T.; Matsuyama, T.; Yoshimura, S.; Nakagomi, T. Early Reperfusion Following Ischemic Stroke Provides Beneficial Effects, Even After Lethal Ischemia with Mature Neural Cell Death. Cells 2020, 9, 1376. [Google Scholar] [CrossRef]
- Nakagomi, T.; Molnar, Z.; Nakano-Doi, A.; Taguchi, A.; Saino, O.; Kubo, S.; Clausen, M.; Yoshikawa, H.; Nakagomi, N.; Matsuyama, T. Ischemia-induced neural stem/progenitor cells in the pia mater following cortical infarction. Stem. Cells Dev. 2011, 20, 2037–2051. [Google Scholar] [CrossRef]
- Tachibana, M.; Ago, T.; Wakisaka, Y.; Kuroda, J.; Shijo, M.; Yoshikawa, Y.; Komori, M.; Nishimura, A.; Makihara, N.; Nakamura, K.; et al. Early Reperfusion after Brain Ischemia Has Beneficial Effects beyond Rescuing Neurons. Stroke 2017, 48, 2222–2230. [Google Scholar] [CrossRef]
- Nakano-Doi, A.; Sakuma, R.; Matsuyama, T.; Nakagomi, T. Ischemic stroke activates the VE-cadherin promoter and increases VE-cadherin expression in adult mice. Histol. Histopathol. 2018, 33, 507–521. [Google Scholar]
- Nakagomi, T.; Tanaka, Y.; Nakagomi, N.; Matsuyama, T.; Yoshimura, S. How Long Are Reperfusion Therapies Beneficial for Patients after Stroke Onset? Lessons from Lethal Ischemia Following Early Reperfusion in a Mouse Model of Stroke. Int. J. Mol. Sci. 2020, 21, 6360. [Google Scholar] [CrossRef] [PubMed]
- Nakano-Doi, A.; Nakagomi, T.; Fujikawa, M.; Nakagomi, N.; Kubo, S.; Lu, S.; Yoshikawa, H.; Soma, T.; Taguchi, A.; Matsuyama, T. Bone marrow mononuclear cells promote proliferation of endogenous neural stem cells through vascular niches after cerebral infarction. Stem. Cells 2010, 28, 1292–1302. [Google Scholar] [CrossRef]
- Nakagomi, T.; Kubo, S.; Nakano-Doi, A.; Sakuma, R.; Lu, S.; Narita, A.; Kawahara, M.; Taguchi, A.; Matsuyama, T. Brain vascular pericytes following ischemia have multipotential stem cell activity to differntiate into neural and vascular lineage cells. Stem. Cells 2015, 33, 1962–1974. [Google Scholar] [CrossRef]
- Santisteban, M.M.; Ahn, S.J.; Lane, D.; Faraco, G.; Garcia-Bonilla, L.; Racchumi, G.; Poon, C.; Schaeffer, S.; Segarra, S.G.; Korbelin, J.; et al. Endothelium-Macrophage Crosstalk Mediates Blood-Brain Barrier Dysfunction in Hypertension. Hypertension 2020, 76, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Matejuk, A.; Ransohoff, R.M. Crosstalk between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Lee, D.H.; Jeong, H.S.; Kim, S.H.; Ye, S.K.; Cho, C.H. STAT3 activation in microglia increases pericyte apoptosis in diabetic retinas through TNF-a/AKT/p70S6 kinase signaling. Biochem. Biophys. Res. Commun. 2022, 613, 133–139. [Google Scholar] [CrossRef]
- Bell, A.H.; Miller, S.L.; Castillo-Melendez, M.; Malhotra, A. The Neurovascular Unit: Effects of Brain Insults during the Perinatal Period. Front. Neurosci. 2019, 13, 1452. [Google Scholar] [CrossRef]
- Shaikh, S.B.; Uy, B.; Perera, A.; Nicholson, L.F. AGEs-RAGE mediated up-regulation of connexin43 in activated human microglial CHME-5 cells. Neurochem. Int. 2012, 60, 640–651. [Google Scholar] [CrossRef]
- Moran, L.B.; Duke, D.C.; Turkheimer, F.E.; Banati, R.B.; Graeber, M.B. Towards a transcriptome definition of microglial cells. Neurogenetics 2004, 5, 95–108. [Google Scholar] [CrossRef]
- Yu, S.; Cheng, L.; Tian, D.; Li, Z.; Yao, F.; Luo, Y.; Liu, Y.; Zhu, Z.; Zheng, M.; Jing, J. Fascin-1 is Highly Expressed Specifically in Microglia after Spinal Cord Injury and Regulates Microglial Migration. Front. Pharmacol. 2021, 12, 729524. [Google Scholar] [CrossRef]
- Gajardo-Gomez, R.; Labra, V.C.; Orellana, J.A. Connexins and Pannexins: New Insights into Microglial Functions and Dysfunctions. Front. Mol. Neurosci. 2016, 9, 86. [Google Scholar] [CrossRef]
- Sanchez, O.F.; Rodriguez, A.V.; Velasco-Espana, J.M.; Murillo, L.C.; Sutachan, J.J.; Albarracin, S.L. Role of Connexins 30, 36, and 43 in Brain Tumors, Neurodegenerative Diseases, and Neuroprotection. Cells 2020, 9, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerr, N.M.; Johnson, C.S.; de Souza, C.F.; Chee, K.S.; Good, W.R.; Green, C.R.; Danesh-Meyer, H.V. Immunolocalization of gap junction protein connexin43 (GJA1) in the human retina and optic nerve. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4028–4034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.F.; Sato, T.; Haimovici, R.; Okamoto, T.; Roy, S. High glucose alters connexin 43 expression and gap junction intercellular communication activity in retinal pericytes. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5376–5382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi-Taura, A.; Okinaka, Y.; Takeuchi, Y.; Ogawa, Y.; Maeda, M.; Kataoka, Y.; Yasui, T.; Kimura, T.; Gul, S.; Claussen, C.; et al. Bone Marrow Mononuclear Cells Activate Angiogenesis via Gap Junction-Mediated Cell-Cell Interaction. Stroke 2020, 51, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi-Taura, A.; Okinaka, Y.; Saino, O.; Takeuchi, Y.; Ogawa, Y.; Kimura, T.; Gul, S.; Claussen, C.; Boltze, J.; Taguchi, A. Gap junction-mediated cell-cell interaction between transplanted mesenchymal stem cells and vascular endothelium in stroke. Stem. Cells 2021, 39, 904–912. [Google Scholar] [CrossRef]
- Dyne, E.; Cawood, M.; Suzelis, M.; Russell, R.; Kim, M.H. Ultrastructural analysis of the morphological phenotypes of microglia associated with neuroinflammatory cues. J. Comp. Neurol. 2021, 530, 1263–1275. [Google Scholar] [CrossRef]
- Kim, S.; Son, Y. Astrocytes Stimulate Microglial Proliferation and M2 Polarization in vitro through Crosstalk between Astrocytes and Microglia. Int. J. Mol. Sci. 2021, 22, 8800. [Google Scholar] [CrossRef]
- Jiang, C.T.; Wu, W.F.; Deng, Y.H.; Ge, J.W. Modulators of microglia activation and polarization in ischemic stroke (Review). Mol. Med. Rep. 2020, 21, 2006–2018. [Google Scholar] [CrossRef] [Green Version]
- Mander, P.K.; Jekabsone, A.; Brown, G.C. Microglia proliferation is regulated by hydrogen peroxide from NADPH oxidase. J. Immunol. 2006, 176, 1046–1052. [Google Scholar] [CrossRef] [Green Version]
- Banks, W.A.; Kovac, A.; Morofuji, Y. Neurovascular unit crosstalk: Pericytes and astrocytes modify cytokine secretion patterns of brain endothelial cells. J. Cereb. Blood Flow. Metab. 2018, 38, 1104–1118. [Google Scholar] [CrossRef]
- Hoptak-Solga, A.D.; Nielsen, S.; Jain, I.; Thummel, R.; Hyde, D.R.; Iovine, M.K. Connexin43 (GJA1) is required in the population of dividing cells during fin regeneration. Dev. Biol. 2008, 317, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Dere, E.; Zlomuzica, A. The role of gap junctions in the brain in health and disease. Neurosci. Biobehav. Rev. 2012, 36, 206–217. [Google Scholar] [CrossRef]
- Banciu, D.D.; Cretoiu, D.; Cretoiu, S.M.; Banciu, A.; Popa, D.; David, R.; Berghea-Neamtu, C.S.; Cipaian, C.R.; Negrea, M.O.; Gheonea, M.; et al. Telocytes’ Role in Modulating Gut Motility Function and Development: Medical Hypotheses and Literature Review. Int. J. Mol. Sci. 2022, 23, 7017. [Google Scholar] [CrossRef]
- Nikolakopoulou, A.M.; Montagne, A.; Kisler, K.; Dai, Z.; Wang, Y.; Huuskonen, M.T.; Sagare, A.P.; Lazic, D.; Sweeney, M.D.; Kong, P.; et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat. Neurosci. 2019, 22, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Ogura, S.; Kurata, K.; Hattori, Y.; Takase, H.; Ishiguro-Oonuma, T.; Hwang, Y.; Ahn, S.; Park, I.; Ikeda, W.; Kusuhara, S.; et al. Sustained inflammation after pericyte depletion induces irreversible blood-retina barrier breakdown. JCI Insight. 2017, 2, e90905. [Google Scholar] [CrossRef] [Green Version]
- Uemura, M.T.; Maki, T.; Ihara, M.; Lee, V.M.Y.; Trojanowski, J.Q. Brain Microvascular Pericytes in Vascular Cognitive Impairment and Dementia. Front. Aging Neurosci. 2020, 12, 80. [Google Scholar] [CrossRef] [Green Version]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell. Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Y.; Betz, A.L. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke 1994, 25, 1658–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.S.; Kim, S.H.; Lee, J.E.; Ahn, K.J.; Kim, D.J.; Choi, H.S.; Kim, J.; Shin, N.Y.; Lee, S.K. Effects of agmatine on blood-brain barrier stabilization assessed by permeability MRI in a rat model of transient cerebral ischemia. AJNR Am. J. Neuroradiol. 2015, 36, 283–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiel, A.; Heiss, W.D. Imaging of microglia activation in stroke. Stroke 2011, 42, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Nakata, M.; Nakagomi, T.; Maeda, M.; Nakano-Doi, A.; Momota, Y.; Matsuyama, T. Induction of Perivascular Neural Stem Cells and Possible Contribution to Neurogenesis Following Transient Brain Ischemia/Reperfusion Injury. Transl. Stroke Res. 2017, 8, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Nishie, H.; Nakano-Doi, A.; Sawano, T.; Nakagomi, T. Establishment of a Reproducible Ischemic Stroke Model in Nestin-GFP Mice with High Survival Rates. Int. J. Mol. Sci. 2021, 22, 12997. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Takagi, Y.; Kaneko, S.; Kurosawa, T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 2011, 60, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Onishi, S.; Tatsumi, Y.; Wada, K.; Yang, H.J.; Sugiura, Y.; Setou, M.; Yoshikawa, H. Sulfatide accumulation in the dystrophic terminals of gracile axonal dystrophy mice: Lipid analysis using matrix-assisted laser desorption/ionization imaging mass spectrometry. Med. Mol. Morphol. 2013, 46, 160–165. [Google Scholar] [CrossRef]
- Nakagomi, N.; Nakagomi, T.; Kubo, S.; Nakano-Doi, A.; Saino, O.; Takata, M.; Yoshikawa, H.; Stern, D.M.; Matsuyama, T.; Taguchi, A. Endothelial cells support survival, proliferation, and neuronal differentiation of transplanted adult ischemia-induced neural stem/progenitor cells after cerebral infarction. Stem. Cells 2009, 27, 2185–2195. [Google Scholar] [CrossRef]
- Yamaoka, N.; Kawasaki, Y.; Xu, Y.; Yamamoto, H.; Terada, N.; Okamura, H.; Kubo, S. Establishment of in vivo fluorescence imaging in mouse models of malignant mesothelioma. Int. J. Oncol. 2010, 37, 273–279. [Google Scholar]
- Sakuma, R.; Takahashi, A.; Nakano-Doi, A.; Sawada, R.; Kamachi, S.; Beppu, M.; Takagi, T.; Yoshimura, S.; Matsuyama, T.; Nakagomi, T. Comparative Characterization of Ischemia-Induced Brain Multipotent Stem Cells with Mesenchymal Stem Cells: Similarities and Differences. Stem. Cells Dev. 2018, 27, 1322–1338. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.Y.; Appan, D.; Chang, K.W.; Chou, C.H.; Lin, C.Y.; Gau, S.S.; Huang, H.S. Mouse hybrid genome mediates diverse brain phenotypes with the specificity of reciprocal crosses. FASEB J. 2022, 36, e22232. [Google Scholar] [CrossRef]
- Nagata, Y.; Yamagishi, M.; Konno, T.; Nakanishi, C.; Asano, Y.; Ito, S.; Nakajima, Y.; Seguchi, O.; Fujino, N.; Kawashiri, M.A.; et al. Heat Failure Phenotypes Induced by Knockdown of DAPIT in Zebrafish: A New Insight into Mechanism of Dilated Cardiomyopathy. Sci. Rep. 2017, 7, 17417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzio, E.; Badisa, R.; Mack, N.; Cassim, S.; Zdralevic, M.; Pouyssegur, J.; Soliman, K.F.A. Whole-transcriptome Analysis of Fully Viable Energy Efficient Glycolytic-null Cancer Cells Established by Double Genetic Knockout of Lactate Dehydrogenase A/B or Glucose-6-Phosphate Isomerase. Cancer Genom. Proteom. 2020, 17, 469–497. [Google Scholar] [CrossRef] [PubMed]


| Categories | GO Term | Enrichment Score by “MGs with ACs/MGs Alone” Analysis | Enrichment Score by “MGs with PCs/MGs Alone” Analysis | Enrichment Score by “MGs with ECs/MGs Alone” Analysis |
|---|---|---|---|---|
| cell proliferation | GO:0008283 | 2.93 | ND | ND |
| cell adhesion | GO:0007155 | 1.55 | ND | 2.81 |
| regulation of cell proliferation | GO:0042127 | 1.77 | ND | 2.52 |
| regulation of cell growth | GO:0001558 | 2.19 | ND | ND |
| regulation of growth | GO:0040008 | 2.02 | ND | ND |
| regulation of cell death | GO:0010941 | 1.74 | ND | 3.06 |
| regulation of apoptotic DNA fragmentation | GO:1902510 | ND | 153.2 | ND |
| regulation of cell adhesion | GO:0030155 | 1.95 | ND | 1.59 |
| regulation of cell differentiation | GO:0045595 | 1.6 | ND | 1.79 |
| positive regulation of apoptotic DNA fragmentation | GO:1902512 | ND | 229.8 | 2907.5 |
| positive regulation of cell adhesion | GO:0045785 | 4.0 | ND | ND |
| positive regulation of cell differentiation | GO:0045597 | 1.84 | ND | ND |
| negative regulation of intrinsic apoptotic signaling pathway in response to DNA damage | GO:1902230 | 10.9 | ND | ND |
| negative regulation of cell differentiation | GO:0045596 | 1.62 | ND | ND |
| Gene Symbol | Gene Name | ID | Fold Change (MGs with ACs/MGs Alone) |
|---|---|---|---|
| Gja1 | gap junction protein, alpha 1 | 1415800_at | 1130.13 |
| Cnn3 | calponin 3, acidic | 1436759_x_at | 565.68 |
| Gja1 | gap junction protein, alpha 1 | 1438945_x_at | 378.16 |
| Fstl1 | follistatin-like 1 | 1416221_at | 374.04 |
| Serpinh1 | serine (or cysteine) peptidase inhibitor, clade H, member 1 | 1450843_a_at | 248.39 |
| Gpr27 | G protein-coupled receptor 27 | 1434848_at | 240.42 |
| Bgn | biglycan | 1437889_x_at | 215.44 |
| Fstl1 | follistatin-like 1 | 1448259_at | 199.03 |
| Fscn1 | fascin homolog 1, actin bundling protein (Strongylocentrotus purpuratus) | 1416514_a_at | 183.35 |
| Gja1 | gap junction protein, alpha 1 | 1437992_x_at | 159.71 |
| Gene Symbol | Gene Name | ID | Fold Change (MGs with ACs/MGs Alone) |
|---|---|---|---|
| Csprs; Gm15433; Gm2666; Gm7609; LOC100041903; LOC100503923; LOC101055758 | component of Sp100-rs; predicted pseudogene 15433; predicted gene 2666; predicted pseudogene 7609; proteinase-activated receptor 1-like | 1435792_at | 209.5 |
| Serpinb2 | serin (or cysteine) peptidase inhibitor, clade B, member 2 | 1419082_at | 150.18 |
| Cnn3 | calponin 3, acidic | 1436759_x_at | 115.21 |
| Igfbp3 | insulin-like growth factor binding protein 3 | 1423062_at | 97.18 |
| Fstl1 | follistatin-like 1 | 1416221_at | 88.44 |
| Fscn1 | fascin homolog 1, actin bundling protein (Strongylocentrotus purpuratus) | 1416514_a_at | 72.77 |
| Serpinh1 | serin (or cysteine) peptidase inhibitor, clade H, member 1 | 1450843_a_at | 65.99 |
| Gja1 | gap junction protein, alpha 1 | 1415800_at | 59.89 |
| Cxd5 | chemokine (C-X-C motif) ligand 5 | 1419728_at | 56.8 |
| Col6a1 | collagen, type VI, alpha 1 | 1448590_at | 47.36 |
| Gene Symbol | Gene Name | ID | Fold Change (MGs with ACs/MGs Alone) |
|---|---|---|---|
| Igfbp3 | insulin-like growth factor binding protein 3 | 1423062_at | 7877.89 |
| Fscn1 | fascin homolog 1, actin bundling protein (Strongylocentrotus purpuratus) | 1416514_a_at | 1522.45 |
| Igfbp3 | insulin-like growth factor binding protein 3 | 1458268_s_at | 948.35 |
| Gja1 | gap junction protein, alpha 1 | 1415800_at | 584.05 |
| Ednrb | endothelin receptor type B | 1437347_at | 559.98 |
| Cnn3 | calponin 3, acidic | 1436759_x_at | 470.72 |
| Wwtr1 | WW domain containing transcription regulator 1 | 1417818_at | 379.8 |
| Armcx2 | armadillo repeat containing, X-linked 2 | 1456739_x_at | 347.99 |
| Gng11 | guanine nucleotide binding protein(G protein), gamma 11 | 1448942_at | 337.17 |
| Lyve1 | lymphatic vessel endothelial hyaluronan receptor 1 | 1429379_at | 301.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano-Doi, A.; Kubo, S.; Sonoda, E.; Taguchi, A.; Nakagomi, T. Different Contacted Cell Types Contribute to Acquiring Different Properties in Brain Microglial Cells upon Intercellular Interaction. Int. J. Mol. Sci. 2023, 24, 1774. https://doi.org/10.3390/ijms24021774
Nakano-Doi A, Kubo S, Sonoda E, Taguchi A, Nakagomi T. Different Contacted Cell Types Contribute to Acquiring Different Properties in Brain Microglial Cells upon Intercellular Interaction. International Journal of Molecular Sciences. 2023; 24(2):1774. https://doi.org/10.3390/ijms24021774
Chicago/Turabian StyleNakano-Doi, Akiko, Shuji Kubo, Emiko Sonoda, Akihiko Taguchi, and Takayuki Nakagomi. 2023. "Different Contacted Cell Types Contribute to Acquiring Different Properties in Brain Microglial Cells upon Intercellular Interaction" International Journal of Molecular Sciences 24, no. 2: 1774. https://doi.org/10.3390/ijms24021774
APA StyleNakano-Doi, A., Kubo, S., Sonoda, E., Taguchi, A., & Nakagomi, T. (2023). Different Contacted Cell Types Contribute to Acquiring Different Properties in Brain Microglial Cells upon Intercellular Interaction. International Journal of Molecular Sciences, 24(2), 1774. https://doi.org/10.3390/ijms24021774






